Abstract
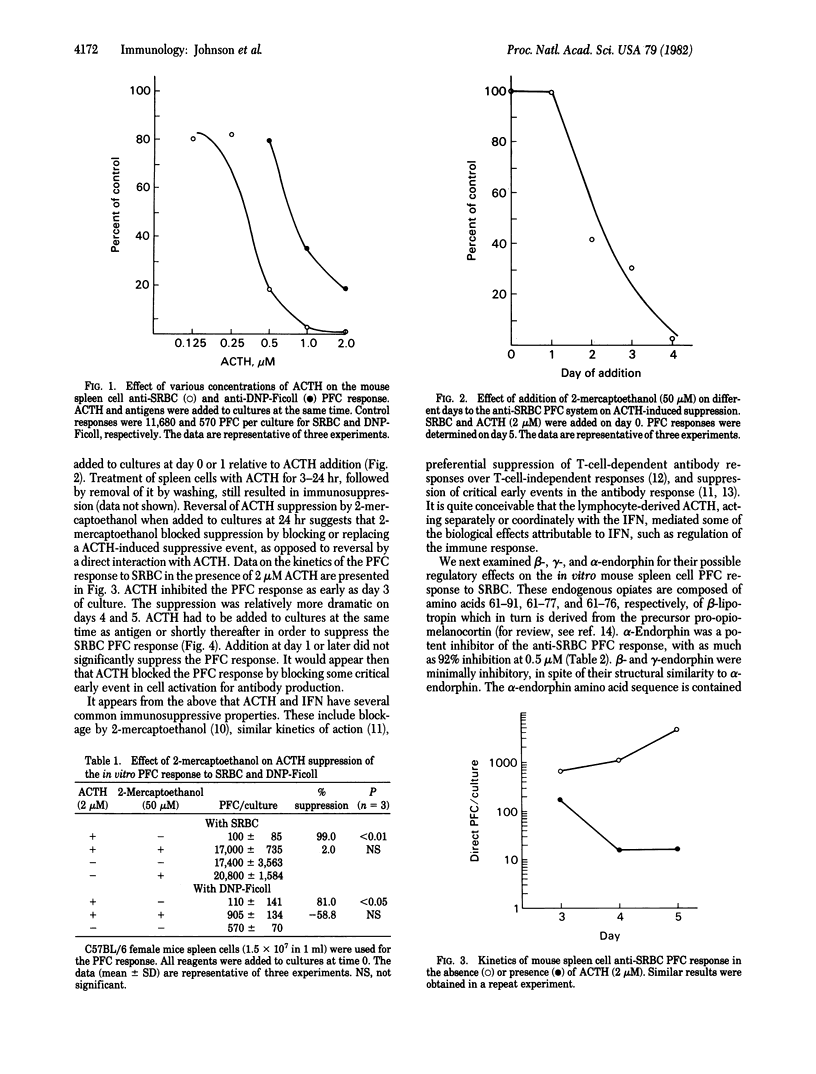
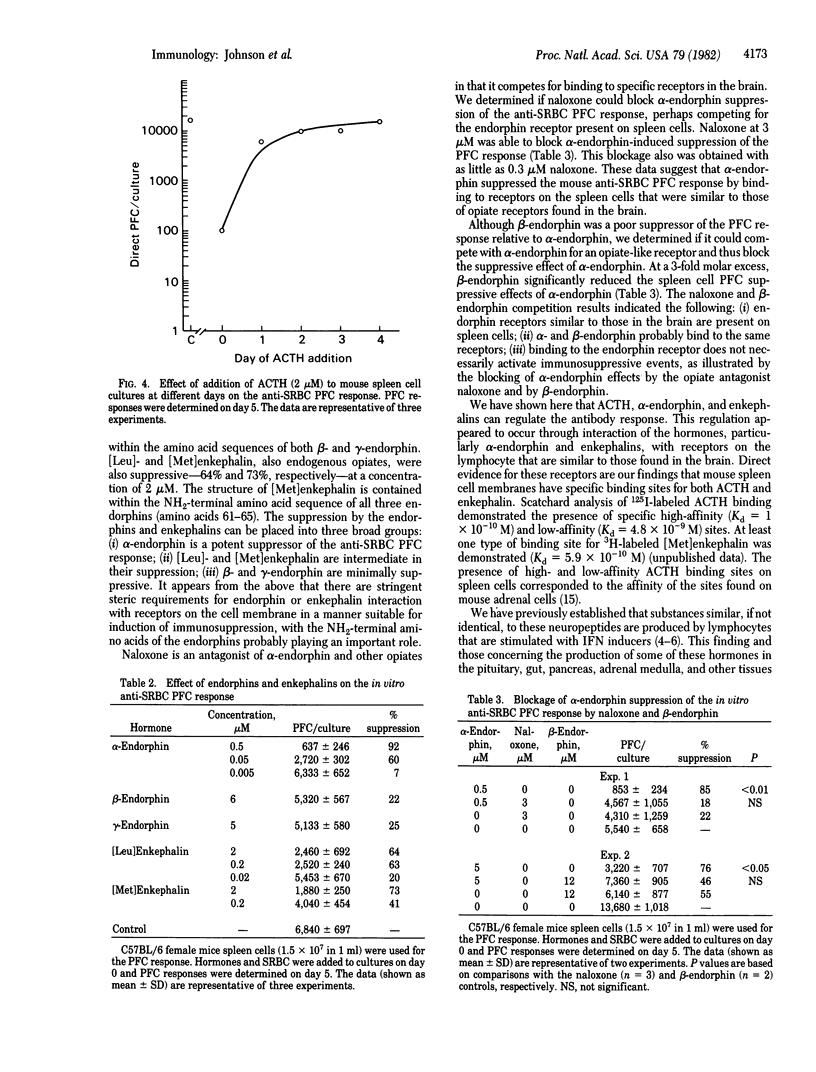
Treatment of lymphocytes with inducers of interferon α (IFN-α) results in the production of corticotropin (ACTH) and endorphin-like activities. The pro-opiomelanocortin-derived hormones ACTH and α-, β-, and γ-endorphin and the structurally related hormones [Leu]- and [Met]enkephalin were therefore tested for their effects on the in vitro antibody response of mouse spleen cells. ACTH and α-endorphin were potent inhibitors (≥80% suppression) of the antibody response to the T-cell-dependent antigen sheep erythrocytes at a concentration of 0.5 μM. [Met]- and [Leu]enkephalin were moderate inhibitors (approximately 60% suppression) at 0.2-2 μM, and β- and γ-endorphin were minimal inhibitors (approximately 20% suppression) at 5-6 μM. At higher concentrations ACTH also inhibited the antibody response to the T-cell-independent antigen dinitrophenyl-Ficoll, suggesting that T-cell function was more sensitive to blockage by these hormones than was B-cell function. ACTH and IFN had similar suppression properties; thus, the hormone-like activities associated with IFN-α may play a role in IFN-induced immunosuppression. α-Endorphin immunosuppression was blocked by naloxone, which suggested that α-endorphin exerted its effects through binding to opiate-like receptors on the spleen cells. The failure of β-endorphin to suppress the immune response significantly was not due to its failure to bind to the opiate-like receptors because it blocked α-endorphin-induced suppression. Direct evidence for both opiate and ACTH receptors on the spleen cells was obtained in binding studies with labeled enkephalin and ACTH. Such studies revealed the presence of both high- and low-affinity receptors. The data show that neuroendocrine polypeptide hormones can regulate the immune response.
Keywords: corticotropin, endorphins, enkephalins, pro-opiomelanocortin, immunoendocrinology
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blalock J. E., Harp C. Interferon and adrenocorticotropic hormone induction of steroidogenesis, melanogenesis and antiviral activity. Arch Virol. 1981;67(1):45–49. doi: 10.1007/BF01314600. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Smith E. M. Human leukocyte interferon (HuIFN-alpha): potent endorphin-like opioid activity. Biochem Biophys Res Commun. 1981 Jul 30;101(2):472–478. doi: 10.1016/0006-291x(81)91284-5. [DOI] [PubMed] [Google Scholar]
- Blalock J. E., Smith E. M. Human leukocyte interferon: structural and biological relatedness to adrenocorticotropic hormone and endorphins. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5972–5974. doi: 10.1073/pnas.77.10.5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock J. E., Stanton J. D. Common pathways of interferon and hormonal action. Nature. 1980 Jan 24;283(5745):406–408. doi: 10.1038/283406a0. [DOI] [PubMed] [Google Scholar]
- Booth R. J., Booth J. M., Marbrook J. Immune conservation: a possible consequence of the mechanism of interferon-induced antibody suppression. Eur J Immunol. 1976 Nov;6(11):769–772. doi: 10.1002/eji.1830061103. [DOI] [PubMed] [Google Scholar]
- Chany C., Rousset S., Bourgeade M. F., Mathieu D., Grégoire A. Role of receptors and the cytoskeleton in reverse transformation and steroidogenesis induced by interferon. Ann N Y Acad Sci. 1980;350:254–265. doi: 10.1111/j.1749-6632.1980.tb20626.x. [DOI] [PubMed] [Google Scholar]
- Chused T. M., Kassan S. S., Mosier D. E. Macrophage requirement for the in vitro response to TNP Ficoll: a thymic independent antigen. J Immunol. 1976 Jun;116(6):1579–1581. [PubMed] [Google Scholar]
- Guillemin R. Beta-lipotropin and endorphins: implications of current knowledge. Hosp Pract. 1978 Nov;13(11):53–60. doi: 10.1080/21548331.1978.11707431. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Bukovic J. A., Baron S. Interferon inhibition of the primary in vitro antibody response to a thymus-independent antigen. Cell Immunol. 1975 Nov;20(1):104–109. doi: 10.1016/0008-8749(75)90089-1. [DOI] [PubMed] [Google Scholar]
- Johnson H. M. Differentiation of the immunosuppressive and antiviral effects of interferon. Cell Immunol. 1978 Mar 15;36(2):220–230. doi: 10.1016/0008-8749(78)90266-6. [DOI] [PubMed] [Google Scholar]
- Johnson H. M., Smith B. G., Baron S. Inhibition of the primary in vitro antibody response by interferon preparations. J Immunol. 1975 Jan;114(1 Pt 2):403–409. [PubMed] [Google Scholar]
- McIlhinney R. A., Schulster D. Studies on the binding of 125I-labelled corticotrophin to isolated rat adrenocortical cells. J Endocrinol. 1975 Jan;64(1):175–184. doi: 10.1677/joe.0.0640175. [DOI] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Blalock J. E. Human lymphocyte production of corticotropin and endorphin-like substances: association with leukocyte interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7530–7534. doi: 10.1073/pnas.78.12.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]


