Abstract
In endothelium, calcium (Ca2+) influx through plasma membrane Ca2+-permeable channels plays a fundamental role in several physiological functions and in the pathogenesis of cardiovascular disease. Current knowledge on the influence of Ca2+ influx in signaling events associated to endothelial dysfunction has grown significantly over recent years, particularly after identification of members of the Transient Receptor Potential Canonical (TRPC) family of channel forming proteins as prominent mediators of Ca2+ entry in endothelial cells. Among TRPC members TRPC3 has been at the center of many of these physiopathological processes. Progress in elucidating the mechanism/s underlying regulation of endothelial TRPC3 and characterization of signaling events downstream TRPC3 activation are of most importance to fully appreciate the role of this peculiar cation channel in cardiovascular disease and its potential use as a therapeutic target. In this updated review we focus on TRPC3 channels, revising and discussing current knowledge on channel expression and regulation in endothelium and the roles of TRPC3 in cardiovascular disease in relation to endothelial dysfunction.
Keywords: TRPC3 channels, endothelial dysfunction, cardiovascular disease, cation channels, Ca2+ influx
INTRODUCTION
Calcium (Ca2+) influx through plasma membrane Ca2+-permeable channels exerts a myriad of roles in Ca2+-dependent signaling associated to endothelial cell physiology and pathophysiology (reviewed in [1–4]). Indeed, changes in intracellular Ca2+ levels associated to receptor-regulated Ca2+ influx have a profound impact on diverse endothelial functions [1,5,6] including short and long term cellular responses, such as activation of kinases, proteases, synthesis and release of vasoactive molecules, inflammatory signaling and gene transcription.
Thrombin, nucleotides, bradykinin, prostaglandins and vascular endothelial growth factor (VEGF), among many others, bind to their receptors on the endothelial cell membrane to promote Ca2+ influx, in most instances as part of a biphasic Ca2+ response composed of Ca2+ release from endogenous stores and Ca2+ influx from the outside. Among those, VEGF is well recognized for its transcendental role in regulating several aspects of endothelial cell function, such as proliferation, migration and nitric oxide generation, to name a few, not only under physiological conditions but also during the course of diseases for which endothelial dysfunction is a hallmark of the associated vascular complication, such as diabetes, atherosclerosis and metabolic syndrome (see [7] and references therein). The Ca2+ influx phase can take place immediately or after a short delay following receptor stimulation and it can be required early, late or continuously throughout the signaling process in order to attain a proper Ca2+-dependent response. Modulation of the expression level and/or function of Ca2+-permeable channels is followed by changes in critical endothelial functions such as regulation of vascular tone, permeability and angiogenesis. This is not surprising considering the wide repertoire of signaling events that can directly or indirectly be affected by Ca2+ influx. Transient Receptor Potential Canonical (TRPC) channels are now recognized among the most important Ca2+-permeable cation channels in vascular endothelium. Regardless of the activating mechanism for each TRPC member (TRPC1-7, vide infra) there is no question about their participation in Ca2+ influx associated with endothelial cell physiology [8,9]. Importantly, a significant amount of experimental evidence indicates that TRPCs take part in the pathogenesis of cardiovascular disease (CVD; [10–12]).
Whereas understanding the mechanism/s underlying activation of endothelial TRPC channels under physiological conditions is critical to define their roles in cardiovascular physiology and disease, identifying and characterizing Ca2+-dependent events downstream to TRPC-mediated Ca2+ entry is of equal importance. In our first review on this topic [13] we discussed the existing evidence regarding the role of members of the TRPC3/6/7 subfamily in CVD within the context of endothelial dysfunction. In this updated review, we center our discussion on TRPC3, including most recent findings indicating a key role of this channel in inflammatory vascular disease.
STRUCTURAL AND FUNCTIONAL FEATURES OF TRPC3 CHANNELS
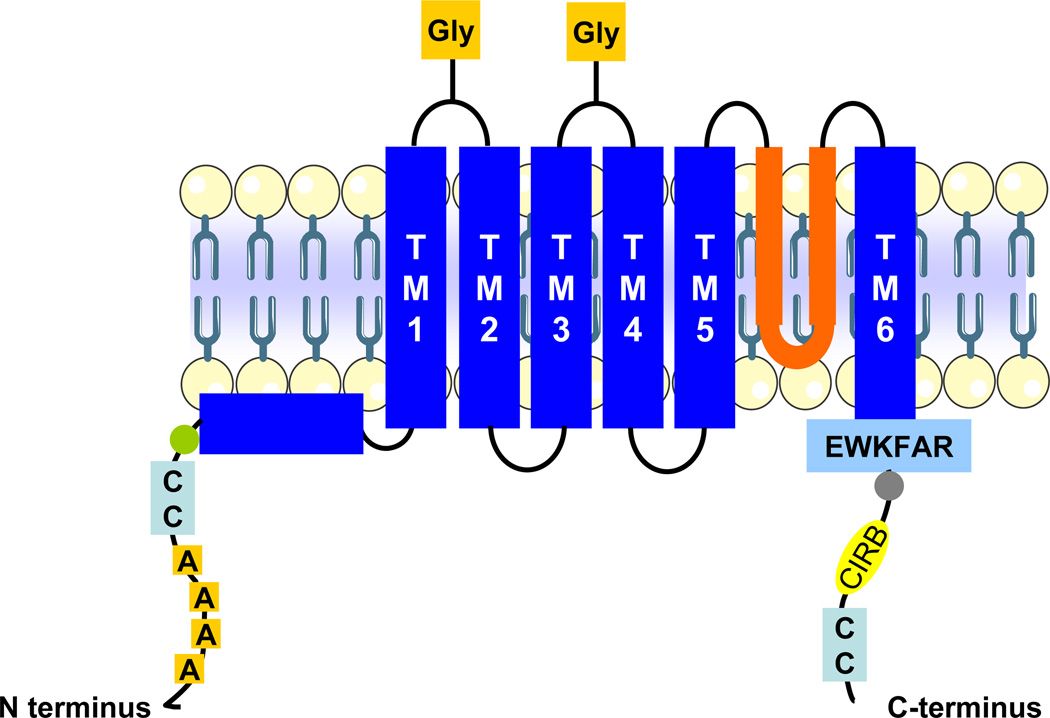
TRPC3 and its close relatives TRPC6 and 7 form a subcategory of the TRPC family of cation channel forming proteins, which are part of the larger TRP superfamily ([14–16] and references therein). Subdivision of the TRPC group into TRPC1, TRPC2, TRPC3/6/7 and TRPC4/5, is for the most part based on structural and pharmacological similarities. TRPC2 forms functional channels in rodents and other mammals but is a pseudogene in humans, old world monkeys and apes. Similarly to other members in the TRPC group, TRPC3 exhibits a general membrane topology that includes six transmembrane domains (TM1-TM6) between the cytoplasmic N- and C-termini, and a putative pore region between TM5 and TM6 (see Figure 1) [17,18]. Four ankyrin repeats in the N-terminus precede a coiled-coil region and a caveolin binding domain. Ankyrin repeats fold into a helix-loop-helix-beta-hairpin/loop structure with a stack of several repeats that form a single domain with a protein-binding interface that fits a variety of binding partners; these mediate proper oligomeric arrangement of TRPC3. Indeed, deletion of ankyrin repeats results in accumulation of TRPC3 in intracellular compartments ([16] and references therein).
Figure 1.
Membrane topology of TRPC3 showing salient structural features. This topological and structural features are shared by all three TRPC3/6/7 proteins, with six predicted transmembrane domains (TM1-TM6), cytoplasmic N- and C-termini and a putative pore region (“pore”, delineated by the re-entry loop between TM5-TM6). The ankyrin repeats (“A” in boxes) on the N-terminus precede the coiled-coil (“CC”) and caveolin binding (circle) regions. Salient features on the C-terminus include the TRP signature motif (EWKFAR sequence), a highly conserved proline rich motif (circle), a CIRB (calmodulin/IP3 receptor binding) domain and another coiled-coil region. The glycosylation site (Gly) in the first extracellular loop is present in TRPC3/6/7 whereas that in the second extracellular loop is present in TRPC6/7. Functional TRPC3 channels are formed by homo- or hetero-tetrameric arrangements of four TRPC proteins (see text for details).
The C-terminus exhibits the conserved EWKFAR sequence (TRP signature motif), a conserved proline rich motif, a calmodulin/IP3 receptor (CIRB) binding domain and a coiled-coil region. Both in native and heterologous expression systems functional TRPC3 channels are made of homo- or hetero (v.g., with TRPC6 or 7)-tetrameric arrangements of four TRPC subunits [16,17,19]; protein-protein interactions occur between specific domains located in the N-terminus, pore region and C-terminal domains [20,21]. Although certain specificity of assembly for different TRPC members has been shown –v.g., TRPC3, 6 and 7 tend to preferentially interact among them rather than with other TRPC members ([16] and references therein)-, other combinations do occur. For instance, in porcine aortic endothelial cells (PAECs) native TRPC3 and 4 associate to form functional cation channels [22]. As is the case with other properties (vide infra) combinatorial alternatives under native expression conditions do not necessarily recapitulate those observed in overexpression systems. The possibility that yet to be known ancillary molecules are required in the assembling process makes it likely that homo- or hetero-tetrameric arrangements of TRPC3 occur in different cell types depending on expression level and/or repertoire of both TRPC and ancillary proteins.
TRPC3 EXPRESSION IN ENDOTHELIAL CELLS
Most endothelial cells express all members of the TRPC family, i.e., TRPC1 through 7 [9,23]; TRPC2 is a pseudogene in humans and thus is not expressed in endothelial cells of that origin. Nevertheless, most studies evaluated the presence of RNA message (mRNA) and a systematic examination at the protein level is not available. Yip et al. [24] provided the first expression analysis of TRPC homologs in different sized human intact vessels. Using in situ hybridization these authors showed that TRPC1, 3–6 are abundantly expressed in endothelium and tunica media from cerebral and coronary arteries, with TRPC7 being present in endothelium but not in smooth muscle cells. A similar expression pattern was observed in small sized coronary arterioles and coronary artery vasa vasorum [24] and importantly, the mRNA expression pattern for TRPC1, 3–6 was confirmed at the protein level by immunohistochemistry of arterial cross sections. Recent work from our lab confirmed, at the protein level, expression of all TRPCs in primary cultures of human coronary artery endothelial cells (HCAECs, [25]) in line with the findings of Yip et al. [24] in intact coronary vessels. A thorough evaluation of the expression of twenty two TRP genes has recently been conducted in mouse by means of RT-PCR and in situ hybridization [26]. While a systematic analysis of vessels from different vascular beds was not performed, that study shows that TRPC3 is particularly abundant in aortic tunica muscularis and endothelium of C57BL/10, Balb/c and NOD mice. Information on mRNA levels however cannot be directly extrapolated to protein abundance, and sometimes mRNA profiles poorly reflect the channel protein repertoire of the plasma membrane. Differences in the turn-over rates of message versus protein and/or regulation of channel trafficking and membrane insertion (vide infra) may affect TRPC abundance in the plasma membrane at a given time. Information at the protein level has also been somewhat limited and/or questionable by the variable success attained with commercially available antibodies. At any rate, whenever available, information on protein expression of TRPC3 varies significantly depending upon the cell line and/or the vascular bed where cells are derived from. The differential expression pattern of TRPC3 and other TRPC proteins in human endothelial cells from different regional locations in the vascular tree has been recently discussed by us in [13] (see also [27]). Of relevance to the potential roles of TRPC3 in coronary artery disease, coronary artery endothelial cells of human origin (HCAECs), despite the presence of message for all TRPCs [24,25] show prominent levels of TRPC3 protein compared to other members of the TRPC family [25]. Another variable relates to the fact that most studies using primary endothelial cell cultures do not state the passage number of the cells used for experimentation. This is important, as the possibility that primary cells become adoptive to the culture conditions and therefore, that changes in TRPC expression pattern occur, should be considered.
We have observed this in HCAECs, where TRPC6 expression varies dramatically with passage number and culture conditions, while TRPC1 is downregulated to almost undetectable levels when cells are exposed to pro-inflammatory conditions [28]. This is of particular importance when using endothelial cells isolated from diseased arteries, as culture-dependent changes in TRPC expression may mask differences that originally existed between normal and pathological cells in the intact tissue. In that context, immunohistochemical analysis of TRPC3 expression in tissue sections from normal versus diseased subjects are likely to provide more relevant information regarding in vivo expression levels than immunoblotting studies on lysates from cultured cells, serving as well as a reference to validate in vitro studies on cell cultures.
REGULATION OF ENDOTHELIAL TRPC3 CHANNELS
G-protein coupled (GPCR) or receptor tyrosine kinase (RTK) receptors can promote Ca2+ responses through activation of phosphoinositide-specific phospholipase C (PI-PLC) of the β or γ type, respectively. This results in generation of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG); IP3 induces Ca2+ release from endogenous Ca2+ stores and a transient increase in cytosolic Ca2+ concentration. In most instances, this is accompanied or followed by Ca2+ entry through plasma membrane Ca2+ channels what provides a source for sustained elevations in cytosolic Ca2+ levels. As is the case in most non-excitable cells, Ca2+ entry in endothelial cells occurs, for the most part, by two major routes: store-operated Ca2+ (SOC) entry (SOCE, triggered by depletion of Ca2+ stores) and/or non-store-operated Ca2+ (non-SOC) entry (non-SOCE; [1,7]). TRPC3 is activated downstream PI-PLC and can mediate both SOCE and non-SOCE under physiological conditions of receptor stimulation [14]. Consequently, TRPC3 participates in a diversity of receptor modulated, Ca2+-dependent endothelial functions, such as nitric oxide production, secretion, proliferation and apoptosis [7–9]. Under physiological conditions TRPC3 can also permeate Na+ and thus can mediate changes in membrane potential modulating the driving force for Ca2+ entry and/or the activity of voltage-gated Ca2+ channels. Indeed, TRPC3-mediated Na+ influx has been shown to trigger the reverse mode of the Na+/Ca2+ exchanger (NCX) in HEK293 cells and rat cardiac myocytes, indirectly altering intracellular Ca2+ levels [29,30]. In the axial component of the transverse tubular system in rat ventricular myocytes TRPC3 forms a signaling complex with NCX and Na+/K+ ATPase [31]; however, operation of similar mechanisms in endothelial cells has not yet been reported.
The discussion below is focused on mechanisms that, directly or indirectly, are known to influence TRPC3 function in endothelium. Regulatory aspects of other TRPCs in endothelial and other cell types have been thoroughly discussed by us [13] and others [15,16,32,33], respectively.
Store-dependent and -independent mechanisms in regulation of TRPC3
In native and heterologous expression systems TRPC3 forms non-voltage gated, non-selective cation channels activated downstream stimulation of PI-PLCβ or PI-PLCγ; although scarcely studied, PLD may also exert a role in receptor-dependent regulation of TRPC3 (discussed by us in [34]). This feature makes TRPC3 –and most TRPCs for that matter- an excellent candidate to mediate SOCE or non-SOCE under physiological conditions of receptor stimulation. While theoretically obvious, this concept has been subject of debate for more than fifteen years, and TRPC3 has been at the center of the controversy [14,16,35,36]. It is however likely that under physiological conditions and depending upon the signaling repertoire of a particular cell type, TRPC3 is subject to modulation by combinations of the two aforementioned mechanisms.
Overall, great deal of our knowledge on the role of TRPCs in endothelial SOCE derives from studies on TRPC1 and 4 ([5,8,37–39], but see [40]). Nevertheless, regulation of endothelial TRPC3 is less clear despite its important role in providing sustained Ca2+ entry in endothelium. In human umbilical vein endothelial cells (HUVECs) for example, SOC currents induced through active (IP3) or passive (thapsigargin) Ca2+ store depletion are abrogated by overexpression of a dominant-negative N-terminal fragment of TRPC3, suggesting that native TRPC3 proteins are part of SOC channels [41]. However, in the HUVEC-derived Ea.hy926 cells, endogenous cation currents with TRPC3-like features are clearly activated by receptor stimulation but not store-depletion [42]. In the latter, adaptation to culture conditions during establishment of the HUVEC-derived cell line may in part account for differences in channel assembly and/or regulation. Comparing SOCE and non-SOCE in uterine artery endothelial cells (UAECs) from pregnant versus non-pregnant ewes, Bird et al. [43] showed that store-operated Ca2+ influx is increased in UAECs derived from pregnant animals compared to non-pregnant controls through a mechanism involving augmented interaction between TRPC3 and type 2 IP3 receptor with no changes in TRPC3 expression [43,44]. If similar changes in regulated Ca2+ signaling and/or TRPC3 coupling mechanism occur in human UAECs remains unknown.
The archetypical store-operated Ca2+-release activated Ca2+ (CRAC) channel results from the integrated function of stromal interaction molecule 1 (STIM1), a Ca2+-binding protein which functions as the sensor of Ca2+ store content, and Orai1 functioning as the channel itself ([45,46] and references therein). Even in instances where a clear store-operated behavior of TRPC3 has been shown, the general channel properties are far from recapitulating those of CRAC channels. But SOC channels represent a heterogeneous group in regards to cation selectivity and pharmacological features [47], and CRAC channels are just a particular case within the SOC category. It is then reasonable that TRPC3, and TRPCs in general, may indeed be part of SOC channels other than CRAC. Notably, some recent studies suggest that STIM1 and Orai proteins interact with TRPCs in instances where the latter are found to be genuinely store-operated ([48–51] and vide infra). [48,51–54]. Whereas those findings relate to the behavior of overexpressed TRPCs in cell types other than endothelial, the recent identification of STIM1 and Orai1 as the fundamental components of SOC/CRAC channels in HUVECs [55] invites to explore the role of these proteins in specific endothelial functions and the possibility that interactions between TRPCs, STIM1 and/or Orais may also take part in endothelium. Liao et al. [51,52] showed that TRPCs functionally interact with Orai1–3 and STIM1 to form SOC channels whose CRAC-like properties seemed determined by the expression level of Orai1. Based on these observations Liao et al. [51,52] proposed that Orai1 is the rate limiting step in determining the store-operated behavior of TRPC1, 3, 6 or 7, which carries the implicit idea that when TRPCs are expressed in excess of the cell’s available Orai1, they function in non-store operated mode [51]. This illustrates the concept introduced by Vazquez et al. [56] that the expression level of a TRPC protein can determine the channel’s ability to operate as SOC or non-SOC. Contrarily, using HEK293 cells stably overexpressing TRPCs DeHaven et al. [57] showed that TRPC1, 3, 5–7 channels are activated in a PI-PLC dependent manner with not participation of STIM1 or Orai1. Clearly, studies aimed at examining potential interactions between STIM1, Orai1 and TRPCs under native expression conditions are needed to validate the relevance of findings derived from overexpression systems.
Regulation by diacylglycerol and phosphorylation
The role of receptor-dependent, PI-PLC-mediated generation of DAG in the activation of members of TRPC3 –and its close relatives TRPC6 and 7– is the most widely accepted –and somewhat assumed- elemental signaling event underlying receptor-dependent activation of TRPC3 [34,36]. Whereas other mechanisms have been proposed, such as store-operation (vide supra), IP3, Ca2+ itself, phosphatidylinositol 4,5-bisphosphate, or regulated membrane trafficking, their operation has not been as consistently established as that of DAG [34,50,58]. Nevertheless, most mechanisms examined so far seem to be severely affected by expression conditions (i.e., native vs. overexpression), experimental assay (v.g., real-time fluorescence vs. patch-clamp) and cell type (i.e., primary vs. immortalized cell lines) among others. The existence of multiple potential phosphorylation sites in the primary sequence of TRPC3 - including consensus motifs for protein kinases A, C, G, myosin light chain kinase and tyrosine kinases- has also pointed to phosphorylation/dephosphorylation as a regulatory mechanism (reviewed in [16]). As mentioned above, there is compelling evidence favoring the notion that PI-PLC-derived DAG is the activating signal for TRPC3 (see also [59] and references therein). Despite the fact that the fatty acid composition of OAG (1-oleoyl-2-acetyl-sn-glycerol) poorly resembles that from endogenous PI-PLC-derived DAG species (predominantly 1-stearoyl-2-arachidonyl-sn-glycerol; recently discussed by us in [34]), this membrane permeable DAG analogue is a traditional pharmacological tool to activate ectopically expressed TRPC3. Notably, in most cell types known to express TRPC3, native channels are generally unresponsive to exogenous OAG [16,33,34]. For instance, whereas HCAECs express TRPC3 there is no detectable cation influx by simply challenging the cells with OAG [60]. The simplest interpretation is that native channels are insensitive to DAG. However, TRPC3 –and its close relatives TRPC6 and 7- is potently inhibited by PKC (discussed by us in [34]) which is massively activated by OAG, masking the ability of OAG to activate native channels. In line with this, when HCAECs are pre-treated with a cocktail of PKC inhibitors, OAG promotes a robust cation influx that is not observed without PKC inhibition [60]. This also suggests that if DAG derived from receptor-stimulated PI-PLC activates native TRPC3 channels, in order to prevent concomitant inhibition by PKC either considerable compartmentalization of the signaling route exists under physiological conditions, or the lipid composition of native DAG species allows for a more fine spatiotemporal control of channel and kinase activation. This has been recently proposed by us in a model where TRPC3’s lipid microenvironment determines the early DAG-dependent activation of the channel and its subsequent PKC-mediated negative modulation [34].
Membrane trafficking
Regulated translocation to and insertion into the plasma membrane of TRPC channels is another regulatory mechanism (reviewed in [61]) and it has been shown to be operational in endothelial cells for TRPC1 [62], TRPC5 [63] and TRPC6 [64,65]; reviewed by us in [13]. It remains to be determined if a similar trafficking mechanism contributes to regulation of TRPC3 in endothelium.
Expression level and constitutive activity
From studies aimed at understanding the discrepancy regarding the ability of TRPC3 to operate as either SOC or non-SOC channel, it became evident that changes in the level of TRPC3 protein within the cell may switch the coupling mechanism underlying channel activation ([56,66], also discussed in [67]). Ectopic expression of TRPC3 in avian B lymphocytes renders channels that can operate as either SOCs or non-SOCs depending on the amount of channel protein. While store-operation was observed only at low TRPC3 expression [56,66], high TRPC3 levels favored the receptor-regulated mode of TRPC3 activation [56,68]. These findings added a novel concept: a TRPC protein functioning in two distinct ways depending on expression level. Recent evidence suggests that such changes may have pathophysiological significance (vide infra). We propose that this may be particularly relevant when one considers channels endowed with significant constitutive, or non-regulated activity (v.g., TRPC3, 7), as increased abundance of the channel in the plasma membrane would impose a gain in constitutive influx that may result in pathological Ca2+-dependent signaling, independently of receptor stimulation. Despite high structural and functional similarity, TRPC3 differs significantly from its close relatives TRPC6 and 7 in regards to constitutive activity. TRPC6 is a tightly regulated receptor-activated cation channel, while TRPC3 exhibits robust constitutive function; to a much lesser extent, TRPC7 is also endowed with measurable constitutive activity [16,36,59]. Channel protein N-glycosylation is a major determinant for differences in constitutive activity, at least between TRPC3 and 6 [69]. Introduction of an additional glycosylated site in the second extracellular loop of TRPC3, which mimics the glycosylation status of TRPC6, markedly reduces TRPC3 constitutive activity, while channel responsiveness to agonists or OAG is preserved [69]. Conversely, removal of a glycosylation site from TRPC6 is sufficient to confer this channel with constitutive activity indistinguishable from that of TRPC3. Are there in vivo examples of biological consequences of TRPC3 constitutive activity? The best available case derives from studies on TRPC6 knockout mice, in which compensatory upregulated expression of TRPC3 results in elevated contractility of aorta and cerebral arteries [70]. In mouse skeletal myocytes TRPC3 expression is upregulated by neuromuscular activity in a calcineurin-dependent manner, and enhanced expression of TRPC3 facilitates NFAT activity [71] in the absence of receptor stimulation, suggesting that the gain in constitutively active channels drives Ca2+-dependent activation of NFAT. Finally, in GABA projection neurons of the substantia nigra pars reticulata from C57BL/6 mice [72] Na+ permeation through constitutively active TRPC3 mediates the membrane depolarization that triggers the firing of action potentials. Are there similar examples in endothelial cells? In HCAECs activation of TNFα or P2Y2 receptors [25,28] induces expression of TRPC3, and the subsequent gain in TRPC3-dependent constitutive cation influx is obligatory in the signaling underlying regulated expression of vascular cell adhesion molecule-1 (VCAM-1; vide infra).
AN OVERVIEW ON TRPC3 CHANNELS IN CARDIOVASCULAR DISEASE
A significant amount of experimental data derived from in vitro and in vivo studies shows that TRPC3 participates in several signaling events associated with the pathogenesis of CVD (reviewed by us in [13,73]). Ca2+ entry through TRPC3 contributes to the Ca2+ signaling associated to cardiac hypertrophy (reviewed in [74]. TRPC3 has been proposed to participate in immune responses mediated by B and T lymphocytes [66,75] what might be of relevance to the pathogenesis of inflammatory vascular disease. Expression of TRPC3, by yet to be known mechanisms, is upregulated in monocytes from spontaneously hypertensive rats [76,77] and this correlates with increased regulated Ca2+ influx. Similarly, increased store-operated and DAG-regulated Ca2+ influx parallel augmented expression of TRPC3 -and 5- in circulating monocytes from patients with essential hypertension [78,79]. Constitutively active TRPC3 is upregulated in TRPC6 knockout mice and is responsible for enhanced contractility of aorta and cerebral arteries and elevated blood pressure [70]. The section below revises existing evidence linking TRPC3 to CVD as it relates to endothelial dysfunction. We recently reviewed literature documenting the role of TRPC6 and 7 in CVD [13] and information on the role of TRPs in general and TRPCs other than TRPC3/6/7 in CVD can be found in [9,12,80,81].
TRPC3 AND ENDOTHELIAL DYSFUNCTION
The endothelium is an extremely versatile and multifunctional tissue committed to an enormous variety of functions. Endothelial cells participate in regulation of vascular tone, anti- and pro-thrombotic events, and regulation of inflammation, immunity and neovascularization, to name a few [82]. Furthermore, the endothelium exhibits considerable phenotypic variability depending upon anatomic site, and dynamic adaptation to local environmental factors. Endothelial dysfunction is defined as an imbalance of one or more of the endothelial duties required for maintenance of vascular homeostasis, and occurs in most, if not all, states of CVD. Because most endothelial functions are interconnected and interdependent, the dysfunctional state is usually manifested as an altered phenotype that revolves around a combination of oxidative stress, inflammation, thrombosis and impaired nitric oxide (NO) production and/or bioavailability.
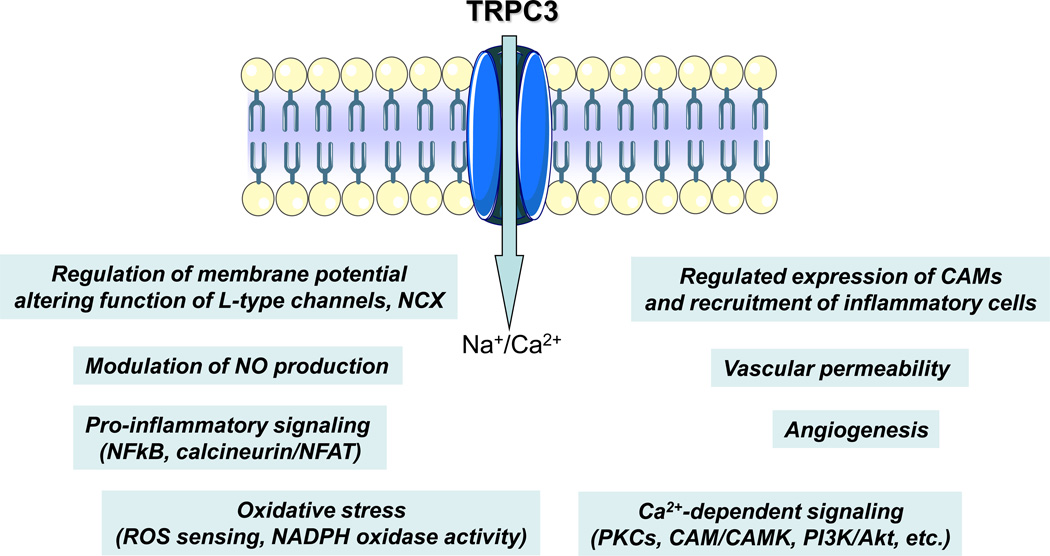
Expression and function of endothelial TRPC3 has been associated, directly or indirectly, to a variety of signaling processes associated to endothelial function, dysfunction and endothelial-related vascular disease (Table 1; see also Figure 2). There is evidence indicating that endothelial TRPC3 participates not only in normal redox functions but also in oxidative stress, which is known to link vascular inflammation and redox imbalance. Using a dominant-negative N-terminal fragment of TRPC3, Balzer et al. [83] showed that native TRPC3 proteins in pulmonary artery endothelial cells (PAECs) form, or are part of, channels mediating oxidative-stress. The studies by Balzer et al. [83] also suggest that TRPC3 is likely the protein responsible for the molecular make up of a redox-sensitive channel identified in calf pulmonary artery endothelial cells, where a tert-butylhydroperoxide-activated non-selective cation conductance mediates Na+ influx and the subsequent breakdown of membrane potential [84,85]. Subsequent work from the same group suggests that in PAECs native TRPC3 associates with TRPC4 to form the redox-sensitive channel [22]. The mechanism by which oxidative stress activates TRPC3-based channels remains largely unknown, but redox-dependent modifications of lipids in the channel’s microdomains or oxidation of critical sulfhydryls on the channel protein, are some possibilities [83,86]. For a discussion on the role of other TRP channels in oxidative stress see [87] and references therein.
| Endothelial cell type or vascular bed |
Role of TRPC3 | Reference |
|---|---|---|
| Porcine coronary endothelial (PCAEC); uterine endothelial (UAEC) | Ca2+ influx underlying nitric oxide release | [94, 95] |
| Human pre-glomerular endothelium | Increased expression in patients with malignant hypertension | [92] |
| Human kidney vascular endothelium | Increased expression in renal cell carcinoma patients with history of hypertension | [93] |
| Human coronary artery endothelial (HCAEC) | Regulated expression of VCAM-1; monocyte recruitment; NFkB signaling | [25, 28] |
| Pulmonary aortic endothelial (PAEC) | Forms redox sensitive channels; normal and oxidative stress-induced depolarization | [22, 83] |
| Calf vascular endothelial | Oxidative stress induced membrane depolarization | [85] |
Figure 2.
Summary of most salient endothelial signaling processes known to be, directly or indirectly, affected by the expression/function of TRPC3 channels. NCX: Na+/Ca2+ exchanger; NO: nitric oxide; CAMs: cell adhesion molecules; PKCs: protein kinase C; CAM: calmodulin; CAMK: calmodulin-dependent kinase; PI3K: phosphatidylinositol-3-kinase; NFκB: nuclear factor kappa B; NFAT: nuclear factor of activated T cells; ROS: reactive oxygen species.
Regulated expression of endothelial cell adhesion molecules (CAMs) and recruitment of circulating leukocytes to the arterial intima constitutes one of the earliest molecular/cellular events in the pathogenesis of inflammatory vascular disease such as atherosclerosis [88]. In HCAECs pro-atherogenic actions of ATP include induced expression of VCAM-1, a critical CAM mediating monocyte adhesion and transendothelial migration [89], through a mechanism that requires P2Y2 receptor activation and changes in intracellular Ca2+ levels [90,91]. In a recent study, we showed that in HCAECs native TRPC3 forms, or is part of, native Ca2+-permeable channels that contribute to ATP-stimulated Ca2+ influx [25], and that TRPC3 expression and constitutive function are obligatory for ATP-induced VCAM-1 and monocyte adhesion, underscoring a potential novel function of TRPC3 in atherogenesis. TNFα, an atherorelevant cytokine that promotes proinflammatory signaling in HCAECs but without inducing regulated-Ca2+ influx, also exhibits an obligatory requirement for TRPC3 expression, confirming that it is the constitutive, non-regulated function of the channel what contributes to the signaling underlying regulated-expression of VCAM-1 and monocyte recruitment [28]. A negative correlation between TRPC3 expression and VEGF-dependent proliferation of HUVECs and the HUVEC-derived cell line EAhy926 was recently described [42].
Information on the role of endothelial TRPC3 in endothelial dysfunction related to hypertension is scarce. A positive correlation has been found in the expression of TRPC3 with increased store-operated and DAG-regulated Ca2+ influx in monocytes isolated from patients with essential hypertension [77,79]. This prompted the authors to examine if TRPC3 levels were altered in malignant hypertension. Essential hypertension is indeed an independent risk factor for malignant hypertension, which develops in approximately one percent of patients with essential hypertension. Thilo et al. [92] evaluated expression of endothelial TRPC3 and 6 proteins by immunostaining cross sections from pre-glomerular arterioles obtained from six patients with malignant hypertension. They found that TRPC3, but not TRPC6, was significantly higher in malignant hypertension specimens compared to samples from patients with diarrhea-associated hemolytic-uremic syndrome, a disease that also presents with endothelial injury/dysfunction but of different pathogenesis [92]. More recently, the same group examined expression of TRPC3 in vascular endothelium of normal renal tissue obtained from patients with renal cell carcinoma and found that TRPC3 is augmented in patients with a history of systolic blood pressure (SBP) >140 mm Hg but not in those with SBP ≤140 mm Hg [93]. Although these findings suggest an association between TRPC3 expression and hypertension, the question remains whether increased TRPC3 is a contributing factor to the pathogenesis of the disease rather than the consequence of an altered endothelial phenotype.
Evidence on the modulation of NO bioavailability as a mechanism linking endothelial TRPC3 function with vasorelaxation and blood pressure is not yet available. In a recent study, Huang et al. [94] hypothesized that in endothelium TRPC3-mediated Ca2+ influx contributes to release of NO release and that hypoxia-reoxygenation may impair TRPC3 activity with the subsequent reduction in NO release. Using a combination of electrophysiological and pharmacological approaches, these authors found that blocking TRPC3 function with SKF96365 or Pyr3 reduced bradykinin dependent vasorelaxation in porcine coronary arteries; these inhibitory effects were recapitulated by hypoxia-reoxygenation, which was also shown to reduce bradykinin-induced TRPC3-mediated Ca2+ influx and, notably, surface expression of TRPC3 [94]. These studies showed for the first time that endothelial TRPC3 contributes to NO release and that TRPC3 expression and function are downregulated by hypoxia-reoxygenation, suggesting that TRPC3 might be an attractive new target for endothelial protection in hypoxia-reoxygenation.
As mentioned above, store-operated, TRPC3-mediated Ca2+ influx is increased in UAECs from pregnant ewes compared to non-pregnant animals [43,44]. In a recent follow up study, Bird and colleagues [95] showed that pregnancy-enhanced TRPC3-mediated Ca2+ influx is fundamental to support ATP-dependent stimulation of eNOS and subsequent production of NO.
Altered endothelial permeability is known to contribute to the pathogenesis of CVD. Both transcellular and paracellular pathways are involved in regulation of endothelial barrier function, and TRPCs have been linked to those processes, particularly TRPC1 and 4 ([2]; reviewed in [96]); however, studies on TRPC3 – and of members of the TRPC3/6/7 group in general- are limited.
CONCLUDING REMARKS
Over the last decade our understanding on the role of TRPC channels in vascular function and CVD has grown tremendously, and TRPC3 in particular has been the subject of several in vitro and in vivo studies. However, knowledge on the roles of TRPC3 in pathophysiological processes related to endothelial function is still limited. This altogether with the lack of channel blockers specific enough to be used in vivo, has slowed down progress regarding the roles of TRPC3 in endothelial-related CVD. To fill that gap in knowledge, we need to understand not only the mechanism/s regulating native TRPC3 function in the context of endothelial signaling, but also to identify molecular/cellular events downstream channel activation. Development of endothelial-specific rather than global transgenic and knockout animals will provide a unique opportunity to assess the contribution of TRPC3 to the pathogenesis of endothelial-related CVD within the context of the multiple events that occur in vivo in intact vessels.
ACKNOWLEDGMENTS
Supported by University of Toledo College of Medicine, American Heart Association grant SDG0635250N (to GV) and R01HL111877-01 (to GV).
REFERENCES
- 1.Tran QK, Watanabe H. Calcium signaling in the endothelium. Handb Exp Pharmacol. 2006;176:145. doi: 10.1007/3-540-32967-6_5. [DOI] [PubMed] [Google Scholar]
- 2.Minshall RD, Malik AB. Transport across the endothelium: regulation of endothelial permeability. Handb. Exp. Pharmacol. 2006;176:107. doi: 10.1007/3-540-32967-6_4. [DOI] [PubMed] [Google Scholar]
- 3.Tran Q-K, Ohashi K, Watanabe H. Calcium signaling in endothelial cells. Cardiovasc. Res. 2000;48:13. doi: 10.1016/s0008-6363(00)00172-3. [DOI] [PubMed] [Google Scholar]
- 4.Isshiki M, Anderson RGW. Calcium signal transduction from caveolae. Cell Calcium. 1999;26:201. doi: 10.1054/ceca.1999.0073. [DOI] [PubMed] [Google Scholar]
- 5.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vasc. Pharmacol. 2002;39:173. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 6.Nilius B, Droogmans G. Ion Channels and Their Functional Role in Vascular Endothelium. Physiol. Rev. 2001;81:1415. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- 7.Petrovic D. The role of vascular endothelial growth factor gene as the genetic marker of atherothrombotic disorders and in the gene therapy of coronary artery disease. Cardiovasc Hematol Agents Med Chem. 2010;8:47. doi: 10.2174/187152510790796183. [DOI] [PubMed] [Google Scholar]
- 8.Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium. 2003;10:5. doi: 10.1080/10623320303356. [DOI] [PubMed] [Google Scholar]
- 9.Yao X, Garland CJ. Recent Developments in Vascular Endothelial Cell Transient Receptor Potential Channels. Circ. Res. 2005;97:853. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 10.Nilius B, Owsianik G, Voets T, Peters JA. Transient Receptor Potential Cation Channels in Disease. Physiol. Rev. 2007;87:165. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- 11.Abramowitz J, Birnbaumer L. Physiology and pathophysiology of canonical transient receptor potential channels. FASEB J. 2009;23:297. doi: 10.1096/fj.08-119495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietrich A, Kalwa H, Fuchs B, Grimminger F, Weissmann N, Gudermann T. In vivo TRPC functions in the cardiopulmonary vasculature. Cell Calcium. 2007;42:233. doi: 10.1016/j.ceca.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Tano JY, Smedlund K, Vazquez G. Endothelial TRPC3/6/7 proteins at the edge of cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2010;8:76. doi: 10.2174/187152510790796138. [DOI] [PubMed] [Google Scholar]
- 14.Birnbaumer L. The TRPC Class of Ion Channels: A Critical Review of Their Roles in Slow, Sustained Increases in Intracellular Ca2+ Concentrations. Ann. Rev. Pharmacol. Toxicol. 2009;49:395. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- 15.Gudermann T, Hofmann T, Mederos y Schnitzler M, Dietrich A. Activation, subunit composition and physiological relevance of DAG-sensitive TRPC proteins. Novartis Found. Symp. 2004;258:103. [PubMed] [Google Scholar]
- 16.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney J, James W. The mammalian TRPC cation channels. Biochim. Biophys. Acta - Mol Cell Res. 2004;1742:21. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Birnbaumer L, Zhu X, Jiang M, Boulay G, Peyton M, Vannier B, Brown D, Platano D, Sadeghi H, Stefani E, Birnbaumer M. On the molecular basis and regulation of cellular capacitative calcium entry: Roles for Trp proteins. Proc. Natl. Acad. Sci. USA. 1996;93:15195. doi: 10.1073/pnas.93.26.15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vannier B, Zhu X, Brown D, Birnbaumer L. The membrane topology of human transient receptor potential 3 as inferred from glycosylation-scanning mutagenesis and epitope immunocytochemistry. J. Biol. Chem. 1998;273:8675. doi: 10.1074/jbc.273.15.8675. [DOI] [PubMed] [Google Scholar]
- 19.Mio K, Ogura T, Hara Y, Mori Y, Sato C. The non-selective cation-permeable channel TRPC3 is a tetrahedron with a cap on the large cytoplasmic end. Biochem. Biophys. Res. Commun. 2005;333:768. doi: 10.1016/j.bbrc.2005.05.181. [DOI] [PubMed] [Google Scholar]
- 20.Lepage PK, Lussier MP, Barajas-Martinez H, Bousquet SM, Blanchard AP, Francoeur N, Dumaine R, Boulay G. Identification of Two Domains Involved in the Assembly of Transient Receptor Potential Canonical Channels. J. Biol. Chem. 2006;281:30356. doi: 10.1074/jbc.M603930200. [DOI] [PubMed] [Google Scholar]
- 21.Lepage PK, Lussier MP, McDuff F-O, Lavigne P, Boulay G. The self-association of two N-terminal interaction domains plays an important role in the tetramerization of TRPC4. Cell Calcium. 2009;45:251. doi: 10.1016/j.ceca.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Poteser M, Graziani A, Rosker C, Eder P, Derler I, Kahr H, Zhu MX, Romanin C, Groschner K. TRPC3 and TRPC4 Associate to Form a Redox-sensitive Cation Channel: evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J. Biol. Chem. 2006;281:13588. doi: 10.1074/jbc.M512205200. [DOI] [PubMed] [Google Scholar]
- 23.Kwan HY, Huang Y, Yao X. TRP channels in endothelial function and dysfunction. Biochim. Biophys. Acta. 2007;1772:907. doi: 10.1016/j.bbadis.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Yip H, Chan WY, Leung PC, Kwan HY, Liu C, Huang Y, Michel V, Yew DT, Yao X. Expression of TRPC homologs in endothelial cells and smooth muscle layers of human arteries. Histochem. Cell Biol. 2004;122:553. doi: 10.1007/s00418-004-0720-y. [DOI] [PubMed] [Google Scholar]
- 25.Smedlund K, Vazquez G. Involvement of Native TRPC3 Proteins in ATP-Dependent Expression of VCAM-1 and Monocyte Adherence in Coronary Artery Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:2049. doi: 10.1161/ATVBAHA.108.175356. [DOI] [PubMed] [Google Scholar]
- 26.Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H. Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics. 2006;7:159. doi: 10.1186/1471-2164-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietrich A, Kalwa H, Gudermann T. TRPC channels in vascular cell function. Thromb. Haemost. 2010;103:262. doi: 10.1160/TH09-08-0517. [DOI] [PubMed] [Google Scholar]
- 28.Smedlund K, Tano JY, Vazquez G. The Constitutive Function of Native TRPC3 Channels Modulates VCAM-1 Expression in Coronary Endothelial Cells through NFkappaB Signaling. Circ Res. 2010;106:1479. doi: 10.1161/CIRCRESAHA.109.213314. [DOI] [PubMed] [Google Scholar]
- 29.Rosker C, Graziani A, Lukas M, Eder P, Zhu MX, Romanin C, Groschner K. Ca2+ signaling by TRPC3 involves Na+ entry and local coupling to the Na+/Ca2+ exchanger. J. Biol. Chem. 2004;279:13696. doi: 10.1074/jbc.M308108200. [DOI] [PubMed] [Google Scholar]
- 30.Eder P, Probst D, Rosker C, Poteser M, Wolinski H, Kohlwein SD, Romanin C, Groschner K. Phospholipase C-dependent control of cardiac calcium homeostasis involves a TRPC3-NCX1 signaling complex. Cardiovasc. Res. 2007;73:111. doi: 10.1016/j.cardiores.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Goel M, Zuo CD, Sinkins WG, Schilling WP. TRPC3 channels colocalize with Na+/Ca2+ exchanger and Na+ pump in axial component of transverse-axial tubular system of rat ventricle. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H874. doi: 10.1152/ajpheart.00785.2006. [DOI] [PubMed] [Google Scholar]
- 32.Bird GS AO, Lievremont JP, Wedel BJ, Trebak M, Vazquez G, Putney JW., Jr Mechanisms of phospholipase C-regulated calcium entry. Curr. Mol. Med. 2004;4:291. doi: 10.2174/1566524043360681. [DOI] [PubMed] [Google Scholar]
- 33.Trebak M, Lemonnier L, Smyth J, Vazquez G, Putney J. Phospholipase C-Coupled Receptors and Activation of TRPC Channels. Transient Receptor Potential (TRP) Channels. 2007:593–614. doi: 10.1007/978-3-540-34891-7_35. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez G, Tano JY, Smedlund K. On the potential role of source and species of diacylglycerol in phospholipase-dependent regulation of TRPC3 channels. Channels (Austin) 2010;4:232. doi: 10.4161/chan.4.3.12058. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen SF, Owsianik G, Nilius B. TRP channels: An overview. Cell Calcium. 2005;38:233. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 36.Trebak M, Vazquez G, Bird GS, Putney JW., Jr The TRPC3/6/7 subfamily of cation channels. Cell Calcium. 2003;33:451. doi: 10.1016/s0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- 37.Freichel M, Vennekens R, Olausson J, Stolz S, Philipp SE, Weissgerber P, Flockerzi V. Functional role of TRPC proteins in native systems: implications from knockout and knock-down studies. J. Physiol. (Lond) 2005;567:59. doi: 10.1113/jphysiol.2005.092999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4-/- mice interferes with increase in lung microvascular permeability. Circ. Res. 2002;91:70. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- 39.Bair AM, Thippegowda PB, Freichel M, Cheng N, Ye RD, Vogel SM, Yu Y, Flockerzi V, Malik AB, Tiruppathi C. Ca2+ entry via TRPC channels is necessary for thrombin-induced NF-kB activation in endothelial cells through AMP-activated protein kinase and protein kinase Cdelta. J. Biol. Chem. 2009;284:563. doi: 10.1074/jbc.M803984200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potier M, Trebak M. New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflugers Arch. 2008;457:405. doi: 10.1007/s00424-008-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Groschner K, Hingel S, Lintschinger B, Balzer M, Romanin C, Zhu X, Schreibmayer W. Trp proteins form store-operated cation channels in human vascular endothelial cells. FEBS Lett. 1998;437:101. doi: 10.1016/s0014-5793(98)01212-5. [DOI] [PubMed] [Google Scholar]
- 42.Kamouchi MMA, Droogmans G, Nilius B. Nonselective cation channels in endothelial cells derived from human umbilical vein. J. Membr. Biol. 1999;169:29. doi: 10.1007/pl00005898. [DOI] [PubMed] [Google Scholar]
- 43.Gifford SM, Yi FX, Bird IM. Pregnancy-enhanced Ca2+ responses to ATP in uterine artery endothelial cells is due to greater capacitative Ca2+ entry rather than altered receptor coupling. J. Endocrinol. 2006;190:373. doi: 10.1677/joe.1.06635. [DOI] [PubMed] [Google Scholar]
- 44.Gifford SM, Yi FX, Bird IM. Pregnancy-enhanced store-operated Ca2+ channel function in uterine artery endothelial cells is associated with enhanced agonist-specific transient receptor potential channel 3-inositol 1,4,5-trisphosphate receptor 2 interaction. J. Endocrinol. 2006;190:385. doi: 10.1677/joe.1.06773. [DOI] [PubMed] [Google Scholar]
- 45.Vig M, Kinet JP. The long and arduous road to CRAC. Cell Calcium. 2007;42:157. doi: 10.1016/j.ceca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potier M, Trebak M. New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflugers Arch. 2008;457:405. doi: 10.1007/s00424-008-0533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parekh AB, Putney JW., Jr Store-Operated Calcium Channels. Physiol. Rev. 2005;85:757. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 48.Worley PF, Zeng W, Huang GN, Yuan JP, Kim JY, Lee MG, Muallem S. TRPC channels as STIM1-regulated store-operated channels. Cell Calcium. 2007;42:205. doi: 10.1016/j.ceca.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as storeoperated channels. Nat. Cell Biol. 2007;9:636. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smyth JT, DeHaven WI, Jones BF, Mercer JC, Trebak M, Vazquez G, Putney JW. Emerging perspectives in store-operated Ca2+ entry: Roles of Orai, Stim and TRP. Biochim. Biophys. Acta – Mol. Cell Res. 2006;1763:1147. doi: 10.1016/j.bbamcr.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 51.Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong DL, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc. Natl. Acad. Sci. USA. 2007;104:4682. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liao Y, Erxleben C, Abramowitz J, Flockerzi V, Zhu MX, Armstrong DL, Birnbaumer L. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc. Natl. Acad. Sci. 2008;105:2895. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan JP, Kim MS, Zeng W, Shin DM, Huang G, Worley PF, Muallem S. TRPC channels as STIM1-regulated SOCs. Channels (Austin) 2009;3:221. doi: 10.4161/chan.3.4.9198. [DOI] [PubMed] [Google Scholar]
- 54.Potier M, Gonzalez JC, Motiani RK, Abdullaev IF, Bisaillon JM, Singer HA, Trebak M. Evidence for STIM1- and Orai1-dependent storeoperated calcium influx through Icrac in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 2009;23:2425. doi: 10.1096/fj.09-131128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdullaev IF, Bisaillon JM, Potier M, Gonzalez JC, Motiani RK, Trebak M. Stim1 and Orai1 Mediate CRAC Currents and Store-Operated Calcium Entry Important for Endothelial Cell Proliferation. Circ. Res. 2008;103:1289. doi: 10.1161/01.RES.0000338496.95579.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vazquez G, Wedel BJ, Trebak M, St John Bird G, Putney JW., Jr Expression level of the canonical transient receptor potential 3 (TRPC3) channel determines its mechanism of activation. J. Biol. Chem. 2003;278:21649. doi: 10.1074/jbc.M302162200. [DOI] [PubMed] [Google Scholar]
- 57.DeHaven W, Jones B, Petranka J, Smyth J, Tomita T, Bird G, Putney JW., Jr RPC channels function independently of STIM1 and Orai1. J. Physiol. 2009;587:2275. doi: 10.1113/jphysiol.2009.170431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lemonnier L, Trebak M, Putney JW., Jr Complex regulation of the TRPC3, 6 and 7 channel subfamily by diacylglycerol and phosphatidylinositol-4,5-bisphosphate. Cell Calcium. 2008;43:506. doi: 10.1016/j.ceca.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dietrich A, Kalwa H, Rost BR, Gudermann T. Pflugers Archiv. The diacylgylcerol-sensitive TRPC3/6/7 subfamily of cation channels: functional characterization and physiological relevance. Eur. J. Physiol. 2005;451:72. doi: 10.1007/s00424-005-1460-0. [DOI] [PubMed] [Google Scholar]
- 60.Vazquez G, Putney JW., Jr Role of Canonical Transient Receptor Potential Channels (TRPC) in Receptor-Dependent Regulation of Vascular Cell Adhesion Molecule-1 In Human Coronary Artery Endothelium. Arterioscler. Thromb. Vasc. Biol. 2006;26:93. [Google Scholar]
- 61.Cayouette S, Boulay G. Intracellular trafficking of TRP channels. Cell Calcium. 2007;42:225. doi: 10.1016/j.ceca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 62.Mehta D, Ahmmed GU, Paria BC, Holinstat M, Voyno- Yasenetskaya T, Tiruppathi C, Minshall RD, Malik AB. RhoA Interaction with Inositol 1,4,5-Trisphosphate Receptor and Transient Receptor Potential Channel-1 Regulates Ca2+ Entry: role in signaling increased endothelial permeability. J. Biol. Chem. 2003;278:33492. doi: 10.1074/jbc.M302401200. [DOI] [PubMed] [Google Scholar]
- 63.Chaudhuri P, Colles SM, Bhat M, Van Wagoner DR, Birnbaumer L, Graham L. Elucidation of a TRPC6-TRPC5 Channel Cascade That Restricts Endothelial Cell Movement. Mol. Biol. Cell. 2008;19:3203. doi: 10.1091/mbc.E07-08-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singh I, Knezevic N, Ahmmed GU, Kini V, Malik AB, Mehta D. G{alpha}q-TRPC6-mediated Ca2+ Entry Induces RhoA Activation and Resultant Endothelial Cell Shape Change in Response to Thrombin. J. Biol. Chem. 2007;282:7833. doi: 10.1074/jbc.M608288200. [DOI] [PubMed] [Google Scholar]
- 65.Fleming I, Rueben A, Popp R, Fisslthaler B, Schrodt S, Sander A, Haendeler J, Falck JR, Morisseau C, Hammock BD, Busse R. Epoxyeicosatrienoic acids regulate Trp channel dependent Ca2+ signaling and hyperpolarization in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2007;27:2612. doi: 10.1161/ATVBAHA.107.152074. [DOI] [PubMed] [Google Scholar]
- 66.Vazquez G, Lievremont J-P, St. J Bird G, Putney JW., Jr Human Trp3 forms both inositol trisphosphate receptor-dependent and receptor-independent store-operated cation channels in DT40 avian B lymphocytes. Proc. Natl. Acad. Sci. USA. 2001;98:11777. doi: 10.1073/pnas.201238198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Putney JW., Jr The enigmatic TRPCs: multifunctional cation channels. Trends Cell Biol. 2004;14:282. doi: 10.1016/j.tcb.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Venkatachalam K, Ma H-T, Ford DL, Gill DL. Expression of Functional Receptor-coupled TRPC3 Channels in DT40 Triple Receptor InsP3 knockout Cells. J. Biol. Chem. 2001;276:33980. doi: 10.1074/jbc.C100321200. [DOI] [PubMed] [Google Scholar]
- 69.Dietrich A, Mederos y Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J. Biol. Chem. 2003;278:47842. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- 70.Dietrich A, Mederos Y Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, L B. Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol. Cell Biol. 2005;25:6980. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenberg P, Hawkins A, Stiber J, Shelton JM, Hutcheson K, Bassel-Duby R, Shin DM, Yan Z, Williams RS. TRPC3 channels confer cellular memory of recent neuromuscular activity. Proc. Natl. Acad. Sci. USA. 2004;101:9387. doi: 10.1073/pnas.0308179101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou F-W, Matta SG, Zhou F-M. Constitutively Active TRPC3 Channels Regulate Basal Ganglia Output Neurons. J. Neurosci. 2008;28:473. doi: 10.1523/JNEUROSCI.3978-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vazquez G. TRPC Channels as Prospective Targets in Atherosclerosis: terra incognita. Front. Biosci. (Schol Ed) 2012;1:157. doi: 10.2741/258. [DOI] [PubMed] [Google Scholar]
- 74.Nishida M, Kurose H. Roles of TRP channels in the development of cardiac hypertrophy. Naunyn Schmiedebergs Arch. Pharmacol. 2008;378:395. doi: 10.1007/s00210-008-0321-8. [DOI] [PubMed] [Google Scholar]
- 75.Philipp S, Strauss B, Hirnet D, Wissenbach U, Mery L, Flockerzi V, Hoth M. TRPC3 mediates T-cell receptor-dependent calcium entry in human T-lymphocytes. J. Biol. Chem. 2003;278:26629. doi: 10.1074/jbc.M304044200. [DOI] [PubMed] [Google Scholar]
- 76.Liu D, Scholze A, Zhu Z, Kreutz R, Wehland-von-Trebra M, Zidek W, Tepel M. Increased Transient Receptor Potential Channel TRPC3 Expression in Spontaneously Hypertensive Rats. Am. J. Hypertens. 2005;18:1503. doi: 10.1016/j.amjhyper.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 77.Liu DY, Scholze A, Kreutz R, Wehland-von-Trebra M, Zidek W, Zhu ZM, Tepel M. Monocytes from spontaneously hypertensive rats show increased store-operated and second messenger-operated calcium influx mediated by transient receptor potential canonical Type 3 channels. Am. J. Hypertens. 2007;20:1111. doi: 10.1016/j.amjhyper.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 78.Liu D, Scholze A, Zhu Z, Krueger K, Thilo F, Burkert A, Streffer K, Holz S, Harteneck C, Zidek W, Tepel M. Transient receptor potential channels in essential hypertension. J. Hypertens. 2006;24:1105. doi: 10.1097/01.hjh.0000226201.73065.14. [DOI] [PubMed] [Google Scholar]
- 79.Liu DY, Thilo F, Scholze A, Wittstock A, Zhao ZG, Harteneck C, Zidek W, Zhu ZM, Tepel M. Increased store-operated and 1-oleoyl-2- acetyl-sn-glycerol-induced calcium influx in monocytes is mediated by transient receptor potential canonical channels in human essential hypertension. J. Hypertens. 2007;25:799. doi: 10.1097/HJH.0b013e32803cae2b. [DOI] [PubMed] [Google Scholar]
- 80.Liu D, Zhu Z, Tepel M. The role of transient receptor potential channels in metabolic syndrome. Hypertens. Res. 2008;31:1989. doi: 10.1291/hypres.31.1989. [DOI] [PubMed] [Google Scholar]
- 81.Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ. Res. 2006;99:119. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 82.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am. J. Physiol. Heart Circ. Physiol. 2006;291:H985. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 83.Balzer M, Lintschinger B, Groschner K. Evidence for a role of Trp proteins in the oxidative stress-induced membrane conductances of porcine aortic endothelial cells. Cardiovasc. Res. 1999;42:543. doi: 10.1016/s0008-6363(99)00025-5. [DOI] [PubMed] [Google Scholar]
- 84.Koliwad SK, Elliott SJ, Kunze DL. Oxidized glutathione mediates cation channel activation in calf vascular endothelial cells during oxidant stress. J. Physiol. 1996;495:37. doi: 10.1113/jphysiol.1996.sp021572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koliwad SK, Kunze DL, Elliott SJ. Oxidant stress activates a non-selective cation channel responsible for membrane depolarization in calf vascular endothelial cells. J. Physiol. 1996;491:1. doi: 10.1113/jphysiol.1996.sp021191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Groschner K, Rosker C, Lukas M. Role of TRP channels in oxidative stress. Novartis Found. Symp. 2004;258:222. [PubMed] [Google Scholar]
- 87.Miller BA, Zhang W. TRP channels as mediators of oxidative stress. Adv Exp Med Biol. 2011;704:531. doi: 10.1007/978-94-007-0265-3_29. [DOI] [PubMed] [Google Scholar]
- 88.Hansson GK. Inflammation, Atherosclerosis, and Coronary Artery Disease. N. Engl. J. Med. 2005;352:1685. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 89.Galkina E, Ley K. Vascular Adhesion Molecules in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2007;27:2292. doi: 10.1161/ATVBAHA.107.149179. [DOI] [PubMed] [Google Scholar]
- 90.Seye CI, Yu N, Jain R, Kong Q, Minor T, Newton J, Erb L, Gonzalez FA, Weisman GA. The P2Y2 Nucleotide Receptor Mediates UTP-induced Vascular Cell Adhesion Molecule-1 Expression in Coronary Artery Endothelial Cells. J. Biol. Chem. 2003;278:24960. doi: 10.1074/jbc.M301439200. [DOI] [PubMed] [Google Scholar]
- 91.Allen S, Khan S, Tam S.-p, Koschinsky M, Taylor P, Yacoub M. Expression of adhesion molecules by Lp(a): a potential novel mechanism for its atherogenicity. FASEB J. 1998;12:1765. doi: 10.1096/fasebj.12.15.1765. [DOI] [PubMed] [Google Scholar]
- 92.Thilo F, Loddenkemper C, Berg E, Zidek W, Tepel M. Increased TRPC3 expression in vascular endothelium of patients with malignant hypertension. Mod. Pathol. 2009;22:426. doi: 10.1038/modpathol.2008.200. [DOI] [PubMed] [Google Scholar]
- 93.Thilo F, Baumunk D, Krause H, Schrader M, Miller K, Loddenkemper C, Zakrzewicz A, Krueger K, Zidek W, Tepel M. Transient receptor potential canonical type 3 channels and blood pressure in humans. J. Hypertens. 2009;27:1217. doi: 10.1097/HJH.0b013e32832a5a9f. [DOI] [PubMed] [Google Scholar]
- 94.Huang JH, He GW, Xue HM, Yao XQ, Liu XC, Underwood MJ, Yang Q. TRPC3 channel contributes to nitric oxide release: significance during normoxia and hypoxia-reoxygenation. Cardiovasc. Res. 2011;91:472. doi: 10.1093/cvr/cvr102. [DOI] [PubMed] [Google Scholar]
- 95.Yi FX, Boeldt DS, Gifford SM, Sullivan JA, Grummer MA, Magness RR, Bird IM. Pregnancy enhances sustained Ca2+ bursts and endothelial nitric oxide synthase activation in ovine uterine artery endothelial cells through increased connexin 43 function. Biol. Reprod. 2010;82:66. doi: 10.1095/biolreprod.109.078253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ahmmed GU, Malik AB. Functional role of TRPC channels in the regulation of endothelial permeability. Pflugers Arch. 2005;451:131. doi: 10.1007/s00424-005-1461-z. [DOI] [PubMed] [Google Scholar]