Abstract
Numerous studies indicate that morphine suppresses pain-evoked activities in both spinal and supraspinal regions. However, little is known about the effect of morphine on the basal brain activity in the absence of pain. The present study was designed to assess the effects of single-dose morphine on the spontaneous discharge of many simultaneously recorded single units, as well as their functional connections, in the lateral pain pathway, including the primary somatosensory cortex (SI) and ventral posterolateral thalamus (VPL), and medial pain pathway, including the anterior cingulate cortex (ACC) and medial dorsal thalamus (MD), in awake rats. Morphine (5mg/kg) was administered intraperitoneally before the recording. Naloxone plus morphine and normal saline injections were performed respectively as controls. The results showed that morphine administration produced significant changes in the spontaneous neuronal activity in more than one third of the total recorded neurons, with primary activation in the lateral pathway while both inhibition and activation in the medial pathway. Naloxone pretreatment completely blocked the effects induced by morphine. In addition, the correlated activities between and within both pain pathways was exclusively suppressed after morphine injection. These results suggest that morphine may play different roles in modulating neural activity in normal versus pain states. Taken together, this is the first study investigating the morphine modulation of spontaneous neuronal activity within parallel pain pathways. It can be helpful for revealing neuronal population coding for the morphine action in the absence of pain, and shed light on the supraspinal mechanisms for preemptive analgesia.
Keywords: morphine, spontaneous activity, the primary somatosensory cortex, the anterior cingulate cortex, thalamus
1. Introduction
Morphine is commonly used to treat severe pain in clinical practice. Although a number of studies have investigated morphine analgesia in peripheral and spinal nervous tissue, less has been studied at the supraspinal level. In recent years, there has been an increasing interest in the supraspinal antinociceptive mechanism of morphine. Functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) scan studies in rats have shown that systemic injection of morphine reduced formalin pain-induced cerebral metabolic increases in the anterior cingulate cortex (ACC), the primary somatosensory cortex (SI), frontal cortex, thalamus [16]. Additionally, morphine-induced decrease in glutamate concentrations has been observed in the ACC and ventral posterolateral thalamic nucleus (VPL) in naïve rats [5] or rats with inflammation [1]. Using multiple single unit recordings, recent studies have demonstrated similar results that morphine can inhibit noxious-evoked neuronal responses of higher brain centers including the ACC, SI, VPL thalamus, and medial dorsal thalamus (MD) [2,17].
In contrast to the large amount of information available on the morphine-mediated antinociceptive mechanism [2,4], little is known about the effect of single-dose morphine administration on the spontaneous brain activity in the absence of pain. It has been established that pre-injury injection of morphine produces preemptive analgesic effects in postoperative patients as well as in experimental pain models [10,15]. Thus, instead of relieving ongoing pain, the preemptive analgesia takes its effect through changing the basal activity of central network. Investigating the effects of morphine on the basal neuronal activity at supraspinal level will provide important new insights into the mechanism of preemptive analgesia.
In the present study, we recorded the neuronal activity within thalamocortical circuits in rats using a multichannel single unit recording technique. We specifically focused on four brain regions in the lateral and the medial pain systems, including the SI, ACC, VPL, and MD, to assess the effects of single-dose morphine on the spontaneous discharge of single units as well as the functional connections between these regions.
2. Materials and methods
2.1 Animals and surgery
The experiments were carried out on ten male Sprague-Dawley rats weighing 300-350 g. Animals were housed individually under a 12:12 h reversed light/dark cycle (light on at 7:00 PM) and were allowed to food and water ad libitum. Rats were handled twice a day and were acclimatized to the experimental apparatus for 30 min before each recording procedure. Experiments were conducted in accordance with the Institutional Review Board for Animal Care and Use of the Institute of Psychology, Chinese Academy of Sciences.
Animals were anaesthetized by intraperitoneal injection of ketamine (100 mg/kg, i.p.). After deeply anesthesia, rats were mounted to a Kopf stereotaxic apparatus in a flat skull position. Four arrays of eight stainless steel Teflon-insulated microwires (50-μm diameter, Biographics, Winston-Salem, NC) were stereotaxically implanted into primary somatosensory cortex (SI, 1.0 mm posterior to bregma (−1.0 A), 2.0 mm lateral to midline (L) and 2.0 mm ventral to the skull surface (V); anterior cingulate cortex (ACC, 3.2 A, 0.8 L and 2.8 V); medial dorsal thalamus (MD, −2.3 A, 0.8 L and 5.5 V); and ventral posterolateral thalamus (VPL thalamus, −3.0 A, 3.0 L and 6.0 V) based on the atlas of Paxinos and Watson. The microelectrode arrays were cemented to the skull using six anchoring screws and dental cement. Penicillin (60,000 U, i.m.) was injected before surgery to prevent infection. All rats were given at least a 1-week recovery period before electrophysiological recording began.
2.2 Electrophysiological recording
For neuronal signal recordings, rats were placed in a plastic chamber (44 × 44 × 44 cm3) in a quiet room maintained at 22 ± 1 °C and allowed to move freely during the entire recording session. Extracellular signal were acquired by the chronically implanted microwire assemblies which are connected to a preamplifier via a headstage plug and two light-weight cables. The outputs of the preamplifier were filtered (0.5 and 5 kHz, 6 dB cut-off) and sent to a multichannel spike-sorting device (Biographics, Inc) for online signal processing. Single units were isolated by setting multiple time-voltage windows using a PC-based software Magnet (Biographics, Inc.). Time stamps of these waveforms and the occurrence of treatment were then stored on a personal computer for off-line analyses. Graphical capture of waveforms, inter-spike interval histograms and autocorrelograms were used to validate the on-line sorting of single units.
2.3 Drugs and procedure
All rats received two experimental sessions: a morphine injection session (n=10) and a saline (n=6) or naloxone plus morphine (n=4) injection session. The order of injections was balanced across animals, with an interval of at least 2 days between different injections. The neuronal activities were recorded continuously for 75 minutes (−15 min ~ 60 min) before and after the drug administration.
Morphine Hydrochloride (purchased from Qinghai Pharmaceutical Factory Co., Ltd, China) was dissolved in normal saline (5 mg/ml) and injected intraperitoneally with a dose of 5 mg/kg. Naloxone Hydrochloride (4 mg/kg, Sigma) was given intraperitoneally 10 min prior to the administration of morphine.
2.4 Data analysis
Discharge of each neuron were calculated in 20-s bin, and analysis program NeuroExplorer (Plexon, Dallas, TX) was used to construct peri-event time histograms with a time range from 15 min before to 60 min after morphine administration. Then the data was exported to Matlab in spreadsheet. Firing rates in each bin were transferred to z scores according to the following formula: z = (x - m) / s, where x is the raw firing rate in the given bin, and m and s are the mean and deviation of pre-treatment basal firing rates. An increase or decrease of firing rates in a given bin over two-fold deviation of the baseline for at least five successive bins was detected as excitatory or inhibitory response. The percentage of neurons with excitatory or inhibitory response over the entire observation period was counted. Cluster analysis (k-means, SPSS) was used to sort the neuronal response patterns according to the similarities of the normalized firing rates (z scores).
Cross-correlation histograms were used to evaluate the correlation of neuronal activities with a time lag. One pair of neurons was selected, with one neuron as the reference neuron and the other as the target neuron. The time of occurrence of spikes from the reference neuron was set at 0 s and the target neuron’s firing 0.5 s before and after the reference neuron’s spike was plotted using 5-ms bin size. The significance level of the cross-correlograms was tested using 95% confidence level in the Nex program. Only peaks beyond 95% confidence limit for at least five successive bins were considered significant. Chi-square tests were used to detect the difference in percentage of correlated neuronal pairs between different treatments.
2.5 Histology
After termination of the experiment, rats were overdosed with ketamine. The recording sites were marked by electrophoretically deposited iron (10 – 20 μA DC current, 10 – 20 s duration, anode current) at the tips of selected wires. Animals were perfused through the heart with the solution of 5% potassium ferricyanide / 4% paraformaldehyde. Then the brain was sectioned at 40 μm in the coronal plane. The sections were mounted on glass slides and observed under a light microscope. The markings of recording sites were easily identified as blue dots.
3. Results
3.1 Effects of morphine on the spontaneous activity of single neurons
The numbers of simultaneously recorded neurons were depicted as follows: a total of 280 neurons in morphine-injected rats (80 ACC, 70 MD, 76 SI, 54 VPL), 161 neurons in NS-injected rats (47 ACC, 41 MD, 43 SI, 30 VPL), and 101 neurons in naloxone plus morphine-injected rats (31 ACC, 22 MD, 28 SI, 20 VPL).
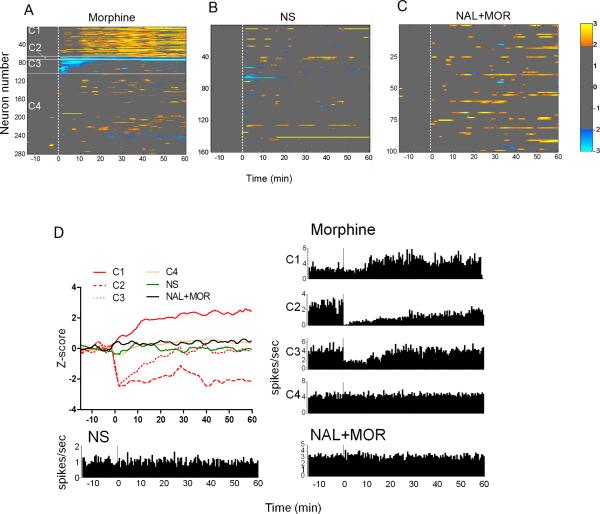
Cluster analysis revealed the response patterns of the cortical and thalamic neurons to morphine administration. As shown in Fig. 1A, morphine resulted in alternations of spontaneous activity in 37.4% of the recorded neurons (C1-C3). About 23.2% of neurons (C1) exhibited delayed excitatory responses, which started 10 min after morphine injection and lasted to the end of the observation period (60 min). In contrast, only 14.2% of neurons (C2 & C3) displayed inhibitory responses, with 3.2% responding immediately and persistently (C2) while 11.1% showing transient inhibitory responses that lasted no longer than 15 min (C3). No significant changes were found in the NS-treated condition (Fig. 1B). Naloxone, as a mu-opioid receptor antagonist, blocked the morphine-induced alterations in the spontaneous neuronal activity (Fig. 1C), leaving very few neurons with discrete excitatory responses. The population responses and single-neuron examples for each cluster and different treatments were illustrated in Fig. 1D.
Fig. 1.
The changes of spontaneous neuronal activity after administration of morphine, normal saline (NS), or naloxone plus morphine (NAL+MOR). (A) Cluster plot depicting different neuronal response patterns (C1-C4) following morphine injection. (B) & (C) Cluster plot showing the neuronal response after NS and NAL+MOR treatments. (D) Summed population data (top let) as well as time-histogram examples of individual neurons for each cluster and during different treatments. The firing rates were transferred into z-scores, as indicated by the colorbar (light yellow for highest and light blue for lowest). Clusters with z-scores > 2 were accepted as significantly excited and < -2 as significantly inhibited.
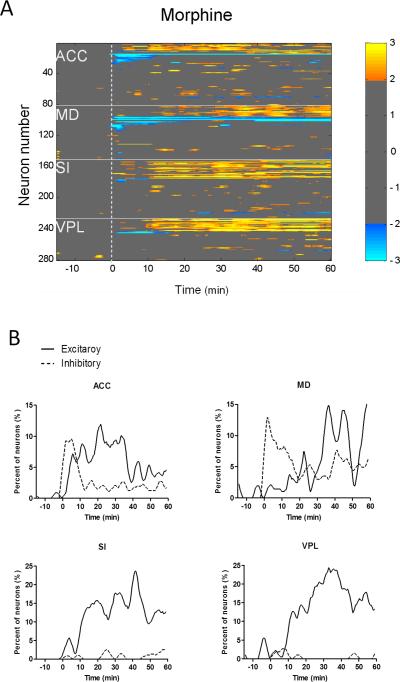
Sorting neurons in each area according to clusters showed a difference in the morphine-induced neuronal responses among different brain areas (Fig. 2A). Neurons that increased their firing rates following morphine treatment were mainly found in the lateral pain pathway (SI cortex and VPL thalamus). Interestingly, the medial pathway (ACC and MD) neurons showed both inhibitory and excitatory responses to morphine administration. Fig. 2B illustrated the percentage of neurons presenting inhibitory and excitatory responses over time. For the medial pathway (ACC and MD) neurons, inhibitory response was dominant within the first 15 min while excitatory response became evident in the 15-60 min. By contrast, the lateral pathway (SI and VPL) neurons exhibited exclusively excitatory responses during the whole 60-min observation period (Fig. 2B). These results suggest that morphine alter the resting activity of lateral and medial pathway neurons in different ways.
Fig. 2.
Temporal response patterns of medial and lateral pathway neurons following morphine administration. (A) Neurons in the medial pathway (ACC and MD) showed both excitatory and inhibitory responses to morphine administration. In contrast, the neuronal response in the lateral pathway (SI and VPL) was predominantly excitatory. (B) Percentage of neurons exhibiting excitatory or inhibitory responses to morphine administration over time. Morphine was injected at time zero.
3.2 Changes in the functional correlations between recorded regions
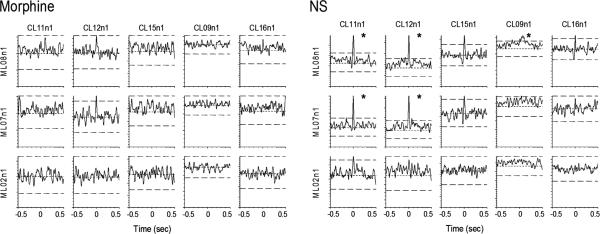
The cross-correlation analysis revealed the changes in correlated activity between neuronal pairs within the parallel pain pathways (Table 1). Morphine administration significantly weakened the thalamo-cortical functional connection within both pain pathways as compared with NS treatment (23.0% vs. 35.8% for SI-VPL correlation, P = 0.011; 1.3% vs. 5.6% for MD-ACC correlation, P = 0.011; see Table 1). In addition, the correlated neuronal activity between the medial and lateral thalamic nuclei (3.7% vs. 16.6% for MD-VPL correlation, P < 0.001) was also diminished by the single dose of morphine. Fig. 3 shows examples of cross-correlograms constructed between ACC and MD neuronal pairs during the morphine or NS sessions.
Table 1.
Comparisons of percentage of correlated neuronal pairs after morphine and NS administration
| Correlation between areas |
Morphine | NS | Total neuron pairs |
Chi-square value |
|---|---|---|---|---|
| MD and VPL | 3.7%*** | 16.6% | 163 | 14.870 |
| SI and VPL | 23.0%* | 35.8% | 165 | 6.439 |
| ACC and MD | 1.3%* | 5.6% | 233 | 6.472 |
| SI and ACC | 13.4% | 10.1% | 238 | 1.295 |
| SI and MD | 3.1% | 7.0% | 229 | 3.708 |
| ACC and VPL | 11.6% | 7.6% | 172 | 1.642 |
P < 0.05
P < 0.001, compared to the NS group, Chi-square test.
Fig. 3.
Cross-correlogram plots of eight simultaneously recorded neurons from the ACC and MD thalamus (5 ACC and 3 MD) during morphine (left) and NS (right) sessions. Five significant correlations were found during NS session (indicated by asterisks). Morphine administration eliminated the correlated activity seen in NS session.
3.3 Histological localization of recording sites
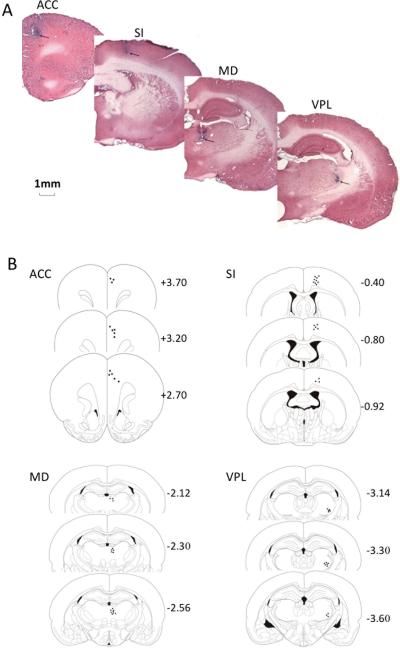
The recording sites were labeled by the iron deposits at the tips of selected microwires (Fig. 4). Electrophysiological data from electrodes that missed the targets were excluded from the analysis.
Fig. 4.
Histological verification (A) as well as schematic illustration (B) of the recording electrode tips. Arrows indicate the recording sites (blue dots) in each area.
4. Discussion
The current study was undertaken to investigate the morphine modulation of basal neuronal activity within the lateral and medial pain systems. The main findings of this study were that: (1) morphine had both increased and decreased effects on the resting neuronal activity in the parallel pain systems, with primary activation in the lateral system while inhibition and activation in the medial system; (2) morphine administration inhibited the correlated neuronal activity between and within both pain pathways.
It is well accepted that one of the mechanisms underlying morphine analgesia owes to the suppression of central neural responses to noxious stimulation. In the present study, we found that morphine induced almost exclusive activation in the sensory pathway. In other words, in contrast to the entire decrease in nociceptive-evoked neural responses under morphine analgesia, the effect of morphine on the resting neuronal activity was predominantly excitatory. Several lines of studies have elucidated the activation of morphine in the sensory pathway. For instance, increased BOLD signal in the SI and thalamus after remifentanil injection were observed in healthy humans [8]. Animal studies also showed that morphine alone produced an increase in cerebral glucose utilization of SI [14], and microinjection of morphine into SI enhanced the local spontaneous neural activity [6].
However, the neural basis and physiological implications are still unknown for the excitatory effect of morphine on the sensory thalamocortical neurons. One possible explanation is the disinhibition of sensory neurons via inhibition of GABAergic interneurons by morphine. Many in vitro and in vivo studies have shown that opiates and opioid peptides suppressed basal extracellular GABA release in regions such as cerebral cortex [9]. There is a hypothesis that the response of a population of neurons in a network is dependent not only on the characteristics of the external stimulus but also on the dynamic changes in the internal state of the network [3].Thus, it is possible that the alternation of basal neuronal activity by morphine may interfere with the patterns of neuronal response to the incoming nociceptive inputs. Further more, cortical activation induced by morphine may lead to suppressed spinal sensory transmission via descending modulation [6].
The present study also found a dual role of morphine in the medial pathway, suggesting an even more complicated mechanism. The medial pain pathway has been considered to be implicated in analgesia besides its role in processing pain unpleasantness [13]. Opioid receptors are found more prominent within the medial thalamic nuclei and the cingulate gyrus than within the lateral structures [19]. The morphine-induced activation may be limited to the neurons related to antinociceptive response, whereas inhibition occurs in neurons involved in pain processing. Nevertheless, our data contrast with some other studies which reported that morphine alone had no effect on both the spontaneous neuronal activity [20] and c-fos mRNA expression in the medial thalamus [12]. The inconsistency may be due to the methodological differences, such as awake vs. anesthetized rats or intravenous vs. intraperitoneal administration, among these studies.
Although morphine caused predominantly excitatory effect on the basal neuronal activity in the lateral and medial pathways, the correlated neuronal activity between and within both pathways was reduced following morphine injection. The decreased correlation suggests that morphine inhibit the synchronous activity within thalamocortical pain network. Enhanced synaptic transmission in ACC [18] and changes of synaptic structure in SI [7] have been observed in the experimental models of inflammatory and neuropathic pain. The fact that morphine and opioids suppressed glutamate release supports the role of morphine in blocking the formation of long-term potentiation (LTP) [11]. Given the fact that morphine pretreatment before surgery can significantly reduce post-surgery pain (i.e., preemptive analgesia) in the clinical settings, the morphine-induced inhibition of synaptic transmission may be a plausible explanation for this effect.
In summary, this is the first study using multiple single neuron recordings to investigate the morphine modulation of neuronal activity within parallel pain pathways in the absence of pain. The results showed that morphine had primary excitatory effect on the basal spontaneous neuronal activity while inhibitory effect on the inter-neuron correlations within thalamocortical pain network. This study may improve our understanding of morphine action, and shed light on the supraspinal mechanisms for the preemptive analgesia.
Research highlight.
▶ Morphine had primary excitatory effect on the basal spontaneous neuronal activity within thalamocortical pain network.
▶ Morphine altered the resting activity of lateral and medial pain pathway neurons in distinct ways.
▶ Morphine inhibited the correlated neuronal activity between and within both pain pathways
Acknowledgement
This work was funded by the Knowledge Innovation Project (KSCX2-EW-Q-18) from the Chinese Academy of Sciences to J.Y.W.; NNSF grants (30770688, 30970959, 61033011, and 31171067), the Knowledge Innovation Project (YZ200944, KSCX2-YW-R-254, and KSCX2-EW-J-8) of the Chinese Academy of Sciences, and a grant from NIH Fogarty International Center (R03 TW008038) to F.L.
Abbreviations
- ACC
the anterior cingulate cortex
- fMRI
functional magnetic resonance imaging
- LTP
long-term potentiation
- MD
medial dorsal thalamus
- PET
positron emission tomography
- SI
the primary somatosensory cortex
- VPL
ventral posterolateral thalamic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abarca C, Silva E, Sepulveda MJ, Oliva P, Contreras E. Neurochemical changes after morphine, dizocilpine or riluzole in the ventral posterolateral thalamic nuclei of rats with hyperalgesia. Eur J Pharmacol. 2000;403:67–74. doi: 10.1016/s0014-2999(00)00502-1. [DOI] [PubMed] [Google Scholar]
- [2].Abdul Aziz AA, Finn DP, Mason R, Chapman V. Comparison of responses of ventral posterolateral and posterior complex thalamic neurons in naive rats and rats with hindpaw inflammation: mu-opioid receptor mediated inhibitions. Neuropharmacology. 2005;48:607–616. doi: 10.1016/j.neuropharm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- [3].Buonomano DV, Maass W. State-dependent computations: spatiotemporal processing in cortical networks. Nat Rev Neurosci. 2009;10:113–125. doi: 10.1038/nrn2558. [DOI] [PubMed] [Google Scholar]
- [4].Chang C, Shyu BC. A fMRI study of brain activations during non-noxious and noxious electrical stimulation of the sciatic nerve of rats. Brain Research. 2001;897:71–81. doi: 10.1016/s0006-8993(01)02094-7. [DOI] [PubMed] [Google Scholar]
- [5].Hao Y, Yang JY, Guo M, Wu CF, Wu MF. Morphine decreases extracellular levels of glutamate in the anterior cingulate cortex: an in vivo microdialysis study in freely moving rats. Brain Res. 2005;1040:191–196. doi: 10.1016/j.brainres.2005.01.072. [DOI] [PubMed] [Google Scholar]
- [6].Hernandez A, Soto-Moyano R. Effect of morphine-induced cortical excitation on spinal sensory transmission. J Neurosci Res. 1986;15:217–222. doi: 10.1002/jnr.490150211. [DOI] [PubMed] [Google Scholar]
- [7].Kim SK, Kato G, Ishikawa T, Nabekura J. Phase-specific plasticity of synaptic structures in the somatosensory cortex of living mice during neuropathic pain. Mol Pain. 2011;7:87. doi: 10.1186/1744-8069-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leppa M, Korvenoja A, Carlson S, Timonen P, Martinkauppi S, Ahonen J, Rosenberg PH, Aronen HJ, Kalso E. Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage. 2006;31:661–669. doi: 10.1016/j.neuroimage.2005.12.019. [DOI] [PubMed] [Google Scholar]
- [9].Nicoll RA, Alger BE, Jahr CE. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980;287:22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- [10].Onal SA, Keles E, Toprak GC, Demirel I, Alpay HC, Avci L. Preliminary findings for preemptive analgesia with inhaled morphine: Efficacy in septoplasty and septorhinoplasty cases. Otolaryng Head Neck. 2006;135:85–89. doi: 10.1016/j.otohns.2006.02.012. [DOI] [PubMed] [Google Scholar]
- [11].Ostermeier AM, Schlosser B, Schwender D, Sutor B. Activation of mu- and delta-opioid receptors causes presynaptic inhibition of glutamatergic excitation in neocortical neurons. Anesthesiology. 2000;93:1053–1063. doi: 10.1097/00000542-200010000-00029. [DOI] [PubMed] [Google Scholar]
- [12].Panegyres PK, Hughes J. Activation of c-fos mRNA in the brain by the kappa-opioid receptor agonist enadoline and the NMDA receptor antagonist dizocilpine. Eur J Pharmacol. 1997;328:31–36. doi: 10.1016/s0014-2999(97)83023-3. [DOI] [PubMed] [Google Scholar]
- [13].Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia - Imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- [14].Quelven I, Roussin A, Zajac JM. Functional consequences of neuropeptide FF receptors stimulation in mouse: a cerebral glucose uptake study. Neuroscience. 2004;126:441–449. doi: 10.1016/j.neuroscience.2004.03.052. [DOI] [PubMed] [Google Scholar]
- [15].Reichert JA, Daughters RS, Rivard R, Simone DA. Peripheral and preemptive opioid antinociception in a mouse visceral pain model. Pain. 2001;89:221–227. doi: 10.1016/s0304-3959(00)00365-1. [DOI] [PubMed] [Google Scholar]
- [16].Tuor UI, Malisza K, Foniok T, Papadimitropoulos R, Jarmasz M, Somorjai R, Kozlowski P. Functional magnetic resonance imaging in rats subjected to intense electrical and noxious chemical stimulation of the forepaw. Pain. 2000;87:315–324. doi: 10.1016/S0304-3959(00)00293-1. [DOI] [PubMed] [Google Scholar]
- [17].Wang JY, Huang J, Chang JY, Woodward DJ, Luo F. Morphine modulation of pain processing in medial and lateral pain pathways. Mol Pain. 2009;5:60. doi: 10.1186/1744-8069-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wei F, Zhuo M. Potentiation of sensory responses in the anterior cingulate cortex following digit amputation in the anaesthetised rat. J Physiol. 2001;532:823–833. doi: 10.1111/j.1469-7793.2001.0823e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yaksh TL. Pharmacology and mechanisms of opioid analgesic activity. Acta Anaesthesiol Scand. 1997;41:94–111. doi: 10.1111/j.1399-6576.1997.tb04623.x. [DOI] [PubMed] [Google Scholar]
- [20].Yang SW, Follett KA, Piper JG, Ness TJ. The effect of morphine on responses of mediodorsal thalamic nuclei and nucleus submedius neurons to colorectal distension in the rat. Brain Research. 1998;779:41–52. doi: 10.1016/s0006-8993(97)01053-6. [DOI] [PubMed] [Google Scholar]