Abstract
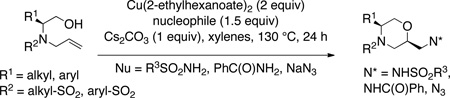
A new copper(II) 2-ethylhexanoate promoted addition of an alcohol and an amine across an alkene (oxyamination) is reported. The alcohol addition is intramolecular while coupling with the amine occurs intermolecularly. Several 2-aminomethyl morpholines were synthesized in good to excellent yields and diastereoselectivities.
The simultaneous addition of oxygen and nitrogen across an alkene (aminooxygenation, oxyamination) is a useful way to install the vicinal aminoalcohol moiety.1 Vicinal amino alcohols are useful in a number of applications including catalysis and medicinal chemistry.2 A number of reactions have been invented to enable this important transformation1 including those that construct a heterocycle in the process.3 While the majority of ring-forming oxyaminations construct nitrogen heterocycles,3a,b,d–k few reports of oxygen heterocycle synthesis have appeared3c,l and only one of them is thought to initiate with addition of oxygen to the alkene.3l An alkyne oxyamination that likely initiates with intramolecular addition of the alcohol to the alkyne has recently been reported.4
We report herein a new copper(II) 2-ethylhexanoate promoted alkene oxyamination reaction that provides 2-aminomethyl functionalized morpholines in good to excellent yields and with generally high levels of diastereoselectivity. This reaction likely proceeds by initial addition of the alcohol moiety to the alkene (vide infra). Morpholines5 are frequently found in bioactive compounds, thus new methods for their efficient and stereoselective synthesis are desirable. Substituted morpholines in particular are useful scaffolds and property-enhancing functional groups in drug discovery. In the past twenty years, a number of concise and inventive methods for the synthesis of variously substituted morpholines have been reported.6 Despite their demonstrated utility in drug discovery,7 however, no direct methods for the synthesis of aminomethyl morpholines from alkenols have appeared.6c
We have recently reported that copper(II) carboxylates promote and catalyze intramolecular alkene aminooxygenation and diamination reactions.8,9 In these reactions, nitrogen heterocycles are formed via a mechanism involving cis-aminocupration across the alkene.3k In these studies, all of the alkene substrates were tethered to amine nucleophiles. More recently, we have begun exploring copper-promoted and catalyzed intramolecular additions of alcohols to alkenes.10 Both carboetherification and hydroetherification reactions have been achieved where the reaction is thought to initiate with a cis-oxycupration of the alkene.10 This letter describes an expansion of this method to the alkene oxyamination reaction, and both five- and six-membered ring oxygen-containing heterocycles have resulted (vide infra).
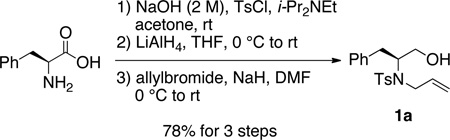
Our interest in the synthesis of morpholines led us to explore the copper(II) 2-ethylhexanoate promoted cyclization/amination reactions of β-hydroxy N-allylsulfonamide 1a. Substrate 1a was synthesized from L-phenylalanine in three steps and 78% overall yield as illustrated in Eq 1.
 |
(1) |
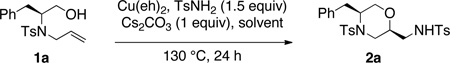
Upon treatment with copper(II) 2-ethylhexanoate (3 equiv) in the presence of TsNH2, β-hydroxy N-allylsulfonamide 1a underwent oxyamination to provide morpholine 2a as a single diastereomer (>20:1 dr) in 45% yield (Table 1, entry 1). Bouyed by this promising initial result, we optimized the product yield and copper(II) loading in this reaction (Table 1). Copper(II) 2-ethylhexanoate [Cu(eh)2] was chosen as the copper(II) source as it is very soluble in non-polar organic solvents where analogous alkene difunctionalization reactions tend to perform well.3i–k,10c
Table 1.
Optimization of the alkene oxyaminationa
 | |||
|---|---|---|---|
| entry | equiv Cu(eh)2 | solvent | yield (%)b |
| 1c | 3 | PhCF3 | 45d |
| 2 | 3 | PhCF3 | 50d |
| 3 | 3 | xylenes | 85 |
| 4 | 2 | xylenes | 87 |
| 5 | 1.5 | xylenes | 50d |
| 6c | 2 | xylenes | 80d |
Conditions: Substrate 1a (0.116 mmol) in solvent (0.1 M) was treated with the specified amount of Cu(eh)2, Cs2CO3 (1 equiv) and TsNH2 (1.5 equiv) at 130 °C for 24 h unless otherwise noted. Only one diastereomer (dr >20:1) was observed by 1H NMR.
Isolated yield unless otherwise noted.
Reaction run at 120 °C.
% conversion based on 1H NMR analysis of the crude mixture. The remainder of the material balance is starting substrate. Cu(eh)2 = Copper(II) 2-ethylhexanoate.
In this reaction, we found that the higher boiling xylenes proved a better solvent than PhCF3 at 130 °C, the temperature these reactions performed best at (Table 1, entries 1–3). We found the copper(II) loading could be reduced from 3 equiv to 2 equiv without diminishing the isolated yield (Table 1, compare entries 3 and 4). Decreasing the copper(II) loading to 1.5 equiv resulted in diminished conversion (Table 1, entry 5).
Lowering the reaction temperature from 130 to 120 °C resulted in lower conversion as well (Table 1, compare entries 4 and 6). No conversion to product was observed under conditions where a catalytic amount of Cu(2-ethylhexanoate)2 (20 mol%) and a stoichiometric amount of MnO2 (3 equiv) was used as oxidant under otherwise optimal reaction conditions (130 °C in xylenes). We have previously used MnO2 successfully in analogous reactions to turnover catalytic amounts of Cu(II).8,9c,10
Using the optimized reaction conditions (Table 1, entry 4) we examined the copper(II)-promoted oxyamination reaction of a number of β-hydroxy-N-allylsulfonamides (Table 2, entries 1–8). Alkyl, phenyl, silyloxymethyl and benzylsulfidomethyl substrates were all tolerated in the reaction abeit the sulfide’s conversion was lower (Table 2, entry 5) and the phenyl substituted substrate 1i (Table 2, entry 8) gave a comparably lower diastereoselectivity (dr = 6:1). N-nosyl and N-mesyl containing substrates 1g and 1h reacted with similar efficiencies as the N-tosyl substrate 1a (Table 2, entries 6 and 7).
Table 2.
Morpholine Synthesis Scopea
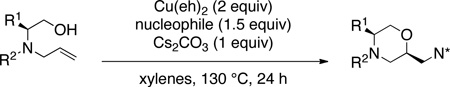 | ||||
|---|---|---|---|---|
| entry | substrate | nucleophile | product (dr)b | yieldc |
| 1 | 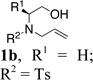 |
TsNH2 | 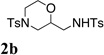 |
80% |
| 2 | 1c, R1 = CH3, R2 = Ts | TsNH2 | 73% | |
| 3 | 1d, R1 = i-Pr, R2 = Ts | TsNH2 | 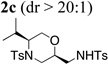 |
82% |
| 4 | 1e, R1 = CH2OTBS, R2 = Ts | TsNH2 | 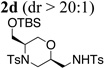 |
72% |
| 5 | 1f, R1 = CH2SBn, R2= Ts | TsNH2 | 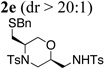 |
42% |
| 6 | 1g, R1 = Bn,R2 = Ns | TsNH2 | 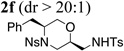 |
83% |
| 7 | 1h, R1 = Bn,R2 = Ms | TsNH2 | 73% | |
| 8 | 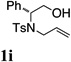 |
TsNH2 | 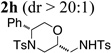 |
67% |
| 9 | 1a, R1 = Bn; R2 = Ts | NsNH2 | 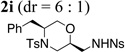 |
82% |
| 10 | 1a | PMBSNH2 | 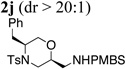 |
82% |
| 11d | 1a | MsNH2 | 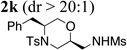 |
43% |
| 12 | 1a | SESNH2 | 98% | |
| 13d | 1a | PhC(O)NH2 | 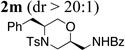 |
87% |
| 14d | 1a | NaN3 | 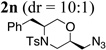 |
53% |
| 15e | 1c | PhC(O)NH2 | 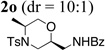 |
73% |
| 16f | 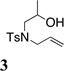 |
TsNH2 | 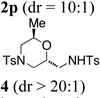 |
52% |
Same conditions as Table 1, entry 4 unless otherwise noted.
Diastereoselectivity obtained by analysis of the crude 1H NMR spectra.
Isolated yields.
Reaction was run using 4 equiv Cu(eh)2.
Reaction was run using 3 equiv of Cu(eh)2.
Reaction was run at 150 °C.
Various nitrogen nucleophiles were explored as summarized in Table 2, entries 9–15. Sulfonamide nucleophiles including the easily de-sulfonylated nosyl and 2-trimethylsilylethylsulfonamide generally performed well (Table 2, entries 9–12) but the reaction with methanesulfonamide gave a lower conversion and the copper(II) loading had to be increased to 4 equiv for a reasonable isolated yield (Table 2, entry 11). The benzamide nucleophile also underwent the coupling, however, increased copper(II) loading (3–4 equiv) was critical to obtaining a good diastereomeric ratio (Table 2, entries 13 and 15 and see Supporting Information for diastereoselectivity as a function of copper loading). Sodium azide could also be used as nucleophile under the higher copper(II) loading conditions (Table 2, entry 14). Anilines failed to function as the amine component in these reactions (no reaction observed, reactions not shown).
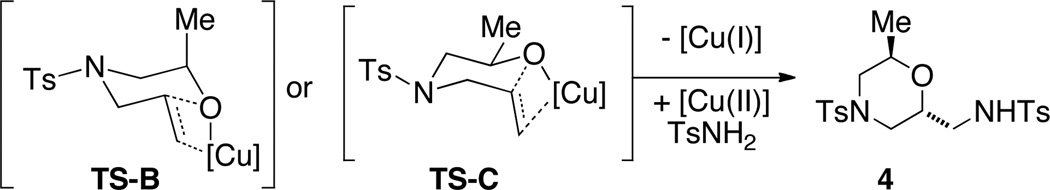
While most of the examples provide 2,5-disubstituted morpholines 2, a brief examination using 2-substituted substrate 3 indicated that 2,6-disubstituted morpholines 4 can also be synthesized, albeit the reaction temperature had to be raised to 150 °C to obtain moderate conversion (Table 2, entry 16).
The 1,1-disubstituted alkene 5 also underwent efficient conversion, giving the highly substituted morpholine 6 in 91% yield albeit as a 2:1 diastereomeric mixture (Eq. 2).
 |
(2) |
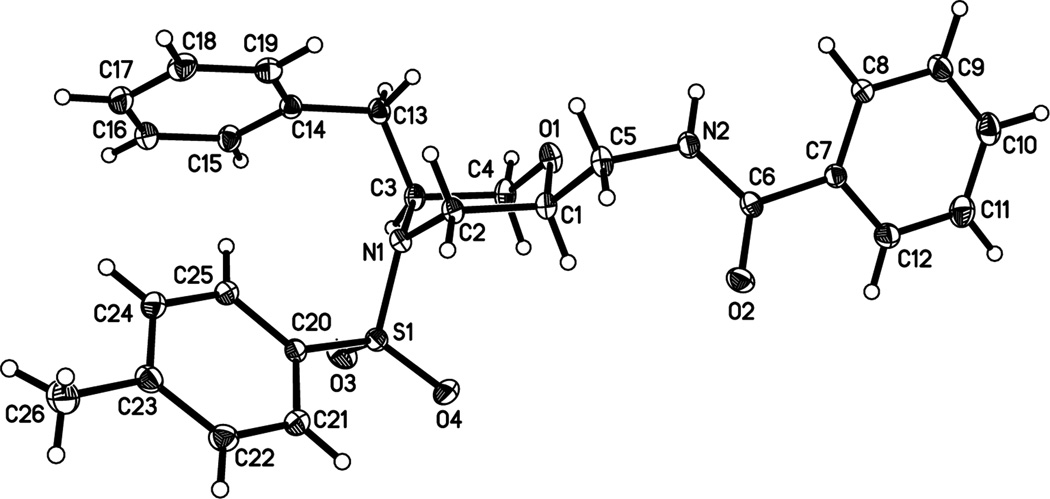
The relative stereochemistry of the major diastereomer of morpholine 2n was assigned as 2,5-cis by X-ray crystallography (Fig. 1). All other 2,5-disubstituted morpholine (major) products were assigned by analogy. The relative stereochemistry of the 2,6-trans-disubstituted morpholine 4 was assigned by nOe (see Supporting Information).
Figure 1.
X-ray crystallography structure of 2,5-cis-2n.
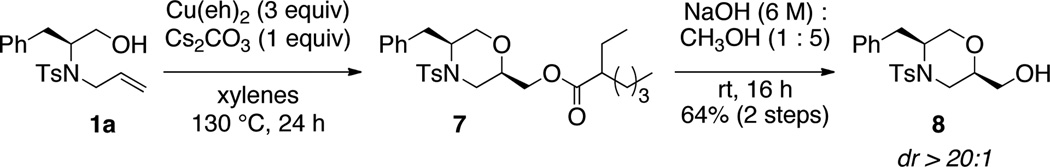
In the absence of an amine nucleophile, dioxygenation of the alkene occurs to give a 1:1 mixture of 2-ethylhexanoic esters 7 (Scheme 1). Suponification of this ester mixture provided the 2-hydroxymorpholine 8 as a single diastereomer (dr = >20:1), confirming the diastereomeric mixture originated from the ester configuration.
Scheme 1.
Dioxygenation in the absence of amine nucleophile
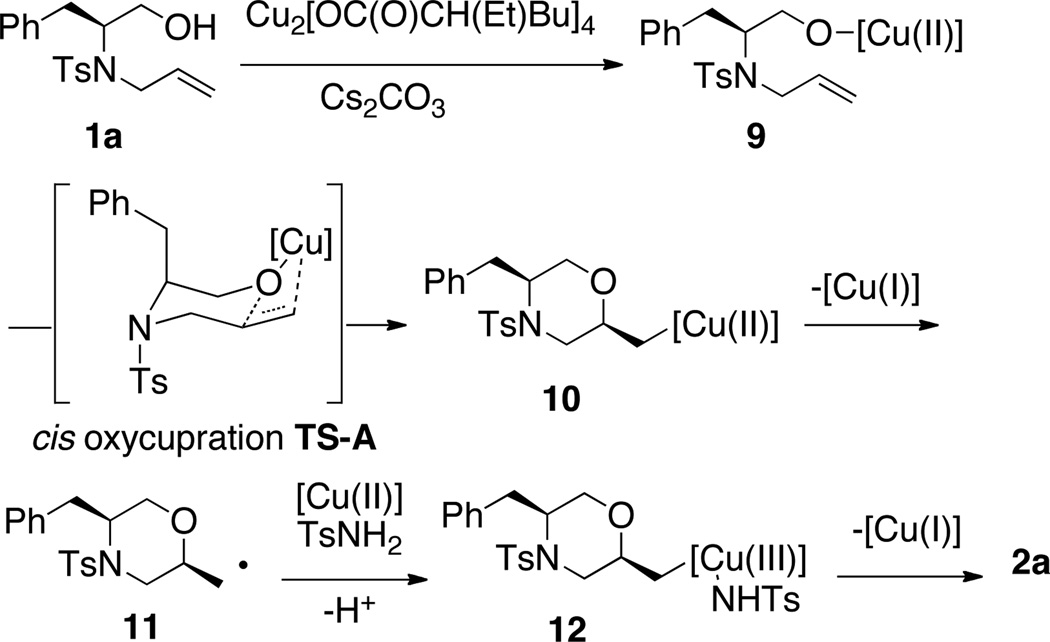
A proposed mechanism that accounts for the observed diastereoselectivity is illustrated in Scheme 2. Coordination of alcohol 1a to the copper(II) 2-ethylhexanoate dimer in the presence of Cs2CO3 likely results in monomer 9. This first step is an analogy to similar reactions of the copper(II) carboxylate with alkenylsulfonamides and the copper(II) ligation is undefined as either one or two carboxylates may be attached at the copper center.3k Thermally promoted cis-oxycupration via transition state A then provides the unstable organocopper(II) intermediate 10. The diastereoselectivity is rationalized by a chair-like transition state where the vicinal tosyl and benzyl substituents adopt pseudoaxial positions,11 much like in the final product 2 (e.g., Fig. 1). Intermediate 10 then undergoes C-Cu(II) homolysis to give carbon radical 11.3k,10 Recombination of the radical with a [Cu(II)] in the presence of TsNH2 then gives an organocopper(III) intermediate 12 that can undergo reductive elimination to form the C-N bond of 2a.9 In the absence of amine nucleophile, a carboxylate ligand is transferred, giving the dioxygenated product 7 (Scheme 1).
Scheme 2.
Proposed oxyamination reaction mechanism
It is possible that some of the amine nucleophiles could coordinate to the copper(II) prior to oxycupration, which may explain the dependence of the reaction diastereoselectivity on the amount of copper(II) 2-ethylhexanoate promoter in the reaction with benzamide. Such coordination could also explain why anilines fail to couple with 1a via oxyamination. (Under the reaction conditions, aniline might complex too tightly with Cu(eh)2 and render it unreactive.) Alternatively, the rate of C-N bond forming reductive elimination (e.g. from 12, Scheme 2) is likely different with different amine nucleophiles and could effect the observed reaction diastereoselectivity (by allowing ring-opening and unselective ring-closing when slow).10
The 2,6-trans diastereoselectivity observed in morpholine 4 can be rationalized by either TS-B or TS-C where either the methyl or the alkene substituent must adopt a pseudo-axial position on the chair-like transition state (Scheme 3). The reaction of the α-methyl substituted alcohol 3 was less efficient than that of 1a, and the temperature of the reaction had to be raised to 150 °C for increased conversion, indicating steric demands proximal to the oxycupration bond formation in TS-B or TS-C decrease reactivity.
Scheme 3.
Rationalization of 2,6-trans selectivity
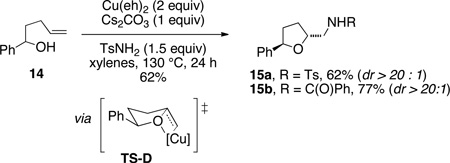
The synthesis of 2-aminomethyltetrahydrofurans was briefly examined. 4-Pentenol underwent oxyamination with TsNH2 and PhC(O)NH2, providing tetrahydrofurans 13a and 13b in 83% and 85% yield, respectively (Eq 3). 1-Phenyl-4-pentenol 14 underwent oxyamination with TsNH2 to provide the 2,5-trans-tetrahydrofuran 15 with >20:1 diastereoselectivity (Eq 4), presumably via TS-D.
In summary, a new method for the stereoselective synthesis of oxygen-containing heterocycles via alkene oxyamination has been developed. This method offers direct entry into aminomethyl-functionalized morpholines and tetrahydrofurans and is a significant extension of copper(II)-promoted alkene difunctionalization chemistry both in the oxyamination transformation and in the ability to synthesize morpholines. Use of these new synthons in drug discovery should now be facile.
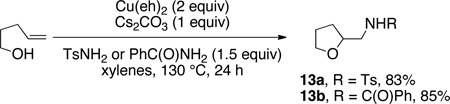 |
(3) |
 |
(4) |
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (GM078383) for support of this research and William W. Brennessel and the X-ray Crystallography Facility at the University of Rochester for obtaining the crystal structure of 2n.
Footnotes
Supporting Information Available: Procedures and characterization data, NMR spectra for all new products and cif data for 2n. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Reviews: Donohoe TJ, Callens CKA, Flores A, Lacy AR, Rathi AH. Chem. Eur. J. 2011;17:58–76. doi: 10.1002/chem.201002323. Bodkin JK, McLeod MD. J. Chem. Soc. Perkin Trans. 1. 2002:2733–2746. Recent intermolecular aminooxygenations: (c) Liu G, Stahl SS. J. Am. Chem. Soc. 2006;128:7179–7181. doi: 10.1021/ja061706h. Michaelis DJ, Williamson KS, Yoon TP. Tetrahedron. 2009;65:5118–5124. doi: 10.1016/j.tet.2009.03.012. Williamson KS, Yoon TP. J. Am. Chem. Soc. 2010;132:4570–4571. doi: 10.1021/ja1013536. Nakanishi M, Minard C, Retailleau P, Cariou K, Dodd RH. Org. Lett. 2011;13:5792–5795. doi: 10.1021/ol202367d. de Haro T, Nevado C. Angew. Chem. Int. Ed. 2011;123:936–940. Ma Z, Naylor BC, Loertscher BM, Hafen DD, Li JM, Castle SL. J. Org. Chem. 2012;77:1208–1214. doi: 10.1021/jo202375a. Williamson KS, Yoon TP. J. Am. Chem. Soc. 2012
- 2.(a) Ager DJ, Prakash I, Schaad DR. Chem. Rev. 1996;96:835–875. doi: 10.1021/cr9500038. [DOI] [PubMed] [Google Scholar]; (b) Bergmeier SC. Tetrahedron. 2000;56:2561–2576. [Google Scholar]
- 3.(a) Donohoe TJ, Churchill GH, Wheelhouse KMP, Glossop PA. Angew. Chem. Int. Ed. 2006;45:8025–8028. doi: 10.1002/anie.200603240. [DOI] [PubMed] [Google Scholar]; (b) Alexanian EJ, Lee C, Sorensen EJ. J. Am. Chem. Soc. 2005;127:7690–7691. doi: 10.1021/ja051406k. [DOI] [PubMed] [Google Scholar]; (c) Desai LV, Sanford MS. Angew. Chem. Int. Ed. 2007;46:5737–5740. doi: 10.1002/anie.200701454. [DOI] [PubMed] [Google Scholar]; (d) Szolcsanyi P, Gracza T. Chem. Commun. 2005:3948–3950. doi: 10.1039/b506731f. [DOI] [PubMed] [Google Scholar]; (e) Fuller PH, Kim J-W, Chemler SR. J. Am. Chem. Soc. 2008;130:17638–17639. doi: 10.1021/ja806585m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Cochran BM, Michael FE. Org. Lett. 2008;10:5039–5042. doi: 10.1021/ol8022165. [DOI] [PubMed] [Google Scholar]; (g) Lovick HM, Michael FE. J. Am. Chem. Soc. 2010;132:1249–1251. doi: 10.1021/ja906648w. [DOI] [PubMed] [Google Scholar]; (h) Mancheno DE, Thornton AR, Stoll AH, Kong AD, Blakey SB. Org. Lett. 2010;12:4110–4113. doi: 10.1021/ol101702w. [DOI] [PubMed] [Google Scholar]; (i) Paderes MC, Chemler SR. Org. Lett. 2009;11:1915–1918. doi: 10.1021/ol9003492. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Paderes MC, Chemler SR. Eur. J. Org. Chem. 2011:3679–3684. doi: 10.1002/ejoc.201100444. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Paderes MC, Belding L, Fanovic B, Dudding T, Keister JB. Chem. Eur. J. 2012;18:1711–1726. doi: 10.1002/chem.201101703. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Schmidt VA, Alexanian EJ. J. Am. Chem. Soc. 2011;133:11402–11405. doi: 10.1021/ja204255e. [DOI] [PubMed] [Google Scholar]
- 4.Hirano K, Satoh T, Miura M. Org. Lett. 2011;13:2395–2397. doi: 10.1021/ol200651r. [DOI] [PubMed] [Google Scholar]
- 5.Morpholine review: Wijtmans R, Vink MKS, Schoemaker HE, van Delft FL, Blaauw RH, Rutjes FPJT. Synthesis. 2004:641.
- 6.(a) Lai J-Y, Shi X-X, Gong Y-S, Dai L-X. J. Org. Chem. 1993;58:4775–4777. [Google Scholar]; (b) Uozumi Y, Tanahashi A, Hayashi T. J. Org. Chem. 1993;58:6826–6832. [Google Scholar]; (c) Wilkinson MC. Tetrahedron Lett. 2005;46:4773–4775. [Google Scholar]; (d) Lanman BA, Myers A. Org. Lett. 2004;6:1045–1047. doi: 10.1021/ol049861t. [DOI] [PubMed] [Google Scholar]; (e) Dave R, Sasaki NA. Org. Lett. 2004;6:15–18. doi: 10.1021/ol035998s. [DOI] [PubMed] [Google Scholar]; (f) Pedrosa R, Andres C, Mendiguchia P, Nieto J. J. Org. Chem. 2006;71:8854–8863. doi: 10.1021/jo061547k. [DOI] [PubMed] [Google Scholar]; (g) Leathen ML, Rosen BR, Wolfe JP. J. Org. Chem. 2009;74:5107–5110. doi: 10.1021/jo9007223. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Chowdhury C, Mukherjee S, Das B, Achari B. J. Org. Chem. 2009;74:3612–3615. doi: 10.1021/jo900428j. [DOI] [PubMed] [Google Scholar]; (i) Ghorai MK, Shukla D, Das K. J. Org. Chem. 2009;74:7013–7022. doi: 10.1021/jo901297d. [DOI] [PubMed] [Google Scholar]; (j) Albanese D, Landini D, Penso M, Tagliabue A, Carlini E. Org. Proc. Res. & Dev. 2010;14:705–711. [Google Scholar]; (k) Bornholdt J, Felding J, Kristensen JL. J. Org. Chem. 2010;75:7454–7457. doi: 10.1021/jo101339g. [DOI] [PubMed] [Google Scholar]; (l) Ritzen B, Hoekman S, Verdasco DD, van Delft FL, Rutjes FPJT. J. Org. Chem. 2010;75:3461–3464. doi: 10.1021/jo1003295. [DOI] [PubMed] [Google Scholar]; (m) Bera S, Panda G. ACS Comb. Sci. 2012;14:1–4. doi: 10.1021/co200129t. [DOI] [PubMed] [Google Scholar]; (n) Lu Z, Stahl SS. Org. Lett. 2012;14:1234–1237. doi: 10.1021/ol300030w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) O’Reilly MC, Lindsley CW. Org. Lett. 2012;14:2910–2913. doi: 10.1021/ol301203z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ancliff RA, Cook CM, Eldred CD, Gore PM, Harrison ST, Judd DB, Keeling SE, Lewell XQ, Mills G, Robertson GM, Swanson S, Walker AJ, Wilkinson M. 7,622,464. Glaxo Group Limited; US Patent. 2009 Nov.
- 8.Chemler SR. J. Organomet. Chem. . 2011;696:150–158. doi: 10.1016/j.jorganchem.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Zabawa TP, Kasi D, Chemler SR. J. Am. Chem. Soc. 2005;127:11250–11251. doi: 10.1021/ja053335v. [DOI] [PubMed] [Google Scholar]; (b) Zabawa TP, Chemler SR. Org. Lett. 2007;9:2035–2038. doi: 10.1021/ol0706713. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sequeira FC, Turnpenny BW, Chemler SR. Angew. Chem. Int. Ed. 2010;49:6365–6368. doi: 10.1002/anie.201003499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller Y, Miao L, Hosseini A, Chemler SR. J. Am. Chem. Soc. 2012;134:12149–12156. doi: 10.1021/ja3034075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The SO2R group of sulfonamides in six-membered rings prefer to be equatorial except when adjacent to a carbon bearing an alkyl substituent, presumably due to mimimization of A1,3-strain: Modarresi-Alam AR, Amirazizi HA, Bagheri H, Rijanzadeh H-R, Kleinpeter E. J. Org. Chem. 2009;74:4740–4746. doi: 10.1021/jo900454a. Toumieux S, Compain P, Martin OR, Selkti M. Org. Lett. 2006;8:4493–4496. doi: 10.1021/ol061649x. Ragoussi ME, Walker SM, Piccanello A, Kariuki BM, Horton PN, Spencer N, Snaith JS. J. Org. Chem. 2010;75:7347–7357. doi: 10.1021/jo101631y.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.