Abstract
Background South Asian children and adults have a more adipose body composition compared with those of European ancestry. This is thought to be related to their increased risk of metabolic disorders. However, little is known about how early in life such differences are manifest.
Objective To determine whether there are differences in fat mass (FM) and fat-free mass (FFM) between UK-born South Asians and White Europeans in infancy.
Design A cross-sectional study of 30 South Asian and 30 White European infants aged 6–12 weeks. Mothers were recruited from clinics in London, and infants’ FM and FFM were determined using air-displacement plethysmography (PeaPod®).
Results In early infancy South Asians had less FFM than White Europeans [0.34 kg less, 95% confidence interval (CI): 0.15, 0.52], with a considerably weaker indication of them also having more FM (0.02 kg more, 95% CI: −0.14, 0.18). These differences persisted when the overall smaller body size of South Asian infants was taken into account. For a given total infant weight, the balance of body composition of South Asians was shifted by 0.16 kg (95% CI: 0.06, 0.25) from FFM to FM. The ethnic differences in the amount of FFM were almost completely accounted for by ethnic differences in the rate of growth in utero and length of gestation.
Conclusions The characteristic differences in body composition observed between adult South Asians and White Europeans are apparent in early infancy. Of particular note is that this is the first study to demonstrate that South Asians compared with White Europeans have reduced FFM in infancy. The early manifestation of this phenotype suggests that it is either genetic and/or determined through exposure to maternal physiology, rather than a consequence of behaviours or diet in childhood or at older ages.
Keywords: Infant body composition, ethnicity, fat mass, fat-free mass, South Asian
Introduction
Rates of type 2 diabetes (T2DM) and cardiovascular disease are high among adults of South Asian ancestry living in the Indian subcontinent. This increased risk relative to most other ethnic groups is also seen in South Asian migrant populations around the world.1 Metabolic profiles suggestive of increased risk of these diseases in adulthood have also been found in school-age children of South Asian ancestry,2 as well as in infants.3,4
Studies comparing South Asians with Whites of European descent have suggested that ethnic differences in predisposition to T2DM are related to differences in body composition.5,6 For any given body mass index, adult South Asians have been found to have less fat-free mass (FFM) and more fat mass (FM).7–12 Differences in body composition among Asian children aged 9–15 years compared with White European children have also been observed.13,14
Three studies of adiposity in early life have been conducted in which Indian infants measured in India have been compared with European infants measured in the UK.4,14–16 These studies suggest that Indian-born babies have a more adipose body composition compared with White babies. However, these infant studies have several limitations. Two measured adiposity using skinfold thicknesses,4,15 whereas the third measured adipose tissue volume using MRI16 and focused on the anatomical distribution of adipose tissue in different depots. Thus, none of these studies has directly differentiated the relative contributions of FM and FFM to body mass. Second, although attention was paid in both studies to make the measurement protocols as similar as possible, e.g. all skinfold measurements in the UK and India were undertaken by the same pair of observers using the same skinfold calipers, in the case of the MRI study, different instrumentation was used. Finally, although the focus of these studies has been on ethnic differences in adiposity (i.e. greater FM), no study to date has paid direct attention to whether there are absolute ethnic differences in the amount of FFM and FM, each of which contributes to future risk of metabolic disorders.17
Determining whether the characteristic ethnic differences in body composition between adult Europeans and South Asians are visible in infancy is important for identifying how early in life interventions targeting body composition might need to be initiated.
In this article, we present the results of the first study to examine the differences in FM and FFM between UK-born South Asian and White European infants using a technology that is based on the direct estimation of body density. Compared with others, this study has the advantage of providing a measure of both FFM and FM.
Subjects and methods
Subjects
The London Mother and Baby Study (LMABS) was a cross-sectional observational study designed to test the hypothesis that UK-born infants (6–12 weeks old) of South Asian ancestry differ in their body composition from infants of White European origin. Sixty mother–infant pairs (30 South Asian; 30 White) were recruited from midwife-led antenatal and health visitor-led well-baby clinics in Northwest London between July 2007 and August 2008. Of the 30 South Asians, 26 were Indian and 4 Pakistani. Ethnic groups were categorized based on self-reported classification as used in the 2001 UK Census. Only infants with mother and father both classified as either ‘Asian or Asian British’ or ‘White’ were eligible to participate. These restrictions minimized the potential influence of mixed ethnic background on infant body composition. Only infants born at 37–42 completed weeks of gestation were included. Infants were excluded if they had a congenital malformation or if their mothers had any medical condition during pregnancy known to influence birthweight and/or body composition such as chronic hypertension, pre-eclampsia or gestational diabetes. This information was obtained by post-natal questionnaire and verified by the Personal Child Health Record (Redbook). Body composition and anthropometry measurements were performed at Northwick Park Hospital when the infants were between 6 and 12 weeks of age.
Body composition
Body weight, FM and FFM of infants were determined using a single air-displacement plethysmography device designed for infants called the PeaPod® (Life Measurements Inc., Concord, CA, USA). The estimates of FM and FFM were taken directly from the output of the PeaPod. The details of the operating principles of the PeaPod have been described previously.18,19 Briefly, measurements of infant mass and volume, determined by the PeaPod, are used to estimate infant whole-body density (i.e. mass/volume). By using standard assumptions about the densities of fat and fat-free tissue, the fraction of fat (Ffat, %) in body weight is derived using the following formula:
where D is whole-body density, and C1 and C2 are calculated from the density of fat (constant) and the density of fat-free tissue (age specific) as specified by Siri.20 Infant values for the densities of FM and FFM have been obtained from multi-component studies21 and have been recently confirmed in a study of Swedish infants.22 In this article, we assume that these values do not vary by ethnicity. Although we were unable to test this assumption in this study, to date, there is no evidence that it is incorrect.
All measurements were performed by the same observer (K.S.) and were done in the same air-conditioned room. The device was calibrated each day it was used according to the manufacturer’s instructions. Two PeaPod measurements at the same clinic visit were performed for about half of the infants (27/60), with the baby being taken out of the device between measurements.
Anthropometry
Anthropometric measures were performed by the same trained observer (K.S.) using standard methods.23 Maternal standing and sitting heights were measured to the nearest 0.1 cm using a Leicester portable height measure (Chasmors, London, UK), and maternal weight was measured to the nearest 0.1 kg with a calibrated Seca mechanical column scale (Chasmors, London, UK). Infant crown-to-heel length was measured to the nearest 0.1 cm using a Rollameter 60 (Harlow Healthcare, UK), and infant weight was measured to the nearest 0.1 kg on the integrated electronic scale of the PeaPod as described above. All maternal and infant circumferences (occipitofrontal, abdominal, chest, mid-upper arm) were measured to the nearest 0.1 cm using a non-stretch fibreglass anthropometry tape (Chasmors, London, UK), and skinfold thicknesses were measured to the nearest 0.2 mm using Holtain skinfold calipers (Harlow Healthcare, UK). All anthropometric measurements were performed in triplicate except for infant length, which was performed once for each infant.
Gestational age- and sex-specific birthweight z-scores were determined using the LMSgrowth Excel add-in program from the Medical Research Council, UK, 2002, based on the British 1990 growth reference.24,25 To assess the impact of different rates of post-natal growth on body composition, we used ordinary linear regression to generate a conditional weight gain variable (kg) that measured how far the weight of the infant deviated from that predicted, given their birthweight, sex and age at the study visit.
Other data
Information regarding date of birth of mother, self-defined ethnicity, country of birth, education, marital status, family medical history, income, parity and duration of breastfeeding was obtained by a questionnaire completed by the mother. Infant birthweight and gestational age (based on date of last menstrual period) and date of birth of the infant were extracted from the Personal Child Health Record (Redbook).
Ethics
The LMABS was given ethical approval by the Central Office for Research Ethics Committees (REC: 06/Q0508/67), the London School of Hygiene & Tropical Medicine and local National Health Service (NHS) trusts (i.e. Northwest London, Barnet/Chase Farm and Chelsea & Westminster NHS trusts). Informed written consent was obtained from all participating mothers.
Statistical methods
Body composition outcome variables were treated as continuous. Ethnicity was treated as a binary exposure variable, either White British/European or South Asian. Mixed-effects linear regression was used to evaluate the effect of ethnic group on FM and FFM outcomes to make full appropriately weighted use of the duplicate body composition data available for almost half the infants. Specifically, for each outcome variable, a linear mixed model with fixed covariate effects of age- and gender- and subject-specific random effects was fitted to the data on all subjects. This allowed us to use all acquired duplicate measurements of outcome when available, together with data from individuals where only one PeaPod measurement was taken. The statistical significance of ethnic differences in the frequency distributions of other variables was investigated using Fisher’s exact test (for categorical variables) and t-tests for continuous variables other than birthweight in kilograms, for which linear regression was used to adjust for gestational age and sex.
A series of adjustments was made to the basic linear (t-test) and linear mixed models for pre- and post-natal infant characteristics (birthweight z-score, gestational age, body weight, length and conditional weight) that were identified, based on the literature, as potential confounders of the association of ethnic group with infant body composition.26,27
Sample size calculations were estimated from work in neonates, suggesting that there is ∼8.0% [standard deviation (SD): 1.13%] total fat in healthy term neonates (N Modi, personal communication). Using these values, it was estimated statistically that ∼28 babies from each ethnic group would be required to provide a statistical power of 90% to detect differences in FM of 1% or more between groups that would be significant at the 5% level.
All data were analysed using the STATA v11.0 (Statacorp, College Station, TX, USA) statistical package.
Results
Background parental characteristics
Table 1 presents the parental characteristics of the infants included in the study. Almost all of the mothers in the White European group were born in the United Kingdom; and one-third of South Asian mothers were born in the United Kingdom, more than half were born in the Indian subcontinent and two were born in East Africa. A similar pattern was observed among fathers in this study. Although both ethnic groups had a mean age of 30 years at delivery, there was a difference in the age distribution, with more White European mothers being under the age of 25 or older than 35 years compared with South Asian mothers. The proportion of women who were primiparous was the same in both ethnic groups. There was an indication that a higher proportion of South Asian mothers had a first degree or higher. Substantially fewer South Asian mothers had ever drunk alcohol during their first 3 months of pregnancy compared with White mothers. Only two of the mothers (both White European) reported smoking during pregnancy.
Table 1.
Maternal and infant characteristics by ethnic group
| Characteristics | White | South Asian | P-value |
|---|---|---|---|
| Mother’s country of birth | 30 (100%) | 30 (100%) | |
| UK/Ireland | 29 (96.7) | 10 (33.3) | |
| India/Pakistan | 0 (0.0) | 18 (60.0) | |
| Other | 1 (3.3) | 2 (6.7) | <0.001 |
| Father’s country of birth | |||
| UK/Ireland | 29 (96.7) | 9 (30.0) | |
| India/Pakistan | 0 (0.0) | 14 (46.7) | |
| Other | 1 (3.3) | 7 (23.3) | <0.001 |
| Maternal age (years) | |||
| <25 | 5 (16.7) | 0 (0.0) | |
| 25–29 | 4 (13.3) | 16 (53.5) | |
| 30–34 | 15 (50) | 11 (36.7) | |
| 35+ | 6 (20) | 3 (10) | 0.002 |
| Parity | |||
| Primiparous | 17 (56.7) | 17 (56.7) | |
| Multiparous | 13 (43.3) | 13 (43.3) | 1.000 |
| Maternal educationa | |||
| None | 1 (3.3) | 0 (0) | |
| O/A levels | 6 (20) | 5 (16.7) | |
| College/first degree | 13(43.3) | 17 (56.7) | |
| Higher degree | 4 (13.3) | 7 (23.3) | |
| Other qualifications (e.g. NVQ) | 6 (20) | 1 (3.3) | 0.175 |
| Smoking during pregnancy | |||
| Yes | 2 (6.7) | 0 (0) | |
| No | 28 (93.3) | 30 (100) | 0.492 |
| Alcohol consumption (first trimester) | |||
| Yes | 28 (93.3) | 8 (26.7) | |
| No | 2 (6.7) | 22 (73.3) | <0.001 |
| Diet | |||
| Vegetarian | 1 (3.3) | 14 (46.7) | |
| Non-vegetarian | 28 (93.3) | 16 (53.3) | |
| Other | 1 (3.3) | 0 | <0.001 |
| Infant characteristics | |||
| Age at examination (weeks) | 7.4 (1.1) | 8.03 (1.3) | 0.062 |
| Sex (male) | 17 (56.7) | 16 (53.3) | 1.000 |
| Birthweight (kg) | 3.56 (0.43) | 3.06 (0.34) | <0.001 |
| Birthweight z-score | 0.15 (1.00) | 0.65 (0.62) | <0.001 |
| Gestational age (weeks) | 40.0 (1.2) | 39.4 (1.2) | 0.038 |
Results are presented as number (%), mean (standard deviation) for continuous variables. For categorical variables, P-values were calculated using Fisher’s exact test. For continuous variables, P-values were calculated from t-tests other than for birthweight (kg), for which linear regression was used to adjust for gestational age and sex.
aCategories as derived from Census 2001.
NVQ, National Vocational Qualification.
Almost half of South Asian mothers were vegetarian compared with only one White European mother. A similar proportion of South Asian and White European mothers reported a family history of both cardiovascular disease (7/30 White European; 9/30 South Asian; P = 0.771) and high blood pressure (15/30 White European; 17/30 South Asian; P = 0.796). There was equivocal evidence that a greater proportion of South Asian mothers (15/30) had a family history of diabetes compared with White European mothers (7/30) (P = 0.06).
Infant characteristics
Infants from both ethnic groups were of similar mean age at examination, and there was an almost identical distribution by sex. As expected, South Asian infants were, on average, 500 g lighter at birth and born 4.4 days earlier compared with their White European counterparts. The birthweight for gestational age z-score for South Asians was almost one SD lower than for White Europeans (Table 1).
A greater proportion of South Asian infants were breastfed beyond 1 month of age compared with those from the White European group, but no infant from either group had begun eating solid foods at the time of the study visit. South Asian and White European infants were similar with respect to the age at which formula milk was introduced and in the proportion of infants who had begun drinking formula milk at the time of examination. A similar number of infants from both ethnic groups reported feeding problems or were reported to be sick the week of the examination. There were no differences between ethnic groups with respect to mode of delivery or age at study visit (data not shown).
Maternal anthropometry at study visit
South Asian mothers were shorter, had smaller head circumferences and weighed less than their White European counterparts when measured at the study visit (Table 2). However, South Asian mothers had greater triceps and subscapular skinfold thicknesses than their White European counterparts.
Table 2.
Maternal anthropometry at examination by ethnic group (mean and SD)
| South Asian–White (adjusted for age) |
||||
|---|---|---|---|---|
| Maternal anthropometry | White (n = 30) Mean (SD) | South Asian (n = 30) Mean (SD) | Difference (95% CI) | P-value |
| Body weight (kg) | 75.4 (18.4) | 65.1 (10.8) | −10.2 (−18.1 to −2.4) | 0.012 |
| Height (cm) | 166.3 (5.8) | 158.6 (5.7) | −7.6 (−10.6 to −4.6) | <0.001 |
| Leg length (cm) | 78.6 (4.9) | 75.6 (3.7) | −3.0 (−5.3 to −0.7) | 0.011 |
| Head circumference (cm) | 55.7 (1.5) | 53.8 (2.0) | −1.9 (−2.8 to −0.9) | <0.001 |
| Triceps skinfolds (mm) | 22.2 (6.7) | 26.5 (5.9) | 4.5 (1.2 to 7.7) | 0.008 |
| Subscapular skinfolds (mm) | 17.0 (5.4) | 21.0 (6.9) | 4.3 (0.8 to 7.7) | 0.018 |
Linear regression was used to compare anthropometry and body composition differences between ethnic groups adjusted for maternal age at examination.
Infant anthropometry and body composition at study visit
At the study visit, South Asian infants were lighter in weight, had lower FFM, were shorter in length and had smaller head and abdominal circumferences, but had larger subscapular skinfold thicknesses compared with White European infants (Table 3). There was a suggestion that South Asian infants also had slightly greater FM.
Table 3.
Infant anthropometry and body composition by ethnic group
| South Asian—White (adjusted for age and sex) |
||||
|---|---|---|---|---|
| Anthropometry and body composition | White British (n = 30) Mean (SD) | South Asian (n = 30) Mean (SD) | Difference (95% CI) | P-value |
| Body weight (kg) | 5.10 (0.59) | 4.88 (0.67) | −0.32 (−0.60 to −0.03) | 0.033 |
| Fat-free mass (kg) | 4.12 (0.45) | 3.85 (0.43) | −0.34 (−0.52 to −0.15) | 0.001 |
| Fat mass (kg) | 0.98 (0.29) | 1.03 (0.31) | 0.02 (−0.14 to 0.18) | 0.789 |
| Crown-to-heel length (cm) | 57.7 (2.1) | 56.9 (2.0) | −1.2 (−2.0 to −0.3) | 0.012 |
| Head circumference (cm) | 39.3 (1.3) | 38.5 (1.3) | −1.1 (−1.7 to −0.5) | 0.001 |
| Chest circumference (cm) | 39.3 (2.0) | 38.8 (2.0) | −0.8 (−1.7 to 0.2) | 0.105 |
| Abdominal circumference (cm) | 39.8 (3.0) | 38.2 (2.6) | −1.9 (−3.3 to −0.5) | 0.008 |
| Mid-upper arm circumference (mm) | 13.0 (1.0) | 12.9 (1.1) | −0.2 (−0.7 to 0.4) | 0.600 |
| Triceps skinfolds (mm) | 8.0 (1.6) | 8.6 (1.5) | 0.5 (−0.3 to 1.3) | 0.213 |
| Subscapular skinfolds (mm) | 6.8 (1.4) | 8.1 (1.6) | 1.2 (0.4 to 2.0) | 0.005 |
Mixed-effects linear regression was used to compare differences in body composition (body weight, FFM and FM) between ethnic groups, adjusted for infant age at examination and sex. Linear regression was used to compare differences in anthropometry between ethnic groups, adjusted for infant age at examination and sex.
As already noted, South Asian infants were smaller than White European infants. Differences in other aspects of anthropometry and body composition seen in Table 3 might therefore be a consequence of differences in absolute size or relative shape. To investigate this, we adjusted the differences in infant anthropometry and body composition for measures of overall body size: infant weight or length. The results of this analysis (Table 4) show that regardless of whether adjusted for weight or length, South Asian infants still had less FFM. In addition, they had smaller head circumferences but larger subscapular skinfolds.
Table 4.
Ethnic difference in infant anthropometry and body composition adjusted for overall body size (body mass or length)
| Anthropometry and body composition | Difference (South Asian–White) adjusted for age, sex and infant body weight |
Difference (South Asian–White) adjusted for age, sex and infant body length |
||
|---|---|---|---|---|
| Difference (95% CI) | P-value | Difference (95% CI) | P-value | |
| Body weight (kg) | NA | −0.13 (−0.40 to 0.14) | 0.341 | |
| Fat-free mass (kg) | −0.16 (−0.25 to −0.06) | 0.002 | −0.20 (−0.36 to −0.04) | 0.016 |
| Fat mass (kg) | 0.16 (0.06 to 0.25) | 0.002 | 0.07 (−0.09 to 0.23) | 0.391 |
| Crown-to-heel length (cm) | −0.67 (−1.47 to 0.14) | 0.102 | NA | |
| Head circumference (cm) | −0.67 (−1.16 to −0.17) | 0.009 | −0.69 (−1.25 to −0.13) | 0.016 |
| Chest circumference (cm) | 0.09 (−0.44 to 0.62) | 0.737 | −0.42 (−1.37 to 0.54) | 0.385 |
| Abdominal circumference (cm) | −0.72 (−1.63 to 0.18) | 0.114 | −1.69 (−3.17 to −0.22) | 0.025 |
| Mid-upper arm circumference (mm) | 0.38 (0.04 to 0.71) | 0.029 | 0.09 (−0.50 to 0.68) | 0.754 |
| Triceps skinfolds (mm) | 1.07 (0.40 to 1.74) | 0.002 | 0.66 (−0.20 to 1.53) | 0.131 |
| Subscapular skinfolds (mm) | 1.73 (1.08 to 2.39) | <0.001 | 1.20 (0.34 to 2.07) | 0.007 |
Mixed-effects linear regression was used to compare differences in body composition (body weight, FFM and FM) between ethnic groups, adjusted for infant age, sex and either infant body mass or infant body length. Linear regression was used to compare differences in anthropometry between ethnic groups, adjusted for infant age, sex and either infant body mass or infant body length.
NA, not applicable.
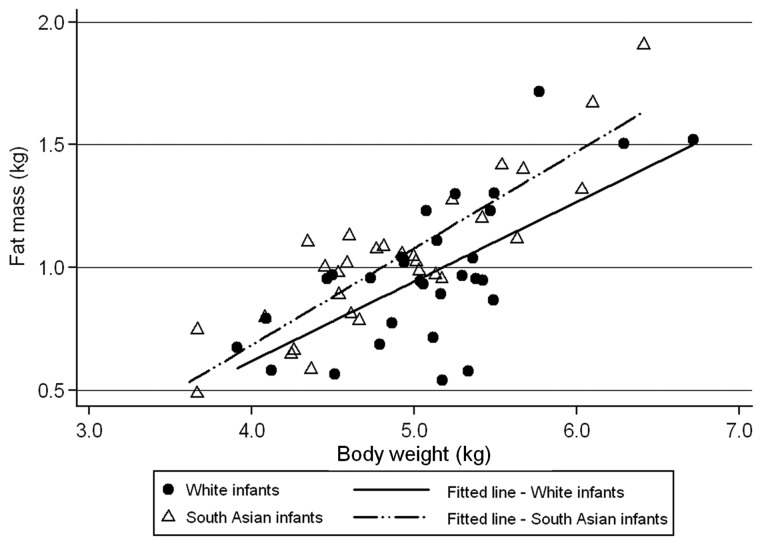
The persistence of strong evidence for South Asians having less FFM than White Europeans even after adjustment for infant body length demonstrates unequivocally that this is not simply a consequence of South Asians being smaller in all respects. In contrast, the observed ethnic differences in FM and FFM after adjustment for total body mass need to be interpreted more carefully. This is because of their obvious interdependence, such that the ethnic differences for the two components of body composition adjusted for weight are the mirror image of each other. Nevertheless, this adjusted difference can be interpreted as showing that for a given total infant weight, on average, the balance of body composition is shifted by 0.16 kg from FFM to FM, i.e. South Asians have a more adipose body composition for a given weight. This is also evident in Figure 1, which graphically illustrates that, at all infant weights, South Asians tend to have more FM and, therefore, less FFM than European infants.
Figure 1.
The relationship of infant body weight with fat mass at 8 weeks of age. Average values for FM (kg) and body weight (kg) are plotted. Fitted lines are generated for each ethnic group separately, accounting for the variable number of duplicate observations
Influence of pre- and post-natal growth on body composition
To explore possible explanations for these ethnic differences in body composition, the influence of fetal growth rate (birthweight z-score) and time in utero (gestational age) and rate of post-natal growth (conditional weight) were evaluated with adjustment for infant length (Table 5). The lower FFM in South Asian relative to White European infants was attenuated by adjustment for birthweight z-score, with or without gestational age. However, adjustment for birthweight z-score slightly augmented the association of ethnic group with FM. In contrast, adjustment for conditional weight did not appreciably influence the basic association of ethnicity with FFM, but did attenuate the ethnic difference in FM.
Table 5.
Ethnic difference in fat mass and fat-free mass adjusted for pre- and post-natal growth, gestational age and infant length at study visit
| South Asian–White | ||||
|---|---|---|---|---|
| Infant characteristics included in each model | Fat mass (kg) difference (95% CI) | P-value | Fat-free mass (kg) difference (95% CI) | P-value |
| Sex, age and length at examination | 0.07 (−0.09 to 0.23) | 0.391 | −0.20 (−0.36 to −0.04) | 0.016 |
| Sex, age and length at examination plus birthweight z-score | 0.10 (−0.07 to 0.27) | 0.235 | −0.14 (−0.30 to 0.03) | 0.109 |
| Sex, age and length at examination plus gestational age | 0.07 (−0.10 to 0.23) | 0.423 | −0.18 (−0.34 to −0.02) | 0.029 |
| Sex, age and length at examination plus birthweight z-score and gestational age | 0.11 (−0.07 to 0.30) | 0.219 | −0.03 (−0.19 to 0.13) | 0.679 |
| Sex, age and length at examination plus conditional weight | 0.00 (−0.10 to 0.10) | 0.992 | −0.25 (−0.38 to −0.12) | <0.001 |
Mixed-effects linear regression adjusted for infant age and sex. Birthweight for gestational age z-score was based on the British 1990 Growth Reference Charts (using LMSgrowth Excel add-in program from the Medical Research Council, UK, 2002). Conditional weight variable is based on the residuals from the regression of body weight (kg) on birthweight (kg) after adjustment for infant age and sex.
Inclusion of maternal age, height, education, diet or drinking habits into the models did not have any appreciable influence on the ethnic differences in infant FFM or FM (data not shown).
Discussion
In this first study designed to measure the absolute amount of FM and FFM in South Asian and White European infants born in the same country, we found strong evidence that South Asian infants had less FFM than White European infants, even when account was taken of their overall smaller size. As a consequence, in relative terms, South Asian infants were more adipose in their body composition, consistent with our finding that they also had larger subscapular skinfold thicknesses. These results extend back to early post-natal life, the point at which the more adipose body composition of South Asians is first evident. Moreover, our finding that after adjustment for infant body length South Asians still have lower FFM relative to White Europeans is consistent with the parallel observations in adults that South Asians have a more adipose body composition than White Europeans for any given level of body mass index.28
The hypothesis of a relatively more adipose body composition among South Asian babies was first put forward in a study in which Indian-born South Asian neonates were compared with UK-born White infants.4 Similar to what we have observed, Indian neonates were lighter in weight and had smaller abdominal, mid-arm and head circumferences compared with White UK-born babies. Interestingly, however, Indian neonatal subscapular skinfold thicknesses, but not triceps skinfold thicknesses, were larger than those of their White UK counterparts, a finding we have replicated in this study. This particular fat patterning was interpreted by authors to be an indicator of greater ‘central or truncal’ fat deposition.
These results were expanded on in the Pune Maternal Nutrition Study, which was a large two-centre population-based study of rural Indian women and their newborns in Pune, India, that compared them with White European mothers and infants born in Southampton, UK.15 When compared with the neonatal measurements of Southampton babies with comparable birthweights (2800–3300 g), South Asian babies born in Pune, India, were longer. However, their subscapular skinfold thicknesses were larger or ‘preserved’, indicating a relatively more adipose body composition. A similar body shape and fat patterning were also observed among low birthweight babies (<2500 g). Whole body MRI studies also suggested a greater amount of central adipose tissue in a similar Indian-born neonate population compared with UK-born White European newborns.16
The present study expands on this work to provide direct estimates not only of FM but also, importantly, of FFM in UK-born South Asian and White European infants at 8 weeks of age. In this study, the greater relative adiposity of South Asian infants is primarily a consequence of their lower average FFM. Moreover, at any given total body mass, South Asians have less FFM and greater FM. These observations may have important implications for our understanding of the development of insulin resistance. Much attention has been placed in the literature on the metabolic and pro-inflammatory action of central or visceral fat in adolescents and adults as an important risk factor for the development of T2DM and cardiovascular disease.29–31 However, skeletal muscle insulin resistance has also been hypothesized to have a pathophysiological role in the development of metabolic syndrome, whereby insulin-resistant skeletal muscle reduces glucose uptake and glycogen synthesis and results in hepatic lipogenesis leading to the atherogenic dyslipidaemia that is associated with the metabolic syndrome.17 In this context, Lear et al. (2009)32 measured body composition by dual energy x-ray absorbiometry (DXA) in adults from several ethnic groups and showed that the greater insulin resistance of South Asians, measured by homeostasis model assessment, was explained by their high ratio of FM to FFM. Taken together with our results, these studies suggest that a lifelong tendency for people of South Asian ancestry to have both more FM and less FFM relative to those of White European ancestry may play a key role in the increased risk of insulin resistance and T2DM.
Interestingly, in the current study, the lower in utero growth rate and slightly shorter gestations of South Asian infants statistically accounted for the observed ethnic differences in FFM, but not of FM. These observations are consistent with published reports showing birthweight as a stronger predictor of FFM than of FM.33 In contrast, adjustment for a measure of post-natal growth uncorrelated with birthweight (conditional weight) had no appreciable effect on FFM but had an attenuating effect on FM, providing some evidence that post-natal weight gain had a greater determining effect on resultant FM of 8-week-old infants. This is also broadly consistent with what has been observed in infant growth in the first year of life.34,35
With respect to maternal determinants of these ethnic differences, it is notable that the South Asian mothers, like their offspring, had greater skinfold thicknesses, despite weighing 10 kg less. These differences imply reduced lean mass in the South Asian mothers, although we did not directly measure this. Thus, the body composition differences of the offspring appear to replicate those in the mothers, indicating a transgenerational effect.
The main limitation of this study is its relatively small size, with the consequent lack of precision in some of the associations of interest. Nevertheless, it is important to note that the ethnic differences in birthweight and gestational age we have observed are consistent with the findings of a recent analysis of all births in England and Wales.36 Moreover, the standardized birthweight z-score generated using the 1990 British Reference population shows that birthweights of White European infants in this study were similar to this reference population, although, as expected, birthweights of the South Asians were more than half a SD lower than the reference population [White European: +0.15 z-score (−0.22, +0.53); South Asian: −0.65 z-score (−0.88, −0.41)]. These comparisons with national data suggest that our study sample is reasonably representative of the broader population from which it is drawn in terms of birthweight and gestational age.
The study has several strengths that enable it to make a novel contribution to the literature on ethnic differences in body composition. Firstly, unlike previous studies, all the mothers and infants were measured using the same device by the same investigator. Secondly, this first use of the PeaPod device to investigate this issue enabled direct estimation of the mass of fat and fat-free tissue. Thirdly, all the infants measured were born in the same country (UK), unlike other studies that compared Indian-born with UK-born infants. This is important in that it reduces any influence of differences in maternal environment in pregnancy and in early post-natal life.
In summary, we provide the most direct evidence to date of the lower FFM and more adipose body composition of South Asian infants born in the UK. These data contribute to our understanding of body composition in early infancy and also present some evidence that these ethnic differences originate in utero. The mounting evidence that ethnic differences in body composition are evident in the first months of post-natal life indicates either a genetic aetiology or the consequences of exposure to maternal physiology. However, large longitudinal studies correlating the changes in FM and FFM from birth to adulthood with the development of precursors to insulin resistance and T2DM are still required to categorically demonstrate the significance of these early differences. Equally important will be further studies aimed at understanding ethnic differences in birthweight and gestation.
The existence of such ethnic differences in early life suggests that they are either genetic or determined primarily through in utero exposure to maternal physiology,37 rather than being a consequence of behaviours or diet in childhood or at older ages. This insight concerning the early origins of these differences needs to be taken into account when developing interventions aimed at reducing later-life ethnic differences in adiposity and the subsequent risk of T2DM.
Funding
The LMABS was funded by a British Heart Foundation Studentship awarded to K.M.S. (FS/05/074). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
KEY MESSAGES.
This study confirms that the differences in body composition observed between adult South Asians and White Europeans are apparent in early infancy.
Its unique contribution is that it is the first study to demonstrate that in infancy, South Asians compared with White Europeans have reduced fat-free mass.
The early manifestation of this phenotype suggests that it is either genetic and/or determined through exposure to maternal physiology, rather than being a consequence of behaviours or diet in childhood or at older ages. This needs to be taken into account when developing interventions aimed at reducing later-life ethnic differences in adiposity and the subsequent risk of T2DM.
Acknowledgements
The authors thank all the mother and baby study participants for their involvement in the study. The authors also thank the midwives, health visitors and GP practices in Northwest London for facilitating the conduct of this study (Trixie McAree, Richard Nicholl, Alison Spiro, Tanya Dennis and Shirley Jenkins). The authors’ responsibilities were as follows—K.M.S.: design and conduct of the study, data analyses, data interpretation and manuscript writing; J.C.W.: design of study and data interpretation; M.S.F.: design of study and data interpretation; C.F.: statistical methods; D.A.L.: design of study and data interpretation. All authors were involved in the critical review of the manuscript.
Conflict of interest: None declared.
References
- 1.Abate N, Chandalia M. Ethnicity, type 2 diabetes & migrant Asian Indians. Indian J Med Res. 2007;125:251–58. [PubMed] [Google Scholar]
- 2.Whincup PH, Gilg JA, Owen CG, Odoki K, Alberti KG, Cook DG. British South Asians aged 13-16 years have higher fasting glucose and insulin levels than Europeans. Diabet Med. 2005;22:1275–77. doi: 10.1111/j.1464-5491.2005.01587.x. [DOI] [PubMed] [Google Scholar]
- 3.Boon MR, Karamali NS, de Groot CJ, et al. E-selectin is elevated in cord blood of South Asian neonates compared with Caucasian neonates. J Pediatr. 2012;160:844–48.e1. doi: 10.1016/j.jpeds.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 4.Yajnik CS, Lubree HG, Rege SS, et al. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab. 2002;87:5575–80. doi: 10.1210/jc.2002-020434. [DOI] [PubMed] [Google Scholar]
- 5.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337:382–86. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 6.Shah A, Hernandez A, Mathur D, Budoff MJ, Kanaya AM. Adipokines and body fat composition in South Asians: results of the Metabolic Syndrome and Atherosclerosis in South Asians Living in America (MASALA) study. Int J Obes (Lond) 2012;36:810–16. doi: 10.1038/ijo.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yajnik CS, Yudkin JS. The Y-Y paradox. Lancet. 2004;363:163. doi: 10.1016/S0140-6736(03)15269-5. [DOI] [PubMed] [Google Scholar]
- 8.Banerji MA, Faridi N, Atluri R, Chaiken RL, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:137–144. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 9.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–46. doi: 10.1046/j.1467-789x.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg-Yap M, Schmidt G, van Staveren WA, Hautvast JG, Deurenberg P. Body fat measurement among Singaporean Chinese, Malays and Indians: a comparative study using a four-compartment model and different two-compartment models. Br J Nutr. 2001;85:491–98. doi: 10.1079/bjn2000276. [DOI] [PubMed] [Google Scholar]
- 11.McKeigue PM. Metabolic consequences of obesity and body fat pattern: lessons from migrant studies. Ciba Found Symp. 1996;201:54–64. doi: 10.1002/9780470514962.ch4. [DOI] [PubMed] [Google Scholar]
- 12.Chandalia M, Lin P, Seenivasan T, et al. Insulin resistance and body fat distribution in South Asian men compared to Caucasian men. PLoS One. 2007;2:e812. doi: 10.1371/journal.pone.0000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haroun D, Taylor SJ, Viner RM, et al. Validation of bioelectrical impedance analysis in adolescents across different ethnic groups. Obesity (Silver Spring) 2010;18:1252–59. doi: 10.1038/oby.2009.344. [DOI] [PubMed] [Google Scholar]
- 14.Nightingale CM, Rudnicka AR, Owen CG, Cook DG, Whincup PH. Patterns of body size and adiposity among UK children of South Asian, black African-Caribbean and white European origin: Child Heart And health Study in England (CHASE Study) Int J Epidemiol. 2011;40:33–44. doi: 10.1093/ije/dyq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yajnik CS, Fall CH, Coyaji KJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–80. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- 16.Modi N, Thomas EL, Uthaya SN, Umranikar S, Bell JD, Yajnik C. Whole body magnetic resonance imaging of healthy newborn infants demonstrates increased central adiposity in Asian Indians. Pediatr Res. 2009;65:584–87. doi: 10.1203/pdr.0b013e31819d98be. [DOI] [PubMed] [Google Scholar]
- 17.Jornayvaz FR, Samuel VT, Shulman GI. The role of muscle insulin resistance in the pathogenesis of atherogenic dyslipidemia and nonalcoholic fatty liver disease associated with the metabolic syndrome. Annu Rev Nutr. 2010;30:273–90. doi: 10.1146/annurev.nutr.012809.104726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr. 2007;85:90–95. doi: 10.1093/ajcn/85.1.90. [DOI] [PubMed] [Google Scholar]
- 19.Urlando A, Dempster P, Aitkens S. A new air displacement plethysmograph for the measurement of body composition in infants. Pediatr Res. 2003;53:486–92. doi: 10.1203/01.PDR.0000049669.74793.E3. [DOI] [PubMed] [Google Scholar]
- 20.Siri WE. Body composition from fluid space and density. In: Brozek J, Hanschel A, editors. Techniques for Measuring Body Composition. Washington, DC: National Academy of Sciences; 1961. pp. 223–44. [Google Scholar]
- 21.Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35(5 Suppl):1169–75. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- 22.Eriksson B, Lof M, Eriksson O, Hannestad U, Forsum E. Fat-free mass hydration in newborns: assessment and implications for body composition studies. Acta Paediatr. 2011;100:680–86. doi: 10.1111/j.1651-2227.2011.02147.x. [DOI] [PubMed] [Google Scholar]
- 23.Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Publishers; 1988. [Google Scholar]
- 24.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–19. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 25.Cole TJ. Conditional reference charts to assess weight gain in British infants. Arch Dis Child. 1995;73:8–16. doi: 10.1136/adc.73.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modi N, Murgasova D, Ruager-Martin R, et al. The influence of maternal body mass index on infant adiposity and hepatic lipid content. Pediatr Res. 2011;70:287–91. doi: 10.1203/PDR.0b013e318225f9b1. [DOI] [PubMed] [Google Scholar]
- 27.Gale C, Logan KM, Santhakumaran S, Parkinson JR, Hyde MJ, Modi N. Effect of breastfeeding compared with formula feeding on infant body composition: a systematic review and meta-analysis. Am J Clin Nutr. 2012;95:656–69. doi: 10.3945/ajcn.111.027284. [DOI] [PubMed] [Google Scholar]
- 28.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 29.Carr DB, Utzschneider KM, Hull RL, et al. Intra-abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes. 2004;53:2087–94. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 31.Sonnenberg GE, Krakower GR, Kissebah AH. A novel pathway to the manifestations of metabolic syndrome. Obes Res. 2004;12:180–86. doi: 10.1038/oby.2004.24. [DOI] [PubMed] [Google Scholar]
- 32.Lear SA, Kohli S, Bondy GP, Tchernof A, Sniderman AD. Ethnic variation in fat and lean body mass and the association with insulin resistance. J Clin Endocrinol Metab. 2009;94:4696–702. doi: 10.1210/jc.2009-1030. [DOI] [PubMed] [Google Scholar]
- 33.Wells JC, Chomtho S, Fewtrell MS. Programming of body composition by early growth and nutrition. Proc Nutr Soc. 2007;66:423–34. doi: 10.1017/S0029665107005691. [DOI] [PubMed] [Google Scholar]
- 34.Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A, Fewtrell MS. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87:1776–84. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 35.Ekelund U, Ong K, Linne Y, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83:324–30. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 36.Moser K, Stanfield KM, Leon DA. Birthweight and gestational age by ethnic group, England and Wales 2005: introducing new data on births. Health Stat Q. 2008;22–31(Autumn):34–55. [PubMed] [Google Scholar]
- 37.Wells JC. The thrifty phenotype as an adaptive maternal effect. Biol Rev Camb Philos Soc. 2007;82:143–72. doi: 10.1111/j.1469-185X.2006.00007.x. [DOI] [PubMed] [Google Scholar]