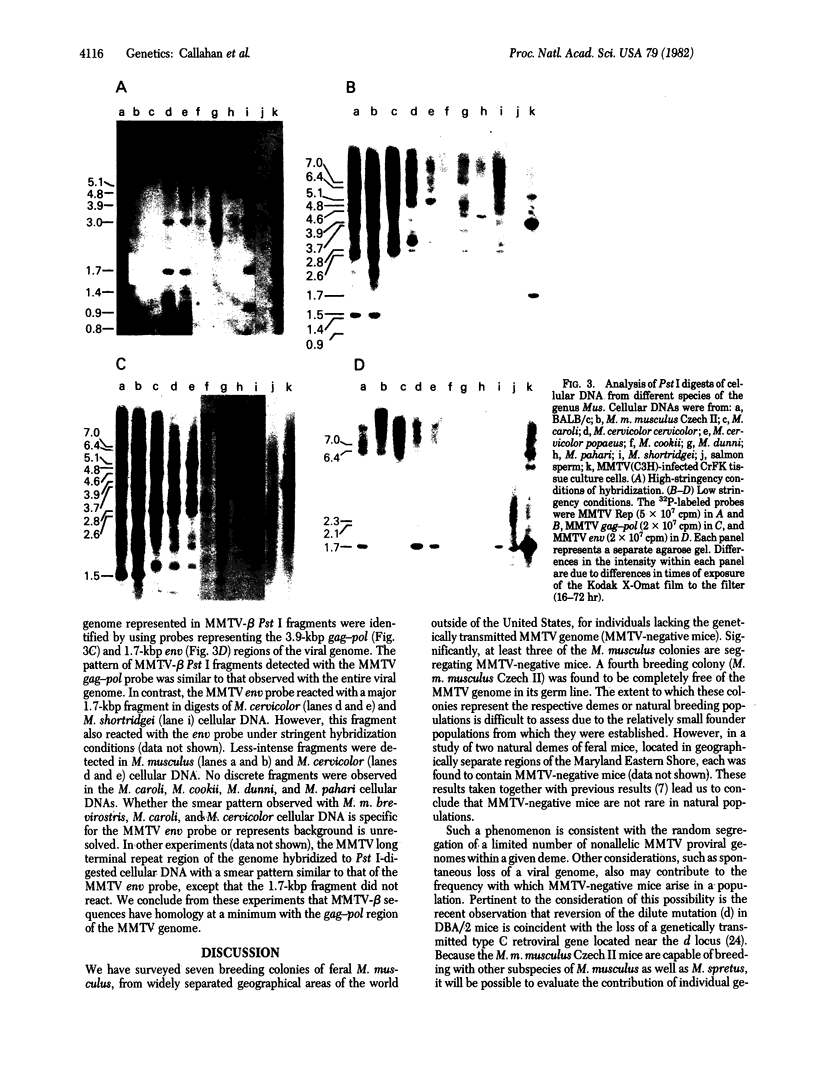
Abstract
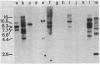
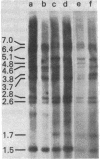
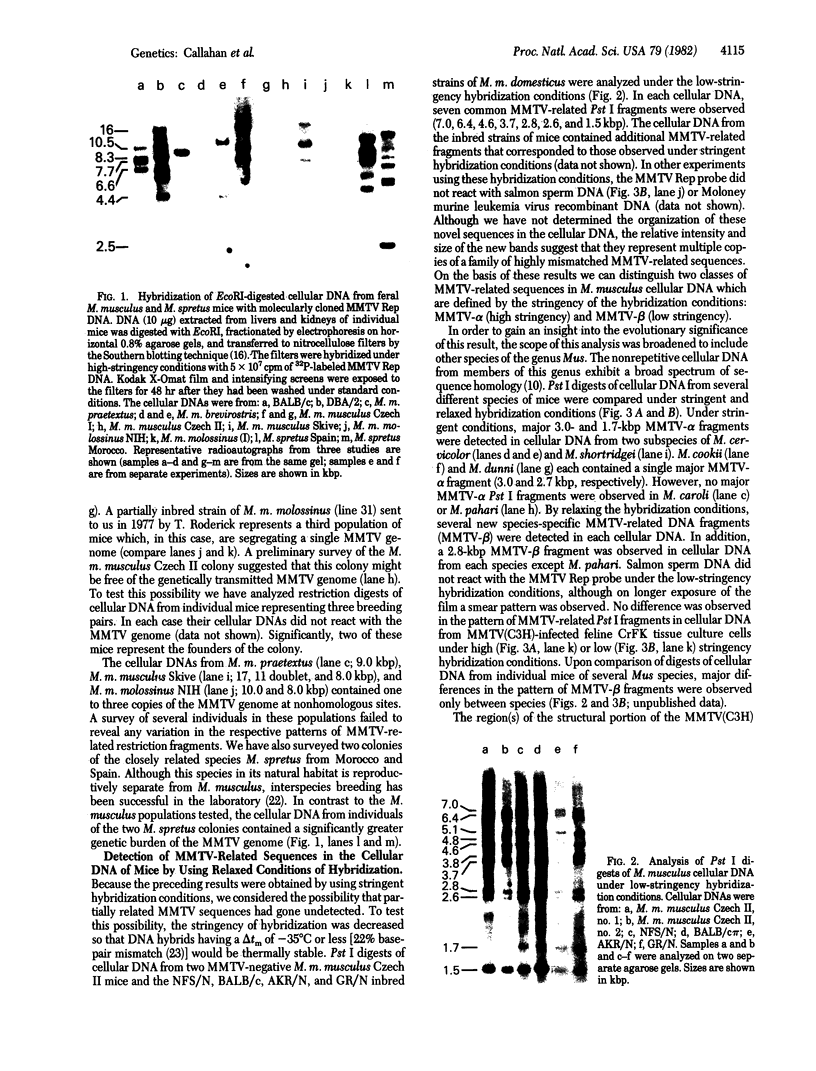
Mice in breeding colonies of feral Mus musculus brevirostris (Azrou, Morocco), M. m. musculus (Studenec, Czechoslovakia), and M. m. molossinus (Fukuoka, Japan) were found to lack the mouse mammary tumor virus (MMTV-alpha) proviral genome in their germ line. MMTV-alpha proviral genomes have been found in all inbred strains of M. musculus by using high-stringency nucleic acid hybridization conditions. We conclude that feral mice in these colonies are heterozygous for a limited number of MMTV-alpha proviral genomes and that those lacking them arose as a result of random chromosomal segregation. All mice in another breeding colony of feral M. m. musculus (Sladeckovce, Czechoslovakia) lack MMTV proviral genes. By relaxing the conditions of nucleic acid hybridization, MMTV-related sequences (designated MMTV-beta) were detected in restricted cellular DNA from MMTV-negative mice and all other inbred strains and feral species of the genus Mus. The apparent ubiquity of the MMTV-beta DNA sequences in the genus Mus and the lack of variation in the pattern of restriction fragments containing these sequences within a species distinguishes them from MMTV-alpha. These results suggest that the MMTV-beta DNA sequences either are the evolutionary progenitors of the infectious MMTV genome or represent an accumulation of evolutionarily divergent MMTV-alpha insertions into the mouse germ line.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Callahan R., Sherr C. J., Chapman V., Todaro G. J. Two distinct endogenous type C viruses isolated from the asian rodent Mus cervicolor: conservation of virogene sequences in related rodent species. J Virol. 1977 Mar;21(3):849–862. doi: 10.1128/jvi.21.3.849-862.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme F., Martin S., Thaler L. Hybridation en laboratoire de Mus musculus L. et Mus spretus Lataste. Experientia. 1978 Sep 15;34(9):1140–1141. doi: 10.1007/BF01922917. [DOI] [PubMed] [Google Scholar]
- Callahan R., Kuff E. L., Lueders K. K., Birkenmeier E. Genetic relationship between the Mus cervicolor M432 retrovirus and the Mus Musculus intracisternal type A particle. J Virol. 1981 Dec;40(3):901–911. doi: 10.1128/jvi.40.3.901-911.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R., Meade C., Todaro G. J. Isolation of an endogenous type C virus related to the infectious primate type C viruses from the Asian rodent Vandeleuria oleracea. J Virol. 1979 Apr;30(1):124–131. doi: 10.1128/jvi.30.1.124-131.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan H. W., Bryan T., Moore J. L., Staal S. P., Rowe W. P., Martin M. A. Identification of ecotropic proviral sequences in inbred mouse strains with a cloned subgenomic DNA fragment. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5779–5783. doi: 10.1073/pnas.77.10.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lander M. R., Rands E., Lowy D. R. Structure of endogenous murine leukemia virus DNA in mouse genomes. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5774–5778. doi: 10.1073/pnas.77.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Gillespie S., Gallo R. C., East J. L., Dmochowski L. Genetic origin of RD114 and other RNA tumour viruses assayed by molecular hybridization. Nat New Biol. 1973 Jul 11;244(132):51–54. doi: 10.1038/newbio244051a0. [DOI] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Howard D. K., Schlom J. Isolation of host-range variants of mouse mammary tumor viruses that efficiently infect cells in vitro. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5718–5722. doi: 10.1073/pnas.75.11.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. B., Little C. C. THE EXISTENCE OF NON-CHROMOSOMAL INFLUENCE IN THE INCIDENCE OF MAMMARY TUMORS IN MICE. Science. 1933 Nov 17;78(2029):465–466. doi: 10.1126/science.78.2029.465. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J., Benveniste R. E., Callahan R., Coon H. G. Isolation from the asian mouse Mus caroli of an endogenous type C virus related to infectious primate type C viruses. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2315–2319. doi: 10.1073/pnas.72.6.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueders K. K., Kuff E. L. Intracisternal A-particle genes: identification in the genome of Mus musculus and comparison of multiple isolates from a mouse gene library. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3571–3575. doi: 10.1073/pnas.77.6.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Bryan T., McCutchan T. F., Chan H. W. Detection and cloning of murine leukemia virus-related sequences from African green monkey liver DNA. J Virol. 1981 Sep;39(3):835–844. doi: 10.1128/jvi.39.3.835-844.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Bryan T., Rasheed S., Khan A. S. Identification and cloning of endogenous retroviral sequences present in human DNA. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4892–4896. doi: 10.1073/pnas.78.8.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConaughy B. L., Laird C. D., McCarthy B. J. Nucleic acid reassociation in formamide. Biochemistry. 1969 Aug;8(8):3289–3295. doi: 10.1021/bi00836a024. [DOI] [PubMed] [Google Scholar]
- Michalides R., Schlom J. Relationship in nucleic acid sequences between mouse mammary tumor virus variants. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4635–4639. doi: 10.1073/pnas.72.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris V. L., Kozak C., Cohen J. C., Shank P. R., Jolicoeur P., Ruddle F., Varmus H. E. Endogenous mouse mammary tumor virus DNA is distributed among multiple mouse chromosomes. Virology. 1979 Jan 15;92(1):46–55. doi: 10.1016/0042-6822(79)90213-7. [DOI] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M. Murine mammary tumor cell clones with varying degrees of virus expression. Virology. 1973 Sep;55(1):163–173. doi: 10.1016/s0042-6822(73)81018-9. [DOI] [PubMed] [Google Scholar]
- Quint W., Quax W., van der Putten H., Berns A. Characterization of AKR murine leukemia virus sequences in AKR mouse substrains and structure of integrated recombinant genomes in tumor tissues. J Virol. 1981 Jul;39(1):1–10. doi: 10.1128/jvi.39.1.1-10.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Kozak C. A. Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4871–4874. doi: 10.1073/pnas.77.8.4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Srinivasan A., Reddy E. P., Aaronson S. A. Abelson murine leukemia virus: molecular cloning of infectious integrated proviral DNA. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2077–2081. doi: 10.1073/pnas.78.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Bishop J. M., Nowinski R. C., Sarker N. H. Mammary tumour virus specific nucleotide sequences in mouse DNA. Nat New Biol. 1972 Aug 9;238(84):189–191. doi: 10.1038/newbio238189a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura F. K., Breda M. Lack of AKR ecotropic provirus amplification in AKR leukemic thymuses. J Virol. 1981 Sep;39(3):808–815. doi: 10.1128/jvi.39.3.808-815.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]