Abstract
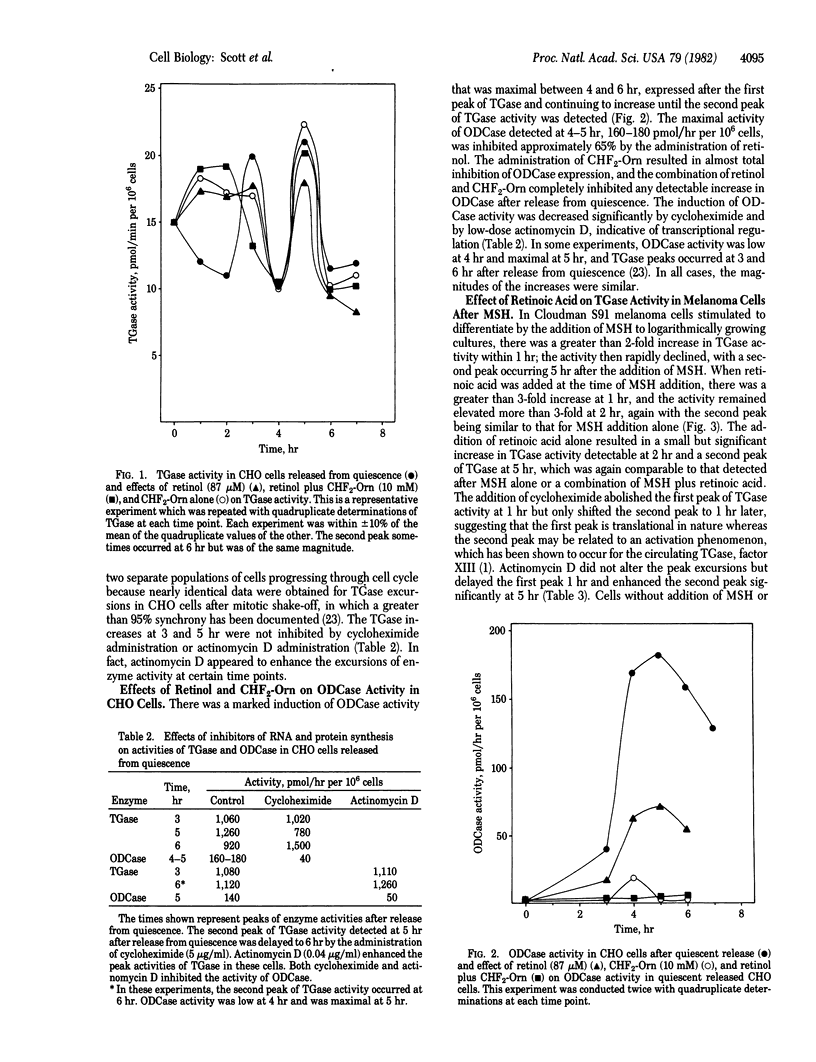
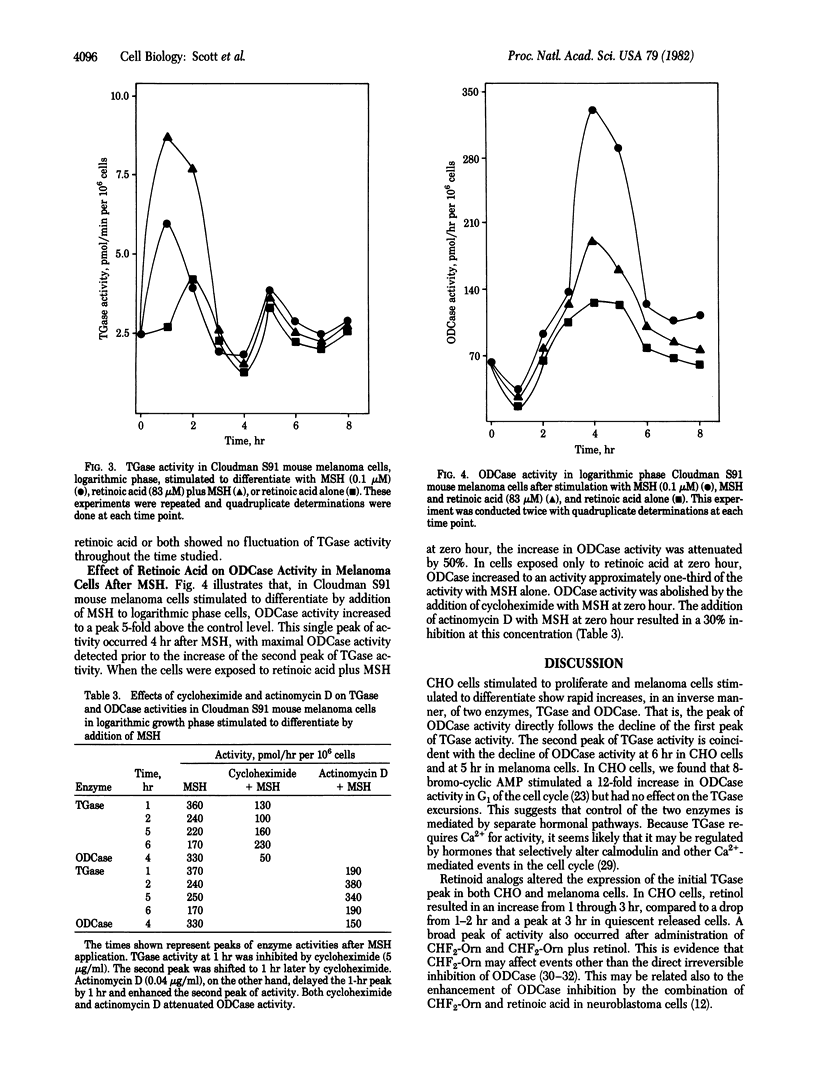
Transglutaminase (TGase; R-glutaminyl-peptide:amine gamma-glutamyltransferase, EC 2.3.2.13) and ornithine decarboxylase (ODCase; L-ornithine carboxy-lyase, EC 4.1.1.17) activities were measured after the addition of retinoid analogs to Chinese hamster ovary (CHO) cells released from quiescence and Cloudman S91 (CCL 53.1) mouse melanoma cells stimulated to differentiate with alpha-melanocyte-stimulating hormone (MSH, melanotropin). In both cell culture lines, we detected a biphasic increase in TGase activity and a single peak of ODCase activity within 7 hr after release or stimulation. Retinoid analogs altered the expression of the initial TGase peak in both CHO and melanoma cells. Retinol increased the activity of TGase 1 hr after release in CHO cells, and the activity remained elevated until hr 4. A broad peak of TGase activity also occurred after the addition of alpha-difluoromethylornithine, an irreversible inhibitor of ODCase, and after addition of alpha-difluoromethylornithine plus retinol. In mouse melanoma cells, retinoic acid plus MSH markedly enhanced the activity of the initial TGase peak compared to MSH alone. Retinoic acid alone also increased TGase activity biphasically in these cells without the addition of MSH. These studies suggest that retinoid effects that increase TGase activity may alter the ODCase expression in proliferation and differentiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchi C. J., Nathan H. C., Hutner S. H., McCann P. P., Sjoerdsma A. Polyamine metabolism: a potential therapeutic target in trypanosomes. Science. 1980 Oct 17;210(4467):332–334. doi: 10.1126/science.6775372. [DOI] [PubMed] [Google Scholar]
- Birckbichler P. J., Orr G. R., Conway E., Patterson M. K., Jr Transglutaminase activity in normal and transformed cells. Cancer Res. 1977 May;37(5):1340–1344. [PubMed] [Google Scholar]
- Bollag W. Vitamin A AND VITAMIN A acid in the prophylaxis and therapy of epithelial tumours. Int Z Vitaminforsch. 1970;40(3):299–314. [PubMed] [Google Scholar]
- Chapman S. K. Antitumor effects of vitamin A and inhibitors of ornithine decarboxylase in cultured neuroblastoma and glioma cells. Life Sci. 1980 Apr 21;26(16):1359–1366. doi: 10.1016/0024-3205(80)90097-1. [DOI] [PubMed] [Google Scholar]
- Chytil F., Ong D. E. Mediation of retinoic acid-induced growth and anti-tumour activity. Nature. 1976 Mar 4;260(5546):49–51. doi: 10.1038/260049a0. [DOI] [PubMed] [Google Scholar]
- Fish L. A., Baxter C. S., Bash J. A. Murine lymphocyte comitogenesis by phorbol esters, and its inhibition by retinoic acid and inhibitors of polyamine biosynthesis. Toxicol Appl Pharmacol. 1981 Mar 30;58(1):39–47. doi: 10.1016/0041-008x(81)90113-7. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Chung S. I. Molecular and catalytic properties of transglutaminases. Adv Enzymol Relat Areas Mol Biol. 1973;38:109–191. doi: 10.1002/9780470122839.ch3. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Haddox M. K., Russell D. H. Cell cycle-specific locus of vitamin A inhibition of growth. Cancer Res. 1979 Jul;39(7 Pt 1):2476–2480. [PubMed] [Google Scholar]
- Haddox M. K., Russell D. H. Differential conjugation of polyamines to calf nuclear and nucleolar proteins. J Cell Physiol. 1981 Dec;109(3):447–452. doi: 10.1002/jcp.1041090310. [DOI] [PubMed] [Google Scholar]
- Haddox M. K., Russell D. H. Increased nuclear conjugated polyamines and transglutaminase during liver regeneration. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1712–1716. doi: 10.1073/pnas.78.3.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddox M. K., Scott K. F., Russell D. H. Retinol inhibition of ornithine decarboxylase induction and G1 progression in Chinese hamster ovary cells. Cancer Res. 1979 Dec;39(12):4930–4938. [PubMed] [Google Scholar]
- Kensler T. W., Verma A. K., Boutwell R. K., Mueller G. C. Effects of retinoic acid and juvenile hormone on the induction of ornithine decarboxylase activity by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1978 Sep;38(9):2896–2899. [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lotan R. Effects of vitamin A and its analogs (retinoids) on normal and neoplastic cells. Biochim Biophys Acta. 1980 Mar 12;605(1):33–91. doi: 10.1016/0304-419x(80)90021-9. [DOI] [PubMed] [Google Scholar]
- Mamont P. S., Duchesne M. C., Grove J., Bey P. Anti-proliferative properties of DL-alpha-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978 Mar 15;81(1):58–66. doi: 10.1016/0006-291x(78)91630-3. [DOI] [PubMed] [Google Scholar]
- Meyskens F. L., Jr Vitamin A and cancer. Ariz Med. 1980 Feb;37(2):84–86. [PubMed] [Google Scholar]
- Paranjpe M. S., De Larco J. E., Todaro G. J. Retinoids block ornithine decarboxylase induction in cells treated with the tumor promotor TPA or the peptide growth hormones, EGF and SGF. Biochem Biophys Res Commun. 1980 May 30;94(2):586–591. doi: 10.1016/0006-291x(80)91272-3. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M. Factors regulating growth and pigmentation of melanoma cells. J Invest Dermatol. 1976 Apr;66(4):201–209. doi: 10.1111/1523-1747.ep12482134. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Haddox M. K. Antiproliferative effects of retinoids related to the cell cycle-specific inhibition of ornithine decarboxylase. Ann N Y Acad Sci. 1981 Feb 27;359:281–297. doi: 10.1111/j.1749-6632.1981.tb12754.x. [DOI] [PubMed] [Google Scholar]
- Russell D. H. Posttranslational modification of ornithine decarboxylase by its product putrescine. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1167–1172. doi: 10.1016/0006-291x(81)90741-5. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B. Retinoids and carcinogenesis. Nutr Rev. 1977 Apr;35(4):65–69. doi: 10.1111/j.1753-4887.1977.tb06541.x. [DOI] [PubMed] [Google Scholar]
- Stubblefield E., Klevecz R. Synchronization of Chinese hamster cells by reversal of colcemid inhibition. Exp Cell Res. 1965 Dec;40(3):660–664. doi: 10.1016/0014-4827(65)90244-2. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Rice H. M., Shapas B. G., Boutwell R. K. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced ornithine decarboxylase activity in mouse epidermis by vitamin A analogs (retinoids). Cancer Res. 1978 Mar;38(3):793–801. [PubMed] [Google Scholar]
- Verma A. K., Shapas B. G., Rice H. M., Boutwell R. K. Correlation of the inhibition by retinoids of tumor promoter-induced mouse epidermal ornithine decarboxylase activity and of skin tumor promotion. Cancer Res. 1979 Feb;39(2 Pt 1):419–425. [PubMed] [Google Scholar]
- Williams-Ashman H. G., Beil R. E., Wilson J., Hawkins M., Grayhack J., Zunamon A., Weinstein N. K. Transglutaminases in mammalian reproductive tissues and fluids: relation to polyamine metabolism and semen coagulation. Adv Enzyme Regul. 1980;18:239–258. doi: 10.1016/0065-2571(80)90018-7. [DOI] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Canellakis Z. N. Transglutaminase-mediated covalent attachment of polyamines to proteins: mechanisms and potential physiological significance. Physiol Chem Phys. 1980;12(5):457–472. [PubMed] [Google Scholar]
- Wong G., Pawelek J., Sansone M., Morowitz J. Response of mouse melanoma cells to melanocyte stimulating hormone. Nature. 1974 Mar 22;248(446):351–354. doi: 10.1038/248351a0. [DOI] [PubMed] [Google Scholar]



