Abstract
The immediate early response gene X-1 (IEX-1) is involved in regulation of various cellular processes including proliferation, apoptosis in part by controlling homeostasis of reactive oxygen species (ROS) at mitochondria. The present study demonstrates reduced inflammatory responses and colorectal cancer in IEX-1 knockout (KO) mice treated with azoxymethane (AOM)/dextran sulfate sodium (DSS). However, DSS induced worse colitis in RAG−/−IEX-1−/− double KO mice than in RAG and IEX-1 single KO mice, underscoring an importance of T cells in IEX-1 deficiency-induced protection against colon inflammation. Lack of IEX-1 promoted the differentiation of IL-17-producing T cells, concomitant with up regulation of Gαi2 expression, a gene that is well-documented for its role in the control of inflammation in the colon. In accordance with this, Th17 cell differentiation was compromised in the absence of Gαi2, and deletion of Gαi2 in T cells alone aggravated colon inflammation and colorectal cancer development after AOM/DSS treatment. Null mutation of IEX-1 also enhanced both proliferation and apoptosis of intestinal epithelial cells (IECs) after injury. A potential impact of this altered IEC turnover on colon inflammation and cancer development is discussed. These observations provide a linkage of IEX-1 and Gαi2 expression in the regulation of Th17 differentiation and suggest a previously unappreciated role for IEX-1 in the control of colon epithelial homeostasis.
Introduction
Prolonged intestinal inflammation or chronic inflammatory bowel disease (IBD) often results in colorectal cancer development, the second most common cancer in the developed countries, responsible for estimated 51,370 deaths in the United States in 2010 alone (1, 2) (www.cancer.gov). Chronic IBD is characterized by a mucosal damage and ulceration, involving rectum and colon (3). It manifests as bloody mucoid, diarrhea, concomitant with DNA damage, epithelial dysplasia, and intestinal inflammation, followed by episodes of disease inactivity. Ulcerative colitis (UC) can be induced by oral administration of dextran sulfate sodium (DSS) to rodents, which mimics human colon inflammation occurring in the entire length of the colon (4). When repetitive DSS administration was combined with a procarcinogen, azoxymethane (AOM), a nearly 100% incidence of carcinoma was induced in the colon, arguing strongly a connection between recurrent inflammation and cancer development (5, 6).
The nuclear transcription factor-kappa B (NF-κB) is known as a master inflammatory mediator and controls the expression of many genes linked to inflammation. One of its targets is the immediate early response gene X-1 (IEX-1) that is known for its involvement in the control of cell growth, apoptosis, and intracellular reactive oxygen species (ROS) homeostasis (7, 8). Its expression can be quickly induced, in response to a variety of stressors, in epithelial, vasculature, and endocrine tissues, as well as inflammatory cells (9). Intracellularly, IEX-1 is localized to the endoplasmic reticulum, nuclei and mitochondria. In mitochondria, it targets the F1F0-ATPase inhibitor (IF1) for degradation, resulting in elevated F1F0-ATPase activity and reduced mitochondrial ROS (mROS) production (8). In contrast, its null mutation increases mROS production that appears to augment Th17 cell differentiation and protect against DSS-induced colitis (10). The protective role of IL-17A is consistent with its ability to fortify tight junctions between intestine epithelial cells (11) and its neutralization with anti-IL17A antibody worsening DSS-induced colitis in mice (10, 12).
The present investigation shows that null mutation of IEX-1 prevents mice from not only colitis but also cancer development after DSS or AOM/DSS treatment. The prevention was primarily attributed to altered differentiation or function of T cells, in part by up regulation of Gαi2 expression. Accordingly, deletion of Gαi2 in T cells alone aggravated colon inflammation and tumor formation. Although IEX-1 deficient animals also displayed a significantly higher level of proliferation and apoptosis in intestine epithelial cells (IECs) than wild type (WT) IECs, the fast turnover of IECs might exert both beneficial and detrimental effects on colon inflammation and cancer development and it might not be as critical as T cells in IEX-1 deficiency-mediated protection against colitis or colon cancer development.
Materials and Methods
Animals
IEX-1 knockout (IEX-1 KO) and WT control mice on the mixed 129Sv/C57BL/6 background (F1) were generated by gene targeting (13). Gαi2-floxed (Gαi2flx/flx) mice in which loxP sites were introduced by homologous recombination before exon2 and after exon4 of the Gnai2 gene were generated as detailed in supporting materials. To specifically delete Gαi2 in T cells, Gαi2flx/flx-CD4-Cre mice were made by back crossing Gαi2-floxed mice on the mixed 129Sv/C57BL/6 background (F1) with CD4-Cre transgenic mice (C57BL/6NTacTgN/CD4-Cre, line # 4196) for three generations (F3) and then used to generate CD4-Cre, Gαi2flx/flx and Gαi2flx/flx-CD4-Cre littermates for the study. CD4-Cre mouse breeding pairs were distributed by Taconic under NIAID contract N01-AO-02740 (14). IEX-1−/−Rag−/− double KO mice on C57BL/6 background were generated by crossbreeding IEX-1-deficient C57BL/6 mice with Rag1-deficient C57BL/6 animals (Jackson laboratory, ME). All mice were housed in conventional cages at the animal facilities of Massachusetts General Hospital in compliance with institutional guidelines. The Institutional Animal Care and Use Committee for Massachusetts General Hospital approved all animal experiments.
Colitis and tumor induction
Acute colitis was induced in the mice by DSS treatment (15), which is a commonly used experimental model of IBD (16). Briefly, mice at 8-10 weeks of age were given 3.5% DSS (molecular weight 36,000 to 50,000; MP Biomedicals) in drinking water for 9 days, followed by 5 days of regular drinking water. The mice were weighed daily, and the value was expressed as a percentage of a body weight decrease relatively to the initial body weight on day 0. Colon cancer was induced by a combination of AOM with DSS following a published protocol (17). The mice were first injected intraperitoneally (i.p.) with 12.5mg/kg AOM (Sigma), followed by 3 cycles of DSS ingestion. The first cycle was administrated 5 days after AOM injection and consisted of 5 days of 2.5% DSS ingestion followed by 16 days of regular water. The second cycle was identical to the first one. The third cycle consisted of 4 days of 2% DSS followed by 10 days of regular water. The animals were sacrificed on the final day of the protocol, and colons were removed for analysis.
Morphological and histological analysis
The treated or untreated control animals were sacrificed at indicated times. Their colons were removed, opened longitudinally, rinsed free of feces with PBS, pinned to the surface of a board at both ends to maintain a linear shape, and fixed in 10% formalin neutral buffer solution. On the following day, the colons were photographed at high resolution and tumors were counted in each individual colon. The colon samples were then embedded in paraffin, cut at 5μm, and stained with hematoxylin and eosin (H&E).
BrdU staining
Mice were i.p. injected with 1ml (1mg/ml) 5-bromo2′-deoxyuridine (BrdU) and sacrificed 2 hr later. Colons were removed, fixed, and sectioned as above. BrdU staining was performed using a Zymed BrdU labeling kit (Zymed, San Francisco, CA) per the manufacturer’s instructions. BrdU-positive cells were counted in a total of 75 crypts in each group, and an average number of positive cells per crypt was presented.
Apoptosis in IECs
To measure apoptosis in IECs, IEX-1 KO and WT littermates at 8~10 weeks of age were given 3.5% DSS in drinking water for 3 days. The mice were sacrificed and their distal colons were dissected, flushed to remove feces with cold Ca2+- and Mg2+-free Hank’s buffer containing 100U/ml penicillin, 100 μg/ml streptomycin sulfate, and 25 μg/ml gentamicin. The colons were opened longitudinally and cut laterally into small pieces. The colon pieces were then incubated at 4°C for 1 hr in 50-ml tube containing cold Hank’s buffer supplemented with 0.02% EDTA and 10 mM dithiothreitol (DTT) and then vortexed for 2 min to dislodge IECs. The vortex procedure was repeated once after collecting the cells released from the colons. The resultant cells were pooled and layered over a 5%, 15%, 40% discontinuous Percoll gradient in Hank’s buffer followed by centrifugation for 30 min at 560 × g at 4°C. Epithelial cells were recovered at the interface between 5% and 15% Percoll, washed, and collected for intracellular staining with epithelial specific antibody against cytokeratin (pan-Cytokerain, C-11, Santa Cruz Bio) and an In Situ Cell Death Detection Kit (TUNEL) according to the manufacturer’s instruction (Roche Applied Science, Indianapolis, IN). The stained cells were evaluated on FACSAria and the flow data were analyzed in flowJo software (Tree Star Inc, Ashland, OR). Apoptotic index (%) was expressed as the percentage of apoptotic cells (TUNEL+) relative to the number of cytokeratin-positive cells examined.
Th17 polarization and flow cytometric analysis
Single-cell suspensions were prepared from spleens and treated with a mixture of rat anti-mouse antibodies (Abs) against CD19, CD32 and CD8 followed by depletion of Ab-bound cells with BioMag goat anti-rat IgG (Polyscience) per manufacturer’s instructions. The freshly isolated CD4+ T cells were plated in 6-well plate at 0.6 × 106/well in 2 ml of complete medium and stimulated with 0.5 μg/ml anti-CD3 and 1 μg/ml anti-CD28 in the presence of 1ng/ml TGF-β and 50 ng/ml IL-6 (PeproTech). After 4 days of incubation, the cells were restimulated with 50 μg/ml PMA and 750 μg/ml ionomycin for 4 hours in the presence of 1 μg/ml Golgi Plug (BD Biosciences). The stimulated cells were stained first with PE-anti-CD4 Ab and then permeabilized in a permeabilizing buffer followed by intracellular staining with anti-IL-17A-Alexa Fluor 700 Ab (TC11-18H10.1, BioLegend). The stained cells were evaluated on FACSAria, and the flow data were analyzed in flowJo software as above.
Quantitative real-time RT-PCR of inflammatory cytokines and Gαi2
WT and IEX-KO mice at 8~10 weeks of age were given with drinking water with or without 3.5% DSS for 36 hr. The mice were sacrificed and colons were dissected, rinsed thoroughly with ice cold Hank’s buffer, and opened longitudinally to expose the intestinal mucosa. The mucosal layers were harvested by gentle scraping with a glass slide. Total RNA was extracted from the resultant scraping samples using a Trizol reagent (Invitrogen) per the manufacturer’s instructions followed by treatment with DNase-I (bovine pancreas; Sigma) for 1 hr at room temperature and re-extraction with phenol/chloroform/isoamyl alcohol mixture to remove DNase. The purified RNA was reverse-transcribed, and amplified by real-time PCR using a SYBR Green PCR kit (Applied Biosystems, Foster City, CA) on an Mx4000TM Multiplex Quantitative PCR System (Stratagene). The primers used were: forward, CCCTCACACTCAGATCATCTTCT and reverse, GCTAC-GACGTGGGCTACAG for TNFα; forward, GCAACTGT-TCCTGAACTCAACT and reverse, ATCTTTTGGGGTC-CGTCAACT for IL-1β; forward, TAGTCCTTCCTACCC-CAATTTCC and reverse, TTGGTCCTTAGCCACTCCTTC for IL-6; forward, TTAAAAACCTGGATCGGAACCAA and reverse, GCATTAGCTTCAGATTTACGGGT for CCL2; forward, AGTGAACTGCGCTGTCAATGC, and reverse, AGGCAAACTTTTTGACCGCC for MIP-2; and forward, GGCTGTATTCCCCTCCATCG and reverse, CCAG-TTGGTAACAATGCCATGT for β-actin. For quantifying Gαi2 mRNA in T cells, T cells isolated from indicated mice were subject to RNA extraction, reverse transcription, real-time PCR as described above using Gαi2 specific primers: forward 5′-CAATGACTCAGCCGCTTACTAC-3′ and reverse, 5′-TGAAGTGTGTTTCCACGATGC-3′. No PCR products were generated from non reverse-transcribed samples run in parallel. Fold induction of mRNA was determined from the threshold cycle (Ct) values normalized for β-actin expression and then normalized to the value derived from controls.
Statistical analyses
The statistical analysis was based on the calculation of arithmetic mean and standard deviation (SD). The difference between two means was compared by two-tailed unpaired Student’s t test assuming equal variances. One-way ANOVA was used for multiple group comparisons. A p-value of less than 0.05 was considered statistically significant.
Results
IEX-1-deficiency prevents from colon tumor formation
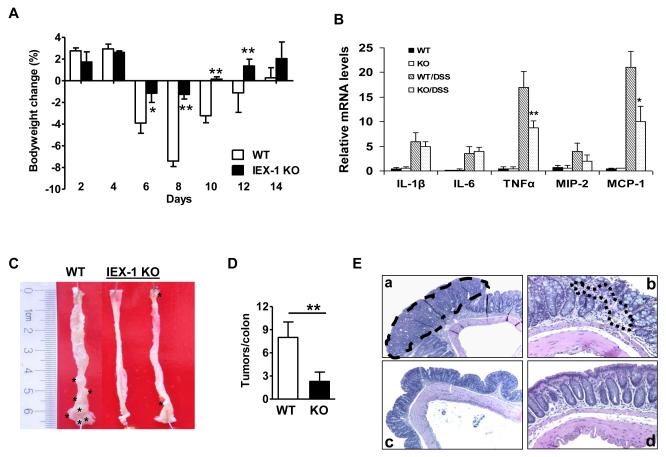
The toxic reagent, DSS directly damages mucosal epithelium and causes an acute intestinal inflammation (15). When given drinking water containing 3.5% DSS over a 9-day period of time, WT animals suffered from a body weight loss up to 7.4 ± 1.5% by day 8, concurrent with colonic bleeding in some mice. The animals gained a body weight gradually and reached a pre-DSS treatment level by day 4 after switching to regular drinking water (Figure 1A). In contrast, IEX-1−/− animals did not experience a significant body weight loss (1.26 ± 0.4%), with almost no fluctuations over a 9-day course of DSS treatment (Figure 1A). Similar protection against colitis induced by 5% DSS was also demonstrated in the absence of IEX-1(10). Consistent with reduced inflammation in the absence of IEX-1, inflammatory cytokine TNFα and chemokine MCP1 (CCL2), but not IL-1β, IL-6 or MIP-2 (CCL3), were produced in the colon mucosa at a significantly lower level in IEX-1 KO mice than in WT control mice treated with 3.5% DSS for 36 hr (figure 1B). To determine whether lack of IEX-1 also protected mice from colon cancer, the mice were i.p. injected with a single dose of AOM followed by 3 cycles of DSS administration as previously described (18). AOM is a procarcinogen structurally similar to cycasin, a naturally-occurring compound. It causes the formation of O6-methylguanine adducts in DNA, leading to G→A transition after replication and tumor formation in the distal colon of rodents. Repeated administration of DSS after AOM treatment greatly enhances the incidence of AOM-induced tumors (17). The treatment gave rise to 8 ± 2.5 tumors per colon in WT animals, but only 2 ± 1 in IEX-1−/− mice, in agreement with reduced colitis in the mice (Figure 1D, p<0.01). The majority of the tumors in WT animals were localized at the distal colon, with fewer tumors in the proximal colon, whereas tumors in IEX-1−/− animals were located primarily in the distal colon (Figure 1C). Microscopic analysis of H&E-stained sections from WT animals demonstrated the presence of colonic adenomas with a high degree of dysplasia, a loss of entire crypts and surface epithelium, and massive infiltration of leukocytes into mucosa and submucosal edema (Figure 1E). In contrast, IEX-1−/− mice displayed much less damage to the epithelium and lacked significant morphological changes in crypt architecture, confirming a protective role of IEX-1-deficiency in colon inflammation and tumor development.
Figure 1.
IEX-1 deficiency protects from DSS-induced colitis and AOM/DSS-induced tumor formation. A. IEX-1 KO mice are relatively refractory to DSS-induced colitis. IEX-1 KO and WT control mice were administered 3.5% DSS in drinking water for 9 days, followed by 5 days of regular drinking water. Body weights are expressed as mean percentages ± standard deviation (SD) of the initial weights. *, p<0.05; **, p<0.01; and n = 6. B. Real-time RT-PCR analysis of inflammatory cytokines or chemokines in indicated mice treated with DSS for 36 hr. *, ** p< 0.05 or 0.01, respectively, compared in the presence or absence of IEX-1, n= 8 in each group. C. Representative macroscopic images of colons showing tumors (marked by *). D. Summary of tumor growth in the presence or absence of IEX-1. **, p<0.01 and n = 9 for WT or 11 for KO mice. E. Representative microscopic images of H&E stained sections of colon tumor from WT (a, b) and IEX-1 KO (c, d) animals (5X for a and c or 20X magnification for b and d). The tumor (dashed line in a) and leukocyte infiltration (dotted line in b) are encircled.
Aggravated colitis in Rag−/−IEX-1−/− mice
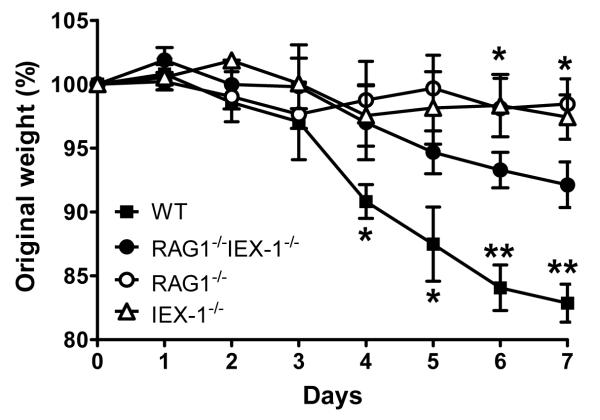
We previously showed that adoptive transfer of IEX-1 KO CD4+CD45RBhigh cells caused delayed and less severe colitis in Rag−/− mice compared to adoptive transfer of WT CD4+CD45RBhigh cells (10). While this observation corroborated diminished pathogenicity of CD4+ T cells lacking IEX-1, it could not rule out that intestine epithelium or other inflammatory, non-T cells were also involved in the disease resistance in IEX-1 KO mice. We thus generated Rag−/−IEX-1−/− double KO mice to address whether diminished inflammation in IEX-1 KO mice could be ascribed to altered function of T cells alone or both T cells and non-T cells. As shown in figure 2, Rag−/− and IEX-1−/− single KO animals both experienced much less severe colitis than Rag−/−IEX-1−/− double KO mice or WT mice upon DSS exposure. The less severe colitis in Rag−/− mice or IEX-1−/− mice than WT mice probably resulted in part from either a lack of pathogenic T cells in Rag−/− mice or increased differentiation of IL-17-producing cells in IEX-1−/− mice as previously demonstrated (10, 19). However, worse colitis in Rag−/−IEX-1−/− double KO mice than single KO mice stresses a T cell-independent mechanism involved, reinforcing a predominant role of T cells in the attenuated colitis and cancer development in IEX-1 KO mice. Despite worse colitis in Rag−/−IEX-1−/− double KO mice compared to single KO mice, the disease in Rag−/−IEX-1−/− double KO mice was till significantly less severe than that of WT mice presumably due to a lack of pathological T cells. Rag−/−IEX-1−/− double KO mice suffered from an 8% decrease in their body weight, compared to an 18% of a body weight loss in WT animals (Figure 2), which had to be sacrificed in accordance with institutional guidelines, resulting in a premature termination of the study. Notably, these groups of mice were on C57BL/6 genetic background and C57BL/6 mice were more susceptible to DSS-induced colitis than the mice on mixed 129Sv/C57BL/6 background as previously described (20).
Figure 2.
IEX-1−/− Rag−/− mice develop worse colitis than IEX-1 single KO mice after DSS treatment. IEX-1−/− Rag−/−, IEX-1−/−, Rag−/− and WT control mice were administered 3.5% DSS in drinking water for 7 days. Body weights were monitored daily and expressed as mean percentages ± SD of the initial weights. *, p<0.05 between double KO mice and single KO mice; and **, p<0.01 between double KO mice and WT mice (n = 6 in each group).
Increased proliferation and apoptosis in IECs in IEX-1 KO animals
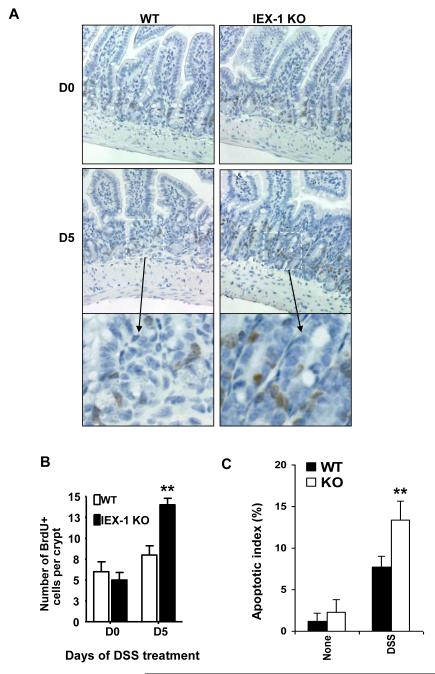
We next investigated whether lack of IEX-1 altered intestinal homeostasis. Normally, the intestinal epithelium of adult mammals undergoes continuous self-renewal in which stem cells at the bottom of the crypts give rise to progenitor cells that proliferate and differentiate as they move up the crypt-surface (21). This process can be tracked by intraperitoneal injection of BrdU 2 hr before colon harvest, which labels proliferating cells. In untreated (day 0) WT and IEX-1 KO animals, BrdU-labeled cells were located primarily within the bottom third of crypts in the colon (Fig. 3A). On average, 7 ± 3 and 5 ± 2 cells were BrdU-positive in the control and IEX-1 KO animals, respectively, which was without statistical significance. By day 5 of DSS treatment, labeled cells were seen extending into the midregions and throughout the colonic crypts in IEX-1 KO animals, whereas WT epithelial cells did not undergo a similar expansion. Counting of BrdU-positive cells demonstrated a higher number of proliferating cells per crypt in IEX-1−/− mice than in WT animals (Figure 3B, p<0.01). Strikingly, the increased IEC proliferation was concurrent with increasing apoptosis in the cells (Figure 3C). The altered proliferation and apoptosis of IECs may exert both detrimental and beneficial effects on colon inflammation and cancer development.
Figure 3.
Increased proliferation and apoptosis in IEX-1 KO IECs. Colon sections were labeled with BrdU antibody (A). Magnification 20X except for the bottom panels that are the enlarged (40x) areas of D5 marked by a white dashed line rectangle. An average number ± SD of BrdU-positive (brown) cells per 1 crypt was presented in (B). **, p<0.01 and n = 75. C. Apoptotic index in IECs. The indicated mice were treated with or without 3.5% DSS for 3 days, after which IECs were isolated and intracellularly stained by anti-cytokeratin and TUNEL following by flow cytometric analysis. The apoptotic index was expressed as % of TUNEL+ apoptotic cells over cytokeratin-positive cells by flow cytometry. n = 6 in each group.
A high level of Gαi2 in IEX-1 KO cells is concurrent with increased Th17 differentiation
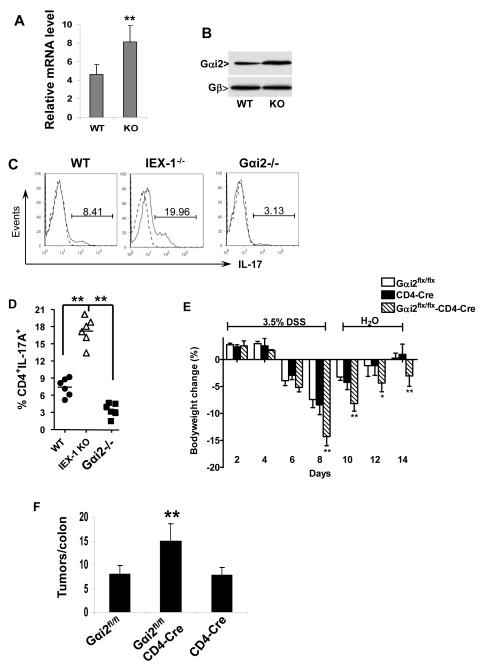
A worse colitis in Rag−/−IEX-1−/− double KO mice compared to IEX-1 KO mice confirmed an importance of T cells in IEX-1 deficiency-induced protection against colon inflammation. Our laboratory previously showed that lack of IEX-1 enhanced Gαi2 expression in the vasculature (13). Interestingly, an absence of this gene in mice produced spontaneous IBD, with a Th1-skewed autoimmunity in the colon (22). In light of reciprocal regulation of Th17 and Th1 subsets, Gαi2 might be up regulated in IEX-1-deficient T cells, favoring Th17 over Th1 cell differentiation. To determine this, Gαi2 expression was analyzed in T cells isolated from WT and IEX-1-deficient mice by real time RT-PCR (Figure 4A) or western blotting (Figure 4B). We found an approximate 60% increase in Gαi2 expression in IEX-1 KO T cells compared to WT counterparts (*p<0.01). Moreover, CD4+ T cells isolated from the spleen of Gαi2−/− mice developed significantly fewer Th17 cells than WT CD4+ T cells under Th17-palorizing conditions (Figure 4C and 4D). Interestingly, an opposite trend was obtained with IEX-1-deficient CD4+ T cells that produced a two-fold higher number of Th17 cells under a similar condition (figure 4, C and D). The observation suggests an indispensible role for Gαi2 in the regulation of Th17 cell differentiation.
Figure 4.
Increased expression of Gαi2 in IEX-1 KO T cells and aggravated colitis in Gαi2flx/flx-CD4-Cre animals. Gαi2 expression was analyzed by real-time RT-PCR (A) and Western blotting (B) in WT and IEX-1 KO T cells (**, p<0.01, n = 5). One representative result of three similar experiments performed and Gβ for equal cell membrane protein loading control in B. C and D. Reduced ability of Gαi2−/− CD4+ T cells to differentiate into Th17 subset in vitro. After 4 days of differentiation under Th17 polarizing conditions, intracellular levels of IL-17A in WT, IEX-1 KO and Gαi2−/− CD4+ T cells were determined by flow cytometry. One representative experiment of flow cytometry is shown in C and percentages of CD4+IL-17A+ producing cells from individual mice are summarized in D (**p<0.01). E. Deletion of Gαi2 in T cells alone results in severe colitis symptoms in the DSS model. Gαi2flx/flx, CD4-Cre, and Gαi2flx/flx-CD4-Cre animals were treated with 3.5% DSS as figure 1. Body weights are expressed as mean percentages ± SD of the initial weights. *p<0.05; ** p<0.01; and n = 9 in each group. F. Tumors were counted in each individual colon and presented as mean ± SD. **, p<0.01 and n = 9.
Absence of Gαi2 in T cells alone aggravates colon inflammation and cancer development
Gαi2−/− mice developed spontaneous colitis, characterized by a Th1-predominant autoimmunity in the colon (22), but it was not clear whether a defect in T cells alone could cause the disease, despite altered function and differentiation of the cells (22, 23, 24). We generated Gαi2flx/flx-CD4-Cre mice to delete Gαi2 specifically in T cells in order to address this issue. Unlike Gαi2−/− mice, Gαi2flx/flx-CD4-Cre mice were healthy and did not show any sign of a gross defect. However, when the mice were subject to DSS-induced colitis, Gαi2flx/flx-CD4-Cre mice lost more weight (14.3 ± 1.7%) than Gαi2flx/flx (7.4 ± 1.5%) or CD4-Cre (8.4 ± 1.8%) control mice on day 8 (Fig, 4E, p<0.01), with slower weight restoration on days 10, 12, and 14. In accordance with severer colitis, the tumor number was also significantly higher in Gαi2flx/flx-CD4-Cre animals (14.9 ± 3.7) than in control animals (8±1.8 in Gαi2flx/flx mice or 7.8 ± 1.5 in CD4-Cre mice) after treatment with DSS/AOM (Figure 4F, **p<0.01). The tumors were predominantly distributed to distal colon, concurrent with signs of rectal prolapse in 80% of animals, corroborating the pathogenicity of Gαi2-deficient T cells.
Discussion
Inflammation is one of attributing factors to carcinogenesis, as has been demonstrated in many studies connecting these two pathological conditions (1, 2). Our previous investigation identified that IEX-1 KO mice were resistant to DSS-induced colitis as reflected by unaltered colon length, body weight, and myeloperoxidase activity of the mice before and after DSS treatment (10). This study extends the finding demonstrating that the mice are also protected against AOM/DSS-induced tumor development. Hence, our present and previous studies highlight an importance of IEX-1 in the suppression of Th17 cell differentiation and in the development of inflammation-associated cancer in the colon. Null mutation of IEX-1 increases differentiation of IL-17-producing T cells that play a primary role in protection against colon inflammation and cancer development in IEX-1 KO mice.
Lack of IEX-1 up regulated Gαi2 expression in T cells in association with Th17 differentiation. Gαi2 KO mice developed a Th1-skewed autoimmunity in the colon, characterized by increasing production of IFN-γ, TNFα, IL-1β, IL-6, and IL-12p40 (22). Moreover, CD4+ T cells purified from the mice exhibited an intrinsic propensity to differentiate into Th1 cells in vitro culture (24), whereas CD4+ T cells lacking Gαi2 had compromised Th17 differentiation (Figure 4C and 4D). Mice lacking Gαi2 in T cells alone developed severer colitis and a higher number of tumors than the control mice after exposing to DSS or AOM/DSS. These studies stress a critical role for Gαi2 in a balance between Th17 and Th1 cell differentiation.
Lack of IEX-1 has been shown to elevate mROS production that may augment Gαi2 expression as occurs in IEX-1-deficient vascular smooth muscle cells (13). The promoter of Gnai2 gene encoding Gαi2 contains transcriptional regulatory motifs for the redoxsensitive NF-κB and nuclear factor erythroid 2-related factor transcription factors (25). IEX-1 deficiency-mediated production of mROS may up regulate Gαi2 expression via these redox-sensitive transcription factors, which is currently under investigation. Apart from transcriptional regulation, Nishida et. al. demonstrated that hydrogen peroxide specifically increased Gαi protein activity but not the activity of Gαs in association with the plasma membrane (26). A high level of Gαi2 activity in IEX-1-deficient T cells may increase Th17 cell differentiation and reciprocally decrease Th1 cell differentiation, rendering IEX-1 KO mice refractory to DSS-induced colitis and AOM/DSS-induced color cancer.
A high level of IL-17A in IEX-1 KO T cells but low in Gαi2−/− T cells correlate with a protective role of this cytokine in intestine inflammation, as has been demonstrated in several studies (10, 12, 27). Moreover, elevated levels of IL-17A as well as Th17 cells were found in various tumors (28, 29, 30, 31, 32). Overexpression of IL-17A in cell lines and their subsequent implantation into mice resulted in tumor formation, suggesting its carcinogenic potential (33). However, expression of IL-17A in murine hematopoietic immunogenic tumors and grafting them into syngeneic immunocompetent mice resulted in tumor inhibition via a T-cell dependent mechanism (34). Supporting data was also reported by Muranski et. al. demonstrating that Th17 cells could protect against advanced B16 melanoma (35). Lastly, Th17 cells in a lung melanoma model were able to promote cytotoxic T cell activation causing tumor reduction (36). These observations argue for complex regulation of inflammation and cytotoxic T cells by IL-17, probably depending on the types of cancers as well as a local immune status.
In addition to increased Th17 cell differentiation (10), lack of IEX-1 appears to facilitate both proliferation and apoptosis in IECs. Increased apoptosis in IECs may increase colon inflammation in IEX-1−/− RAG1−/− double KO mice, but it may also hinder tumor development in IEX-1 KO mice as has been demonstrated in several animal models (37, 38, 39, 40). On the other hand, a high level of IEC proliferation could promote epithelial repair, reducing colon inflammation. In support of this, human UC patients in remission have high proliferative rates of epithelial mucosa, indicative of an active regeneration process (41). Moreover, a study by Araki et al. demonstrates that a reduced proliferative rate of intestine epithelial cells is correlated with deterioration of colon inflammation (42). However, increased proliferation can potentially promote tumor growth as has been shown in Gαi2−/− mice and others (43), in contrast to reduced tumor development in IEX-1 KO mice. Perhaps, the high proliferation of IECs is counteracted by increasing apoptosis in the cells. Therefore, the altered proliferation and apoptosis of IECs may have a limit or even adverse impact on the attenuated colon inflammation and cancer development seen in IEX-1 KO mice. It may be also unlikely that other inflammatory, non-T cells like macrophages play a role in the diminished inflammation and colon cancer development in IEX-1 KO mice in light of worse colitis developed in Rag−/−IEX-1−/− double KO mice.
Taken together, the present study demonstrates that lack of IEX-1 attenuates inflammation in DSS-induced acute colitis and reduces colorectal tumor formation mainly by altered function or differentiation of T cells. The study provides novel insight into a role of Gαi2 in regulating a balance between Th1 and Th17 cell differentiation secondarily to IEX-1 expression.
Supplementary Material
Acknowledgments
We thank the members in Dr. Wu groups for stimulating discussions and valuable comments. This work is supported in part by National Institutes of Health (NIH) grants AI050822 and AI070785, and a Senior Research Award from the Crohn’s & Colitis Foundation of America (to M.X.W.) and by the Intramural Research Program of the NIH (Z01-ES-101643 to LB).
Reference List
- 1.Rhodes JM, Campbell BJ. Inflammation and colorectal cancer: IBD-associated and sporadic cancer compared. Trends Mol.Med. 2002;8:10–6. doi: 10.1016/s1471-4914(01)02194-3. [DOI] [PubMed] [Google Scholar]
- 2.Wong NA, Harrison DJ. Colorectal neoplasia in ulcerative colitis-recent advances. Histopathology. 2001;39:221–34. doi: 10.1046/j.1365-2559.2001.01292.x. [DOI] [PubMed] [Google Scholar]
- 3.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 4.Okayasu I, Yamada M, Mikami T, Yoshida T, Kanno J, Ohkusa T. Dysplasia and carcinoma development in a repeated dextran sulfate sodium-induced colitis model. J.Gastroenterol.Hepatol. 2002;17:1078–83. doi: 10.1046/j.1440-1746.2002.02853.x. [DOI] [PubMed] [Google Scholar]
- 5.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 6.Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87–92. doi: 10.1136/gut.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang YH, Wu JY, Zhang Y, Wu MX. Synergistic and opposing regulation of the stress-responsive gene IEX-1 by p53, c-Myc, and multiple NF-kappaB/rel complexes. Oncogene. 2002;21:6819–28. doi: 10.1038/sj.onc.1205854. [DOI] [PubMed] [Google Scholar]
- 8.Shen L, Zhi L, Hu W, Wu MX. IEX-1 targets mitochondrial F1Fo-ATPase inhibitor for degradation. Cell Death.Differ. 2009;16:603–12. doi: 10.1038/cdd.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldmann KA, Pittelkow MR, Roche PC, Kumar R, Grande JP. Expression of an immediate early gene, IEX-1, in human tissues. Histochem.Cell Biol. 2001;115:489–97. doi: 10.1007/s004180100284. [DOI] [PubMed] [Google Scholar]
- 10.Ustyugova IV, Zhi L, Wu MX. Reciprocal regulation of the survival and apoptosis of Th17 and Th1 cells in the colon. Inflamm.Bowel.Dis. 2011;10 doi: 10.1002/ibd.21772. [DOI] [PubMed] [Google Scholar]
- 11.Kinugasa T, Sakaguchi T, Gu X, Reinecker HC. Claudins regulate the intestinal barrier in response to immune mediators. Gastroenterology. 2000;118:1001–11. doi: 10.1016/s0016-5085(00)70351-9. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin.Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Shahid M, Shen L, Seldin DC, Lu B, Ustyugova IV, Chen X, et al. Impaired 3′,5′-cyclic adenosine monophosphate-mediated signaling in immediate early responsive gene X-1-deficient vascular smooth muscle cells. Hypertension. 2010;56:705–12. doi: 10.1161/HYPERTENSIONAHA.110.154880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–74. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 15.Kitajima S, Takuma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp.Anim. 1999;48:137–43. doi: 10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 16.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitisassociated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–62. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–73. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Kim TW, Seo JN, Suh YH, Park HJ, Kim JH, Kim JY, et al. Involvement of lymphocytes in dextran sulfate sodium-induced experimental colitis. World J.Gastroenterol. 2006;12:302–5. doi: 10.3748/wjg.v12.i2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahler M, Bristol IJ, Leiter EH, Workman AE, Birkenmeier EH, Elson CO, et al. Differential susceptibility of inbred mouse strains to dextran sulfate sodium-induced colitis. Am.J.Physiol. 1998;274:G544–G551. doi: 10.1152/ajpgi.1998.274.3.G544. [DOI] [PubMed] [Google Scholar]
- 21.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu.Rev.Physiol. 2009;71:241–60. doi: 10.1146/annurev.physiol.010908.163145. 241-60. [DOI] [PubMed] [Google Scholar]
- 22.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, et al. Gi2 alpha protein deficiency: a model of inflammatory bowel disease. J.Clin.Immunol. 1995;15:101S–5S. doi: 10.1007/BF01540899. [DOI] [PubMed] [Google Scholar]
- 23.Huang TT, Zong Y, Dalwadi H, Chung C, Miceli MC, Spicher K, et al. TCR-mediated hyper-responsiveness of autoimmune Galphai2(−/−) mice is an intrinsic naive CD4(+) T cell disorder selective for the Galphai2 subunit. Int.Immunol. 2003;15:1359–67. doi: 10.1093/intimm/dxg135. [DOI] [PubMed] [Google Scholar]
- 24.Ohman L, Franzen L, Rudolph U, Harriman GR, Hultgren HE. Immune activation in the intestinal mucosa before the onset of colitis in Galphai2-deficient mice. Scand.J.Immunol. 2000;52:80–90. doi: 10.1046/j.1365-3083.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 25.Arinze IJ, Kawai Y. Transcriptional activation of the human Galphai2 gene promoter through nuclear factor-kappaB and antioxidant response elements. J.Biol.Chem. 2005;280:9786–95. doi: 10.1074/jbc.M414006200. [DOI] [PubMed] [Google Scholar]
- 26.Nishida M, Maruyama Y, Tanaka R, Kontani K, Nagao T, Kurose H. G alpha(i) and G alpha(o) are target proteins of reactive oxygen species. Nature. 2000;408:492–5. doi: 10.1038/35044120. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor W, Jr., Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat.Immunol. 2009;10:603–9. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, et al. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J.Immunol. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 29.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–5. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 30.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc.Natl.Acad.Sci.U.S.A. 2008;105:15505–10. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin.Cancer Res. 2008;14:3254–61. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem.Biophys.Res.Commun. 2008;374:533–7. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 33.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 34.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–21. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 35.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–98. doi: 10.1016/j.immuni.2009.09.014. %20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. J.Pathol. 1996;180:152–9. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 38.Schulzke JD, Bojarski C, Zeissig S, Heller F, Gitter AH, Fromm M. Disrupted barrier function through epithelial cell apoptosis. Ann.N.Y.Acad.Sci. 2006;1072:288–99. doi: 10.1196/annals.1326.027. [DOI] [PubMed] [Google Scholar]
- 39.Qiu W, Wu B, Wang X, Buchanan ME, Regueiro MD, Hartman DJ, et al. PUMA-mediated intestinal epithelial apoptosis contributes to ulcerative colitis in humans and mice. J.Clin.Invest. 2011;121:1722–32. doi: 10.1172/JCI42917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiu W, Carson-Walter EB, Kuan SF, Zhang L, Yu J. PUMA suppresses intestinal tumorigenesis in mice. Cancer Res. 2009;69:4999–5006. doi: 10.1158/0008-5472.CAN-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serafini EP, Kirk AP, Chambers TJ. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981;22:648–52. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araki Y, Mukaisyo K, Sugihara H, Fujiyama Y, Hattori T. Increased apoptosis and decreased proliferation of colonic epithelium in dextran sulfate sodium-induced colitis in mice. Oncol.Rep. 2010;24:869–74. doi: 10.3892/or.2010.869. [DOI] [PubMed] [Google Scholar]
- 43.Edwards RA, Wang K, Davis JS, Birnbaumer L. Role for epithelial dysregulation in early-onset colitis-associated colon cancer in Gi2-alpha−/−mice. Inflamm.Bowel.Dis. 2008;14:898–907. doi: 10.1002/ibd.20414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.