Abstract
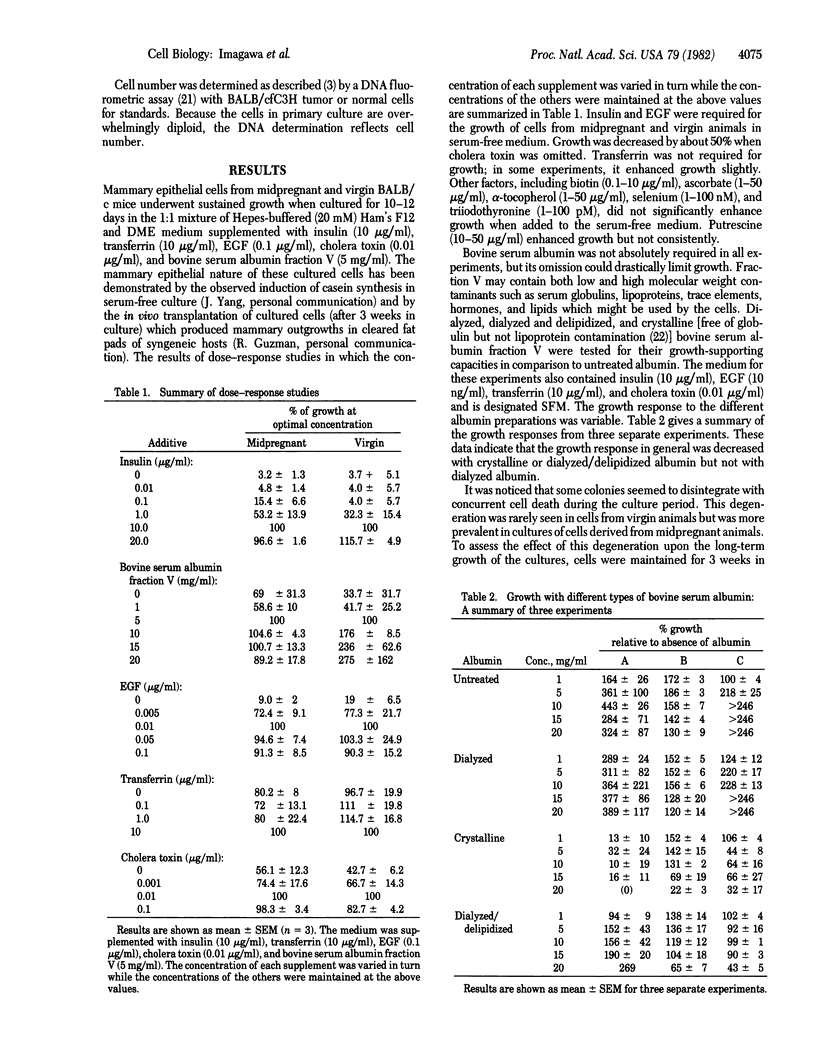
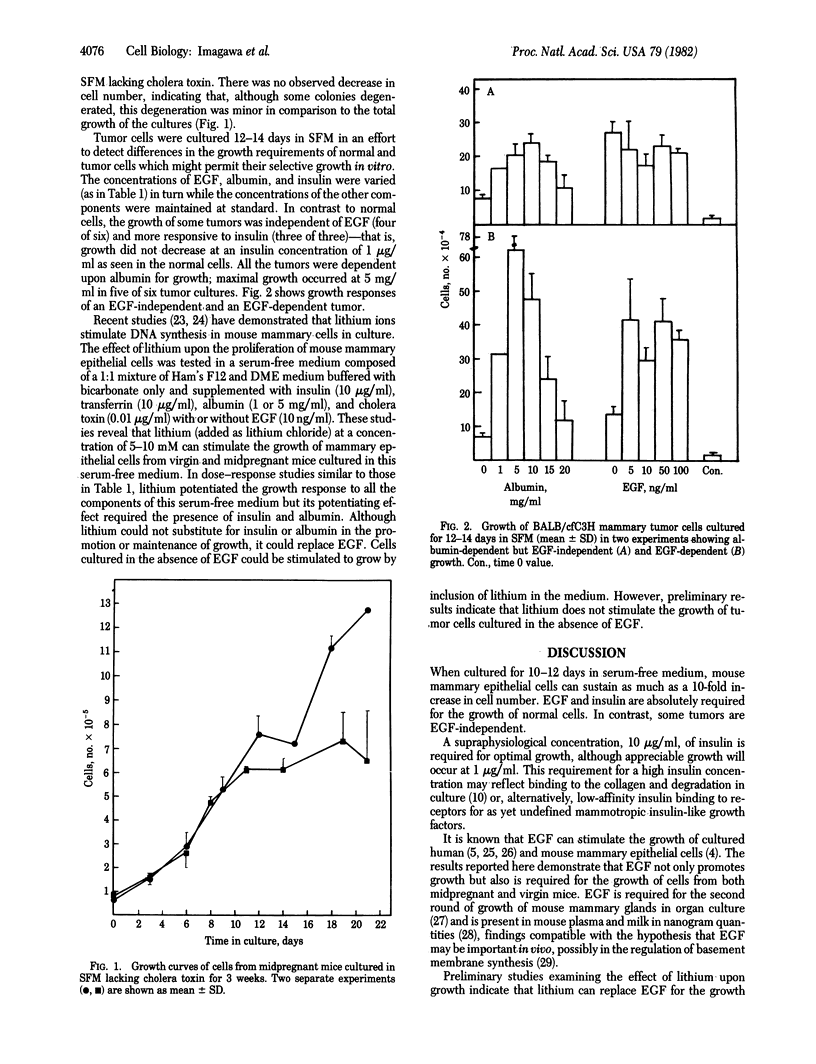
Freshly isolated normal and tumor mouse mammary epithelial cells embedded within a collagen gel matrix undergo sustained growth when cultured for as long as 3 wk in a serum-free medium composed of a 1:1 (vol/vol) mixture of Hepesbuffered Ham's F12 and Dulbecco's modified Eagle's medium supplemented with insulin, epidermal growth factor (EGF), transferrin, bovine serum albumin fraction V, and cholera toxin. Of these additives, only insulin, EGF, and albumin are required for the growth of most normal cells. Albumin is not always an absolute requirement for growth but greatly enhances it. Lithium has been found to stimulate the growth of normal cells and can replace EGF. The collagen matrix culture system allows sustained growth of primary cultures of both normal and neoplastic mammary epithelium in serum-free conditions. This serum-free system will be useful in identifying and investigating the role of hormones, growth factors, and nutritional factors in regulating the growth of mammary epithelial cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allegra J. C., Lippman M. E. Growth of a human breast cancer cell line in serum-free hormone-supplemented medium. Cancer Res. 1978 Nov;38(11 Pt 1):3823–3829. [PubMed] [Google Scholar]
- Barnes D., Sato G. Growth of a human mammary tumour cell line in a serum-free medium. Nature. 1979 Oct 4;281(5730):388–389. doi: 10.1038/281388a0. [DOI] [PubMed] [Google Scholar]
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Bolander F. F., Jr, Nicholas K. R., Topper Y. J. Retention of glucocorticoid by isolated mammary tissue may complicate interpretation of results from in vitro experiments. Biochem Biophys Res Commun. 1979 Nov 14;91(1):247–252. doi: 10.1016/0006-291x(79)90610-7. [DOI] [PubMed] [Google Scholar]
- Byyny R. L., Orth D. N., Cohen S., Doyne E. S. Epidermal growth factor: effects of androgens and adrenergic agents. Endocrinology. 1974 Sep;95(3):776–782. doi: 10.1210/endo-95-3-776. [DOI] [PubMed] [Google Scholar]
- Cham B. E., Knowles B. R. A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res. 1976 Mar;17(2):176–181. [PubMed] [Google Scholar]
- Cherington P. V., Smith B. L., Pardee A. B. Loss of epidermal growth factor requirement and malignant transformation. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3937–3941. doi: 10.1073/pnas.76.8.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L. S., Hamilton R. L., Goerke J., Weinstein J. N., Havel R. J. Interaction of unilamellar liposomes with serum lipoproteins and apolipoproteins. J Lipid Res. 1980 Nov;21(8):993–1003. [PubMed] [Google Scholar]
- Hinegardner R. T. An improved fluorometric assay for DNA. Anal Biochem. 1971 Jan;39(1):197–201. doi: 10.1016/0003-2697(71)90476-3. [DOI] [PubMed] [Google Scholar]
- Honegger P., Lenoir D., Favrod P. Growth and differentiation of aggregating fetal brain cells in a serum-free defined medium. Nature. 1979 Nov 15;282(5736):305–308. doi: 10.1038/282305a0. [DOI] [PubMed] [Google Scholar]
- Hori C., Oka T. Induction by lithium ion of multiplication of mouse mammary epithelium in culture. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2823–2827. doi: 10.1073/pnas.76.6.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosick H. L. Uptake and utilization of free fatty acids supplied by liposomes to mammary tumor cells in culture. Exp Cell Res. 1979 Aug;122(1):127–136. doi: 10.1016/0014-4827(79)90567-6. [DOI] [PubMed] [Google Scholar]
- Iscove N. N., Guilbert L. J., Weyman C. Complete replacement of serum in primary cultures of erythropoietin-dependent red cell precursors (CFU-E) by albumin, transferrin, iron, unsaturated fatty acid, lecithin and cholesterol. Exp Cell Res. 1980 Mar;126(1):121–126. doi: 10.1016/0014-4827(80)90476-0. [DOI] [PubMed] [Google Scholar]
- Kirkland W. L., Yang N. S., Jorgensen T., Longley C., Furmanski P. Growth of normal and malignant human mammary epithelial cells in culture. J Natl Cancer Inst. 1979 Jul;63(1):29–41. [PubMed] [Google Scholar]
- Knauer D. J., Iyer A. P., Banerjee M. R., Smith G. L. Identification of somatomedin-like polypeptides produced by mammary tumors of BALB/c mice. Cancer Res. 1980 Dec;40(12):4368–4372. [PubMed] [Google Scholar]
- Maciag T., Nemore R. E., Weinstein R., Gilchrest B. A. An endocrine approach to the control of epidermal growth: serum-free cultivation of human keratinocytes. Science. 1981 Mar 27;211(4489):1452–1454. doi: 10.1126/science.6970413. [DOI] [PubMed] [Google Scholar]
- Mather J. P., Sato G. H. The use of hormone-supplemented serum-free media in primary cultures. Exp Cell Res. 1979 Nov;124(1):215–221. doi: 10.1016/0014-4827(79)90271-4. [DOI] [PubMed] [Google Scholar]
- Medina D., Oborn C. J. Growth of preneoplastic mammary epithelial cells in serum-free medium. Cancer Res. 1980 Nov;40(11):3982–3987. [PubMed] [Google Scholar]
- Nair B. K., De Ome K. B. A growth-stimulating factor released by cultured mouse mammary tumor cells. Cancer Res. 1973 Nov;33(11):2754–2760. [PubMed] [Google Scholar]
- Nilausen K. Role of fatty acids in growth-promoting effect of serum albumin on hamster cells in vitro. J Cell Physiol. 1978 Jul;96(1):1–14. doi: 10.1002/jcp.1040960102. [DOI] [PubMed] [Google Scholar]
- Ptashne K., Stockdale F. E., Conlon S. Initiation of DNA synthesis in mammary epithelium and mammary tumors by lithium ions. J Cell Physiol. 1980 Apr;103(1):41–46. doi: 10.1002/jcp.1041030107. [DOI] [PubMed] [Google Scholar]
- Rockwell G. A., Sato G. H., McClure D. B. The growth requirements of SV40 virus transformed Balb/c-3T3 cells in serum-free monolayer culture. J Cell Physiol. 1980 May;103(2):323–331. doi: 10.1002/jcp.1041030218. [DOI] [PubMed] [Google Scholar]
- Salomon D. S., Liotta L. A., Kidwell W. R. Differential response to growth factor by rat mammary epithelium plated on different collagen substrata in serum-free medium. Proc Natl Acad Sci U S A. 1981 Jan;78(1):382–386. doi: 10.1073/pnas.78.1.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savion N., Lui G. M., Laherty R., Gospodarowicz D. Factors controlling proliferation and progesterone production by bovine granulosa cells in serum-free medium. Endocrinology. 1981 Aug;109(2):409–420. doi: 10.1210/endo-109-2-409. [DOI] [PubMed] [Google Scholar]
- Sirbasku D. A. Estrogen induction of growth factors specific for hormone-responsive mammary, pituitary, and kidney tumor cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3786–3790. doi: 10.1073/pnas.75.8.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobl J. S., Lippman M. E. Prolonged retention of estradiol by human breast cancer cells in tissue culture. Cancer Res. 1979 Sep;39(9):3319–3327. [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Shearer M., Stoker M. G. Growth requirements of human mammary epithelial cells in culture. Int J Cancer. 1977 Dec 15;20(6):903–908. doi: 10.1002/ijc.2910200613. [DOI] [PubMed] [Google Scholar]
- Tonelli Q. J., Sorof S. Epidermal growth factor requirement for development of cultured mammary gland. Nature. 1980 May 22;285(5762):250–252. doi: 10.1038/285250a0. [DOI] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]
- Yang J., Elias J. J., Petrakis N. L., Wellings S. R., Nandi S. Effects of hormones and growth factors on human mammary epithelial cells in collagen gel culture. Cancer Res. 1981 Mar;41(3):1021–1027. [PubMed] [Google Scholar]
- Yang J., Guzman R., Richards J., Imagawa W., McCormick K., Nandi S. Growth factor- and cyclic nucleotide-induced proliferation of normal and malignant mammary epithelial cells in primary culture. Endocrinology. 1980 Jul;107(1):35–41. doi: 10.1210/endo-107-1-35. [DOI] [PubMed] [Google Scholar]
- Yang J., Richards J., Guzman R., Imagawa W., Nandi S. Sustained growth in primary culture of normal mammary epithelial cells embedded in collagen gels. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2088–2092. doi: 10.1073/pnas.77.4.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]


