Abstract
BACKGROUND
The optimal treatment for locally advanced superior sulcus tumors is not clear. The authors report long-term results of a trial examining the safety and efficacy of surgery followed by concurrent chemoradiation therapy for this disease.
METHODS
Thirty-two patients with resectable or marginally resectable superior sulcus tumors at The University of Texas MD Anderson Cancer Center from 1994 to 2010 were enrolled in a prospective trial. Surgery involved segmentectomy or lobectomy with en bloc resection of the involved chest wall and complete nodal staging; radiation therapy (RT) began 14 to 42 days later to a dose of 60 grays (Gy) in 50 1.2-Gy fractions if surgical margins were negative or 64.8 Gy in 54 1.2-Gy fractions if margins were positive. Two cycles of etoposide (50 mg/ m2) and cisplatin (50 mg/m2) were given during RT, and another 3 cycles were given after RT. Eleven patients underwent prophylactic cranial irradiation (PCI).
RESULTS
The protocol completion rate was 78%. Gross total resection was accomplished in all 32 patients; 28% underwent R1 resection. Operative mortality was 0%. The most common surgical complication was postoperative pneumonia (25%). At a median follow-up time of 53.4 months (range, 2–154 months), the 2-year, 5-year, and 10-year rates of locoregional control were 84%, 76%, and 76%; distant metastasis-free survival, 52%, 48%, and 48%; disease-free survival, 49%, 45%, and 45%; and overall survival, 72%, 50%, and 45%, respectively. The brain was the most common site of distant failure (n =5), but no patient who received PCI experienced brain metastasis.
CONCLUSIONS
Surgery followed by postoperative chemoradiation is safe and effective for the treatment of marginally resectable superior sulcus tumors.
Keywords: Pancoast tumor, lung neoplasms, adjuvant radiotherapy, adjuvant chemotherapy, chemoradiation
Superior sulcus tumors represent a unique subset of nonsmall cell lung malignancies that have been described as early as 1838.1 Over the past several decades, the treatment of superior sulcus tumors has evolved to become multimodal. In a highly cited trial by the Southwest Oncology Group (SWOG), patients with T3-T4N0-N1 superior sulcus tumors were treated with preoperative cisplatin and etoposide and concurrent radiation to 45 grays (Gy), followed by thoracotomy for disease that was stable or responsive to this treatment. Five-year survival rates were promising at 44% for the entire group and >50% for cases in which a complete response was achieved.2,3 As a result, many have advocated preoperative chemoradiation, even for resectable superior sulcus tumors. However, no studies have established that preoperative chemotherapy, with or without radiation treatment, is superior to surgical resection followed by postoperative treatment in cases that are resectable at diagnosis.
In this context, we initiated a prospective phase 2 study of patients with resectable superior sulcus tumors treated with surgical resection followed by adjuvant chemoradiation. Radiation was delivered in twice-daily fractions to minimize long-term toxicity, which is particularly important as these tumors are often close to the brachial plexus, and to minimize tumor-cell repair and repopulation between fractions.4 We report here the long-term results of this study, focusing on survival outcomes, failure patterns, and treatment complications, with the goal of establishing if this treatment approach is safe and effective for this relatively rare but aggressive disease.
MATERIALS AND METHODS
Eligibility
This pilot study (internal protocol ID 92-038) was approved by the appropriate institutional review board of The University of Texas MD Anderson Cancer Center, and subjects were required to provide written informed consent. Patient enrollment began in 1994 and ended in 2007. During this time period, 155 patients underwent surgery for superior sulcus tumors at our institution, and of these patients, 32 were enrolled in this trial. Eligible patients had histologically confirmed, resectable or marginally resectable nonsmall cell carcinoma of the superior sulcus. Reasons for exclusion from the protocol included patient refusal and treatment before arriving at our institution, such as induction chemotherapy.
Patients were staged with a complete history and physical examination, computed tomography (CT) scan of the chest, mediastinal staging such as endobronchial ultrasound or endoscopy, and positron emission tomography staging when it became commonly used for this disease in 2002. Superior sulcus tumors were defined as tumors arising medially in the apex of the lung with or without invasion into the thoracic ribs or vertebra such that the central axis of the tumor was required to be at the first rib or higher; tumors arising more laterally or chest wall lesions not located in the superior sulcus were excluded. Patients with American Joint Committee on Cancer sixth edition stage IIB, IIIA, or IIIB malignancies were eligible; patients with stage IIIA or IIIB disease were eligible only if involvement of the vertebral bodies or mediastinum was considered resectable. Criteria for resectability was left at the discretion of the surgeons at our institution, but included factors such as significant invasion into the spinal cord, nerve roots, brachial plexus, or vertebral bodies such that gross total resection would place the patient at a significant risk for high-grade neurologic dysfunction. During the time period of our study, 81 patients with superior sulcus tumors were not candidates for surgical resection and received radiation therapy for curative intent.
All patients were evaluated for eligibility by multi-disciplinary teams consisting of experts in radiation oncology, thoracic and cardiovascular surgery, and thoracic/ head and neck medical oncology before registration. All eligible patients’ imaging studies and pathologic reports were centrally reviewed at The University of Texas MD Anderson Cancer Center. All patients were required to have a predicted postoperative forced expiratory volume in 1 second of at least 1.0 L and arterial blood gases (on room air) of pO2 ≥60 mm Hg and pCO2 ≤48 mm Hg. Patients with impending cord compression were ineligible for this study. Patient characteristics are listed in Table 1.
Table 1.
Patient Characteristics
| Characteristic | No. of Patients (%) |
|---|---|
| Sex | |
| Male | 20 (63) |
| Female | 12 (37) |
| Karnofsky performance score | |
| 90–100 | 15 (47) |
| 80 | 8 (25) |
| 70 | 3 (9) |
| Unknown | 6 (19) |
| Clinical T classification | |
| T3 | 26 (81) |
| T4 | 6 (18) |
| Clinical N classification | |
| N0 | 27 (84.5) |
| N1 | 2 (6.25) |
| N2 | 2 (6.25) |
| NX | 1 (3) |
| Tumor histology | |
| Adenocarcinoma | 20 (63) |
| Squamous cell carcinoma | 10 (31) |
| Other/unspecified | 2 (6) |
Treatment
Surgery
Patients first underwent surgery, which consisted of a segmentectomy or lobectomy, with en bloc resection of the involved chest wall at the discretion of the operating surgeon. For cases in which vertebral involvement, intraforaminal extension, or extensive brachial plexus involvement was suspected, surgery was performed in conjunction with a neurosurgical team. All patients underwent complete nodal staging including lobar, hilar, subcarinal, tracheobronchial angle, paratracheal, and subaortic nodal stations.
Radiation therapy
Radiation treatment began between 14 and 42 days after surgery. The radiation dose depended on the surgical outcome, being 60 Gy given in 50 fractions of 1.2 Gy each over a course of 5 weeks for patients with negative surgical margins and 64.8 Gy in 54 fractions for patients with positive margins. Patients received radiation therapy on a twice daily regimen. Patients with extranodal extension received 50 Gy in 1.2-Gy fractions in the absence of gross residual disease or positive margins.
The radiation technique consisted of opposed anteroposterior-posteroanterior fields and encompassed all gross disease plus a margin of 2 cm as well as the bilateral supraclavicular regions, the ipsilateral hilum, and the entire mediastinum inferiorly to 5 cm below the carina. If mediastinal lymphadenopathy was present, the contralateral hilum was also treated. After 50 Gy, off-cord oblique fields were used to minimize the dose to the spinal cord. An electron field was used when necessary to supplement the supraclavicular field.
When the protocol was initially opened, prophylactic cranial irradiation (PCI) at a dose of 25 Gy in 2.5-Gy fractions was required at the completion of chest irradiation. However, this requirement was changed during enrollment because of the lack of definitive evidence of its efficacy in improving survival. Therefore, when the protocol was closed, 11 patients had received PCI, and 21 had not.
Chemotherapy
During radiation therapy, patients were given 2 cycles of oral etoposide at 50 mg/m2 daily on days 1 to 5 and 8 to 12 and bolus intravenous cisplatin at 50 mg/m2 on day 1. The dosing schedule for etoposide (daily vs twice daily vs every other day) was determined by the patient’s body surface area, with patients with greater body surface area receiving more frequent doses. After chemoradiation therapy and PCI (when applicable) had been completed, 3 more cycles of the cisplatin and oral etoposide were given, at the same dose, 1 cycle every 4 weeks.
Follow-Up and Reporting
Interval histories were taken and physical examinations performed weekly during treatment, after each treatment event, and at each follow-up visit. After completion of therapy, patients were followed every 3 months for 2 years, every 6 months for 3 years, and yearly thereafter. A chest x-ray and blood tests including a basic metabolic panel and complete blood count were obtained at each visit. Chest and upper abdomen CT, brain magnetic resonance imaging, and bone scans were obtained every 6 months for 2 years and then yearly for a total of 5 years. Additional images and blood tests were obtained at the treating physician’s discretion.
Statistical Considerations
The primary study endpoint was locoregional control (LRC). Secondary clinical endpoints were disease-free survival (DFS), distant metastasis-free survival, and overall survival (OS). For this phase 2 pilot study, it was calculated that 30 patients were needed to estimate local control and survival rates with a standard error of ≤10%. Kaplan-Meier analysis was used to generate 5-year and 10-year estimates of LRC, DFS, distant metastasis-free survival, and OS. All toxicity events occurring while patients were on protocol were considered treatment-related unless a given event could be clearly shown to be the result of tumor progression. Toxicity was scored according to the Common Toxicity Criteria for Adverse Events version 2.0.
RESULTS
Adherence to Treatment Regimen and Surgical Results
Twenty-five patients (78%) completed the entire protocol (surgery, radiation, and chemotherapy), 6 only completed surgery and radiation therapy, and 1 patient completed surgery only. Gross total resection was accomplished in all 32 patients, R0 in 23 (72%), and R1 in 9 (28%). In terms of pathologic classifications, 25 patients (78%) had T3 disease, and 7 (22%) had T4 disease; 25 patients (78%) had N0, 4 (13%) had N1, 1 (3%) had N2, and 2 (6%) had NX disease.
Disease Control and OS
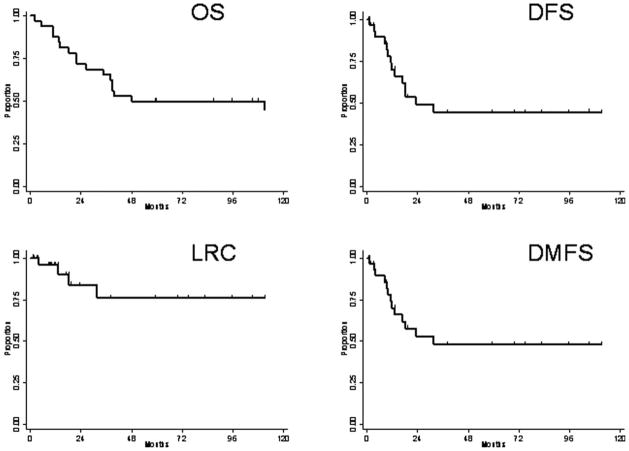
At a median follow-up time of 53.4 months (range, 2–154 months), the LRC rates at 2, 5, and 10 years were 84%, 76%, and 76%, respectively; corresponding distant metastasis-free survival rates were 52%, 48%, and 48%; DFS rates were 49%, 45%, and 45%; and OS rates were 72%, 50%, and 45%. Kaplan-Meier survival curves are shown in Figure 1. The median time to disease progression was 14.8 months (range, 0.8–153 months). Four patients (13%) had experienced locoregional failure at last follow-up, and 14 patients (44%) had distant metastasis (DM). Locoregional failures occurred in years 1997, 1999, 2003, and 2007. Two additional patients were found to have a presumed second lung malignancy. The first patient was 35 years old at diagnosis and 10 years later was found to have contralateral disease with metastatic adenopathy and multiple intracranial metastases. Because of the prolonged time interval and the apparent origin in the contralateral lung, this disease was considered a second malignancy rather than a recurrence. The second patient was 60 years old at diagnosis and approximately 2 years later developed a tumor in the contralateral lung that was consistent with a new primary tumor per pathology review at an outside hospital. The patient received a second course of definitive radiation to this region and then was lost to follow-up.
Figure 1.
Kaplan-Meier curves illustrate overall survival (OS), disease-free survival (DFS), locoregional tumor control (LRC), and distant metastasis-free survival (DMFS).
For the patients who experienced DM, the sites of treatment failure were as follows, with some patients having failure in >1 site: brain (n =5), bone (n =4), adrenal gland (n =4), contralateral lung (n =2), pleura (n =1), and liver (n =1). None of the 11 patients who received PCI had intracranial failure.
Surgical Toxicity
The most common surgical complication was postoperative pneumonia, experienced by 8 patients; 6 patients had pneumothorax, 3 had atrial fibrillation, 2 had urinary retention or bladder spasms, and 5 had other complications (1 dysesthesia, 1 empyema, 1 lung collapse, 1 vocal cord paresis, and 1 wound infection). No patient died from any surgical complication.
Chemoradiation Toxicity
Adverse events attributable to chemoradiation are shown in Table 2. No patient experienced grade 5 toxicity. The most common causes of severe (grade 3–4) chemoradiation-related toxicity were dysphagia (n =10), pneumonitis (n =1), lung fibrosis (n =1), leukopenia (n =2), and granulocytopenia (n =3).
Table 2.
Chemoradiation Complications
| Complication | Severity (No. of Patients [N = 532]) | |||||
|---|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Unknown | |
| Dysphagia | 6 | 6 | 10 | 10 | 0 | 0 |
| Pneumonitis | 31 | 0 | 0 | 1 | 0 | 0 |
| Pulmonary fibrosis | 25 | 1 | 3 | 1 | 0 | 2 |
| Anemia | 21 | 6 | 5 | 0 | 0 | 0 |
| Leukopenia | 23 | 1 | 6 | 2 | 0 | 0 |
| Granulocytopenia | 25 | 1 | 3 | 2 | 1 | 0 |
| Thrombocytopenia | 27 | 4 | 1 | 0 | 0 | 0 |
DISCUSSION
The current standard treatment for locally advanced non-small cell lung cancer is multimodal and includes surgery (for those who are eligible), chemotherapy, and radiation treatment. Although these modalities are the same as those used for superior sulcus tumors, the general approach to this subgroup of lung tumors has differed from that of primary lung malignancies at other sites, largely because superior sulcus tumors often present with intractable pain or neurologic symptoms resulting from involvement of adjacent nerves or bony structures. Local progression of disease in superior sulcus tumors can also lead to invasion of the spinal canal with subsequent spinal cord compression. As a result, immediate local therapy is often indicated.
Previous retrospective reports of surgical resection as definitive local therapy for superior sulcus tumors demonstrated low rates of gross total resection, high rates of locoregional relapse, and low rates of survival. Rusch et al, reporting on 225 patients who underwent thoracotomy as definitive treatment for T3 or T4 superior sulcus tumors from 1974 to 1998 at Memorial Sloan-Kettering Cancer Center, found that gross total resection could be achieved in 50% to 60% of patients with T3 tumors and 40% of patients with T4 tumors, with an operative mortality rate of 0%. Five-year OS rates ranged from 46% for patients with stage IIB tumors to 0% to 13% for patients with stage IIIA or IIIB tumors.5
As a result of findings such as these, the Southwest Oncology Group began a prospective trial in 1995 examining the role of preoperative chemoradiation (cisplatin and etoposide with 45 Gy radiation) followed by surgical resection for patients with T3-T4N0-N1 superior sulcus tumors. Patients who responded or showed stable disease after this induction therapy were offered definitive surgical resection. With this approach, 83 (75%) of 111 patients completed the entire treatment regimen; R0 was possible for 75 patients (90%) and gross total resection (R0 or R1) in 76 patients (92%). Or, put another way, 68% of initially enrolled patients were able to undergo gross total resection.2 In the most recent update of this trial, the 5-year survival rate was 44% for all patients; 17 patients (15%) experienced local relapse, 19 (17%) experienced brain-only relapse, and 19 (17%) experienced relapse in other distant sites.3 A similar trial conducted by the Japan Clinical Oncology Group (JCOG 9806) involved a regimen of 2 cycles of chemotherapy (mitomycin, vindesine, and cisplatin) with concurrent radiation (45 Gy in 25 fractions delivered in a split-course fashion) followed by surgical resection. In that trial, 75% of patients completed the regimen, and the 5-year DFS and OS rates were 45% and 56%, respectively.6 As a result of these trials, preoperative concurrent chemoradiation followed by surgery (with or without adjuvant chemotherapy) is the treatment currently recommended by the National Comprehensive Cancer Network for patients with marginally resectable or resectable N0 or N1 superior sulcus tumors.7
The approach in the present prospective study was different in that patients received surgical resection first, followed by concurrent chemoradiation (up to 64.8 Gy, given twice daily, with cisplatin and etoposide). If this approach can produce high rates of R0/R1 resections with acceptable morbidity, it has theoretical benefits over induction chemoradiation. First, surgical resection may provide more effective palliation of symptoms as well as spinal decompression in cases involving neurologic compromise. Although we did not measure the efficacy of palliation as an endpoint in the current study, prior studies have demonstrated that, particularly in cases of spinal canal compromise, surgical decompression causes more immediate relief of symptoms. For example, in a randomized trial by Patchell et al comparing decompressive surgical treatment followed by radiation therapy in the setting of metastatic disease, the trial was stopped early because an interim analysis demonstrated that patients who received upfront surgery retained the ability to walk significantly longer than those patients receiving radiation initially.8 Second, the preoperative chemoradiation dose in the SWOG 9416 and JCOG 9806 regimens was relatively low, and thus an R1 or R2 resection may need to be followed by further radiation—which would then be delivered in a split-course fashion, a schedule that is suboptimal in terms of maximizing the probability of local control. This latter concern has been addressed in more recent reports of a more intensive preoperative regimen, with preoperative radiation doses approaching 60 Gy with acceptable toxicity.9,10
We have previously demonstrated retrospectively that long-term survival can be achieved with superior sulcus tumors using a variety of approaches. The most common approach for resectable tumors was that of surgery followed by radiation, with or without chemotherapy. The 5-year survival rate of patients who underwent a gross total resection in this study and received postoperative radiation was 82%.11 Accordingly, we have now reported the current prospective study and found that proceeding from immediate surgical resection to postoperative chemoradiation produced rates of adherence to treatment, operative mortality, R0 resections, LRC, and OS that were analogous to those found after induction chemoradiation (Table 3). In addition to these similarities, the nature of the operative complications was similar in the 2 approaches, with several complications being common to all studies: postoperative pneumonia, arrhythmias, empyemas, and wound infections. The findings on operative morbidity and mortality are particularly significant because in the current study, patients would be expected to undergo more extensive surgery to treat disease that had not been downsized by chemoradiation. Furthermore, we did not observe any brachial plexopathy after radiation therapy despite >60 Gy being delivered to this region, which we conclude can be attributed at least in part to our use of a hyperfractionated regimen to minimize late radiation effects.
Table 3.
Outcome Variables After Preoperative Versus Postoperative Concurrent Chemoradiation for Superior Sulcus Tumors
| Outcome Variables | Preoperative Concurrent Chemoradiation | Postoperative Concurrent Chemoradiation, Current Study | |
|---|---|---|---|
| SWOG 94–16 | JCOG 9806 | ||
| Adherence to treatment regimen | 75%a | 76% | 78% |
| Operative mortality rates | 2.4% | 3.5% | 0% |
| R0/R1 resection rates | 92%b | 95% | 100% |
| Locoregional control rates | 85%c | 87%d | 76% |
| 5-year overall survival rates | 44% | 56% | 50% |
Abbreviations: JCOG, Japan Clinical Oncology Group; SWOG, Southwest Oncology Group.
Defined as percentage of patients undergoing induction chemoradiation followed by thoracotomy as a proportion of the total number of eligible patients.
Defined as percentage of patients in whom R0/R1 resection was achieved as a proportion of those patients who proceeded to surgery.
Reported as local control.
Reported as initial site of failure.
Other notable findings were that the rate of brain metastases in our study (15%) was low compared with that of prior reports and that none of the patients who received PCI experienced treatment failure in the brain. PCI has been assessed in several randomized studies, including 3 studies conducted in the 1980s and 1990s demonstrating that PCI could delay and even prevent the development of brain metastases from nonsmall cell lung cancer but did not significantly affect OS.12–14 Preliminary results from a more recent study by the Radiation Therapy Oncology Group assessing the benefit of PCI for 356 patients with stage IIIA or IIIB disease randomized to PCI versus observation indicated that although PCI did not affect the 1-year DFS or OS rates, the relapse rate in the central nervous system was reduced from 18% to 7.7%.15 Thus, based on the lack of a clear survival benefit, PCI is not recommended routinely for patients with locally advanced nonsmall cell lung cancer. However, it has been well established that adenocarcinomas have a higher propensity to metastasize to the brain than do squamous cell carcinomas and that superior sulcus tumors are particularly likely to produce intracranial metastases.16,17 The results of the current study suggest that an individualized treatment approach to PCI should be used, with highly selective criteria based on the location and histology of the tumor, patient age, and baseline neurocognitive deficits.18
The current study had several limitations. First, the number of patients was smaller than that of previous reports, precluding statistical analyses of predictive factors such as extent of resection and TNM classification. Second, the potential advantages of postoperative chemoradiation for superior sulcus tumors are accompanied by some disadvantages, including the possibility of needing a larger radiation field to cover the entire tumor bed rather than a field limited solely by the initial extent of disease. A second potential disadvantage is an increase in operative complications because of the lack of tumor downsizing away from critical structures; however, no such increase in operative morbidity was found in the current study, and no mortalities were attributed to treatment. Third, this study took place over a protracted period of time, from 1994 to 2007. We acknowledge that during this time period, inherent changes in technique and expertise can occur that can affect tumor control. However, in our study, we found that the locoregional recurrence rate was relatively constant over the duration of enrollment, indicating that the treatment year did not influence local recurrence rates.
Finally, it is important to emphasize that the results of this study, although promising, were undoubtedly dependent on patient selection. Indeed, previous retrospective studies demonstrating low rates of LRC with upfront surgery often included substantial percentages of patients for whom gross total resection could not be achieved. In the current study, all of the patients initially deemed to have resectable disease ultimately underwent R0 or R1 resections, highlighting the importance of surgical experience and a referral to a tertiary medical center when possible. Furthermore, we acknowledge that prior studies, and particularly the SWOG study cited above, demonstrated that the long-term survivors were those patients who had R0 resections, which were not achieved in 22% of the patients in our study. However, the difference in the approaches is that, whereas in the SWOG study the goal was to increase resectability rates and thereby reduce the risk of residual disease postoperatively, in the approach from our institution, microscopic disease is further addressed by adjuvant chemoradiation. We do not present these results as superior to those previously published in prospective trials, but rather present upfront surgery as a viable method to achieve similar rates of local control and OS for this disease. Regardless of the timing of surgery, we advocate that this extensive oncologic resection should be performed by an experienced surgeon with this technique, if necessary at a tertiary academic center.
In summary, we demonstrated that surgical resection followed by adjuvant chemoradiation therapy for superior sulcus tumors is a reasonable alternative to pre-operative chemoradiation, with similar survival outcomes and treatment toxicity. We further showed that, with appropriate patient selection and an aggressive chemoradiation regimen, including a radiation fractionation scheme that minimizes late toxicity, 10-year LRC and OS rates were approximately 75% and 50%, respectively, with no treatment-related mortality. Finally, our findings suggest that PCI could be considered for patients with this subset of nonsmall cell lung cancer, as the rate of intracranial failure for all patients (15%) was substantially lower than that of previous reports and was 0% among patients who received PCI. We recognize, however, that the small number of patients limits the strength of this conclusion.
Acknowledgments
FUNDING SOURCES
This research was supported in part by the National Institutes of Health through The University of Texas MD Anderson Cancer Center’s Cancer Center Support Grant CA016672.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Hare E. Tumor involving certain nerves. London Medical Gas. 1838;1:16–18. [Google Scholar]
- 2.Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: initial results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160) J Thorac Cardiovasc Surg. 2001;121:472–483. doi: 10.1067/mtc.2001.112465. [DOI] [PubMed] [Google Scholar]
- 3.Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160) J Clin Oncol. 2007;25:313–318. doi: 10.1200/JCO.2006.08.2826. [DOI] [PubMed] [Google Scholar]
- 4.Arcangeli G, Mauro F, Morelli D, Nervi C. Multiple daily fractionation in radiotherapy: biological rationale and preliminary clinical experiences. Eur J Cancer. 1979;15:1077–1083. doi: 10.1016/0014-2964(79)90123-3. [DOI] [PubMed] [Google Scholar]
- 5.Rusch VW, Parekh KR, Leon L, et al. Factors determining outcome after surgical resection of T3 and T4 lung cancers of the superior sulcus. J Thorac Cardiovasc Surg. 2000;119:1147–1153. doi: 10.1067/mtc.2000.106089. [DOI] [PubMed] [Google Scholar]
- 6.Kunitoh H, Kato H, Tsuboi M, et al. Phase II trial of pre-operative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol. 2008;26:644–649. doi: 10.1200/JCO.2007.14.1911. [DOI] [PubMed] [Google Scholar]
- 7. [Accessed on December 21, 2009];National Comprehensive Cancer Network Clinical Guidelines for Non-Small Cell Lung Cancer. 2010 2 Available at: www.nccn.org. [Google Scholar]
- 8.Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 9.Kwong KF, Edelman MJ, Suntharalingam M, et al. High-dose radiotherapy in trimodality treatment of Pancoast tumors results in high pathologic complete response rates and excellent long-term survival. J Thorac Cardiovasc Surg. 2005;129:1250–1257. doi: 10.1016/j.jtcvs.2004.12.050. [DOI] [PubMed] [Google Scholar]
- 10.Suntharalingam M, Sonett JR, Haas ML, et al. The use of concurrent chemotherapy with high-dose radiation before surgical resection in patients presenting with apical sulcus tumors. Cancer J. 2000;6:365–371. [PubMed] [Google Scholar]
- 11.Komaki R, Roth JA, Walsh GL, et al. Outcome predictors for 143 patients with superior sulcus tumors treated by multidisciplinary approach at the University of Texas M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 2000;48:347–354. doi: 10.1016/s0360-3016(00)00736-7. [DOI] [PubMed] [Google Scholar]
- 12.Cox JD, Stanley K, Petrovich Z, Paig C, Yesner R. Cranial irradiation in cancer of the lung of all cell types. JAMA. 1981;245:469–472. [PubMed] [Google Scholar]
- 13.Russell AH, Pajak TE, Selim HM, et al. Prophylactic cranial irradiation for lung cancer patients at high risk for development of cerebral metastasis: results of a prospective randomized trial conducted by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1991;21:637–643. doi: 10.1016/0360-3016(91)90681-s. [DOI] [PubMed] [Google Scholar]
- 14.Umsawasdi T, Valdivieso M, Chen TT, et al. Role of elective brain irradiation during combined chemoradiotherapy for limited disease non-small cell lung cancer. J Neurooncol. 1984;2:253–259. doi: 10.1007/BF00253278. [DOI] [PubMed] [Google Scholar]
- 15.Gore EM, Bae S, Wong S, et al. A phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small cell lung cancer: initial analysis of Radiation Therapy Oncology Group 0214 [abstract] J Clin Oncol. 2008;27(15 suppl):Abstract 7506. doi: 10.1200/JCO.2010.29.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Detterbeck FC. Pancoast (superior sulcus) tumors. Ann Thorac Surg. 1997;63:1810–1818. doi: 10.1016/s0003-4975(97)00360-3. [DOI] [PubMed] [Google Scholar]
- 17.Komaki R, Roh J, Cox JD, Lopes da Conceicao A. Superior sulcus tumors: results of irradiation of 36 patients. Cancer. 1981;48:1563–1568. doi: 10.1002/1097-0142(19811001)48:7<1563::aid-cncr2820480716>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Topkan E, Yildirim BA, Selek U, Yavuz MN. Cranial prophylactic irradiation in locally advanced non-small cell lung carcinoma: current status and future perspectives. Oncology. 2009;76:220–228. doi: 10.1159/000201933. [DOI] [PubMed] [Google Scholar]