Abstract
Aptamers are synthetic, relatively short (e.g., 20-80 bases) RNA or ssDNA oligonucleotides that can bind targets with high affinity and specificity, similar to antibodies, because they can fold into unique, three-dimensional shapes. For use in various assays and experiments, aptamers have been conjugated with biotin or digoxigenin to form complexes with avidin or anti-digoxigenin antibodies, respectively. In this study, we developed a method to label the 5' ends of aptamers with cotinine, which allows formation of a stable complex with anti-cotinine antibodies for the purpose of providing another affinity unit for the application in biological assays using aptamers. To demonstrate the functionality of this affinity unit in biological assays, we utilized two well-known aptamers: AS1411, which binds nucleolin, and pegaptanib, which binds vascular endothelial growth factor. Cotinine-conjugated AS1411/anti-cotinine antibody complexes were successfully applied to immunoblot, immunoprecipitation, and flow cytometric analyses, and cotinine-conjugated pegaptanib/anti-cotinine antibody complexes were used successfully in enzyme immunoassays. Our results show that cotinine-conjugated aptamer/anti-cotinine antibody complexes are an effective alternative and complementary technique for aptamer use in multiple assays and experiments.
Keywords: aptamers, nucleotide; cotinine; enzyme-linked immunosorbent assay; flow cytometry; immunoblotting; immunoprecipitation
Introduction
Aptamers are synthetic, relatively short (e.g., 20-80 bases) RNA or ssDNA oligonucleotides that can fold into unique, three-dimensional shapes. Aptamers can bind targets with high affinity and specificity and were first described as affinity molecules for protein binding in 1990 (Ellington and Szostak, 1990; Tuerk and Gold, 1990). Following the development of the SELEX (systematic evolution of ligands by exponential enrichment) method, the isolation of aptamers specific to various targets has become more efficient and easier to perform (Oguro et al., 2003; Miyakawa et al., 2006; Ohuchi et al., 2006). Aptamers can form stable and specific complexes with a wide variety of targets, including low molecular compounds such as amino acids (Harada and Frankel, 1995; Yang et al., 1996) and complex protein targets such as cell membrane proteins (Ulrich et al., 1998; Homann and Goringer, 1999; Blank et al., 2001; Ulrich et al., 2002; Guo et al., 2006). Therefore, aptamers have been used in a variety of methods in which antibodies are commonly used, such as in enzyme immunoassays, immunoprecipitation analyses, flow cytometric analyses (Ireson and Kelland, 2006; Ferreira et al., 2008; Sakai et al., 2008), protein microarrays (Chen et al., 2008), magnetic-separation assays (Gao et al., 2007), lateral flow assays (Liu et al., 2007; Shaikh et al., 2007), and biosensor experiments (Backmann et al., 2005; Borisov and Wolfbeis, 2008). For use in such applications, aptamers can be either conjugated to beads or surfaces, or labeled with enzymes or fluorescent dyes. However, because the cross-linking conditions to one enzyme, dye, or sensor cannot be applied to other targets, the determination of specific conditions for aptamer cross-linking to multiple enzymes, dyes, or sensors is a time-consuming process. Therefore, labeling of aptamers with biotin to produce complexes with avidin, streptavidin, or neutravidin in various cross-linked forms has been commonly employed when an aptamer is to be applied to multiple assays (Murphy et al., 2003; Baldrich et al., 2005; Li et al., 2009; Tanaka et al., 2009). Additionally, aptamers have been labeled with digoxigenin to produce complexes with anti-digoxigenin antibodies (Ramos et al., 2007, 2010).
In this report, we introduce cotinine-conjugated aptamer/anti-cotinine antibody complexes as an alternative and complementary platform for the use of aptamers in biological assays. We utilized two well-known aptamers: AS1411 that binds nucleolin (Bates et al., 1999; Dapic et al., 2002, 2003) and pegaptanib that binds vascular endothelial growth factor (VEGF) (Ruckman et al., 1998; Ng and Adamis, 2006). Cotinine-conjugated AS1411/anti-cotinine antibody complexes were successfully applied to immunoblot, immunoprecipitation, and flow cytometric analyses, and cotinine-conjugated pegaptanib/anti-cotinine antibody complexes were successfully used in enzyme immunoassays.
Results
Binding of AS1411-cotinine/anti-cotinine antibody complexes to cell-surface nucleolin
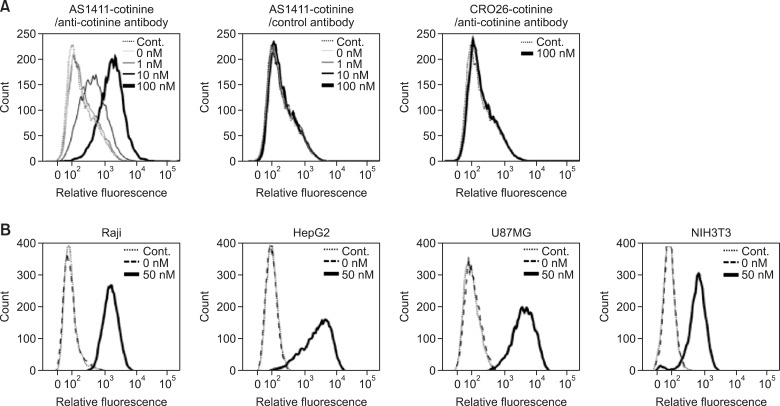
To assess whether AS1411-cotinine/anti-cotinine antibody complexes (Figures 1, 2) bind to nucleolin on cell surfaces, Raji cells were incubated with AS1411-cotinine/anti-cotinine antibody complexes and FITC-labeled anti-human IgG antibodies. With the concentration of anti-cotinine antibody fixed at 100 nM, cotinine-conjugated AS1411 at concentrations of 1, 10, and 100 nM bound to the cell surface in a dose-dependent manner (Figure 3A). As an IgG molecule, an anti-cotinine antibody contains two paratopes and can form a complex with two molecules of AS1411-cotinine. When either CRO26 (Figure 2) or an isotype control for anti-cotinine antibody was used instead of AS1411 or anti-cotinine antibody, respectively, binding of the complex was not observed. CRO26, the negative control of AS1411, is an oligonucleotide in which each dG of AS1411 is replaced by dC, which blocks both the formation of a stable quadruplex structure and nucleolin binding (Soundararajan et al., 2009).
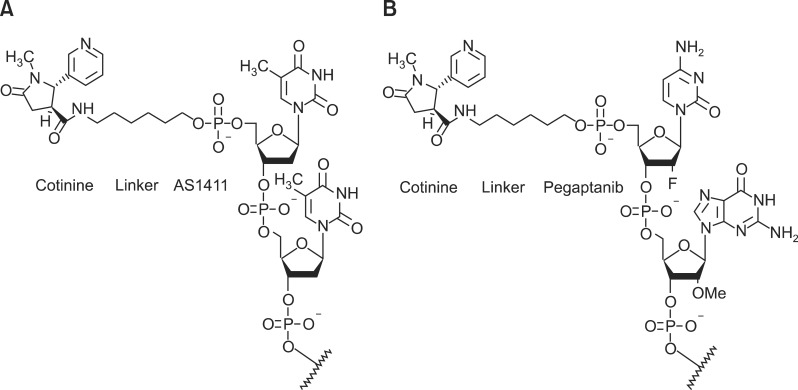
Figure 1.
Aptamers conjugated to cotinine with a linker. (A) AS1411-cotinine. (B) Pegaptanib-cotinine.
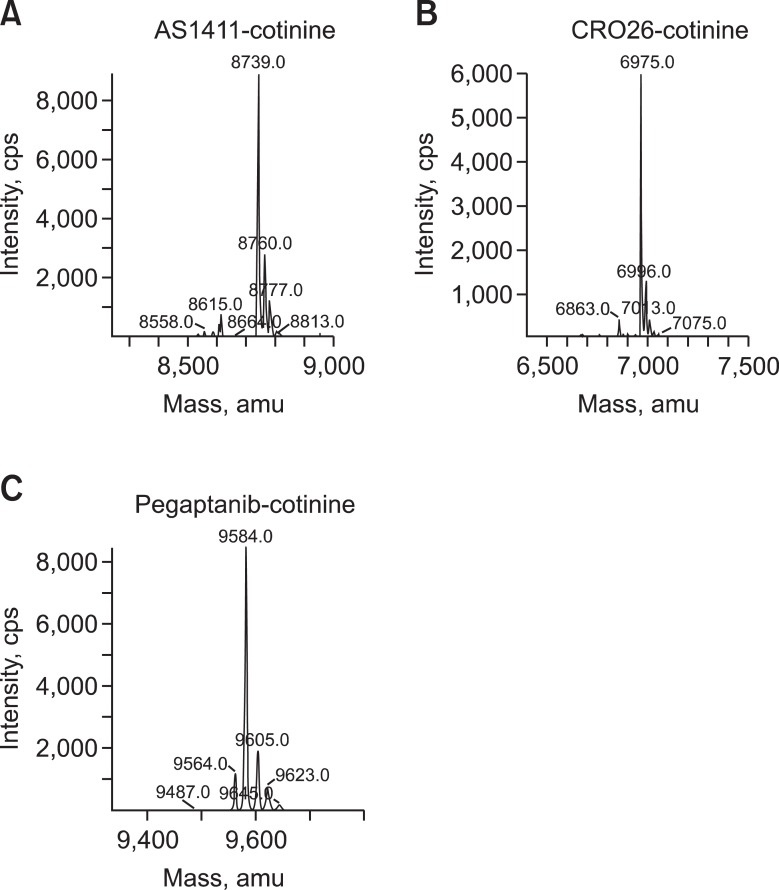
Figure 2.
Mass spectroscopy data of aptamer-conjugated cotinine. The quality of AS1411-cotinine (A), CRO26-cotinine (B), and pegaptanib-cotinine (C) was analyzed using an ion-trap mass spectrometer through electrospray ionization (ESI-IT/MS).
Figure 3.
Flow cytometric analysis with AS1411-cotinine/anti-cotinine antibody complexes. (A) Raji cells were incubated with complexes containing the indicated concentrations of AS1411-cotinine and 100 nM anti-cotinine antibody and subsequently stained with FITC-conjugated anti-human Fc antibody. As a control, CRO26-cotinine and palivizumab were used in place of AS1411-cotinine and anti-cotinine antibody, respectively. Control signal was obtained from cells incubated only with FITC-conjugated anti-human Fc antibody. (B) Raji, HepG2, U87MG, and NIH3T3 cells were incubated with AS1411-cotinine/anti-cotinine antibody complexes and subsequently stained with FITC-conjugated anti-human Fc antibody.
We then performed flow cytometric analysis with AS1411-cotinine/anti-cotinine antibody complexes using three additional cell lines that were reported previously to possess different cell surface expression levels of nucleolin (Semenkovich et al., 1990; Hanakahi et al., 1997; Masumi et al., 2006). With 50 nM AS1411-cotinine and 100 nM anti-cotinine antibody, the complex showed stronger binding to HepG2 and U87MG cells and weaker binding to NIH3T3 cells compared to Raji cells (Figure 3B).
AS1411-cotinine/anti-cotinine antibody complex recognition of denatured nucleolin
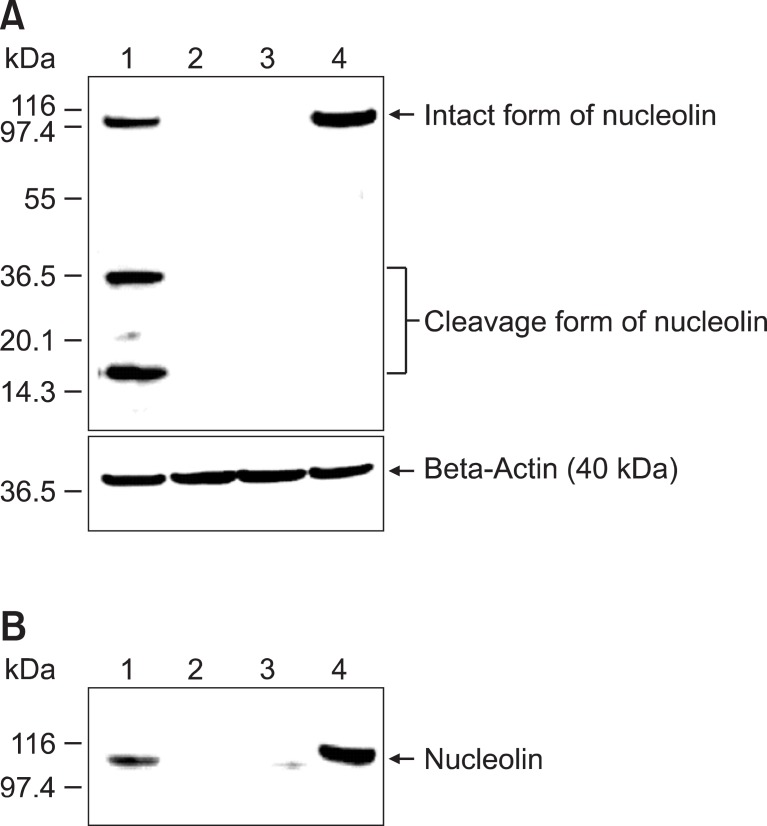
To determine whether AS1411-cotinine/anti-cotinine antibody complexes recognize the denatured form of nucleolin, we performed immunoblot analyses (Figure 4A). Raji cell lysate (50 µg) was subjected to SDS-PAGE, proteins were transferred to a nitrocellulose membrane, and the membrane was incubated sequentially with AS1411-cotinine/anti-cotinine antibody complexes, HRP-conjugated anti-human IgG antibody, and chemiluminescent substrate solution, with intermittent washing with TBST. AS1411-cotinine/anti-cotinine antibody complexes reacted not only to full-length nucleolin (105 kDa) but also to lower molecular mass forms of nucleolin (<40 kDa) that have been previously reported to be generated by nucleolin autolytic activity (Figure 4A) (Chen et al., 1991; Fang and Yeh, 1993). In contrast, mouse anti-nucleolin antibody reacted only to full-length nucleolin. When either CRO26 or palivizumab was used instead of AS1411 or anti-cotinine antibody, respectively, no bands were visualized.
Figure 4.
Immunoblot analysis and immunoprecipitation of nucleolin with AS1411-cotinine/anti-cotinine antibody complexes. (A) Immunoblot analysis. Raji cell lysates were subjected to 4-12% SDS-PAGE, and proteins were transferred onto a nitrocellulose membrane. The membrane was probed with AS1411-cotinine/anti-cotinine antibody complexes (lane 1), AS1411-cotinine/control antibody complexes (lane 2), CRO26-cotinine/anti-cotinine antibody complexes (lane 3), and mouse anti-nucleolin antibody (lane 4). HRP-conjugated mouse anti-human IgG, HRP-conjugated rabbit anti-mouse IgG antibody, and SuperSignal Pico West chemiluminescent substrate were used for visualization of the bands. (B) Immunoprecipitation. Raji cell lysates were incubated with AS1411-cotinine/anti-cotinine antibody complexes (lane 1), AS1411-cotinine/control antibody complexes (lane 2), and CRO26-cotinine/anti-cotinine antibody complexes (lane 3). All lysate mixtures were incubated with immobilized protein A beads, and precipitated proteins were fractionated by SDS-PAGE and probed with mouse anti-nucleolin antibody. In lane 4, the cell lysate was directly loaded onto the gel and probed with mouse anti-nucleolin antibody.
AS1411-cotinine/anti-cotinine antibody complex immunoprecipitation of nucleolin
Raji cell lysate was incubated with AS1411-cotinine/anti-cotinine antibody complexes overnight. Complexes were then immunoprecipitated using protein A beads and subjected to SDS-PAGE. After the proteins were transferred to a nitrocellulose membrane, immunoblot analysis was performed using anti-nucleolin antibody. A protein band with a molecular weight of 105 kDa was visualized, confirming that AS1411-cotinine/anti-cotinine antibody complexes successfully immunoprecipitated nucleolin from Raji cell lysates (Figure 4B). However, when either CRO26 or palivizumab was used instead of AS1411 or anti-cotinine antibody, respectively, no bands were visualized.
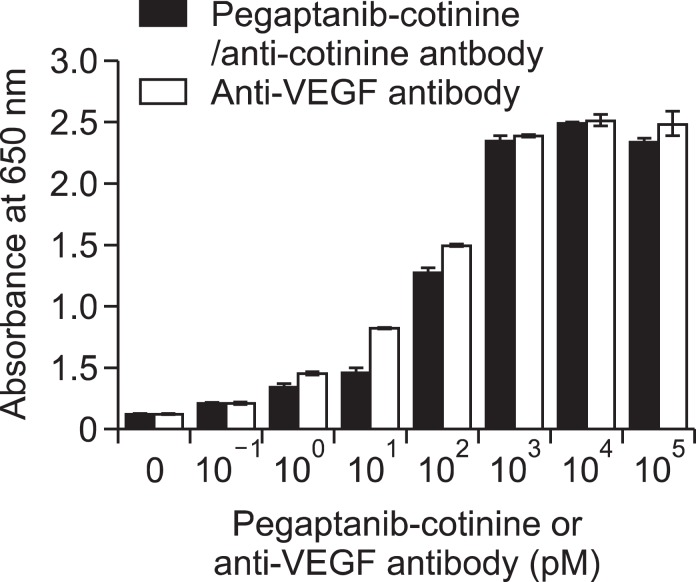
Specific binding of pegaptanib-cotinine/anti-cotinine antibody complexes to VEGF165
To verify whether pegaptanib-cotinine/anti-cotinine antibody complexes (Figures 1, 2) can bind to immobilized VEGF165 on a microtiter plate, we performed an enzyme immunoassay using a VEGF165-coated microtiter plate, cotinine-conjugated pegaptanib/anti-cotinine antibody complexes, and HRP-conjugated anti-human IgG antibody. Cotinine-conjugated pegaptanib/anti-cotinine antibody complexes bound to VEGF165 on the plate in a dose-dependent manner from 10-1 to 103 pM (Figure 5). In a parallel experiment, bevacizumab showed dose-dependent binding to VEGF165.
Figure 5.
Enzyme immunoassay analysis using pegaptanib-cotinine/anti-cotinine antibody complexes. Pegaptanib-cotinine/anti-cotinine antibody complexes were allowed to react with VEGF165 immobilized on a microtiter plate. The concentration of anti-cotinine antibody was maintained as one-half that of pegaptanib-cotinine as the antibody is bivalent. Bevacizumab was used as a positive control. HRP-conjugated rabbit anti-human IgG antibody and 3,3',5,5'-tetramethylbenzidine substrate solution were employed to determine the amount of complex bound to the microtiter plate. The results are expressed as the mean ± standard deviation of triplicate measurements.
Discussion
For application of aptamers in multiple assays and experiments, biotin labeling has been the most commonly adopted option to avoid the need to develop optimal aptamer cross-linking conditions for multiple enzymes, dyes, or sensors individually (Murphy et al., 2003; Baldrich et al., 2005; Li et al., 2009; Tanaka et al., 2009). Because biotin is stable and small (molecular weight of 244.31 kDa), it rarely interferes with the function of labeled molecules. The avidin-biotin detection system allows an aptamer to be easily captured, recovered, immobilized, or detected with a limited number of secondary detection reagents generated by modifying avidin, streptavidin, or neutravidin. A major limitation of this system is that biotin, as vitamin B7, is present in small amounts in all living cells and participates in many biological processes including cell growth and the citric acid cycle (Bender, 1999). Biotin is especially abundant in tissues such as brain, liver, and blood, and endogenous biotin can cause considerable background noise in assays based on biotin binding (Ramos-Vara, 2005).
Digoxigenin also has been used to label aptamers for use in biological assays (Ramos et al., 2007, 2010). It is a steroid with a low molecular weight of 390.51 Da that is found exclusively in the flowers and leaves of plants such as Digitalis purpuea, Digitalis orientalis, and Digitalis lanata. Additionally, digoxigenin is a hapten with high immunogenicity (Holtke et al., 1995). It also has served as a standard immunohistochemical marker for in situ hybridization (Hauptmann and Gerster, 1994). For labeling of atpamers, digoxigenin can be conjugated to a nucleotide triphosphate, and the labeled nucleotide triphosphate is then used in aptamer synthesis. The resulting digoxigenin-labeled aptamer can form a complex with anti-digoxigenin antibody for applications in assays and experiments.
In this study, we aimed to develop an additional hapten-labeled aptamer and anti-hapten antibody system that can be applied to develop multiplex aptamer assays. Based on several of its characteristics, we selected and tested cotinine as a candidate hapten for labeling aptamers. In a previous study, we showed that anti-cotinine antibody can be generated by immunization of animals with cotinine-conjugated carrier proteins (Park et al., 2010). Cotinine is the major metabolite of nicotine found in tobacco smoke, and cotinine-labeled horseradish peroxidase is sufficiently stable to give consistent results in enzyme immunoassays (Park et al., 2010). Because cotinine has a low molecular weight of 176.22 Da, we hypothesized that it would not alter the function of an aptamer upon conjugation. Both cotinine-conjugated AS1411 and cotinine-conjugated pegaptanib retained their reactivity to their original targets in this study. As both cotinine and its binding molecules are not present physiologically in animal and human tissues, minimal background signal was expected. As shown in Figure 4, the AS1411-cotinine/anti-cotinine antibody complex recognized denatured nucleolin with minimal background.
Taken together, our results demonstrate that cotinine-conjugated aptamer/anti-cotinine antibody complexes can be used in applications such as flow cytometric, immunoblot, immunoprecipitation, and enzyme immunoassay analyses, providing a viable alternative for employing cotinine-labeled aptamers in multiple assays and experiments, alone or in combination with biotin- and/or digoxigenin-labeled aptamers. Additionally, the stability of the complex and successful retention of aptamer reactivity make this system suitable for potential in vivo application.
Methods
Preparation of aptamer-cotinine conjugates
The aptamers used in this study were AS1411, 5'-d(GGTGGTGGTGGTTGTGGTGGTGGTGG)-3', an inactive control aptamer (CRO26), 5'-d(CCTCCTCCTCCTTCTCCTCCTCCTCC)-3', and pegaptanib, 5'-pCfpGmpGmpArpArpUfpCfpAmpGmpUfpGmpAmpAmpUfpGmpCfpUfpUfpAmpUfpAmpCfpAmpUfpCfpCfpGm-3'-p-dT. These aptamers were synthesized with an amino C6 linker at the 5'-terminus by ST Pharm Co. (Seoul, South Korea). All the aptamers were conjugated to cotinine using the active ester method as described previously (Park et al., 2010), purified to homogeneity (i.e., >95% purity) in reversed-phase high-pressure liquid chromatography with an Xbridge Prep C18 column (5 µm, 10 × 150 mm, Waters Corp., Milford, MA). The quality of conjugated aptamers was analyzed with an ion-trap mass spectrometer through electrospray ionization (ESI-IT/MS) by Postech Aptamer Initiative (Pohang, South Korea). AS1411-cotinine and CRO26-cotinine conjugates were dissolved in water; pegaptanib-cotinine conjugates were dissolved in diethyl pyrocarbonate-treated water. All aptamer-cotinine conjugates were aliquoted and stored at -20℃. Before use, all the aptamers were denatured at 95℃ for 5 min and slowly cooled to 25℃ over 30 min.
Antibodies
Mouse anti-nucleolin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Palivizumab (Synagis, Abbot Laboratories, Kent, UK) and bevacizumab (Avastin, Genentech Inc, South San Francisco, CA) were used as control antibodies. Fluorescein isothiocyanate (FITC)- and horseradish peroxidase (HRP)-conjugated anti-human Fc antibodies were purchased from Thermo Fisher Scientific (Rockford, IL).
The recombinant rabbit/human chimeric anti-cotinine antibody used in this study was originally generated through a form of scFv for use in an enzyme immunoassay for detecting cotinine in the biological fluids of smokers (Park et al., 2010). For the construction of an expression vector for anti-cotinine IgG, the genes encoding the variable regions of the heavy chain (VH) and light chain (VL) were amplified from an anti-cotinine scFv-Fc expression vector using 5'-ATCCTGTTCCTGGTGGCCACCGCCACCGGCCAGTCGGTGAAGGAGTCC-3' and 5'-ATCCTGTTCCTGGTGGCCACCGCCACCGGCGAGCTCGATCTGACCCAG-3' as the 5' primers and 5'-TGAAGAGATGGTGACCAGGGTGCC-3' and 5'-TAGGATCTCCAGCTCGGTCCCTCC-3' as the 3' primers, respectively (Park et al., 2010). Human VH constant region (CH1-CH3) and human VL constant region (Cκ) were amplified from a human bone marrow cDNA library (Clontech Laboratories, Inc., Palo Alto, CA) using 5'-GTCACCATCTCTTCAGCCTCCACCAAGGGC-3' and 5'-GAGCTCGGATCCCTTGCCGGCCGT-3' as the 5' primers and 5'-GAGCTGGAGATCCTACGGACCGTGGCCGCC-3' and 5'-GCAAGCTCTAGACTAGCACTCGCC-3' as the 3' primers, which contain an annealing site for both VH and VL. Overlap extension polymerase chain reaction (PCR) was performed using 5'-ACATCGGCTAGCCGCCACCATGGGCTGGTCCTGCATCATCCTGTTCCTG-3' and 5'-ACTTAAGCTTGCGCCACCATGGGCTGGTCCTGCATCATCCTGTTCCTG-3' as the 5' primers and 5'-GAGCTCGGATCCCTTGCCGGCCGT-3' and 5'-GCAAGCTCTAGACTAGCACTCGCC-3' as the 3' primers to generate genes encoding the complete VH and VL fragments, respectively. The complete VH and VL DNAs were digested, respectively, with BamHI and NheI and with HindIII and XbaI (New England Biolabs Inc., Beverly, MA) and inserted into an expression vector designed for mammalian secretion of full-length IgGs. The vector encoding anti-cotinine IgG was used to transfect CHO DG44 cells (Invitrogen, Carlsbad, CA) as described previously (Trill et al., 1995). Overexpressed anti-cotinine IgG was purified by protein A-gel affinity chromatography according to the manufacturer's instructions (Repligen Corp., Cambridge, MA).
Cell culture
Adherent CHO DG44 cells were cultivated at 37℃ under an atmosphere of 95% air and 5% CO2 in CD DG44 media (GIBCO, Invitrogen) containing 100 U/ml penicillin and 100 g/ml streptomycin, and supplemented with 10 µM hypoxanthine, 1.6 µM thymidine, 8 mM L-glutamine, and 18 mg/L Pluronic F-68 (GIBCO).
Raji (human Burkitt's lymphoma), HepG2 (human hepatocellular carcinoma), U87MG (human glioblastoma), and NIH3T3 (mouse embryonic fibroblast) cells were obtained from American Type Culture Collection. Cells were grown in RPMI 1640 (GIBCO) culture media containing 10% fetal bovine serum (FBS; GIBCO), 100 U/ml penicillin, and 100 g/ml streptomycin at 37℃ in 5% CO2.
Flow cytometric analysis
Raji, HepG2, U87MG, and NIH3T3 cells (1 × 105 cells/ml) were resuspended in 100 µl flow cytometric assay buffer [1% FBS and 0.02% sodium azide in phosphate-buffered saline (PBS)] and incubated with the indicated concentrations of AS1411-cotinine and 100 nM anti-cotinine antibody at 4℃ for 20 min. As a control, CRO26-cotinine and palivizumab were used in place of AS1411-cotinine and anti-cotinine antibody, respectively. After washing twice with flow cytometric assay buffer, cells were incubated with FITC-labeled anti-human IgG (Thermo Fisher Scientific) at 4℃ for 15 min and washed again with flow cytometric assay buffer. The cells were fixed with PBS containing 2% paraformaldehyde; fluorescence intensity was measured using FACSCanto™ II (BD Bioscience, Heidelberg, Germany) and analyzed with FlowJo data analysis software (Treestar, Ashland, OR).
Immunoblot analysis
Raji cells were harvested by centrifugation at 168 g for 3 min at 4℃ and then washed three times with PBS. The pellet was resuspended in 1 ml lysis buffer (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 0.25 mM synthetic dextrose complete medium, 1 mM PMSF, 1 µg/ml aprotinin, 1 µg/ml leupeptin, and 1 µg/ml pepstatin A) and sonicated for three rounds, 10 s each at an output setting of 7 (Sonic Dismembrator model 500, Thermo Fisher Scientific). The sonicated samples were cleared by centrifugation for 10 min at 17,000 g, and the amount of protein in the supernatants was measured by Bradford assay (Bio-Rad, Hercules, CA). The lysate (50 µg) was dissolved in 4 × SDS loading buffer (50 mM MES, 50 mM Tris-base, 0.1% SDS, 1 mM EDTA, and 50 mM dithiothreitol, pH 7.3) and separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) using 4-12% Bis-Tris gel (Invitrogen) followed by transfer onto a nitrocellulose membrane (Whatman, Dassel, Germany) using an XCell SureLock™ Novex Mini-Cell (Invitrogen) at 40 V for 60 min. The membrane was pre-incubated in TBST (10 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% Tween-20) containing 5% non-fat milk (BD Biosciences Diagnostic Systems, Sparks, MD) at room temperature for 30 min and then incubated with 100 nM AS1411-cotinine/50 nM anti-cotinine antibody complexes, 100 nM AS1411-cotinine/50 nM control antibody complexes, 100 nM CRO26-cotinine/50 nM anti-cotinine antibody complexes, or a 1:100 dilution of mouse anti-nucleolin antibody (Santa Cruz Biotechnology) at room temperature for 2 h. After the membrane was washed three times with TBST, it was incubated with either HRP-conjugated rabbit anti-human IgG antibody (Thermo Fisher Scientific) or HRP-conjugated anti-mouse IgG antibody (Thermo Fisher Scientific) diluted 1:5,000 in TBST at room temperature for 1 h. The membrane was washed three times with TBST, and protein bands were visualized by the addition of SuperSignal Pico West chemiluminescent substrate (Thermo Fisher Scientific) following the manufacturer's instructions.
Oligonucleotide immunoprecipitation
The Raji cell lysate (1 mg protein in 1 ml) was incubated with 40 nM AS1411-cotinine/20 nM anti-cotinine antibody complexes, 40 nM CRO26-cotinine/20 nM anti-cotinine antibody complexes, or 40 nM AS1411-cotinine/20 nM control antibody complexes at 4℃ overnight on an end-over-end rotator. Protein A-sepharose beads (40 µl, Repligen) were added to the lysate mixture and incubated with rotation for 2 h at 4℃. After centrifugation at 800 g for 1 min, the immunoprecipitates were washed three times with wash buffer (20 mM Tris-Cl, pH 7.5, 150 mM NaCl, and 1% Triton X-100), resuspended in 4 × SDS loading buffer, and denatured at 95℃ for 10 min. All samples were analyzed SDS-PAGE using 4-12% Bis-Tris gel and transferred onto a nitrocellulose membrane. The membrane was blocked with 5% nonfat milk in TBST and then treated with mouse anti-nucleolin IgG (1:100; Santa Cruz Biotechnology). After the membrane was washed three times with TBST, it was incubated with HRP-conjugated rabbit anti-mouse IgG diluted 1:5,000 in TBST at room temperature for 1 h. The membrane was washed three times with TBST, and protein bands were visualized by the addition of the SuperSignal Pico West chemiluminescent substrate (Thermo Fisher Scientific) following the manufacturer's instructions.
Enzyme immunoassays
The wells of microtiter plates (Corning Costar Corp., Cambridge, MA) were coated by the addition of 50 ng human VEGF165 (R&D Systems, Minneapolis, MN) dissolved in 20 µl PBS to each well for an overnight incubation at 4℃. After washing with PBS, the wells were incubated with 150 µL PBS containing 3% bovine serum albumin (BSA) for 2 h and then washed with PBS. Subsequently, both the 100 nM pegaptanib-cotinine/50 nM anti-cotinine antibody complex and 100 nM bevacizumab were serially diluted 10-fold in PBS containing 3% BSA and added to each well. The plates were incubated for 1 h at room temperature and then washed five times with 0.05% Tween 20 in PBS (PBST). Subsequently, a 50-µl aliquot of HRP-conjugated rabbit anti-human IgG (Thermo Fisher Scientific), diluted 1:5,000 in PBS with 3% BSA, was added to each well and incubated for 1 h at room temperature. After washing four times with 0.05% PBST, peroxidase activity was detected by the addition of 50 µl 3,3',5,5'-tetramethylbenzidine substrate solution (Thermo Fisher Scientific) to each well. The plates were incubated for 10 min at room temperature, and the absorbance at 650 nm was measured using a Multiskan Ascent instrument (Labsystems, Helsinki, Finland).
Acknowledgements
This work was supported by a grant 2011-0022972 from Ministry of Education, Science and Technology, Republic of Korea.
References
- 1.Backmann N, Zahnd C, Huber F, Bietsch A, Pluckthun A, Lang HP, Guntherodt HJ, Hegner M, Gerber C. A label-free immunosensor array using single-chain antibody fragments. Proc Natl Acad Sci USA. 2005;102:14587–14592. doi: 10.1073/pnas.0504917102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baldrich E, Acero JL, Reekmans G, Laureyn W, O'Sullivan CK. Displacement enzyme linked aptamer assay. Anal Chem. 2005;77:4774–4784. doi: 10.1021/ac0502450. [DOI] [PubMed] [Google Scholar]
- 3.Bates PJ, Kahlon JB, Thomas SD, Trent JO, Miller DM. Antiproliferative activity of G-rich oligonucleotides correlates with protein binding. J Biol Chem. 1999;274:26369–26377. doi: 10.1074/jbc.274.37.26369. [DOI] [PubMed] [Google Scholar]
- 4.Bender DA. Optimum nutrition: thiamin, biotin and pantothenate. Proc Nutr Soc. 1999;58:427–433. doi: 10.1017/s0029665199000567. [DOI] [PubMed] [Google Scholar]
- 5.Blank M, Weinschenk T, Priemer M, Schluesener H. Systematic evolution of a DNA aptamer binding to rat brain tumor microvessels. selective targeting of endothelial regulatory protein pigpen. J Biol Chem. 2001;276:16464–16468. doi: 10.1074/jbc.M100347200. [DOI] [PubMed] [Google Scholar]
- 6.Borisov SM, Wolfbeis OS. Optical biosensors. Chem Rev. 2008;108:423–461. doi: 10.1021/cr068105t. [DOI] [PubMed] [Google Scholar]
- 7.Chen CM, Chiang SY, Yeh NH. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem. 1991;266:7754–7758. [PubMed] [Google Scholar]
- 8.Chen Z, Tabakman SM, Goodwin AP, Kattah MG, Daranciang D, Wang X, Zhang G, Li X, Liu Z, Utz PJ, Jiang K, Fan S, Dai H. Protein microarrays with carbon nanotubes as multicolor Raman labels. Nat Biotechnol. 2008;26:1285–1292. doi: 10.1038/nbt.1501. [DOI] [PubMed] [Google Scholar]
- 9.Dapic V, Bates PJ, Trent JO, Rodger A, Thomas SD, Miller DM. Antiproliferative activity of G-quartet-forming oligonucleotides with backbone and sugar modifications. Biochemistry. 2002;41:3676–3685. doi: 10.1021/bi0119520. [DOI] [PubMed] [Google Scholar]
- 10.Dapic V, Abdomerovic V, Marrington R, Peberdy J, Rodger A, Trent JO, Bates PJ. Biophysical and biological properties of quadruplex oligodeoxyribonucleotides. Nucleic Acids Res. 2003;31:2097–2107. doi: 10.1093/nar/gkg316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 12.Fang SH, Yeh NH. The self-cleaving activity of nucleolin determines its molecular dynamics in relation to cell proliferation. Exp Cell Res. 1993;208:48–53. doi: 10.1006/excr.1993.1221. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira CS, Papamichael K, Guilbault G, Schwarzacher T, Gariepy J, Missailidis S. DNA aptamers against the MUC1 tumour marker: design of aptamer-antibody sandwich ELISA for the early diagnosis of epithelial tumours. Anal Bioanal Chem. 2008;390:1039–1050. doi: 10.1007/s00216-007-1470-1. [DOI] [PubMed] [Google Scholar]
- 14.Gao L, Zhuang J, Nie L, Zhang J, Zhang Y, Gu N, Wang T, Feng J, Yang D, Perrett S, Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 15.Guo KT, SchAfer R, Paul A, Gerber A, Ziemer G, Wendel HP. A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific DNA aptamers. Stem Cells. 2006;24:2220–2231. doi: 10.1634/stemcells.2006-0015. [DOI] [PubMed] [Google Scholar]
- 16.Hanakahi LA, Dempsey LA, Li MJ, Maizels N. Nucleolin is one component of the B cell-specific transcription factor and switch region binding protein, LR1. Proc Natl Acad Sci USA. 1997;94:3605–3610. doi: 10.1073/pnas.94.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada K, Frankel AD. Identification of two novel arginine binding DNAs. EMBO J. 1995;14:5798–5811. doi: 10.1002/j.1460-2075.1995.tb00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauptmann G, Gerster T. Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos. Trends Genet. 1994;10:266. doi: 10.1016/0168-9525(90)90008-t. [DOI] [PubMed] [Google Scholar]
- 19.Höltke HJ, Ankenbauer W, Muhlegger K, Rein R, Sagner G, Seibl R, Walter T. The digoxigenin (DIG) system for non-radioactive labelling and detection of nucleic acids--an overview. Cell Mol Biol (Noisy-le-grand) 1995;41:883–905. [PubMed] [Google Scholar]
- 20.Homann M, Goringer HU. Combinatorial selection of high affinity RNA ligands to live African trypanosomes. Nucleic Acids Res. 1999;27:2006–2014. doi: 10.1093/nar/27.9.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ireson CR, Kelland LR. Discovery and development of anticancer aptamers. Mol Cancer Ther. 2006;5:2957–2962. doi: 10.1158/1535-7163.MCT-06-0172. [DOI] [PubMed] [Google Scholar]
- 22.Li N, Ebright JN, Stovall GM, Chen X, Nguyen HH, Singh A, Syrett A, Ellington AD. Technical and biological issues relevant to cell typing with aptamers. J Proteome Res. 2009;8:2438–2448. doi: 10.1021/pr801048z. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Lin YY, Wang J, Wu H, Wai CM, Lin Y. Disposable electrochemical immunosensor diagnosis device based on nanoparticle probe and immunochromatographic strip. Anal Chem. 2007;79:7644–7653. doi: 10.1021/ac070691i. [DOI] [PubMed] [Google Scholar]
- 24.Masumi A, Fukazawa H, Shimazu T, Yoshida M, Ozato K, Komuro K, Yamaguchi K. Nucleolin is involved in interferon regulatory factor-2-dependent transcriptional activation. Oncogene. 2006;25:5113–5124. doi: 10.1038/sj.onc.1209522. [DOI] [PubMed] [Google Scholar]
- 25.Miyakawa S, Oguro A, Ohtsu T, Imataka H, Sonenberg N, Nakamura Y. RNA aptamers to mammalian initiation factor 4G inhibit cap-dependent translation by blocking the formation of initiation factor complexes. RNA. 2006;12:1825–1834. doi: 10.1261/rna.2169406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy MB, Fuller ST, Richardson PM, Doyle SA. An improved method for the in vitro evolution of aptamers and applications in protein detection and purification. Nucleic Acids Res. 2003;31:e110. doi: 10.1093/nar/gng110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng EW, Adamis AP. Anti-VEGF aptamer (pegaptanib) therapy for ocular vascular diseases. Ann N Y Acad Sci. 2006;1082:151–171. doi: 10.1196/annals.1348.062. [DOI] [PubMed] [Google Scholar]
- 28.Oguro A, Ohtsu T, Svitkin YV, Sonenberg N, Nakamura Y. RNA aptamers to initiation factor 4A helicase hinder cap-dependent translation by blocking ATP hydrolysis. RNA. 2003;9:394–407. doi: 10.1261/rna.2161303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohuchi SP, Ohtsu T, Nakamura Y. Selection of RNA aptamers against recombinant transforming growth factor-beta type IIIreceptor displayed on cell surface. Biochimie. 2006;88:897–904. doi: 10.1016/j.biochi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Park S, Lee DH, Park JG, Lee YT, Chung J. A sensitive enzyme immunoassay for measuring cotinine in passive smokers. Clin Chim Acta. 2010;411:1238–1242. doi: 10.1016/j.cca.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 31.Ramos E, Pineiro D, Soto M, Abanades DR, Martin ME, Salinas M, Gonzalez VM. A DNA aptamer population specifically detects Leishmania infantum H2A antigen. Lab Invest. 2007;87:409–416. doi: 10.1038/labinvest.3700535. [DOI] [PubMed] [Google Scholar]
- 32.Ramos E, Moreno M, Martin ME, Soto M, Gonzalez VM. In vitro selection of Leishmania infantum H3-binding ssDNA aptamers. Oligonucleotides. 2010;20:207–213. doi: 10.1089/oli.2010.0240. [DOI] [PubMed] [Google Scholar]
- 33.Ramos-Vara JA. Technical aspects of immunohistochemistry. Vet Pathol. 2005;42:405–426. doi: 10.1354/vp.42-4-405. [DOI] [PubMed] [Google Scholar]
- 34.Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, Claesson-Welsh L, Janjic N. 2'-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273:20556–20567. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 35.Sakai N, Masuda H, Akitomi J, Yagi H, Yoshida Y, Horii K, Furuichi M, Waga I. RNA aptamers specifically interact with the Fc region of mouse immunoglobulin G. Nucleic Acids Symp Ser (Oxf) 2008:487–488. doi: 10.1093/nass/nrn247. [DOI] [PubMed] [Google Scholar]
- 36.Semenkovich CF, Ostlund RE, Jr, Olson MO, Yang JW. A protein partially expressed on the surface of HepG2 cells that binds lipoproteins specifically is nucleolin. Biochemistry. 1990;29:9708–9713. doi: 10.1021/bi00493a028. [DOI] [PubMed] [Google Scholar]
- 37.Shaikh NA, Ge J, Zhao YX, Walker P, Drebot M. Development of a novel, rapid, and sensitive immunochromatographic strip assay specific for West Nile Virus (WNV) IgM and testing of its diagnostic accuracy in patients suspected of WNV infection. Clin Chem. 2007;53:2031–2034. doi: 10.1373/clinchem.2007.091140. [DOI] [PubMed] [Google Scholar]
- 38.Soundararajan S, Wang L, Sridharan V, Chen W, Courtenay-Luck N, Jones D, Spicer EK, Fernandes DJ. Plasma membrane nucleolin is a receptor for the anticancer aptamer AS1411 in MV4-11 leukemia cells. Mol Pharmacol. 2009;76:984–991. doi: 10.1124/mol.109.055947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tanaka Y, Honda T, Matsuura K, Kimura Y, Inui M. In vitro selection and characterization of DNA aptamers specific for phospholamban. J Pharmacol Exp Ther. 2009;329:57–63. doi: 10.1124/jpet.108.149526. [DOI] [PubMed] [Google Scholar]
- 40.Trill JJ, Shatzman AR, Ganguly S. Production of monoclonal antibodies in COS and CHO cells. Curr Opin Biotechnol. 1995;6:553–560. doi: 10.1016/0958-1669(95)80092-1. [DOI] [PubMed] [Google Scholar]
- 41.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 42.Ulrich H, Ippolito JE, Pagan OR, Eterovic VA, Hann RM, Shi H, Lis JT, Eldefrawi ME, Hess GP. In vitro selection of RNA molecules that displace cocaine from the membrane-bound nicotinic acetylcholine receptor. Proc Natl Acad Sci USA. 1998;95:14051–14056. doi: 10.1073/pnas.95.24.14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulrich H, Magdesian MH, Alves MJ, Colli W. In vitro selection of RNA aptamers that bind to cell adhesion receptors of Trypanosoma cruzi and inhibit cell invasion. J Biol Chem. 2002;277:20756–20762. doi: 10.1074/jbc.M111859200. [DOI] [PubMed] [Google Scholar]
- 44.Yang Y, Kochoyan M, Burgstaller P, Westhof E, Famulok M. Structural basis of ligand discrimination by two related RNA aptamers resolved by NMR spectroscopy. Science. 1996;272:1343–1347. doi: 10.1126/science.272.5266.1343. [DOI] [PubMed] [Google Scholar]