Abstract
PURPOSE
To evaluate RTVue spectral-domain optical coherence tomography (OCT) (Optovue Inc, Fremont, California, USA) reproducibility and to assess agreement with Stratus time-domain OCT (Carl Zeiss Meditec, Dublin, California, USA) measurements.
DESIGN
Observational clinical study.
METHODS
Scans were obtained from both eyes of all participants 3 times using the RTVue nerve head map 4-mm diameter protocol and once using Stratus OCT within the same session. RTVue reproducibility and agreement with Stratus OCT were evaluated for retinal nerve fiber layer (RNFL) and optic disc measurements.
RESULTS
Thirty healthy participants (60 eyes) and 38 glaucoma patients (76 eyes) were included in the study. RTVue reproducibility was good in both healthy participants and patients. For average RNFL thickness, the intraclass correlation coefficients in healthy eyes and patient eyes were 0.97 whereas for rim area they were 0.97 and 0.96, respectively. The correlation between RTVue and Stratus measurements generally was good, especially for average RNFL thickness (healthy eyes and patient eyes, r2 = 0.82 and 0.86, respectively) and rim volume (healthy eyes and patient eyes, r2 = 0.78 and 0.76, respectively). Bland-Altman plots showed good agreement between the instruments, with better agreement for average RNFL thickness (95% limits of agreement in healthy eyes and patient eyes, −8.6 to 12 µm and −5.6 to −14.8 µm, respectively) than optic disc parameters. Cup-to-disc ratio 95% limits of agreement in healthy eyes and patient eyes were −0.3 to 0.4 and −0.2 to 0.3, respectively. Optic disc measurements with RTVue were smaller than those with Stratus OCT (eg, disc area was on average 0.4 mm2 smaller and rim area was 0.3 mm2 smaller with RTVue).
CONCLUSIONS
Reproducibility of RTVue RNFL and optic disc measurements was excellent in both groups. The level of agreement between RTVue and Stratus measurements suggests that RTVue has the potential to detect glaucomatous structural changes.
Optical Coherence Tomography (Oct) is a noninvasive retinal nerve fiber layer (RNFL) and optic disc imaging method that provides micrometer-scale resolution.1– 4 OCT technology has changed considerably in recent years with the incorporation of spectral-domain (SD) imaging that offers significant advantages over the traditional time-domain (TD) OCT techniques.5
Unlike TD-OCT, SD-OCT uses a stationary reference mirror, and the OCT signal is acquired using a spectrometer as a detector.6,7 SD technology currently is capable of an acquisition speed of up to 29,000 A scans per second.8 In addition, SD-OCT offers a higher resolution than TD-OCT2 and can provide a significant reduction in motion artifacts and an increased signal-to-noise ratio compared with TD-OCT.9,10 Until very recently, ophthalmic applications of OCT technology were performed exclusively using TD-OCT (Stratus OCT; Carl Zeiss Meditec, Dublin, California, USA). The recently introduced RTVue (Optovue Inc, Fremont, California, USA) is one of several ultra high-speed, high-resolution OCT retina scanners based on SD-OCT technology. Because of its high frame transfer rate and fast Fourier transform algorithm, RTVue can perform 26,000 A scans per second with a depth resolution of 5 µm (RTVue; RT100 User Manual, Fremont, California, USA).
Before any new device can be widely accepted for use in clinical practice, it is important that its reproducibility, diagnostic accuracy, and ability to detect changes over time are tested. Estimating instrument reproducibility is essential for describing the smallest changes detectable for identifying and monitoring the progression of glaucoma. Additionally, because both RTVue and Stratus OCT were designed to assess anatomic structures of the eye (ie, RNFL and the optic disc features) to help clinicians to identify the presence of glaucomatous structural damage, it is important to understand how measurements from both instruments compare. The objective of the present study was to assess the repeatability of RNFL thickness and optic disc measurements using RTVue in healthy participants and glaucoma patients and to compare these measurements with those acquired with Stratus OCT.
METHODS
All participants from the university of California, San Diego, Diagnostic Innovations in Glaucoma Study, a prospective longitudinal study designed to evaluate optic nerve structure and visual function in glaucoma,11 imaged using RTVue were included in this study. Informed consent was obtained from all participants.
Participants were recruited between June 2007 and April 2008. All participants underwent a complete ophthalmologic examination, including assessment of medical and family history, visual acuity testing with refraction, slit-lamp biomicroscopy including gonioscopy, intraocular pressure (IOP) measurement with Goldmann applanation tonometry, and dilated stereoscopic fundus examination. Visual sensitivity was tested using Humphrey Field Analyzer (Humphrey-Zeiss Systems, Dublin, California, USA) 24-2 Swedish interactive threshold algorithm standard automated perimetry (SAP), and stereophotographs of the optic disc and peripapillary retina were obtained (TRC-SS; Topcon Instruments Corp of America, Paramus, New Jersey, USA).
The inclusion criteria for all participants were a best-corrected visual acuity of 20/40 or better, a spherical refraction within ±5.0 diopters (D), cylinder correction within ±3.0 D, a healthy anterior segment appearance by examination with slit-lamp biomicroscopy, open angles at gonioscopy, good-quality stereophotography of the optic disc, and reliable (false positives, fixation losses, and false negatives of 33% or less with no observable testing artifacts) SAP results, all within 6 months of optical imaging using RTVue and Stratus OCT.
Exclusion criteria were history of ocular surgery (except for uncomplicated glaucoma and cataract surgery), other diseases affecting the visual fields (VF) (eg, pituitary lesions, demyelinating diseases, human immunodeficiency virus seropositivity or acute immune deficiency symptoms, or diabetic retinopathy), secondary causes of IOP increase (eg, iridocyclitis, trauma), or a combination thereof and use of medications known to affect VF sensitivity or diseases affecting color vision.
For the purpose of this study, participants were categorized as healthy participants or glaucoma patients. Healthy participants were those with IOP of less than 22 mm Hg, normal-appearing optic discs and RNFLs by masked stereophotograph assessment, and normal SAP results in both eyes. Glaucoma patients had repeatable abnormal SAP results (pattern standard deviation (SD) with P ≤ 0.05, glaucoma hemifield test results outside normal limits, or both), glaucomatous optic disc appearance by masked stereophotograph assessment (those with cup-to-disc area ratio, rim thinning, or RNFL defects indicative of glaucoma), repeatable IOP of 22 mm Hg or more in at least 1 eye, or a combination thereof.
INSTRUMENTATION
RTVue
RTVue software version 2.0.4.0 (Model RT 100; Optovue Inc) uses a scanning laser diode to emit a scan beam with a wavelength of 840 ± 10 nm to provide images of ocular microstructures. In this study, the 3-dimensional (3D) disc and nerve head map 4-mm diameter (NHM4) RTVue protocols were used. The 3D disc protocol is a 4 × 4-mm raster scan centered on the optic disc and composed of 101 B scans each composed of 512 A scans (acquisition time, 2.2 seconds). The resulting scan provides a 3D image of the optic disc and surrounding area. The en face image generated by this scanning protocol was used to draw the contour line describing the disc margin that is required to generate optic disc parameters from the NHM4 protocol (below). The contour line initially was drawn on the en face image by hand. The position of the contour line then was assessed and, if necessary, corrected by examining the interface between its position and the position of the retinal pigment epithelium (RPE) tips in approximately 8 locations.
The NHM4 protocol is composed of 12 radial scans 3.4 mm in length (452 A scans each) and 6 concentric ring scans ranging from 2.5 to 4.0 mm in diameter (587 or 775 A scans each) all centered on the optic disc (using the previously drawn contour line to ensure scan registration). This scan configuration provides 9510 A scans in 0.39 seconds. Areas between A scans are interpolated. A polar RNFL thickness map and various parameters that describe the optic disc are provided. To compare measurements between the RTVue and Stratus OCT, RNFL thickness measurements were obtained for the 3.45-mm radius ring only, and measurements were described as average RNFL and RNFL in the inferior, temporal, superior, and nasal quadrants. The RNFL thickness parameters were measured by assessing a total of 2,325 data points between the anterior and posterior RNFL borders. The optic cup was defined automatically by RTVue software as the intersection points of the nerve head inner boundary and a parallel line that is 150 µm above the connecting line of the RPE tips. Optic disc parameters obtained were optic disc area, optic cup area, neuroretinal rim area, nerve head volume, cup volume, rim volume, cup-to-disc area ratio, cup-to-disc horizontal ratio, and cup-to-disc vertical ratio.
Stratus Optical Coherence Tomography
For this study, Stratus OCT software version 4.0.7 (OCT Model 3000) fast RNFL thickness and fast optic disc acquisition protocols were used. Details about Stratus OCT are described elsewhere.12 In brief, for the fast RNFL protocol, a total of 3 scans, each composed of 256 A scans, are acquired consecutively using a circle with a diameter of 3.4 mm. An automated computer algorithm delineates the anterior and posterior margins of the RNFL. The RNFL thickness parameters are measured by assessing 768 data points between the anterior and posterior RNFL borders.
For fast optic disc analysis, 6 radial linear scans measuring 4-mm long are centered on the optic disc. After the scans are acquired, an automated algorithm detects the edges of the optic nerve head based on the change in reflectivity determined by the termination points of the RPE layer. For each radial scan, a straight line traced between the 2 reference points defines the disc diameter. The optic cup is defined automatically by Stratus OCT software as the intersection points of the nerve head inner boundary and a parallel line that is 150 µm above the connecting line of the RPE tips. Stratus OCT parameters investigated were average and quadrant specific RNFL thicknesses, disc area, cup area, rim area, vertical integrated rim area (volume), and cup-to-disc area ratio.
For both instruments, good-quality OCT images were obtained without pupil dilation. Criteria for determining scan quality were: signal strength of 30 or more for RTVue and 6 or more for Stratus OCT (as suggested by the manufacturers), a clear fundus image allowing optic disc and the scan circle visibility before and during image acquisition, even and dense color saturation throughout all retinal layers with red color visible in the RPE, and RNFL visible with no interruptions and a continuous scan pattern without missing or blank areas (ie, no algorithm failures). For the RTVue, absence of scan failures and en face OCT image distortions because of blinking or eye movements was required. For the optic nerve head analysis with Stratus OCT, each radial scan was evaluated for the presence of artifacts such as failure to detect the edges of the RPE or improper identification of retinal boundaries. Scans with algorithm failure to detect the edges of the RPE or improper identification of retinal boundaries were excluded from the analysis. One RTVue 3D disc scan, 3 consecutive RTVue NHM4 scans, 1 Stratus fast RNFL scan, and 1 Stratus fast optic disc scan were obtained by an experienced technician within a single session.
STATISTICAL ANALYSIS
To determine the intratest variability of RTVue RNFL and optic disc measurements, the within-subjects SD, the intraclass correlation coefficient, and the coefficient of variation (CV) were calculated. The within-subjects SD was defined as the square root of the within-subject variance (defined as the within-subjects sum of squares divided by its degrees of freedom). Linear regression (r2) was performed to describe the strength of relationships for RNFL and optic disc measurements between instruments. The method of generalized estimating equations was applied to adjust for the correlation between the 2 eyes of each subject.13 Bland-Altman plots were used to evaluate the agreement in the measurements obtained by the 2 instruments. P < .05 was considered to be statistically significant. Statistical analyses were performed using SPSS software version 15.0.0 (SPSS Inc, Chicago, Illinois, USA) and JMP software version 7.0 (SAS Institute, Cary, North Carolina, USA).
RESULTS
All participants satisfying the criteria for inclusion participated in the study. Two subjects (4 eyes) were excluded because of poor-quality scans. Two eyes were excluded because of Stratus OCT signal strength of less than 6, and 2 eyes were excluded because of Stratus OCT algorithm failure to identify retinal boundaries properly. A total of 68 individuals (136 eyes) with a mean age ± SD of 65.3 ± 10.3 years were included in the study. Of these, 30 subjects (60 eyes) were classified as healthy and 38 subjects (76 eyes) were classified as glaucoma patients. Table 1 shows demographics and ocular characteristics of the study population. There were statistically significant differences in SAP mean deviation (P < .001, t test) and pattern SD (P < .001, t test) between healthy and glaucoma patients.
TABLE 1.
Demographic and Clinical Data of Healthy Participants and Glaucoma Patients
| Healthy Participants (n = 30, 60 Eyes) |
Glaucoma Patients (n = 38, 76 Eyes) |
|
|---|---|---|
| Female (n) | 23 | 21 |
| Mean age (yrs) | 63.5 ± 10.2 | 66.7 ± 10.2 |
| MD (dB)a | 0.1 ± 0.84 | −1.85 ± 2.8 |
| PSD (dB)a | 1.53 ± 0.26 | 3.65 ± 3.2 |
| IOP (mm Hg) | 15.7 ± 2.65 | 16.7 ± 4.85 |
dB = decibels; IOP = intraocular pressure; MD = mean deviation; PSD = pattern standard deviation; yrs = years.
P < .001 (analysis of variance).
Table 2 shows RTVue RNFL thickness measurements and describes their reproducibility. CVs for RNFL thickness ranged from 1.54% (average RNFL) to 3.88% (nasal RNFL) for healthy participants and ranged from 1.9% (average RNFL) to 4.72% (temporal RNFL) for glaucoma patients. Table 3 shows optic disc measurements and describes their reproducibility. For healthy participants’ and patients’ optic disc parameters, CVs ranged from 0.27% and 0.4%, respectively, for disc area to 29.1% and 21.3%, respectively, for cup volume.
TABLE 2.
Reproducibility of RTVue Retinal Nerve Fiber Layer Thickness Measurements in Healthy Participants and Glaucoma Patients
| RNFL Parameters |
Healthy Participants | Glaucoma Patients | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | Sw ± 1.96 se | CV % | ICC (95% CI) | Mean (95% CI) | Sw ± 1.96 se | CV % | ICC (95% CI) | |
| TEMP (µm) | 80.6 (77.4 to 83.8) | 2.82 ± 0.52 | 3.54 | 0.92 (0.88 to 0.95) | 71.2 (68.7 to 73.8) | 3.36 ± 0.7 | 4.72 | 0.86 (0.81 to 0.91) |
| SUP (µm) | 120.6 (117 to 124.3) | 3.8 ± 0.57 | 3.16 | 0.91 (0.86 to 0.94) | 103.2 (99.6 to 106.8) | 3.67 ± 0.59 | 3.53 | 0.93 (0.89 to 0.95) |
| NAS (µm) | 75.8 (72.9 to 78.7) | 2.94 ± 0.44 | 3.88 | 0.91 (0.86 to 0.94) | 68.6 (66.2 to 71.1) | 3.22 ± 0.52 | 4.6 | 0.88 (0.83 to 0.92) |
| INF (µm) | 134.3 (129.7 to 138.9) | 3.53 ± 0.55 | 2.65 | 0.95 (0.92 to 0.97) | 113.2 (108.9 to 117.4) | 3.21 ± 0.48 | 2.87 | 0.96 (0.94 to 0.97) |
| AVG (µm) | 102.8 (100.1 to 105.6) | 1.57 ± 0.27 | 1.54 | 0.97 (0.95 to 0.98) | 89.1 (86.5 to 91.7) | 1.69 ± 0.23 | 1.9 | 0.97 (0.96 to 0.98) |
AVG = average quadrant; CI = confidence interval; CV = coefficient of variation; ICC = intraclass correlation coefficient; INF = inferior quadrant; NAS = nasal quadrant; SUP = superior quadrant; Sw = within-subjects standard deviation; RNFL = retinal nerve fiber layer; TEMP = temporal quadrant.
Reproducibility is expressed as the Sw, the ICC, and the CV. Sw is defined as the square root of the within-subject variance (defined as the within-subjects sum of squares divided by its degrees of freedom). CV is calculated as the square root of the residual mean squared values of 3 measures, divided by mean.
TABLE 3.
Reproducibility of RTVue Optic Disc Measurements in Healthy Participants and Glaucoma Patients
| Healthy Participants | Glaucoma Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Optic Disc Parameters | Mean (95% CI) | Sw ± 1.96 se | CV % | ICC (95% CI*) | Mean (95% CI) | Sw ± 1.96 se | CV % | ICC (95% CI) |
| Disc area (mm2) | 1.6 (1.53 to 1.66) | 0 ± 0 | 0.27 | 1 (0.99 to 1) | 1.68 (1.59 to 1.78) | 0.01 ± 0 | 0.4 | 0.99 (0.98 to 0.99) |
| Cup area (mm2) | 0.34 (0.28 to 0.41) | 0.03 ± 0.01 | 23.5 | 0.98 (0.96 to 0.98) | 0.8 (0.67 to 0.93) | 0.05 ± 0.01 | 11.6 | 0.99 (0.98 to 0.99) |
| Rim area (mm2) | 1.25 (1.18 to 1.32) | 0.03 ± 0.01 | 2.68 | 0.97 (0.96 to 0.99) | 0.89 (0.81 to 0.96) | 0.05 ± 0.01 | 6.22 | 0.96 (0.94 to 0.97) |
| Rim volume (mm3) | 0.23 (0.2 to 0.26) | 0.02 ± 0 | 8.96 | 0.96 (0.94 to 0.98) | 0.12 (0.1 to 0.15) | 0.01 ± 0 | 12 | 0.97 (0.95 to 0.98) |
| Nerve head volume (mm3) |
0.38 (0.34 to 0.43) | 0.03 ± 0 | 7.11 | 0.97 (0.95 to 0.98) | 0.22 (0.19 to 0.25) | 0.02 ± 0 | 10.6 | 0.97 (0.95 to 0.98) |
| Cup volume (mm3) | 0.04 (0.03 to 0.06) | 0.01 ± 0 | 29.1 | 0.98 (0.97 to 0.99) | 0.23 (0.15 to 0.31) | 0.02 ± 0.01 | 21.3 | 0.99 (0.99 to 0.99) |
| Horizontal cup-todisc ratio | 0.47 (0.41 to 0.53) | 0.04 ± 0.01 | 14.1 | 0.93 (0.9 to 0.96) | 0.68 (0.63 to 0.73) | 0.04 ± 0.01 | 8.38 | 0.94 (0.91 to 0.96) |
| Vertical cup-todisc ratio | 0.42 (0.36 to 0.49) | 0.03 ± 0.01 | 15.8 | 0.96 (0.93 to 0.97) | 0.67 (0.62 to 0.72) | 0.03 ± 0.01 | 8.19 | 0.95 (0.93 to 0.97) |
| Cup-to-disc ratio | 0.21 (0.17 to 0.25) | 0.02 ± 0 | 22.2 | 0.97 (0.96 to 0.98) | 0.44 (0.39 to 0.49) | 0.03 ± 0.01 | 11.5 | 0.97 (0.96 to 0.98) |
CI = confidence interval; CV = coefficient of variation; ICC = intraclass correlation coefficient; Sw = within-subjects standard deviation.
Reproducibility is expressed as the Sw, the ICC, and the CV. Sw is defined as the square root of the within-subject variance (defined as the within-subjects sum of squares divided by its degrees of freedom). CV is calculated as the square root of the residual mean squared values of 3 measures, divided by mean.
Linear regression (r2) was performed to test the strength of the relationship between RTVue and Stratus OCT measurements. The results are provided in Table 4 for healthy participants and glaucoma patients.
TABLE 4.
Agreement and Correlation between RTVue and Stratus Measurements
| Healthy Participants | Glaucoma Patients | |||
|---|---|---|---|---|
| Optic Disc Parameters | Mean Difference (range) | r2 (P) | Mean Difference (range) | r2 (P) |
| TEMP RNFL (µm) | 7.5 (−13.5 to 28.5) | 0.44 (<.001) | 5.4 (−7.5 to 18.2) | 0.75 (<.001) |
| SUP RNFL (µm) | −3.6 (−23.8 to 16.7) | 0.6 (<.001) | 2.1 (−20.1 to 24.2) | 0.71 (<.001) |
| NAS RNFL (µm) | −3.6 (−25.9 to 18.8) | 0.58 (<.001) | 0.3 (−18.9 to 19.5) | 0.65 (<.001) |
| INF RNFL (µm) | 6.4 (−13.8 to 26.6) | 0.74 (<.001) | 10.8 (−7 to 28.5) | 0.83 (<.001) |
| AVG RNFL (µm) | 1.7 (−8.6 to 12) | 0.81 (<.001) | 4.6 (−5.6 to 14.8) | 0.86 (<.001) |
| Disc area (mm2) | −0.4 (−1 to 0.2) | 0.31 (<.001) | −0.4 (−0.8 to 0) | 0.82 (<.001) |
| Cup area (mm2) | −0.1 (−0.7 to 0.6) | 0.75 (<.001) | −0.1 (−0.6 to 0.4) | 0.84 (<.001) |
| Rim area (mm2) | −0.3 (−1.1 to 0.4) | 0.22 (.002) | −0.3 (−0.7 to 0.1) | 0.67 (<.001) |
| Rim volume (mm3) | −0.3 (−0.8 to 0.1) | 0.78 (<.001) | −0.2 (−0.5 to 0.2) | 0.76 (<.001) |
| Cup-to-disc ratio | 0.0 (−0.3 to 0.4) | 0.65 (<.001) | 0.1 (−0.2 to 0.3) | 0.77 (<.001) |
AVG = average quadrant; INF = inferior quadrant; NAS = nasal quadrant; SUP = superior quadrant; RNFL = retinal nerve fiber layer; TEMP = temporal quadrant.
Agreement is expressed in terms of the mean difference between measurements from the 2 instruments (mean difference) and the 95% limits of agreements between the measurements (range). Correlation is expressed as the r 2 with the level of significance (P value). Mean difference = RTVue minus Stratus.
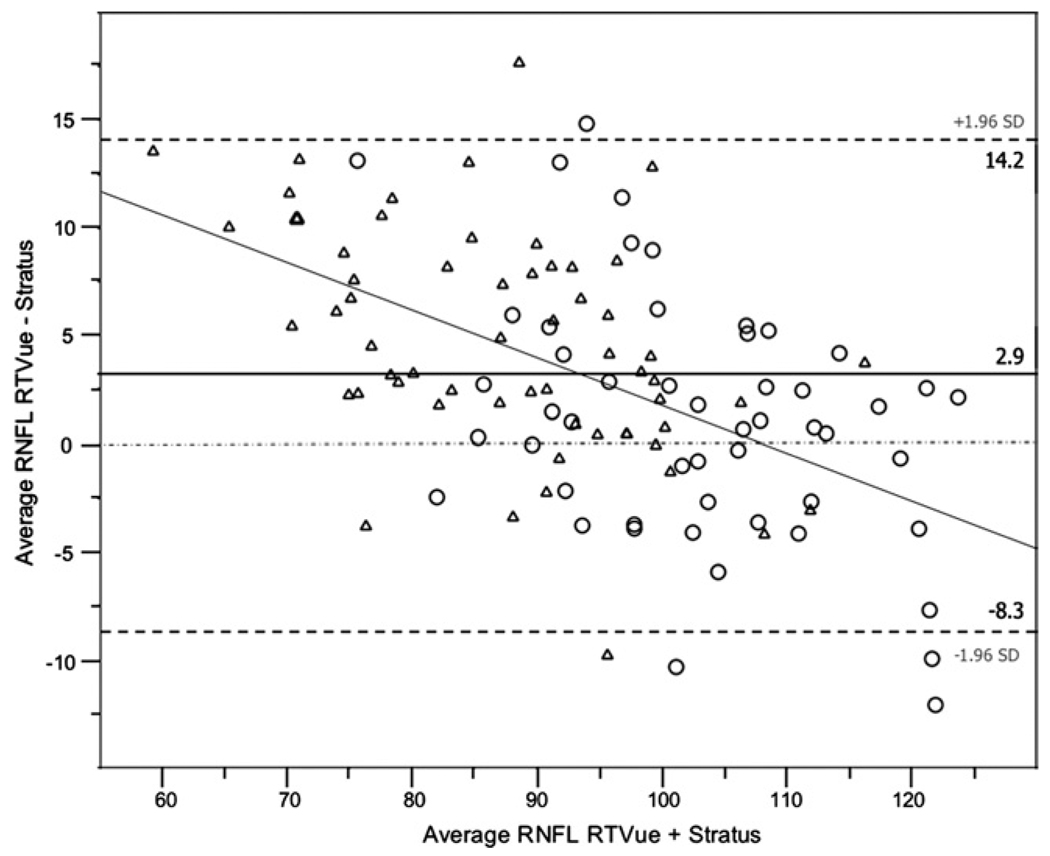
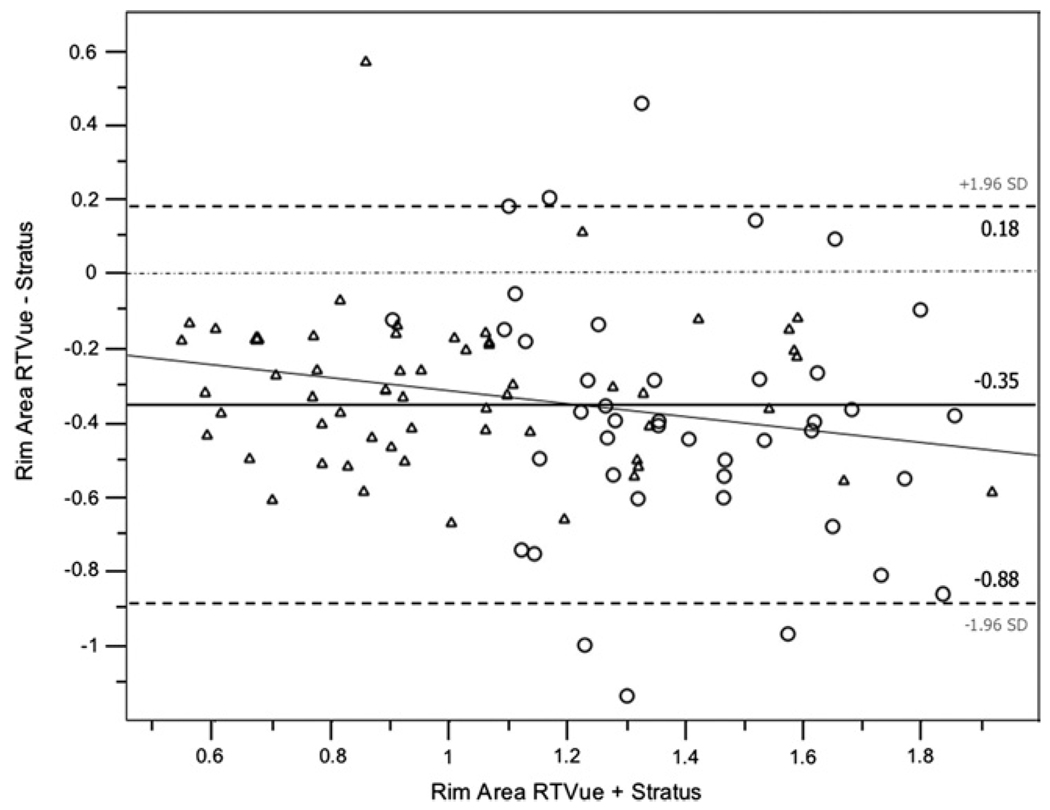
Bland-Altman plots were used to evaluate the agreement between RTVue and Stratus OCT measurements. For constructing the plots, the difference between RTVue and Stratus OCT measurements was used (ie, RTVue measurement minus Stratus OCT measurement; these values are shown in the second and fourth columns of Table 4). Table 4 shows the 95% limits of agreement for RNFL thickness and optic disc measurements in healthy participants and glaucoma patients. Rim area and disc area showed particularly large average differences (25% to 28% of the RTVue mean) between the instruments. Bland-Altman plots also were constructed for the average RNFL thickness (Figure 1) and for the rim area (Figure 2) for healthy and patient eyes combined to assess the agreement between instruments at different ranges of RNFL thickness and rim area, respectively. The slopes calculated were significant for average RNFL thickness (r2 = 0.27; P < .001) indicating that RTVue measurements are larger compared with Stratus OCT measurements for average RNFL thickness values of up to approximately 110 µm (Figure 1). This was not the case for the rim area (r2 = 0.03; P = .08; Figure 2).
FIGURE 1.
Bland-Altman plot showing the average retinal nerve fiber layer thickness (RNFL) agreement between RTVue spectral-domain optical coherence tomography (SD-OCT) and Stratus time-domain optical coherence tomography (TD-OCT) in healthy persons (circle) and glaucoma patients (triangle).
FIGURE 2.
Bland-Altman plot showing the rim area agreement between RTVue SD-OCT and Stratus TD-OCT in healthy persons (circle) and glaucoma patients (triangle).
We also evaluated the signal strength as provided by RTVue and Stratus OCT. The mean signal strength of the RTVue NHM4 scans was 58.22 (95% confidence interval [CI], 55.91 to 60.52) and 54.43 (95% CI, 52.52 to 56.34) in healthy and patient eyes, respectively (F = 6.48; P = .03). In addition, a statistically significant but weak correlation was found between signal strength and RNFL thickness in healthy eyes (r2 = 0.07; P = .04). For Stratus OCT, the mean signal strength of the fast RNFL scans was 8.96 (95% CI, 8.64 to 9.27) and 8.56 (95% CI, 8.29 to 8.83) in healthy and glaucoma patient eyes, respectively (F = 3.89; P = .05). For Stratus OCT, the signal strength of the optic disc image was significantly higher in healthy subject eyes than in glaucoma patient eyes (F = 6.25; P = .01). Specifically, the mean signal strength was 9.2 (95% CI, 8.89 to 9.5) in healthy subject eyes and 8.68 (95% CI, 8.4 to 8.96) in glaucoma patient eyes. In addition, there was a positive association between signal strength and RNFL thickness in healthy eyes (r2 = 0.11; P = .02). There was no association between RTVue and Stratus OCT RNFL and optic disc signal strengths (r2 = 0.02 and P = .08; r2 = 0.02 and P = .12, respectively).
DISCUSSION
In this study, RTVue-measured RNFL thickness and optic disc parameters showed high repeatability and excellent reproducibility in both healthy and glaucoma patient eyes. In addition, good correlation and agreement between RTVue and Stratus OCT was found in both groups. These results suggest the ability for the RTVue to detect glaucomatous structural damage is likely as good as that of the Stratus OCT.12,14–16
Because the reproducibility of Stratus OCT results has been evaluated in previous studies, this study was designed to assess RTVue reproducibility only. Previous studies have shown that Stratus OCT intrasession variability is low, with the tendency for the measurements in glaucomatous eyes to be more variable.17,18 A recent study by Budenz and associates that evaluated the reproducibility of the Stratus OCT fast RNFL thickness protocol in glaucomatous eyes with 3 scans repeated in sequence during the same session (ie, using a method similar to the one applied for this study) found excellent reproducibility, with the CV ranging between 3.8% (intraclass correlation coefficient, 0.98) and 8.6% (intraclass correlation coefficient, 0.89) for average and nasal quadrant RNFL thickness, respectively.18 Because the Stratus OCT fast RNFL protocol is composed of the average of 3 single, immediately consecutive scans obtained within 1.3 seconds, it is possible to estimate reproducibility within our single Stratus RNFL scans. Although a direct comparison with RTVue reproducibility would not be appropriate because RTVue images were single images that were acquired separately, we estimated the CVs for Stratus OCT-measured average RNFL thickness and found the results to be similar to those from RTVue. For healthy eyes, the Stratus OCT CV was 2.33% vs the RTVue CV of 1.54%. For glaucoma patient eyes, the Stratus OCT CV was 2.26% vs the RTVue CV of 1.9%. Slightly better reproducibility of RTVue in part is the result of the increased number of A scans, increased resolution, and robust algorithms used by RTVue for detecting structural damage. However, the study design and the study population have the potential to influence the results. In our study, although mean deviation and pattern SD were significantly different between the 2 groups, patients had only mild (61 eyes, 80%) or moderate (15 eyes, 20%) VF defects based on the Hodapp-Anderson Parrish classification, indicating early disease.19 Previous studies have not characterized the study population adequately. It is possible that the reproducibility of the measurements decreases in eyes with more advanced glaucomatous damage.
As expected, our results indicate that most RTVue and Stratus OCT measurements are highly correlated, although some correlations were moderate at best (eg, disc area in healthy eyes). Correlations generally were lower in healthy eyes than patient eyes, particularly for parameters describing disc topography (eg, for the rim area, r2 was 0.22 in healthy eyes vs 0.67 in glaucoma patient eyes). This may be explained in part by the use of different strategies to identify the RPE tips and to define the optic disc contour line. The Stratus OCT is limited by the number of radial scans used to identify the RPE tips. In the case of healthy discs with small cups, RPE tips can be masked by the presence of vessel shadows, making it difficult for the instrument to delineate the optic disc margin properly. The RTVue allows the operator to identify the RPE tips at any location around the optic disc with the use of the en face OCT image, capable of providing both horizontal and vertical scans at any given point within the map. This may prevent the interference of the vessels in defining the optic disc margin, thus allowing more accurate measurements of the optic disc parameters.
When transitioning to a new and more advanced technology, such as SD-OCT for retina and optic disc imaging, evidence is required to confirm that the new instrument can offer clinicians a significant advantage over previous technologies. In glaucoma, one challenge is to demonstrate that SD-OCT can improve diagnostic accuracy and progression detection compared with TD-OCT. The current study shows that RTVue measurement variability is small, and this finding represents preliminary evidence that SD-OCT has the potential to advance glaucoma diagnosis and management. However, because glaucoma is a slowly progressing disease and follow-up is required to monitor disease progression, it is also important to assess how RNFL thickness and optic disc measurements compare with previous OCT technology that currently may be used by clinicians for the follow-up of their glaucoma patients. In this study, Bland-Altman plots showed good agreement between RTVue and Stratus OCT RNFL measurements. However, in healthy eyes, RTVue average, temporal, and inferior RNFL measurements tended to be thicker than those of Stratus OCT by an average of 1.7, 7.5, and 6.4 µm, respectively (Table 4). In addition, the Bland-Altman plot in Figure 1 shows that RTVue tends to provide average RNFL thickness measurements approximately 3 µm thicker than Stratus OCT and that this effect is more pronounced for lower values of average RNFL thickness. The plot also indicates that for values of average RNFL thickness greater than approximately 110 µm, the relationship tends to invert (ie, Stratus OCT provides greater RNFL thickness measurements than RTVue). Clinicians incorporating RTVue in their clinical practice should be aware of the discrepancies in the measurements between RTVue and Stratus OCT.
Evaluating the presence of systematic discrepancies between the 2 instruments will help to identify factors responsible for the disagreement that can be ruled out in clinical practice. For example, it is possible that differences in signal strength between the 2 instruments explain at least in part the differences between the measurements. Signal strength has been shown to affect RNFL thickness measurements using Stratus OCT (also confirmed by our study).20,21 The current study showed that RTVue RNFL thickness measurements also can be affected by signal strength. It is possible that differences in signal strength between images taken with the 2 instruments influenced the agreement between the measurements. However, only good-quality scans were used for this study, restricting the range of this parameter. In addition, in healthy eyes, RTVue measurements were not consistently greater than Stratus OCT measurements for all sectors, suggesting the presence of other factors responsible for these findings. Differences between the instruments’ scan circle placement and software edge detection algorithms for RNFL detection may influence RNFL thickness measurements.
Our results also showed that RTVue optic disc measurements consistently were smaller than Stratus OCT measurements in healthy and patients eyes. Table 4 shows negative mean values for all disc parameters when subtracting Stratus OCT measurements from RTVue measurements, with values ranging from −0.4 mm2 for disc area to −0.1 mm2 for the cup area. Figure 2 shows the Bland-Altman plot for rim area, with RTVue providing measurements on average 0.3 mm2 lower than Stratus OCT, independent of the size of the rim. Again, this finding may be explained in part by the different strategies applied to detect the RPE tips by the 2 devices. Further studies are needed to determine whether optic disc parameters measured by RTVue with the help of the en face OCT image may provide greater accuracy than the measurements provided by Stratus OCT. In this study, scans were obtained within the same session and by the same operator. It is possible that RTVue may provide a greater intervisit variability and that results may be less repeatable when scans are obtained by different operators over time.
In conclusion, the present study demonstrates excellent reproducibility of RTVue measurements in healthy and patient eyes. RTVue average RNFL measurements generally were thicker and optic disc measurements were smaller than Stratus OCT measurements, suggesting that RNFL and optic disc measurements obtained with RTVue and Stratus OCT are not interchangeable. The former findings suggest that RTVue can detect small changes in RNFL thickness and optic disc topography over time.
Acknowledgments
This study was supported by grants EY011008 and EY008208 from the National Institutes of Health, Bethesda, Maryland. Dr Weinreb is a consultant for Carl Zeiss Meditec, Dublin, California. Drs Weinreb, Zangwill, and Medeiros receive research support from Carl Zeiss Meditec, Optovue Inc, Fremont, California, and Heidelberg Engineering, Dossenheim, Germany. Dr Medeiros also receives research support from Reichert Ophthalmic Instruments, Depew, New York. Dr Bowd receives research support from Lace Elettronica, Pisa, Italy. Involved in design of study (A.O.G.-G., G.V., C.B., L.M.Z., R.N.W.); conduct of study (A.O.G.-G., G.V.); data collection, management, and analysis (A.O.G.-G., G.V., C.B., L.M.Z.); and preparation, review, and approval of the manuscript (A.O.G.-G., G.V., C.B., L.M.Z., F.A.M., R.N.W.). The University of California San Diego Institutional Review Board approved all protocols and adheres to the Declaration of Helsinki. Health Insurance Portability and Accountability Act authorization forms were obtained from all participants.
Biography

Alberto O. González-García, MD, is an Assistant Professor at the Cuban Institute of Neurology and Neurosurgery and Consultant Ophthalmic Surgeon and Clinician at the Cuban Institute of Ophthalmology. Dr González-García has completed a fellowship in neuro-ophthalmology at the Cuban Institute of Neurology and Neurosurgery and a glaucoma research fellowship at the Hamilton Glaucoma Center, University of California San Diego.
REFERENCES
- 1.Blumenthal EZ, Williams JM, Weinreb RN, et al. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology. 2000;107:2278–2282. doi: 10.1016/s0161-6420(00)00341-9. [DOI] [PubMed] [Google Scholar]
- 2.Chen TC, Cense B, Pierce MC, et al. Spectral-domain optical coherence tomography: ultra high-speed, ultra high-resolution ophthalmic imaging. Arch Ophthalmol. 2005;123:1715–1720. doi: 10.1001/archopht.123.12.1715. [DOI] [PubMed] [Google Scholar]
- 3.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimoto JG, Bouma B, Tearney GJ, et al. New technology for high-speed and high-resolution optical coherence tomography. Ann N Y Acad Sci. 1998;838:95–107. doi: 10.1111/j.1749-6632.1998.tb08190.x. [DOI] [PubMed] [Google Scholar]
- 5.Leitgeb R, Hitzenberger CK, Fercher AF. Performance of Fourier-domain vs. time-domain optical coherence tomography. Opt Express. 2003;11:889–894. doi: 10.1364/oe.11.000889. [DOI] [PubMed] [Google Scholar]
- 6.Van Velthoven ME, Faber DJ, Verbraak FD, et al. Recent developments in optical coherence tomography for imaging the retina. Prog Retin Eye Res. 2007;26:57–77. doi: 10.1016/j.preteyeres.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Wojtkowski M, Leitgeb R, Kowalczyk A, et al. In vivo human retinal imaging by Fourier-domain optical coherence tomography. J Biomed Opt. 2002;7:457–463. doi: 10.1117/1.1482379. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Erfurth U, Leitgeb RA, Michels S, et al. Three-dimensional ultra high-resolution optical coherence tomography of macular diseases. Invest Ophthalmol Vis Sci. 2005;46:3393–3402. doi: 10.1167/iovs.05-0370. [DOI] [PubMed] [Google Scholar]
- 9.Nassif N, Cense B, Park BH, et al. In vivo human retinal imaging by ultra high-speed spectral-domain optical coherence tomography. Opt Lett. 2004;29:480–482. doi: 10.1364/ol.29.000480. [DOI] [PubMed] [Google Scholar]
- 10.De Boer JF, Cense B, Park BH, et al. Improved signal-to-noise ratio in spectral-domain compared with time-domain optical coherence tomography. Opt Lett. 2003;28:2067–2069. doi: 10.1364/ol.28.002067. [DOI] [PubMed] [Google Scholar]
- 11.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–1360. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros FA, Zangwill LM, Bowd C, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139:44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 13.Hanley JA, Negassa A, Edwardes MD, et al. Statistical analysis of correlated data using Generalized Estimating Equations: an orientation. Am J Epidemiol. 2003;157:364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 14.Bagga H, Greenfield DS. Quantitative assessment of structural damage in eyes with localized visual field abnormalities. Am J Ophthalmol. 2004;137:797–805. doi: 10.1016/j.ajo.2003.11.060. [DOI] [PubMed] [Google Scholar]
- 15.Jeoung JW, Parkq KH, Kim TW, et al. Diagnostic ability of optical coherence tomography with a normative database to detect localized retinal nerve fiber layer defects. Ophthalmology. 2005;112:2157–2163. doi: 10.1016/j.ophtha.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Budenz DL, Michael A, Chang RT, et al. Sensitivity and specificity of the Stratus OCT for perimetric glaucoma. Ophthalmology. 2005;112:3–9. doi: 10.1016/j.ophtha.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 17.Budenz DL, Chang RT, Huang X, Knighton RW, Tielsch JM. Reproducibility of retinal nerve fiber thickness measurements using the Stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2005;46:2440–2443. doi: 10.1167/iovs.04-1174. [DOI] [PubMed] [Google Scholar]
- 18.Budenz DL, Fredette MJ, Feuer WJ, Anderson DR. Reproducibility of peripapillary retinal nerve fiber thickness measurements with Stratus OCT in glaucomatous eyes. Ophthalmology. 2008;115:661–666. doi: 10.1016/j.ophtha.2007.05.035. [DOI] [PubMed] [Google Scholar]
- 19.Hodapp E, Parrish RK, Anderson DR. Clinical decisions in glaucoma. St Louis, Missouri: Mosby; 1993. p. 53. [Google Scholar]
- 20.Wu Z, Vazeen M, Varma R, et al. Factors associated with variability in retinal nerve fiber layer thickness measurements obtained by optical coherence tomography. Ophthalmology. 2007;114:1505–1512. doi: 10.1016/j.ophtha.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 21.Cheung CY, Leung CK, Lin D, et al. Relationship between retinal nerve fiber layer measurement and signal strength in optical coherence tomography. Ophthalmology. 2008;115:1347–1351. doi: 10.1016/j.ophtha.2007.11.027. [DOI] [PubMed] [Google Scholar]