Abstract
Large numbers of Thy-1-positive cells were observed in cultures of bone marrow cells that had been depleted of T cells and grown for 3 to 4 days in the presence of medium conditioned by concanavalin A-activated spleen cells. Cells bearing levels of Thy-1 comparable with those on the bulk of thymocytes were isolated by using the fluorescence-activated cell sorter. Although many were large blasts, the Thy-1-positive cells failed to grow in response to T-cell growth factor and concanavalin A; about one-third, however, proliferated in the presence of factors stimulating hemopoietic progenitor cells. Furthermore, the Thy-1-positive population included cells capable of forming large colonies of macrophages and granulocytes in agar and cells forming splenic colonies in lethally irradiated mice. The appearance of the Thy-1-positive cells did not correlate with the presence of either T-cell growth factor or T-cell-derived granulocyte/macrophage colony-stimulating factor. These findings indicate that Thy-1 can occur on various murine hemopoietic stem and progenitor cells and myeloid cells; Thy-1 can no longer be regarded as an unambiguous marker of commitment to the T-cell lineage.
Full text
PDF

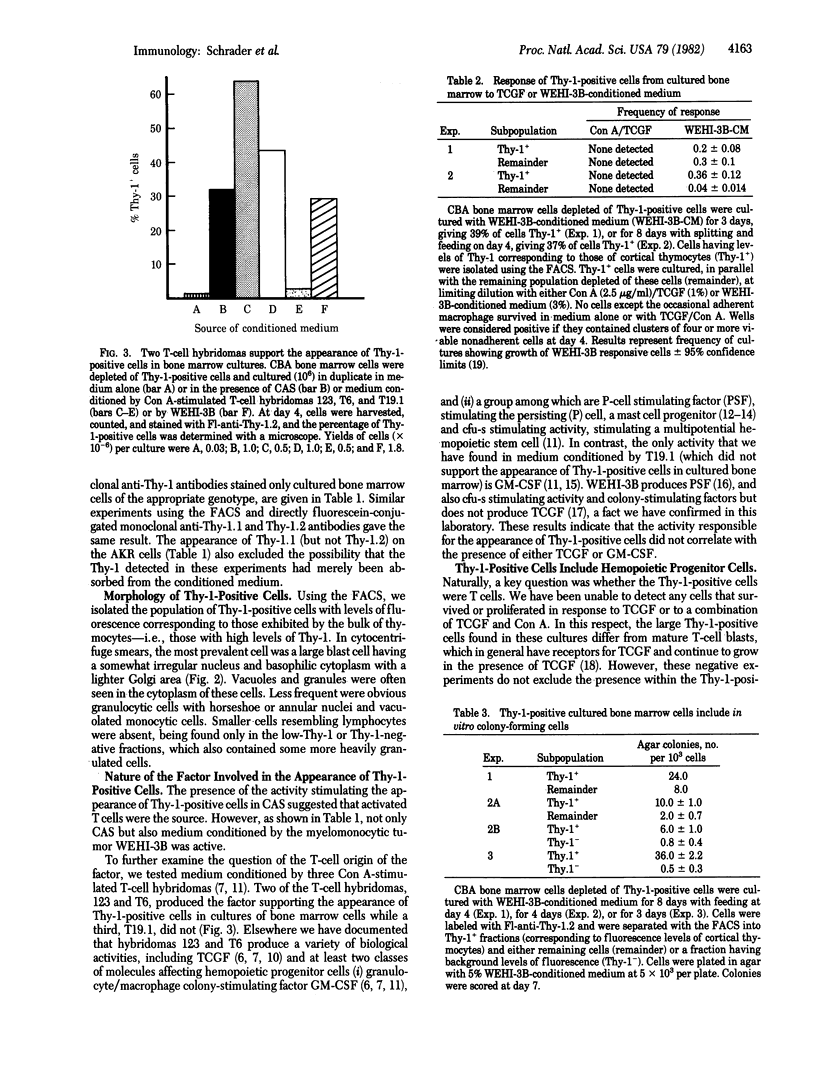


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess A. W., Bartlett P. F., Metcalf D., Nicola N. A., Clark-Lewis I., Schrader J. W. Granulocyte-macrophage colony-stimulating factor produced by an inducible murine T-cell hybridoma: molecular properties and cellular specificity. Exp Hematol. 1981 Oct;9(9):893–903. [PubMed] [Google Scholar]
- Clark-Lewis I., Schrader J. W. Biochemical characterization of regulatory factors derived from T cell hybridomas and spleen cells. I. Separation of T cell growth factor and T cell replacing factor from granulocyte-macrophage colony-stimulating factor. J Immunol. 1982 Jan;128(1):168–174. [PubMed] [Google Scholar]
- Clark-Lewis I., Schrader J. W. P cell-stimulating factor: biochemical characterization of a new T cell-derived factor. J Immunol. 1981 Nov;127(5):1941–1947. [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Nunn M. E., Holden H. T. Low density of Thy 1 antigen on mouse effector cells mediating natural cytotoxicity against tumor cells. J Immunol. 1978 Jul;121(1):304–309. [PubMed] [Google Scholar]
- Hunt S. V., Mason D. W., Williams A. F. In rat bone marrow Thy-1 antigen is present on cells with membrane immunoglobulin and on precursors of peripheral B lymphocytes. Eur J Immunol. 1977 Nov;7(11):817–823. doi: 10.1002/eji.1830071114. [DOI] [PubMed] [Google Scholar]
- Komuro K., Boyse E. A. In-vitro demonstration of thymic hormone in the mouse by conversion of precursor cells into lymphocytes. Lancet. 1973 Apr 7;1(7806):740–743. doi: 10.1016/s0140-6736(73)92127-2. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. The role of mitogenic lectins in T-cell triggering. Nature. 1979 Jul 19;280(5719):239–241. doi: 10.1038/280239a0. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Iscove N. N., Coutinho A. Two distinct factors are required for induction of T-cell growth. Nature. 1980 Feb 14;283(5748):664–666. doi: 10.1038/283664a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- REIF A. E., ALLEN J. M. THE AKR THYMIC ANTIGEN AND ITS DISTRIBUTION IN LEUKEMIAS AND NERVOUS TISSUES. J Exp Med. 1964 Sep 1;120:413–433. doi: 10.1084/jem.120.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Ritter M. A., Morris R. J., Goldschneider I. Hidden Thy-1 antigen in a subpopulation of mouse bone marrow cells. Immunology. 1980 Mar;39(3):375–384. [PMC free article] [PubMed] [Google Scholar]
- Ruscetti F. W., Morgan D. A., Gallo R. C. Functional and morphologic characterization of human T cells continuously grown in vitro. J Immunol. 1977 Jul;119(1):131–138. [PubMed] [Google Scholar]
- Scheid M. P., Goldstein G., Boyse E. A. The generation and regulation of lymphocyte populations: evidence from differentiative induction systems in vitro. J Exp Med. 1978 Jun 1;147(6):1727–1743. doi: 10.1084/jem.147.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Arnold B., Clark-Lewis I. A con A-stimulated T-cell hybridoma releases factors affecting haematopoietic colony-forming cells and B-cell antibody responses. Nature. 1980 Jan 10;283(5743):197–199. doi: 10.1038/283197a0. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Clark-Lewis I. T cell hybridoma-derived regulatory factors. I. Production of T cell growth factor following stimulation by concanavalin A. J Immunol. 1981 Mar;126(3):1101–1105. [PubMed] [Google Scholar]
- Schrader J. W. In in vitro production and cloning of the P cell, a bone marrow-derived null cell that expresses H-2 and Ia-antigens, has mast cell-like granules, and is regulated by a factor released by activated T cells. J Immunol. 1981 Feb;126(2):452–458. [PubMed] [Google Scholar]
- Schrader J. W., Lewis S. J., Clark-Lewis I., Culvenor J. G. The persisting (P) cell: histamine content, regulation by a T cell-derived factor, origin from a bone marrow precursor, and relationship to mast cells. Proc Natl Acad Sci U S A. 1981 Jan;78(1):323–327. doi: 10.1073/pnas.78.1.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh U., Owen J. J. Studies on the maturation of thymus stem cells. The effects of catecholamines, histamine and peptide hormones on the expression of T cell alloantigens. Eur J Immunol. 1976 Jan;6(1):59–62. doi: 10.1002/eji.1830060113. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Walker E. B., Lanier L. L., Warner N. L. Characterization and functional properties of tumor cell lines in accessory cell replacement assays. J Immunol. 1982 Feb;128(2):852–859. [PubMed] [Google Scholar]
- Warner N. L., Moore M. A., Metcalf D. A transplantable myelomonocytic leukemia in BALB-c mice: cytology, karyotype, and muramidase content. J Natl Cancer Inst. 1969 Oct;43(4):963–982. [PubMed] [Google Scholar]
- Yung Y. P., Eger R., Tertian G., Moore M. A. Long-term in vitro culture of murine mast cells. II. Purification of a mast cell growth factor and its dissociation from TCGF. J Immunol. 1981 Aug;127(2):794–799. [PubMed] [Google Scholar]






