Abstract
Purpose
Omitting elective nodal irradiation from planning target volumes does not compromise outcomes in patients with non–small-cell lung cancer, but whether the same is true for those with limited-stage small-cell lung cancer (LS-SCLC) is unknown. Therefore, in the present study, we sought to determine the clinical outcomes and the frequency of elective nodal failure in patients with LS-SCLC staged using positron emission tomography/computed tomography and treated with involved-field intensity-modulated radiotherapy.
Methods and Materials
Between 2005 and 2008, 60 patients with LS-SCLC at our institution underwent disease staging using positron emission tomography/computed tomography before treatment using an intensity-modulated radiotherapy plan in which elective nodal irradiation was intentionally omitted from the planning target volume (mode and median dose, 45 Gy in 30 fractions; range, 40.5 Gy in 27 fractions to 63.8 Gy in 35 fractions). In most cases, concurrent platinum-based chemotherapy was administered. We retrospectively reviewed the clinical outcomes to determine the overall survival, relapse-free survival, and failure patterns. Elective nodal failure was defined as recurrence in initially uninvolved hilar, mediastinal, or supraclavicular nodes. Survival was assessed using the Kaplan-Meier method.
Results
The median age of the study patients at diagnosis was 63 years (range, 39–86). The median follow-up duration was 21 months (range, 4–58) in all patients and 26 months (range, 4–58) in the survivors. The 2-year actuarial overall survival and relapse-free survival rate were 58% and 43%, respectively. Of the 30 patients with recurrence, 23 had metastatic disease and 7 had locoregional failure. We observed only one isolated elective nodal failure.
Conclusions
To our knowledge, this is the first study to examine the outcomes in patients with LS-SCLC staged with positron emission tomography/computed tomography and treated with definitive intensity-modulated radiotherapy. In these patients, elective nodal irradiation can be safely omitted from the planning target volume for the purposes of dose escalation and toxicity reduction.
Keywords: Small-cell lung cancer, Involved field radiation, Positron emission tomography, PET, Intensity-modulated radiotherapy, IMRT
INTRODUCTION
The value of radiotherapy (RT) for local control of small-cell lung cancer (SCLC) confined to the thorax is indisputable (1, 2). Moreover, the use of concurrent chemotherapy with RT has resulted in an increased survival benefit and has become the standard of care for patients with limited-stage small cell lung cancer (LS-SCLC), yie-lding 5-year survival rates of about 28% (3). Nonetheless, most patients still experience recurrence and ultimately death from their disease. Evidence suggests that disease control in SCLC patients can be improved by even more aggressive therapy than that used currently, including radiation dose escalation. For example, in a multisite Phase I study evaluating different accelerated RT regimens with concurrent chemotherapy, the 18-month survival rate for the maximal tolerated dose of 61.2 Gy was 82%, significantly better than the rate achieved at the lowest dose of 50.4 Gy (25%) (4). However, enthusiasm among physicians for dose-escalated RT combined with chemotherapy must be tempered by the potential for significant toxic effects, including esophagitis, pneumonitis, and bone marrow suppression (4–7).
One strategy for escalating radiation doses without increasing toxicity is to diminish the targeted volume by omitting elective radiation fields, including uninvolved nodal stations. This strategy has proved useful for non–small-cell lung cancer and is associated with a minimal incidence of elective nodal recurrence (8–10). More recently, physicians have adopted this strategy for limited-stage SCLC (LS-SCLC); however, published data supporting its use for this disease are sparse. In one study, De Ruysscher et al. (11) found that among patients with LS-SCLC treated with involved-field RT guided by computed tomography (CT), the elective nodal failure (ENF) rate (11%) was unacceptably high. However, a similar study by the same group using positron emission tomography (PET) with CT (PET/CT) for target delineation produced better results, demonstrating an isolated ENF rate of only 3% (12). In the latter study, the investigators did not use four-dimensional (4D) CT-guided target delineation or treatment planning, respiratory gating, or intensity-modulated RT (IMRT); thus, the generalizability of their results is limited. The incorporation of these new technologies, including IMRT to PET/CT staging, co-uld offer an opportunity to further reduce toxicity but carry other risks. For example, incidental irradiation to nontargeted elective nodes might be different in IMRT than for three-dimensional conformal techniques and affect the rate of elective nodal failure. In the present study, we hypothesized that the clinical outcomes and rate of elective nodal failure would be acceptable using the combined approach of PET/CT staging and IMRT planning. Therefore, we conducted a retrospective study of our experience with, and report on the clinical outcomes and patterns of failure of, involved-field RT for LS-SCLC using IMRT guided by PET/CT.
METHODS AND MATERIALS
Patient selection
The clinical records of all consecutive patients with LS-SCLC who had undergone external beam RT initiated at the University of Texas M.D. Anderson Cancer Center between 2005 and 2008 were retrospectively reviewed. LS-SCLC was defined as disease confined to the thorax and regional nodes without malignant pleural effusion. The patients were included in the analysis if they had been diagnosed with histologically proven LS-SCLC that had not been treated previously with RT, had undergone staging using PET/CT before RT, had undergone definitive IMRT for the primary disease, and had complete RT records available. The patients who had been diagnosed initially with LS-SCLC and sc-heduled for induction chemotherapy but who subsequently developed distant metastasis before referral to the radiation oncology department were not included in the present analysis. Most (97%) of the patients received concurrent platinum-based chemotherapy. The M.D. Anderson institutional review board approved the present study.
Radiotherapy
The patients underwent IMRT using the Pinnacle CT-based treatment planning software program (Philips Medical Systems, Andover, MA). They underwent simulation and treatment in the supine position with their arms raised above their heads and were immobilized using a custom-made Vac-Lok cradle (Medtec, Orange City, IA). Most patients (73%) underwent 4D simulation. The gross tumor volume (GTV) included regions of primary disease and nodal metastasis defined by metabolically active regions on the patient’s staging PET/CT scans. Involved nodal regions diagnosed by histologic evaluation of the biopsy samples obtained during mediastinoscopy or bronchoscopy were included in the GTV, regardless of the nodes’ 18F-fluorodeoxyglucose avidity on PET/CT scans. For 4D simulation, an internal GTV was defined as the sum envelope of GTVs extracted from the component images obtained in 10-breath phases (13). Typically, the clinical target volume (CTV) was defined as the internal GTV plus an 8-mm margin. The planning target volume (PTV) was defined as the CTV plus a 5–10-mm margin. In some cases, in lieu of the internal GTV covering the entire respiratory cycle, the treatment volumes were defined at deep inspiration, if necessary, to conform to normal tissue restraints. For the patients who underwent simulation during free breathing, the GTV was expanded by 0.8 cm to define the CTV, except for where the normal anatomic barriers and dose constraints required a smaller expansion. The CTV was then expanded by 1 cm to compensate for setup variability and target motion to generate the PTV. The plans were corrected for tissue inhomogeneity during treatment planning.
Radiation was delivered using photon beams of ≥6 MV from a linear accelerator. During RT, radiation oncologists evaluated the patients weekly to evaluate any acute toxic effects. Staging was repeated about 3 months after RT completion using contrast-enhanced CT. If no disease progression was observed, the patients were offered prophylactic cranial RT to a total dose of 25 Gy in 10 fractions. After completion of all treatment, the patients underwent repeat clinical examinations and imaging every 3 months for 2 years and then every 6 months for 3 years. Tumor recurrences were scored as separate, discrete events if they occurred ≥3 months apart.
Statistical analysis
The clinical endpoints examined included overall survival (OS), relapse-free survival (RFS), and patterns of failure. Recurrences were classified as in-field failures if most of the recurrent tumor volume or an obvious origin of recurrence was located in the treatment field PTV. In contrast, out-of-field failure was defined as a recurrence outside the PTV but within the lungs, pleura, mediastinum, or regional nodes. Elective nodal failure (ENF) represented a subset of out-of-field failure and was defined as a recurrence outside the PTV but within a hilar, mediastinal, or supraclavicular nodal basin. ENF was deemed to be isolated only in the absence of other sites of failure. Survival and the interval-to-failure durations were measured from the date of pathologic diagnosis of LS-SCLC, and the timing of the recurrences was defined as the time of the first imaging or clinical finding of recurrence. Survival probabilities were determined using the Kaplan-Meier method. Differences among the subgroups of patients were evaluated using the log–rank test. Toxicities were graded according to the Radiation Therapy Oncology Group criteria (14, 15).
RESULTS
Patient population and treatment
A total of 60 patients with LS-SCLC treated using IMRT guided by PET/CT between 2005 and 2008 were included in the present study (Table 1). Their median age was 63 years (range, 39–86). Most patients had a good performance status, with 85% of them having an Eastern Cooperative Oncology Group score of 0 or 1. Nearly all the patients (94%) had nodal disease at treatment, and approximately one-third of the patients had Stage N3 disease. Eight patients (13%) had involvement of an ipsilateral or contralateral supraclavicular nodal basin, and two (3%) had direct extension into adjacent bone; in all these cases, the treating oncologists classified the disease as limited stage at treatment planning.
Table 1.
Patient and treatment characteristics
| Characteristic | Value |
|---|---|
| Patients (n) | 60 |
| Age (y) | |
| Median | 63 |
| Range | 38–86 |
| Gender (n) | |
| Male | 25 (42) |
| Female | 35 (58) |
| ECOG performance status (n) | |
| 0 | 6 (10) |
| 1 | 45 (75) |
| 2 | 3 (5) |
| 3 | 0 (0) |
| Not specified | 6 (10) |
| Weight loss before treatment (n) | |
| None | 23 (38) |
| <10 lb | 10 (17) |
| ≥10 lb | 20 (33) |
| Not specified | 7 (12) |
| Primary tumor size (n) | |
| ≤3 cm | 22 (37) |
| >3 cm | 36 (60) |
| Primary tumor not identified | 2 (3) |
| AJCC nodal stage (n) | |
| 0 | 4 (7) |
| 1 | 10 (17) |
| 2 | 24 (40) |
| 3 | 22 (37) |
| Chemotherapy (n) | |
| Induction | 18 (30) |
| Concurrent | |
| Cisplatin/etoposide | 40 (67) |
| Carboplatin/etoposide | 11 (18) |
| Cisplatin/irinotecan | 4 (7) |
| Unknown | 3 (5) |
| None | 2 (3) |
| RT (n) | |
| Simulation | |
| Four-dimensional | 44 (73) |
| Free breathing | 16 (27) |
| Dose (n) | |
| 45 Gy, twice daily | 41 (68) |
| Other | 19 (32) |
| Prophylactic cranial irradiation (n) | |
| Yes | 37 (62) |
| No | 22 (37) |
| Unknown | 1 (2) |
Abbreviations: ECOG = Eastern Cooperative Oncology Group; AJCC = American Joint Committee on Cancer; RT = radiotherapy.
Data in parentheses are percentages.
Eighteen patients (30%) underwent induction chemotherapy, and 58 patients (97%) underwent concurrent chemotherapy, usually with a platinum agent and topoisomerase inhibitor. The most prescribed radiation dose was 45 Gy delivered in 30 twice-daily fractions; 68% of the patients received this treatment. Other prescribed total doses ranged from 40.5 Gy delivered in 27 fractions to 63.8 Gy delivered in a combination of daily and accelerated twice-daily fractions. Thirty-seven patients (62%) subsequently received prophylactic cranial RT after definitive therapy to the primary disease.
Clinical outcomes and patterns of failure
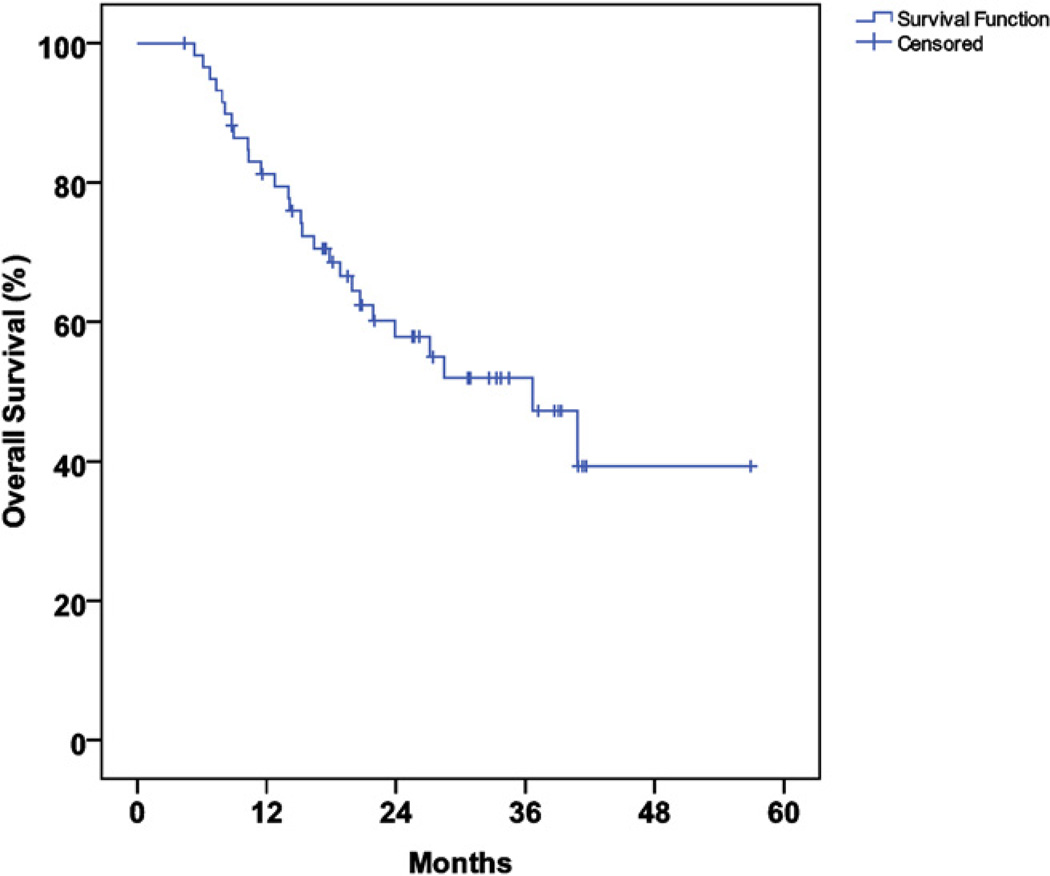
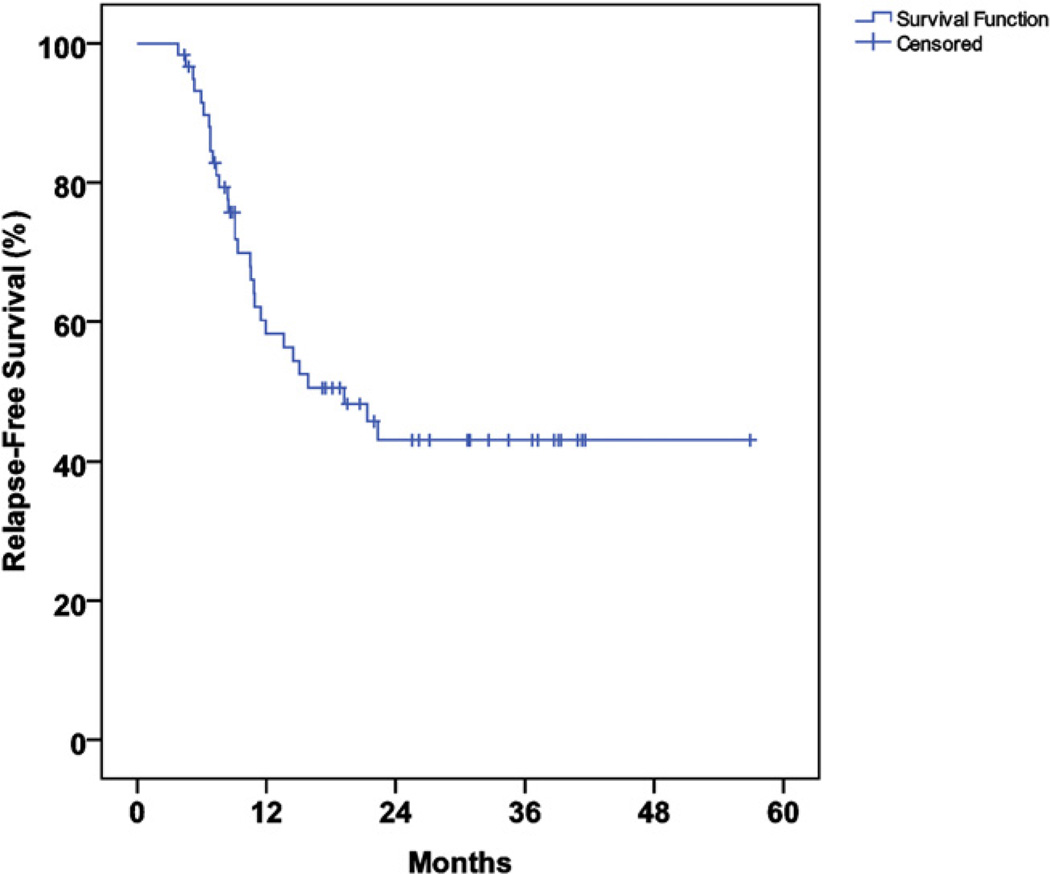
The median follow-up duration was 21 months (range, 4–58) in all patients and 26 months (range, 4–58) in survivors. The median actuarial overall survival time was 36 months (95% confidence interval [CI], 22–51), and the actuarial 2-year OS and RFS rate were 58% and 43%, respectively (Figs. 1 and 2).
Fig. 1.
Actuarial overall survival.
Fig. 2.
Actuarial relapse-free survival.
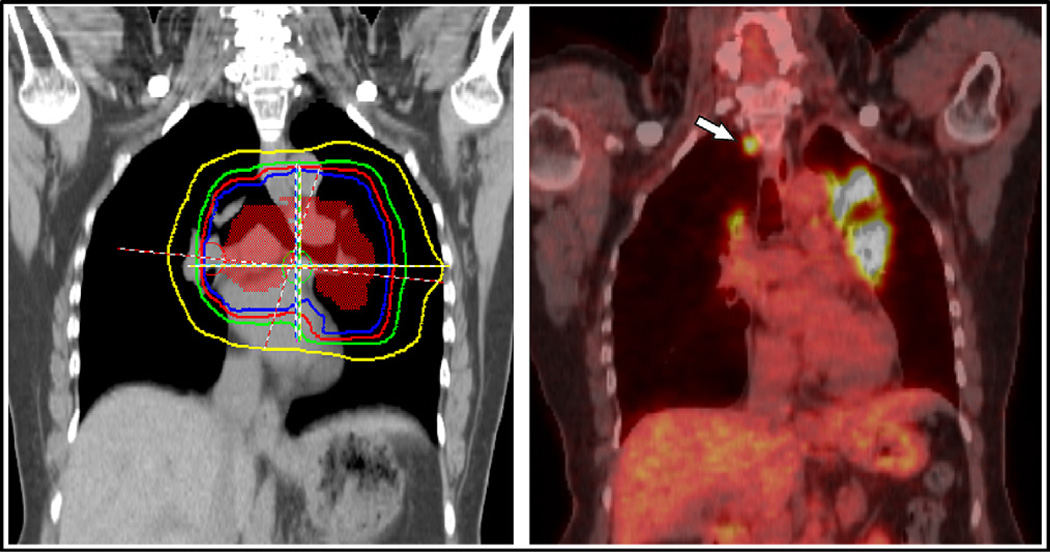
Thirty patients (50%) had an observed recurrence. The leading cause of treatment failure was metastatic disease, which developed in 23 patients (38%) and accounted for 77% of all recurrences. Seven patients (12%) experienced locoregional failure. One patient (2%) had an isolated ENF, which represented 3% of all recurrences. The site of this nodal recurrence had <5 Gy of incidental radiation during the initial treatment (Fig. 3). We observed three other ENFs, with two occurring concurrently with in-field failures and one occurring concurrently with distant metastasis. Two ENFs occurred in the paratracheal nodes, and the other two occurred in the supraclavicular nodes. The sites of recurrence and their frequencies are listed in Table 2.
Fig. 3.
Example of isolated elective nodal failure in our cohort. (Right) Intensity-modulated radiotherapy planning dose volumes for this patient, who received 45 Gy in 30 fractions given twice daily (orange, clinical target volume; blue, 45 Gy isodose line; red, 36 Gy; green, 20 Gy; yellow, 5 Gy). (Left) Elective nodal failure in right paratracheal lymph node (white arrow) observed on surveillance positron emission tomography/computed tomography scan obtained about 3 months after radiotherapy completion. Other areas of 18F-fluorodeoxyglucose avidity on the image indicate inflammation and did not appear on subsequent scans.
Table 2.
Patterns of failure for first recurrence
| Recurrence | Patients with recurrence(n) |
|---|---|
| Total | 30 (100) |
| Locoregional failure | 7 (23) |
| In field | 3 (10) |
| Out of field (includes elective nodes) | 2 (7) |
| In field and out of field (includes elective nodes) | 2 (7) |
| Distant failure | 23 (77) |
| Distant metastases alone | 18 (60) |
| In-field and distant metastases | 2 (7) |
| Out-of-field and distant metastases | 2 (7) |
| In-field, out-of-field, and distant metastases | 1 (3) |
| Elective nodes | 4 (13) |
| Pattern | |
| Isolated elective nodes | 1 (3) |
| In-field and elective nodes | 1 (3) |
| Distant metastases and elective nodes | 2 (7) |
| Location | |
| Paratracheal | 2 (7) |
| Supraclavicular | 2 (7) |
Data in parentheses are percentages.
Toxicity
The rates of esophageal and pulmonary toxicities are presented in Table 3. A total of 14 (23%) and 4 (7%) patients experienced moderately symptomatic (Grade 3) acute radiation esophagitis and pneumonitis, respectively. No patients experienced Grade 4 or 5 acute esophagitis or pneumonitis. Neutropenic fever occurred in 10 patients (17%). No chronic Grade 3 esophageal or pulmonary toxicities were observed; one late Grade 4 toxicity occurred in a chronic smoker who required continuous oxygen by nasal cannula after developing severe pulmonary fibrosis following treatment.
Table 3.
Esophageal and pulmonary toxicities graded using Radiation Therapy Oncology Group criteria
| RTOG grade | ||||||
|---|---|---|---|---|---|---|
| Toxicity | 0 | 1 | 2 | 3 | 4 | 5 |
| Acute (n) | ||||||
| Esophageal | 3 (5) | 15 (25) | 28 (47) | 14 (23) | 0 (0) | 0 (0) |
| Pulmonary | 38 (63) | 12 (20) | 6 (10) | 4 (7) | 0 (0) | 0 (0) |
| Chronic (n) | ||||||
| Esophageal | 54 (90) | 5 (8) | 1 (2) | 0 (0) | 0 (0) | 0 (0) |
| Pulmonary | 40 (67) | 9 (15) | 10 (17) | 0 (0) | 1 (2) | 0 (0) |
Abbreviation: RTOG = Radiation Therapy Oncology Group.
Data in parentheses are percentages.
PET/CT characteristics
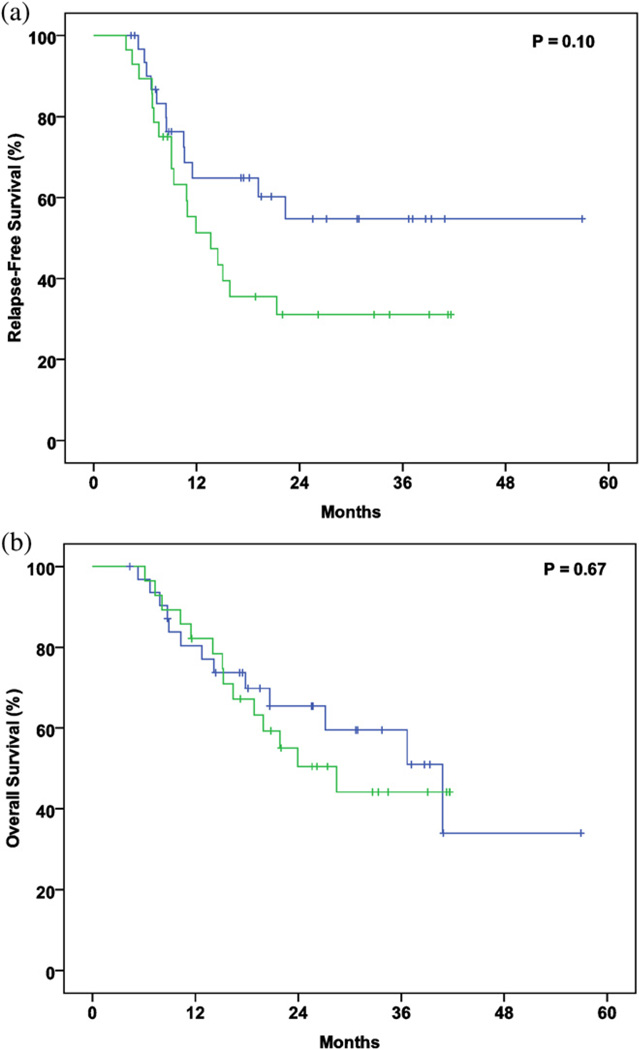
Thirty-five patients (58%) underwent staging using PET/CT at our institution, and the remainder did so at another facility. The mean duration from the date of PET/CT to the start of RT was 38 days (range, 3–153; 95% CI, 30–47). In the patients for whom the standardized uptake value (SUV) was reported, the mean maximal SUV was 13.99 (range, 5.4–36.4; 95% CI, 12.1–15.9). Neither the setting in which the PET was performed nor the maximal SUV correlated with OS or RFS (data not shown). A period of <30 days from the date of PET/CT to the start of RT was associated with a nonsignificant trend toward improved RFS (p = .10) but not improved OS (Fig. 4).
Fig. 4.
(a) Relapse-free survival and (b) overall survival stratified by interval from positron emission tomography/computed tomography staging study to start of radiotherapy (green, ≥30 days; blue, <30 days).
DISCUSSION
In the early 1990s, emerging evidence demonstrated that small radiation target volumes did not adversely affect tumor control in the treatment of LS-SCLC (16–18). In fact, >80% of failures after RT during this period occurred in field, suggesting that inadequate radiation doses, rather than inadequate volumes, were the primary causes of intrathoracic recurrence (19). Therefore, the prevailing trend for RT in LS-SCLC during the past two decades has been to reduce the treatment field size while increasing the radiation dose. Small volumes have the additional benefit of sparing surrounding organs at risk from potentially life-threatening complications. The lungs, in particular, benefit from the reduced volumes, because the rate of pneumonitis approaches 33% once the lung volume receiving ≥20 Gy exceeds 40% (20). Thus, the treatment approach for SCLC at our institution has been to use image-guided RT to deliver escalated radiation doses to conformal treatment volumes (13). To that end, PET/CT scanning for staging and IMRT for treatment planning were incorporated at our institution for the treatment of LS-SCLC in 2005.We retrospectively reviewed the clinical outcomes and failure patterns, including the rate of ENF, in patients treated using these technologies. In our cohort of 60 patients, we observed a promising 2-year actuarial OS and RFS rate of 58% and 43%, respectively. We only observed one isolated elective nodal failure, and the dominant mode of recurrence in our data set was not locoregional failure but rather metastatic disease. The treatment was well tolerated by most patients, with Grade 3 acute esophagitis and pneumonitis occurring in 23% and 7% of patients, respectively. Because of these findings, omitting elective nodal RT from the planning treatment volumes is reasonable and could improve local control without increasing toxicity by allowing dose escalation in a small field.
Positron emission tomography/CT is likely to be the most sensitive noninvasive method of delineating the extent of SCLC. Several series have shown that PET staging alters the presumed distribution of SCLC in 25–30%of patients previously staged using CT only (21–23). Therefore, the use of CT alone for target delineation in patients with LS-SCLC carries the risk of a geographic miss secondary to undetected disease. Confirming this hypothesis, a Phase II study of involved-field RT found a rather high rate of ENF (11%) in patients with SCLC staged using CT alone (11). In contrast, the addition of PET/CT staging to the diagnostic workup in a parallel study by the same investigators found a much improved rate of ENF (3%) (12). Additional improvements in PET techniques and logistics might further improve local control and survival in SCLC.
A natural hypothesis is that staging at or near simulation might result in improved disease coverage by preventing tumor progression in the PET-to-simulation interval. Our data suggest a trend toward improved RFS rates with PET-to-simulation intervals <30 days, making the conclusion that PET/CT ought to be performed at or near simulation a tempting one. However, patients with earlier RT simulations might have been healthier or had a better response to the initial treatment. Moreover, chemotherapy delivery affects the optimal timing of PET/CT. At many institutions, the standard practice is to have patients undergo simulation just before RT, at which time induction chemotherapy or the first dose of concurrent chemotherapy might have already been delivered (24). In these cases, PET/CT staging at simulation might be compromised by a diminished signal as a result of the systemic treatment. Therefore, additional studies are needed to determine the optimal timing of PET/CT in relation to both the delivery of the first dose of chemotherapy and the start of RT.
We did not observe a difference in OS or RFS between the patients whose PET/CT scans were obtained at our institution and those whose scans were obtained at other facilities, despite suggestion in the literature that the quality of PET/CT studies can depend on the practice setting and training experience of the interpreting radiologist (25). Likewise, we did not detect any differences in the OS or RFS rate when we grouped patients according to the maximal SUVs on staging PET scans, which conflicts with recent data indicating that the SUV is prognostically relevant (26). The retrospective nature of our study and the heterogeneity of it with regard to the specific timing of PET/CT in relation to the delivery of chemotherapy might have diluted the effect of SUV on prognosis.
To our knowledge, this is the first study to examine the use of PET/CT and IMRT for involved-field RT for LS-SCLC. However, a recent study by van Loon et al. (12) in The Netherlands reported outcomes for a similar group of 60 patients with LS-SCLC treated using three-dimensional conformal techniques in place of IMRT. Strikingly, our series and theirs had nearly the same incidence of isolated ENF (2% and 3%, respectively), providing compelling corroboration of our primary finding that omission of elective nodal irradiation in pursuit of dose escalation and reduced toxicity is warranted. However, some important differences in outcomes of these two studies warrant attention. The 2-year actuarial OS and RFS rates in our study (58% and 43%, respectively) were greater than those (35% and 17%, respectively) reported by van Loon et al. (12). Our results also represent a modest improvement over that in the landmark Intergroup Trial 0096, in which the 2-year OS and RFS rate in the 45 Gy twice-daily arm was 47% and 29%, respectively.
The disparities in the study population and inherent selection bias were certainly responsible for some of the differences in outcomes seen between these studies. In addition, it is worth exploring other differences in study design and treatment technique that could also have had a bearing on the patient outcomes. Specifically, one explanation for the disparities between our results and those in the Dutch study is the use of different RT techniques, including IMRT and 4D-CT simulation in our study. Previously, we compared IMRT with three-dimensional conformal RT for advanced non–SCLC and found improved target coverage and reduced normal tissue exposure using the IMRT plans. Specifically, IMRT improved target conformality, delivered greater doses to the target, and reduced the mean lung volume receiving >20 Gy (27, 28). Also, most of our patients underwent 4D simulation for treatment planning. Liu et al. (29) previously demonstrated that during the respiratory cycle, one-half of all lung tumors moved >5 mm and one-tenth moved >1 cm. Our use of 4D simulation, therefore, might have reduced the rate of geographic misses during treatment caused by tumor motion. We observed a trend suggesting that patients who underwent 4D simulation had better RFS rates than those who underwent simulation with free breathing (data not shown); however, that analysis was limited because the free breathing scans were done in a small group of patients who underwent treatment at the beginning of our study period before 4D simulation was fully commissioned. We expect that the completion of ongoing lung cancer trials designed to clarify the use of technologies such as IMRT and 4D simulation in the treatment of lung cancer will shed more light on their contribution to improving outcomes.
One other issue worth considering is the potential role of incidental irradiation in the elective nodal failures seen in our study. Other investigators have studied incidental irradiation in three-dimensional conformal therapy of involved-field radiation in advanced non-SCLC and have reported that the median mean dose for thoracic nodal regions (Levels 1–11) was >40 Gy, except for at Levels 1, 3, and 7 in their series (30). It stands to reason that the treatment of LS-SCLC also results in high levels of incidental nodal irradiation, because this disease tends to be more centrally located near mediastinal nodal basins. Although beyond the scope of the present study, future investigations could clarify this issue by measuring the doses to incident nodes and relating them to rates of nodal control. It is notable that the 1 patient in our series with isolated elective nodal failure had negligible radiation dose at the site of paratracheal nodal failure (Fig. 3).
Our study was retrospective and thus had several important limitations that could explain the promising outcomes we observed. The improved sensitivity of PET/CT in detecting distant metastases might have excluded patients who otherwise would have been classified as having LS-SCLC in previous diagnostic eras, thereby improving the mean outcomes in our cohort. This stage-migration phenomenon might be responsible for our improved outcomes compared to Intergroup Trial 0096. Furthermore, because the patients were not prospectively followed up, selection bias and the loss of patients from our tertiary referral center to local follow-up might have contributed to an underestimation of the rates of recurrence and death. Finally, the follow-up in our study was limited both because of the recent advent of this treatment strategy at our institution and the relative rarity of LS-SCLC in the general population.
Despite these limitations, the data from evaluations of image-guided involved-field RT for LS-SCLC are scarce; thus, we believe that the present series of 60 patients is a significant addition to the published data and provides intriguing hypotheses for the future study of treatments of this disease. We hope that future prospective studies using strict protocols for patient stratification, diagnosis, and treatment will verify and build on the outcomes we have reported.
CONCLUSIONS
In the present cohort of 60 patients with LS-SCLC staged using PET/CT and undergoing definitive IMRT, only one isolated ENF occurred. We have concluded that for the purposes of dose escalation and reduced toxicity, elective nodal irradiation can be safely omitted from the PTV in patients who underwent staging and treatment using these techniques.
Footnotes
Presented at the 52nd Annual Meeting of the American Society for Radiation Oncology (ASTRO), San Diego, CA.
Conflict of interest: none.
REFERENCES
- 1.Pignon JP, Arriagada R, Ihde DC, et al. A meta-analysis of thoracic radiotherapy for small-cell lung cancer. N Engl J Med. 1992;327:1618–1624. doi: 10.1056/NEJM199212033272302. [DOI] [PubMed] [Google Scholar]
- 2.Warde P, Payne D. Does thoracic irradiation improve survival and local control in limited-stage small-cell carcinoma of the lung? A meta-analysis. J Clin Oncol. 1992;10:890–895. doi: 10.1200/JCO.1992.10.6.890. [DOI] [PubMed] [Google Scholar]
- 3.Turrisi AT, III, Kim K, Blum R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 4.Komaki R, Swann RS, Ettinger DS, et al. Phase I study of thoracic radiation dose escalation with concurrent chemotherapy for patients with limited small-cell lung cancer: Report of Radiation Therapy Oncology Group (RTOG) protocol 97-12. Int J Radiat Oncol Biol Phys. 2005;62:342–350. doi: 10.1016/j.ijrobp.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Choi NC, Herndon JE, II, Rosenman J, et al. Phase I study to determine the maximum-tolerated dose of radiation in standard daily and hyperfractionated-accelerated twice-daily radiation schedules with concurrent chemotherapy for limited-stage small-cell lung cancer. J Clin Oncol. 1998;16:3528–3536. doi: 10.1200/JCO.1998.16.11.3528. [DOI] [PubMed] [Google Scholar]
- 6.Murray N, Coy P, Pater JL, et al. Importance of timing for thoracic irradiation in the combined modality treatment of limited-stage small-cell lung cancer: The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1993;11:336–344. doi: 10.1200/JCO.1993.11.2.336. [DOI] [PubMed] [Google Scholar]
- 7.Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: A phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 8.De Ruysscher D, Wanders S, van Haren E, et al. Selective mediastinal node irradiation based on FDG-PET scan data in patients with non–small-cell lung cancer: A prospective clinical study. Int J Radiat Oncol Biol Phys. 2005;62:988–994. doi: 10.1016/j.ijrobp.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 9.Rosenzweig KE, Sura S, Jackson A, et al. Involved-field radiation therapy for inoperable non small-cell lung cancer. J Clin Oncol. 2007;25:5557–5561. doi: 10.1200/JCO.2007.13.2191. [DOI] [PubMed] [Google Scholar]
- 10.Sulman EP, Komaki R, Klopp AH, et al. Exclusion of elective nodal irradiation is associated with minimal elective nodal failure in non-small cell lung cancer. Radiat Oncol. 2009;4:5. doi: 10.1186/1748-717X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Ruysscher D, Bremer RH, Koppe F, et al. Omission of elective node irradiation on basis of CT-scans in patients with limited disease small cell lung cancer: a phase II trial. Radiother Oncol. 2006;80:307–312. doi: 10.1016/j.radonc.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 12.van Loon J, De Ruysscher D, Wanders R, et al. Selective nodal irradiation on basis of (18)FDG-PET scans in limited-disease small-cell lung cancer: A prospective study. Int J Radiat Oncol Biol Phys. 2010;77:329–336. doi: 10.1016/j.ijrobp.2009.04.075. [DOI] [PubMed] [Google Scholar]
- 13.Chang JY, Dong L, Liu H, et al. Image-guided radiation therapy for non-small cell lung cancer. J Thorac Oncol. 2008;3:177–186. doi: 10.1097/JTO.0b013e3181622bdd. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2007;2007(57):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 15.Smith BD, Haffty BG, Smith GL, et al. Use of postmastectomy radiotherapy in older women. Int J Radiat Oncol Biol Phys. 2008;71:98–106. doi: 10.1016/j.ijrobp.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Schild SE, Curran WJ., Jr . Small cell lung cancer. In: Gunderson LL, Tepper JE, editors. Clinical radiation oncology. 2nd ed. Philadelphia: Elsevier Churchill Livingstone; 2007. p. 1827. [Google Scholar]
- 17.Kies MS, Mira JG, Crowley JJ, et al. Multimodal therapy for limited small-cell lung cancer: A randomized study of induction combination chemotherapy with or without thoracic radiation in complete responders: and with wide-field versus reduced-field radiation in partial responders: A Southwest Oncology Group Study. J Clin Oncol. 1987;5:592–600. doi: 10.1200/JCO.1987.5.4.592. [DOI] [PubMed] [Google Scholar]
- 18.Liengswangwong V, Bonner JA, Shaw EG, et al. Limited-stage small-cell lung cancer: Patterns of intrathoracic recurrence and the implications for thoracic radiotherapy. J Clin Oncol. 1994;12:496–502. doi: 10.1200/JCO.1994.12.3.496. [DOI] [PubMed] [Google Scholar]
- 19.Brodin O, Rikner G, Steinholtz L, et al. Local failure in patients treated with radiotherapy and multidrug chemotherapy for small cell lung cancer. Acta Oncol. 1990;29:739–746. doi: 10.3109/02841869009092993. [DOI] [PubMed] [Google Scholar]
- 20.Graham MV, Purdy JA, Emami B, et al. Clinical dose volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 21.Bradley JD, Dehdashti F, Mintun MA, et al. Positron emission tomography in limited-stage small-cell lung cancer: A prospective study. J Clin Oncol. 2004;22:3248–3254. doi: 10.1200/JCO.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 22.Niho S, Fujii H, Murakami K, et al. Detection of unsuspected distant metastases and/or regional nodes by FDG-PET [corrected] scan in apparent limited-disease small-cell lung cancer. Lung Cancer. 2007;57:328–333. doi: 10.1016/j.lungcan.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 23.van Loon J, Offermann C, Bosmans G, et al. 18FDG-PET based radiation planning of mediastinal lymph nodes in limited disease small cell lung cancer changes radiotherapy fields: A planning study. Radiother Oncol. 2008;87:49–54. doi: 10.1016/j.radonc.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 24.Spiro SG, James LE, Rudd RM, et al. Early compared with late radiotherapy in combined modality treatment for limited disease small-cell lung cancer: A London Lung Cancer Group multicenter randomized clinical trial and meta-analysis. J Clin Oncol. 2006;24:3823–3830. doi: 10.1200/JCO.2005.05.3181. [DOI] [PubMed] [Google Scholar]
- 25.Blodgett T. Best practices in PET/CT: Consensus on performance of positron emission tomography-computed tomography. Semin Ultrasound CT MR. 2008;29:236–241. doi: 10.1053/j.sult.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Lee YJ, Cho A, Cho BC, et al. High tumor metabolic activity as measured by fluorodeoxyglucose positron emission tomography is associated with poor prognosis in limited and extensive stage small-cell lung cancer. Clin Cancer Res. 2009;15:2426–2432. doi: 10.1158/1078-0432.CCR-08-2258. [DOI] [PubMed] [Google Scholar]
- 27.Liu HH, Wang X, Dong L, et al. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1268–1279. doi: 10.1016/j.ijrobp.2003.09.085. [DOI] [PubMed] [Google Scholar]
- 28.Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1258–1267. doi: 10.1016/j.ijrobp.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 29.Liu HH, Balter P, Tutt T, et al. Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys. 2007;68:531–540. doi: 10.1016/j.ijrobp.2006.12.066. [DOI] [PubMed] [Google Scholar]
- 30.Kimura T, Togami T, Nishiyama Y, et al. Impact of incidental irradiation on clinically uninvolved nodal regions in patients with advanced non–small-cell lung cancer treated with involved-field radiation therapy: Does incidental irradiation contribute to the low incidence of elective nodal failure? Int J Radiat Oncol Biol Phys. 2010;77:337–343. doi: 10.1016/j.ijrobp.2009.05.039. [DOI] [PubMed] [Google Scholar]