Abstract
Glucagon-like peptide-1 (GLP-1) signaling modulates sweet-taste sensitivity in the mouse. Because circumvallate papillae (CVPs) express both GLP-1 and its receptor, a local regulation has been suggested. However, whether dietary lipids are involved in this regulation, as shown in the gut, is unknown. By using a combination of biochemical, immunohistochemical, and behavioral approaches, the present data i) confirm the role of GLP-1 signaling in the attraction for sucrose, ii) demonstrate that minute quantities of long-chain FAs (LCFAs) reinforce the attraction for sucrose in a GLP-1 receptor-dependent manner, iii) suggest an involvement of the LCFA receptor GPR120 expressed in taste buds in this system, and iv) support the existence of a regulation by GLP-1 of the lipid sensing mediated by lingual CD36. Therefore, oro-sensory detection of LCFAs may affect sweet and fatty taste responsiveness by controlling the secretion of lingual GLP-1. This regulatory loop, probably triggered by the LCFA-GPR120 interaction, might contribute to the high palatability of foods rich both in fat and sugar.
Keywords: long-chain fatty acid, eating behavior, obesity risk, health
Substantial evidence supports the existence of a specific detection system devoted to the oro-sensory perception of dietary lipids in both rodents and humans. Long-chain FAs (LCFAs) are the main molecules detected by this system and are thought to play a significant role in the spontaneous preference for fatty foods (1, 2). The plasma membrane glycoprotein CD36 has been the first plausible candidate identified to exert the function of a lipid sensor in the oral cavity (3). Indeed, it displays a very high affinity for LCFAs (4), is specifically found in the gustatory papillae in rat (5), mouse (3), or human (6), and ablation of CD36 gene expression renders mice unable to recognize and prefer LCFAs in a textured solution during two-bottle preference tests (3, 7, 8). In human subjects, the common single-nucleotide polymorphism rs1761667, known to reduce CD36 gene expression (9), is also associated with a deep attenuation of orosensory sensitivity for fat (10).
Two unrelated members of the G protein-coupled receptor family, the free fatty acid receptor 1 (FFAR1, also termed GPR40) and GPR120, have also been recently identified as playing a role in the spontaneous preference for fat in the mouse (11). Such a function is probably indirect for FFAR1, inasmuch as it is not found in taste buds in rat (12) and human (13) and is not systematically detected in circumvallate papillae (CVPs) in the mouse (8, 11), in contrast to GPR120. This last observation raises the question of the respective role(s) played by CD36 and GPR120 in the coding mechanisms for fat taste at the periphery. The fact that CD36 expression is subjected to a short-term lipid-mediated downregulation in mouse taste buds during food intake, whereas GPR120 gene expression remains unchanged (8), is consistent with distinct functions.
A biological action for GPR120 was first identified in the entero-endocrine L cells, in which its activation by LCFA triggers the secretion of the glucagon-like peptide-1 (GLP-1) (14). In addition to its insulinotropic effect, GLP-1 exerts multiple physiological functions, including a role in the regulation of eating behavior (15). Interestingly, GLP-1 and its receptor (GLP-1R) have also been identified in mouse taste buds, suggesting an involvement of this incretin in the sense of taste (16). Consistent with this assumption, it has been shown that GLP-1 signaling modulates taste sensitivity in the mouse, decreasing sour taste but enhancing the responsiveness to sucrose (16). However, mechanisms by which this regulation takes place are not yet determined.
Compelling evidence supports the existence of a functional continuum along the oro-intestinal tract responsible for the permanent analysis and control of ingestion, digestion, absorption, and metabolic fate of energy nutrients. For fat, cells from taste buds and entero-endocrine cells share common lipid sensors (e.g., GPR120), express similar hormones and their respective receptors (e.g., GLP-1, GLP-1R), and are connected to afferent nerve fibers involved in feeding behavior (i.e., gustatory nerves and vagus). A continuum being “a set of elements such that one can pass from one to another continuously,” we propose that fundamental knowledge of the gut can be used to better understand the functional characteristics of the oro-sensory tract, and reciprocally. Consistent with this hypothesis, the goal of the present work was to determine whether LCFA, GPR120, and GLP-1 are functionally linked in the tongue, as found in the gut, and to explore the impact of such a regulatory system on sweet and fatty taste responsiveness.
MATERIALS AND METHODS
Ethics statement
French guidelines for the use and care of laboratory animals were followed, and experimental protocols were approved by the animal ethics committee of Burgundy University (approval codes B1010, B0210, and C1011).
Animals
Animals were housed in a controlled environment (constant temperature and humidity, darkness from 7 PM to 7 AM) and were fed a standard laboratory chow (4RF21; Mucedola, Italy). C57Bl/6J wild-type mice were purchased from Charles River Laboratories (France). CD36 −/− (17) and Glp1r −/− (18) mice with a C57Bl/6J background were bred locally.
Behavioral experiments
CD36 −/− and Glp1r −/− mice were used in the behavioral experiments. Two different tests, offered successively in a randomized manner, were a licking test or, simultaneously, a two-bottle preference test. A control or an experimental solution were used: a licking test that offered successively, in a randomized manner, a control and an experimental solution and a two bottle preference test in which control and experimental solutions were offered simultaneously.
Licking test.
This test consists of subjecting a mouse to the control or experimental solution successively to determine the number of licks given on each bottle using a contact lickometer (Med Associates). Mice were food- and water-deprived for 6 h before the test, which took place 6 h after the beginning of the dark period. After a training period required to learn the procedure, different groups of mice were subjected to different solutions. In a first experiment, mice were randomly subjected to a bottle containing a control solution (62 mM of sucrose; Sigma-Aldrich) or a bottle containing an experimental solution [62 mM of sucrose + 200 µM of oleic acid (OLA) or α-linolenic acid (ALA); Sigma-Aldrich] for 15 min. Then mice were offered the other bottle for an additional 15 min session. OLA and ALA were previously dissolved in ethanol (0.1% final). The same quantity of ethanol was added in the control solution. In a second experiment, mice were randomly subjected to a bottle containing water (control solution) or a bottle containing 62 mM sucrose, 200 µM of OLA, or 200 µM of ALA in water. In a third experiment, mice were randomly subjected to a bottle containing mineral oil (control solution; Cooper, France) or different concentrations of OLA in mineral oil. In each experiment, data were analyzed for 1 min from the first lick to exclude postingestive signals.
Two-bottle preference test.
Mice were subjected for 12 h to a double-choice test. Mice were offered a pair of bottles of water in experimental cages for 1 day. Because rapeseed oil was added in xanthan gum to facilitate solubilization and minimize textural cues, mice were subjected on day 2 to 0.3% xanthan gum (Sigma-Aldrich) alone to avoid neophobia. A double-choice test between control solution (xanthan gum) and experimental solution (xanthan gum + rapeseed oil) was performed on day 3. The position of the bottles (on the right or the left) was changed daily to avoid the development of side preference. Consumption of each solution (in grams) was analyzed for 12 h after the beginning of the test, and preference for the experimental solutions (ratio between the consumption on experimental bottle and the total consumption) was calculated.
Papillae isolation
CVPs from wild-type or Glp1r −/− mice were isolated according to previously published procedures (3). In brief, the lingual epithelium was separated from connective tissue by enzymatic dissociation (elastase and dispase mixture, 2 mg/ml each in Tyrode buffer: 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 1 mM CaCl2, 10 mM glucose, 1 mM MgCl2, 10 mM Na pyruvate, pH 7.4), and papillae were dissected under a microscope. Epithelium surrounding the papillae was also collected to serve as nonsensory control tissue. Samples were snap-frozen in liquid nitrogen and stored at −80°C until RNA or protein extraction, or put in culture.
Tissue culture of CVPs
CVPs of wild-type mice were isolated and incubated at 37°C in an oxygenized medium containing 33 µM FA-free BSA alone (Sigma-Aldrich; control solution), 200 µM ALA, 200 µM OLA, or 50 µM of a GPR120 agonist (GSK137647A). After 2 h of incubation, the medium was collected, and the active GLP-1 release was measured by ELISA (Millipore). We have postulated that secretion of GLP-1 by CVPs might be very low. To be sure to detect active GLP-1 in the incubation medium, 10 pM of pure GLP-1 were systematically added in each experimental well, but not in standard curve, according to the manufacturers’ recommendations. In these conditions, values under 2 pM become resolved. A dipeptidyl peptidase 4 (DPP4) inhibitor (0.1%; Millipore) was added to the medium to prevent GLP-1 degradation.
Compound profiling in recombinant GPR120 receptor assay using intracellular calcium mobilization
U2OS cells (human osteo-sarcoma ATCC HTB-96; American Type Culture Collection) were grown in DMEM/F-12 supplemented with 10% FBS and 2 mM l-glutamine. Recombinant GPR120-expressing cells were generated by transducing U2OS cells with BacMam viruses encoding the respective receptors and the chimeric G-protein Gα16 according to established protocols (19). In brief, cells were plated to a density of 2 × 105 cells/ml in cell culture medium containing human GPR120 (0.25%, v/v), mouse GPR120 (0.5%, v/v), or rat GPR120 (0.8%, v/v) BacMam virus. Gα16 BacMam virus (0.12%, v/v) was also transduced in preparation of recombinant human GPR120 cells to allow efficient coupling of the human GPR120 receptor to the phospholipase C pathway. This solution of cells/virus mixture was then plated at a density of 104 cells/well and cultured at 37°C, 5% CO2, 95% humidity for 24 h. Functional EC50 studies were performed in cells incubated with HBSS containing the cytoplasmic calcium indicator Fluo-4 dye in the acetylmethyl form (4 mM), 2.5 mM probenecid, and 250 μM Brilliant Black at 37°C for 60 min. Compound plates were generated containing 3% DMSO in dye loading buffer. Compounds (i.e., GPR120 agonist GSK137647A or histamine for host untransduced U2OS cells) were added to the cells at a 1:3 dilution, and calcium mobilization was measured using a fluorescence image plate reader (Molecular Devices). Data were converted into normalized responses with respect to assay standards GSK137647A (for GPR120) or histamine (for host U2OS cells). Data were further analyzed using a four-parameter fit to calculate EC50 values.
Real-time RT-PCR
Total RNA from CVPs and surrounding nongustatory epithelium (negative control) was extracted using RNeasy mini-columns (Qiagen). Genomic DNA digestion was performed using the RNase-free DNase set (Qiagen). First-strand cDNA was generated by reverse transcription from total RNA (Omniscript Reverse Transcription; Qiagen). Levels of mRNA transcripts were determined by real-time RT-PCR (StepOnePlus apparatus; Applied Biosystems). RNA levels were normalized against levels of 36B4 RNA transcripts. Primer probe sets were designed with the Primer3 software tool using gene sequences, either from the GenBank database or purchased directly from Applied Biosystems. PCR amplification was done using Sybrgreen (Power SYBR Green PCR Master Mix; Applied Biosystems) or Taqman (Universal Taqman PCR Master Mix; Applied Biosystems) technology. The oligonucleotide sequences of primers and probes are shown in Table 1. The comparative 2-ΔΔCT method was used for relative quantification (20).
TABLE 1.
Sequences and GenBank numbers of primers employed for RT-PCR amplifications
| Gene name | Nucleotides sequences (5′→ 3′) or Applied Biosystems Taqman Assay ID details | PubMed accession number |
| CD36 | Forward: GGCCAAGCTATTGCGACATG | NM_007643 |
| Probe: CACAGACGCAGCCTCCTTTCCACCT | ||
| Reverse: CCGAACACAGCGTAGATAGAC | ||
| GPR120 | Mm01198944_m1 | NM_181748 |
| α-Gustducin | Forward: ACACATTGCAGTCCATCCTAGC | XM_144196 |
| Probe: TGAAGTTGTTCTTGGTCCTCTCGGCTCC | ||
| Reverse: ATCACCATCTTCTAGTGTATTTGCC | ||
| PLCβ2 | Forward: GGCTTGAGTTCATCGTCATT | NM_177568 |
| Reverse: ACTCCCCCTGTCTTTTCCTA | ||
| T1R2 | Forward: CCGCCAGGCTTTCTTCACC | NM_031873.1 |
| Probe: TTGCTTCTCCGTCTGCCTCTCCTGC | ||
| Reverse: AGACGCACACAATCTGGAAGG | ||
| IP3R3 | Forward: ACGGAGCTGCCACATTTAT | NM_080553 |
| Reverse: CTCGTCCTCTTCTACGATCTC | ||
| 36B4 | Forward: GCCACCTGGAGAACAACCC | NM_007475 |
| Probe: AGGTCCTCCTTGGTGAACACGAAGCC | ||
| Reverse: GCCAACAGCATATCCCGAATC | ||
| T1R3 | Mm00473459_g1 | NM_031872.2 |
| SGLT-1 | Mm0041203_m1 | NM_019810.4 |
| TRPM5 | Mm00498453_m1 | NM_020277.2 |
Western blotting
Samples were homogenized using a micro-potter in a tris/sodium/EDTA buffer (50 mM Tris HCl, 150 mM NaCl, 1 mM EDTA, 1% nonidet P.40). Protein concentration in homogenates was assayed using a bicinchoninic acid kit (Perkin Elmer). After being separated by SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane by electroblotting. After being blocked using a TBS buffer containing 5% BSA and 0.05% Tween 20, membranes were incubated overnight at 4°C with an anti-CD36 primary antibody raised in goat (1:1,000 dilution; R&D Systems) or an anti-GPR120 primary antibody raised in rabbit (1:1,000 dilution; MBL International Corp.). After a set of washes, an appropriate peroxidase-conjugated secondary antibody was added (Santa Cruz Biotechnology). Antibody labeling was detected by chemiluminescence (ECL-plus reagent; Perkin Elmer). GAPDH was used as an internal reference protein.
Immunohistochemistry
CVPs from wild-type mice were fixed for 2–3 h in 4% paraformaldehyde, cryoprotected overnight with 30% sucrose in 0.1 M phosphate buffer (pH 7.4), and then embedded in OCT medium (Tissue-Tek; Sakura Finetek). Cryostat sections (10 µm) were air dried for 2 h at room temperature and then rehydrated in 0.1 M PBS (pH 7.4) for 10 min. Rehydrated sections were incubated for 1 h with PBS containing 0.3% Triton X-100, 30 min with PBS 50 mM glycine, and then blocked with 10% FA-free BSA in PBS for 40 min. Next, the slices were incubated overnight at 4°C with an anti-GPR120 primary antibody raised in rabbit (1:500 dilution; MBL). Specificity of the GPR120 antibody has been documented elsewhere (11). After washing, sections were incubated for 1 h at room temperature with a fluorescent anti-rabbit secondary antibody (Alexa 568, 1:1,000 dilution; Invitrogen). After washing, slices were blocked again before adding an anti-CD36 primary antibody raised in goat (1:250 dilution; R&D Systems) or an anti-GLP-1 primary antibody raised in goat (1:100 dilution; Santa Cruz Biotechnology). This GLP-1 antibody was used elsewhere (21). Sections were next incubated with a fluorescent anti-goat secondary antibody (Alexa 488, 1:1,000 dilution; Invitrogen) and then counterstained with Hoechst reactive (0.05 mg/ml; Sigma-Aldrich) to stain the nuclei. Slices were analyzed under a confocal microscope (Leica). In no cases was fluorescent staining observed when the primary antibody was omitted.
Statistics
Results are expressed as mean ± SEM. The significance of differences between groups was evaluated with SigmaStat (Systat Software; Germany). We first checked that the data for each group were normally distributed and that variances were equal and then carried out ANOVA, two-tailed Student's t-test, or Mann-Whitney tests. A P value of less than 0.05 was considered to be statistically significant.
RESULTS
LCFAs enhance the GLP-1-mediated induction of sweet-taste sensitivity
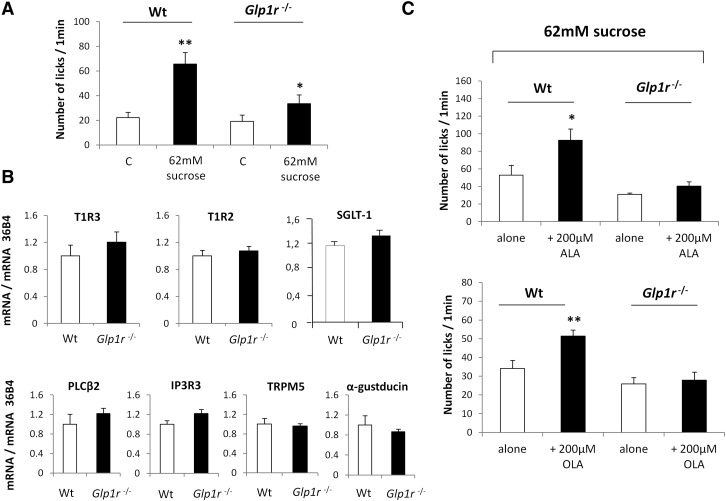
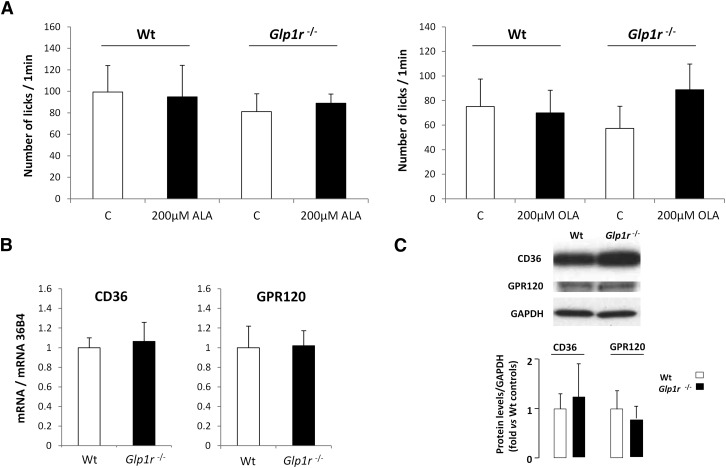
To explore the mechanisms by which GLP-1 can affect sweet-taste sensitivity, drinking behavior of wild-type and Glp1r-null mice was compared using computer-controlled lickometers and a brief access procedure (1 min). Consistent with published data (16), functional disruption of the Glp1r gene led to a decrease in the attraction for sucrose under conditions in which postingestive cues were known to be deeply minimized (Fig. 1A). This effect took place independently of changes in both CVP structure (data not shown) and expression of the key genes responsible for sweet-taste perception (Fig. 1B). Interestingly, addition of a small quantity of ALA or OLA reinforced the avidity for the sucrose solution in wild-type mice, but was without effect in Glp1r-null mice (Fig. 1C), suggesting that LCFAs may modulate sweet-taste sensitivity via the GLP-1 signaling pathway. It is unlikely that the greater preference for the fat-sweet mix was due to an additive effect of these two tastants, inasmuch as the concentration of LCFA used (i.e., 200 µM ≈ 0.005%) was not detected by mice when it was presented alone in a control solution (Fig. 2A). This behavior is independent of any change in relative expression of genes encoding for the gustatory lipid sensors GPR120 and CD36 in Glp1r-null mice (Fig. 2B, C).
Fig. 1.
Minute quantities of LCFA increase sweet-taste sensitivity via the GLP-1 signaling. A: Short-term licking tests (1 min) in wild-type (Wt) and Glp1r −/− mice subjected to a control solution or 62 mM sucrose solution. Mean ± SEM (n = 11–15). * P < 0.05; ** P < 0.01. B: mRNA levels of key genes involved in sweet-taste perception assayed by real-time PCR in CVPs from wild-type (Wt) and Glp1r −/− mice. Each value corresponds to a pool of total RNA from two mice. Mean ± SEM (n = 6). C: Short-term licking tests (1 min) in wild-type (Wt) and Glp1r −/− mice subjected to a 62 mM sucrose solution alone or in the presence of 200 µM ALA or OLA. Mean ± SEM (n = 11–13). * P < 0.05; ** P < 0.01.
Fig. 2.
Glp1r gene disruption does not affect gene expression of CD36 and GPR120 in mouse CVP. A: Short-term licking tests (1 min) in wild-type (Wt) and Glp1r −/− mice subjected to a control solution or a 200 µM ALA or OLA solution. Mean ± SEM (n = 11–13). B: CD36 and GPR120 mRNA levels assayed by real-time PCR in CVPs from wild-type (Wt) and Glp1r −/− mice. Each value corresponds to a pool of total RNA from two mice. Mean ± SEM (n = 6). C: CD36 and GPR120 protein levels assayed by Western blotting in CVPs from wild-type (Wt) and Glp1r −/− mice. A representative blot corresponding to a pool of total proteins from three mice is shown. Mean ± SEM (n = 2–4).
GLP-1 signaling in CVPs is independent of CD36 gene expression
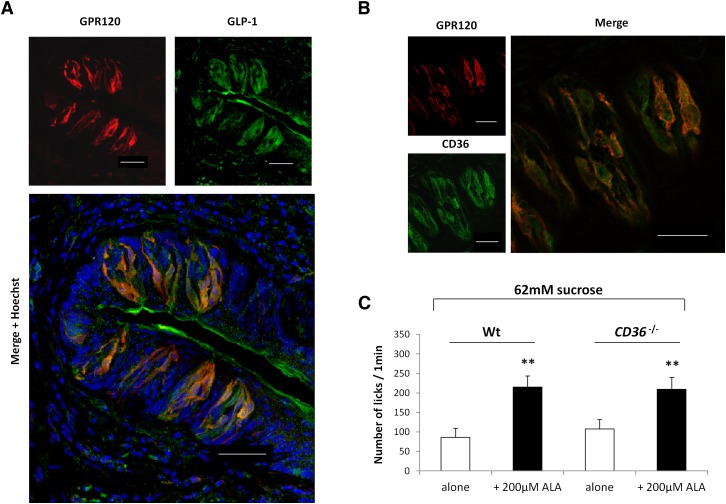
GLP-1 has been found in a few taste bud cells (TBCs) in various species (16, 22), but the mechanisms leading to its secretion by gustatory papillae are not yet fully understood. Because mouse CVPs express both GPR120 and GLP-1, we hypothesized that the activation of GPR120 by LCFA leads to GLP-1 secretion by TBCs, as reported for intestinal entero-endocrine L cells (14). In support of this hypothesis, GPR120 and GLP-1 were found to be coexpressed in a large number of mouse taste cells from mouse CVPs (Fig. 3A). No staining was detected when the GPR120 or the GLP-1 antibodies were omitted (data not shown). The fact that CD36 was also found to be coexpressed with GPR120 in subsets of TBCs (Fig. 3B) raises the possibility of a direct or indirect implication of lingual CD36 in the GLP-1-dependent modulation of avidity for sucrose. To assess this assumption, sucrose licking tests in the presence or absence of 200 μM ALA were performed in CD36-null mice. Interestingly, enhanced attraction for sucrose appeared to be independent of expression of the CD36 gene (Fig. 3C).
Fig. 3.
LCFA-induced sweet-taste sensitivity is independent of the CD36 gene expression. A: Immunolocalization of GPR120 and GLP-1 in mouse CVPs. Nuclei were stained by Hoechst's dye. Scale bar is 40 µm. B: Immunolocalization of GPR120 and CD36 in mouse CVPs. Scale bar is 40 µm. C: Short-term licking tests (1 min) in wild-type (Wt) and CD36-null mice subjected to a 62 mM sucrose solution alone or in the presence of 200 µM ALA. Mean ± SEM (n = 10). ** P < 0.01.
GPR120 is involved in the lipid-mediated release of GLP-1 by mouse CVPs
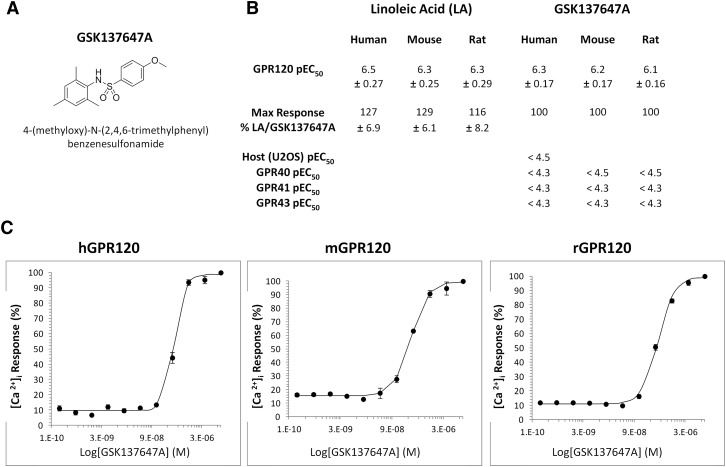
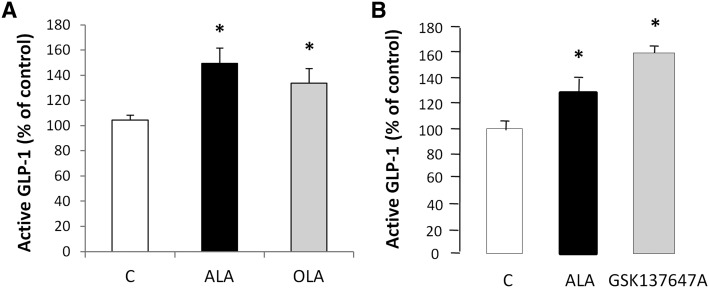
To assess the role of GPR120 in the lipid-mediated activation of GLP-1 signaling in TBCs, freshly isolated mouse CVPs were incubated for 2 h in an oxygenized medium containing anti-DPP4, to prevent GLP-1 degradation, and 200 µM LCFA or 50 µM GSK137647A. This new drug (Fig. 4A) was identified by screening a recombinant GPR120 receptor assay coupled with the calcium imaging as a potent and selective GPR120 agonist in various species (Fig. 4B, C). ALA, which is a potent activator of GPR120 in vitro (14), led to a small but significant rise in active GLP-1 levels in culture medium (2.08 pM ± 0.09 vs. 1.51 pM ± 0.16 in controls without ALA) (data not shown). Because GPR120 is thought to be preferentially a ω3 receptor (23), the effect of ALA on GLP-1 secretion was compared with OLA. As shown in Fig. 5A, addition of ALA and, to a lesser extent, of OLA increased the GLP-1 content of medium. Interestingly, addition of the specific GPR120 agonist GSK137647A fully reproduced the ALA effect, suggesting that GPR120 might be responsible for the LCFA-mediated release of GLP-1 by the mouse CVPs (Fig. 5B).
Fig. 4.
Identification of a selective GPR120 agonist. A: Formula of the specific GPR120 agonist GSK137647A. B: In vitro potency (pEC50) and efficacy (max response) of GPR120 agonists linoleic acid (LA) and GSK137647A for human, mouse, and rat GPR120. C: Calcium-response curves of the GPR120 agonist GSK137647A for human, mouse, and rat GPR120. For B and C, values are means of at least three experiments.
Fig. 5.
LCFA and the selective GPR120 agonist GSK137647A induce active GLP-1 release by mouse CVPs. A: GLP-1 release by freshly isolated CVPs incubated in the presence of 33 µM BSA alone (C, control) or with 200 µM ALA or 200 µM OLA. Each value corresponds to the GLP-1 released by a pool of CVP from three mice. Mean ± SEM (n = 3–4). * P < 0.05. B: GLP-1 release by freshly isolated CVPs incubated in the presence of 33 µM BSA alone (C, control) or with 200 µM ALA or 50 µM of the specific GPR120 agonist GSK137647A. Each value corresponds to the GLP-1 released by a pool of CVPs from three mice. Mean ± SEM (n = 3). * P < 0.05.
Disruption of the Glp1r gene affects the detection threshold for lipids in the oral cavity
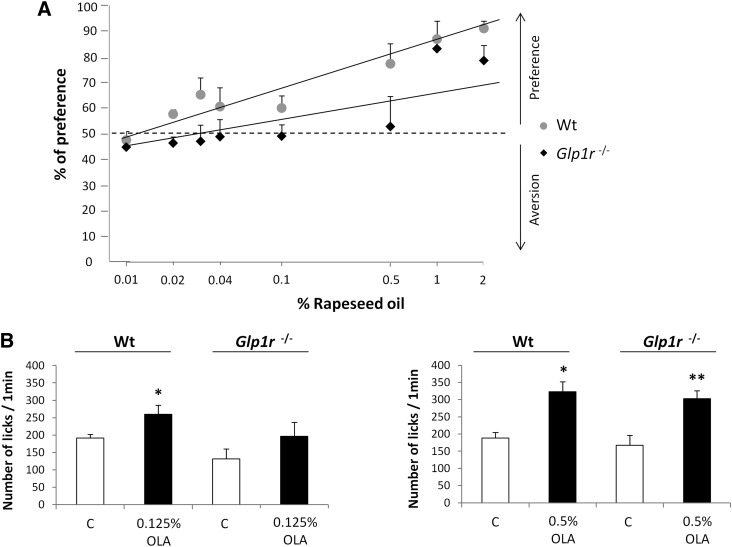
GLP-1 signaling in mouse taste buds modulates sweet-taste sensitivity (16). To determine whether such a regulatory system was also involved in the oro-sensory detection of dietary lipids, wild-type and Glp1r −/− mice were subjected to a set of long-term (12 h) two-bottle preference tests using increasing amounts of rapeseed oil, known to contain both OLA and ALA. Glp1r −/− mice were unable to detect low concentrations (from 0.02% to 0.5% w/v) of oil, contrary to control animals. However, Glp1r −/− mice responded to high-lipid solutions (≥1% w/v) similarly to wild-type mice (Fig. 6A), suggesting that GLP-1 signaling also plays a role in the fatty-taste sensitivity.
Fig. 6.
Disruption of the Glp1r gene affects the lipid detection threshold in the mouse. A: Long-term two-bottle preference tests (12 h) in wild-type (Wt) and Glp1r −/− mice subjected to control solution (0.3% xanthan gum in water) and growing levels of rapeseed oil (0.01–2%) in 0.3% xanthan gum. Xanthan gum was used to minimize textural cues and to emulsify rapeseed oil. Mean ± SEM (n = 10–12). Dotted line represents a lack of preference. B: Short-term licking tests (1 min) in wild-type (Wt) and Glp1r −/− mice subjected to a control solution (mineral oil) and 0.125 or 0.5% OLA in mineral oil. Mean ± SEM (n = 11–20). * P < 0.05; ** P < 0.01.
It has been previously demonstrated that GLP-1 in TBCs may act on local targets in a paracrine manner (16). To confirm that GLP-1-mediated modulation of the detection threshold for lipids took place in the oral cavity, mice were subjected to a computer-controlled lickometer using a brief access procedure (1 min) to minimize postingestive effects. As expected, Glp1r-deficient mice failed to detect small quantities of OLA (0.125% = 4.4 mM), but shared similar high licking responses for the 0.5% OLA solution (= 17.7 mM) compared with responses obtained with wild-type mice, suggesting a higher detection threshold for fat in Glp1r −/− mice (Fig. 6B).
Regulation of lingual CD36 is modulated by GLP-1 signaling
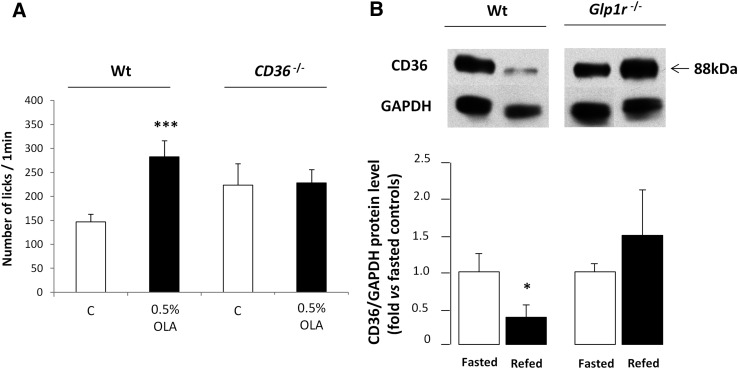
According to previous published data (3, 7, 8), lingual CD36 plays a significant role in the spontaneous preference for fat (Fig. 7A). Indeed, CD36 −/− mice failed to detect large quantities of OLA in a textured solution, in contrast to wild-type mice. It has recently been reported in mouse CVPs that CD36 is a lipid-sensitive receptor whom the downregulation during a meal might lead to progressive sensory-specific satiety for lipid-rich foods (8). The origin of this physiological regulation remains elusive. Because GLP-1 affects the detection threshold for lipids in oral cavity, it was tempting to hypothesize that CD36 expression levels in taste buds might be modulated by the GLP-1 signaling pathway during the postprandial period. To explore this assumption, mice fasted overnight were refed a standard laboratory chow for 2 h, and CD36 expression levels were assayed by Western blotting in wild-type and Glp1r −/− mouse CVPs. As expected, a 2-fold decrease in lingual CD36 protein levels were found in refed wild-type mice. By contrast, no change was detected in Glp1r-null mice (Fig. 7B).
Fig. 7.
Regulation of lingual CD36 is modulated by GLP-1 signaling. A: Short-term licking tests (1 min) in wild-type (Wt) and CD36 −/− mice subjected to a control solution (mineral oil) and 0.5% OLA in mineral oil. Mean ± SEM (n = 7). *** P < 0.001. B: CD36 protein levels determined by Western blotting in CVPs from wild-type (Wt) or Glp1r −/− mice fasted overnight or refed ad libitum with a standard laboratory chow for 2 h. Each point corresponds to a pool of total proteins from three to four mice. Mean ± SEM (n = 4). * P < 0.05.
DISCUSSION
The sense of taste informs the organism about the quality of the food before it is ingested, leading to stereotyped eating behavior (e.g., preference or aversion). Taste buds not only specifically detect tastants responsible for the basic taste modalities, but are also able to modulate gustatory perception in an autocrine or a paracrine manner. This last function, probably related to the body energy balance, is not yet fully understood. A better knowledge of physiological mechanisms modulating gustation is required to explain and, perhaps, predict the ingestive decision circuitry. It is a major health challenge, since it can be thought that a dysfunction of this regulatory system might lead to disturbances in eating behavior.
Subsets of taste bud cells synthesize and secrete gastrointestinal hormones known to be controlled by energy nutrients, including lipids, and involved in the regulation of food intake, as GLP-1. The concomitant presence of the receptor for GLP-1 (GLP-1R) in gustatory mucosa (16) suggests that this hormone is locally active and, thus, might directly affect the basic functions in mouse taste buds. Consistent with this assumption, it has been reported that GLP-1 signaling enhances sweet-taste sensitivity (16). Because GLP-1 was found to be colocalized with the sweet-taste receptor subunit T1R3 and α-gustducin in a subset of type II TBCs in mouse CVPs, it was concluded that GLP-1-positive cells are probably sweet sensitive (16). Data reported herein confirm that the knock-out of the Glp1r gene decreases the attraction for sucrose in the mouse. We show that it is not elicited by changes in the expression of key genes encoding for sweet-taste transduction molecules, including T1R2 and T1R3 taste receptors, glucose/galactose transporter SGLT-1, α-gustducin, phospholipase C-β2, the receptor for inositol 1,4,5-trisphosphate, or the transient receptor potential M5 channel. Therefore, further investigations will be required to elucidate the involved mechanism.
We also show that attraction for the sucrose solution was reinforced by the presence of ALA or OLA, suggesting the existence of an additive lipid-dependent regulatory system. Such an effect has also been found in the rat (24). In our experiments, this phenomenon occurred while the LCFA concentration used (i.e., 200 µM ≈ 0.005%) was undetectable by the mouse when it was presented alone during licking tests. It is consistent with the results of Yoneda et al. (25) showing that mice are unable to properly detect 0.01% LCFA (ALA, OLA, or linoleic acid) during short-term behavioral tests. Therefore, the change in perceived intensity of sweet taste may not be attributed to addition of sucrose and LCFA effects. Interestingly, we have found that salient impact of ALA or OLA was abolished in Glp1r-null mice, bringing the first demonstration that dietary lipids affect the perception threshold of sucrose via the GLP-1 signaling pathway. Because CD36 and GPR120 are lipid sensors expressed in the gustatory epithelium, their implication in this regulation was possible. A role of CD36 seems unlikely because CD36-null mice display a similar attraction for fat-sweet mixture as control mice during short-term licking tests. By contrast, several observations are in favor of an implication of GPR120. First, GPR120 and GLP-1 are found to be colocalized in subsets of TBCs in mouse CVPs. This observation correlated quite well with the fact that GPR120 (11, 26) and GLP-1 (16) are mainly expressed in type II TBCs in the mouse. Second, using an original ex-vivo approach maintaining the morphological and functional integrity of taste buds, we found that LCFAs lead to GLP-1 release by mouse CVPs. ALA, which is known to be a potent activator of the GPR120 receptor (14), appears to be a stronger GLP-1 secretagogue than OLA. Third, use of the specific GPR120 agonist GSK137647A reproduces the secretion of active GLP-1 mediated by LCFAs, especially ALA. Because LCFA, GPR120, and GLP-1 are functionally linked in the entero-endocrine L cells in the gut (14), these results suggest that the activation of lingual GPR120 by LCFAs might induce the release of GLP-1 by TBCs, increasing the attraction for sucrose. This original function for the sense of taste does not exclude a direct implication of GPR120 in the oro-sensory perception of dietary lipids, as proposed by Damak and collaborators (11). Indeed, it is thought that the glucose sensor T1R3 plays a role in both sweet-taste perception and hormone secretion (27, 28).
GLP-1 signaling also appears to be involved in the oro-sensory perception of dietary fat. Invalidation of the Glp1r gene leads to a significant reduction of sensitivity to rapeseed oil in long term (12 h) two-bottle preference tests. Although the preference threshold for oil was 0.02% in age-matched wild-type controls, it was up to 0.5% in Glp1r −/− mice. Mechanisms responsible for this eating behavior mainly take place in the oral cavity. Indeed, similar data were reproduced when wild-type controls and Glp1r-null mice were tested with a computer-controlled lickometer using a brief-access procedure (1 min) known to minimize postingestive cues. We have recently reported that the CD36 protein level in mouse CVPs is subjected to a short-term down-regulation during food intake, contrary to GPR120 (8). It is a very sensitive regulation strictly dependent on the presence of lipid in the diet. Interestingly, direct oil deposition onto the tongue is sufficient to trigger the decrease of CD36 protein in CVP, confirming a local regulation (8). However, the underlying mechanism(s) remain poorly understood. Data reported here demonstrate that GLP-1 signaling plays a significant role in this regulation. Indeed, no decrease in CD36 protein level was observed in CVPs from refed Glp1r-null mice, contrary to wild-type animals. As reported for numerous surface receptors, this negative feedback might constitute a desensitization system during persistent exposure to dietary lipids. Consistent with this assumption, the postprandial downregulation of CD36 in CVPs seems to be sufficient to affect the motivation for fat during a meal, initially high and then gradually decreasing secondary to food intake (8). Therefore, it is tempting to speculate that the lower attraction for fat found in Glp1r −/− mice is related to a dysfunction in the GLP-1 regulatory loop controlling CD36 protein level in CVPs.
The existence of physiological links between oro-sensory perception of lipids, selection of energy-dense foods, and obesity risk is gradually emerging. An inverse correlation between peripheral gustatory sensitivity to PUFAs and preference for lipid-rich foods has been reported in rats (29). In healthy humans, hypersensitivity to lipids seems to be associated with lower energy consumption, fat intake, and body mass index (30). This phenomenon might be related to lipid sensors found in taste buds. The fact that a common genetic polymorphism leading to the reduction of CD36 gene expression produces an attenuation of oro-sensory sensitivity for fat in humans (10) is consistent with this assumption. Studies have also shown synergy between oral fat sensitivity and attraction for sucrose in rodents. The ability of unsaturated LCFAs to inhibit the delayed-rectifying K+ channels in rat TBCs has been the first mechanism identified (31). Indeed, lipid-mediated cellular depolarization added to that triggered by sucrose should increase the sweet-taste perception (24). Present data highlight an alternative mechanism suggesting the involvement of GLP-1 signaling. The relative physiological importance of these two mechanisms remains to be established.
In conclusion, our data support the existence of a functional link between unsaturated LCFAs including ω3, GPR120, and the secretion of GLP-1 by mouse CVP. This system, reminiscent of what happens in the entero-endocrine L cells, modulates the sensitivity thresholds for energy-dense nutrients (sucrose and LCFA). For lipids, it appears to be implicated in a regulatory loop targeting CD36. Because change of CD36 protein level in CVPs modulates the motivation for fat during a meal (8), this LCFA/GPR120/GLP-1 axis might play a significant role in the sensory-specific satiety for lipids. Therefore, it is tempting to speculate that a dysfunction of this regulatory loop might lead to an increased motivation to obtain high-fat foods. A better understanding of the molecular mechanisms responsible for lipid sensing in the gustatory papillae and of their physiological impact on eating behavior should allow the development of new therapeutic and nutritional strategies for mitigating excess food intake and limiting obesity risk.
Footnotes
Abbreviations:
- ALA
- α-linolenic acid
- CVPs
- circumvallate papillae
- DPP4
- dipeptidyl peptidase 4
- FFAR1
- free fatty acid receptor 1
- GLP-1
- glucagon-like peptide-1
- GLP-1R
- GLP-1 receptor
- LCFA
- long-chain FA
- OLA
- oleic acid
- TBC
- taste bud cell
This work was supported by the Burgundy Council and Centre National Interprofessionnel de l'Economie Laitière (CNIEL) (HumanFATaste program to P.B.). C.M. and M.C. are fellows of HumanFATaste program. D.J.D. is supported by a Canada Research Chair in Regulatory Peptides, a Banting and Best Diabetes Centre Novo Nordisk Chair in Incretin Biology, and by Operating Grant OG-3-11-3270-DD from the Canadian Diabetes Association.
REFERENCES
- 1.Fukuwatari T., Shibata K., Iguchi K., Saeki T., Iwata A., Tani K., Sugimoto E., Fushiki T. 2003. Role of gustation in the recognition of oleate and triolein in anosmic rats. Physiol. Behav. 78: 579–583 [DOI] [PubMed] [Google Scholar]
- 2.Chale-Rush A., Burgess J. R., Mattes R. D. 2007. Evidence for human orosensory (taste?) sensitivity to free fatty acids. Chem. Senses. 32: 423–431 [DOI] [PubMed] [Google Scholar]
- 3.Laugerette F., Passilly-Degrace P., Patris B., Niot I., Febbraio M., Montmayeur J. P., Besnard P. 2005. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J. Clin. Invest. 115: 3177–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baillie A. G., Coburn C. T., Abumrad N. A. 1996. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homologue. J. Membr. Biol. 153: 75–81 [DOI] [PubMed] [Google Scholar]
- 5.Fukuwatari T., Kawada T., Tsuruta M., Hiraoka T., Iwanaga T., Sugimoto E., Fushiki T. 1997. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 414: 461–464 [DOI] [PubMed] [Google Scholar]
- 6.Simons P. J., Kummer J. A., Luiken J. J., Boon L. 2011. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 113: 839–843 [DOI] [PubMed] [Google Scholar]
- 7.Sclafani A., Ackroff K., Abumrad N. A. 2007. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293: R1823–R1832 [DOI] [PubMed] [Google Scholar]
- 8.Martin C., Passilly-Degrace P., Gaillard D., Merlin J. F., Chevrot M., Besnard P. 2011. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS ONE. 6: e24014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Love-Gregory L., Sherva R., Schappe T., Qi J. S., McCrea J., Klein S., Connelly M. A., Abumrad N. A. 2011. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum. Mol. Genet. 20: 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepino M. Y., Love-Gregory L., Klein S., Abumrad N. A. 2012. The fatty acid translocase gene, CD36, and lingual lipase influence oral sensitivity to fat in obese subjects. J. Lipid Res. 53: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartoni C., Yasumatsu K., Ohkuri T., Shigemura N., Yoshida R., Godinot N., le Coutre J., Ninomiya Y., Damak S. 2010. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 30: 8376–8382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumura S., Mizushige T., Yoneda T., Iwanaga T., Tsuzuki S., Inoue K., Fushiki T. 2007. GPR expression in the rat taste bud relating to fatty acid sensing. Biomed. Res. 28: 49–55 [DOI] [PubMed] [Google Scholar]
- 13.Galindo M. M., Voigt N., Stein J., van Lengerich J., Raguse J. D., Hofmann T., Meyerhof W., Behrens M. 2012. G protein-coupled receptors in human fat taste perception. Chem. Senses. 37: 123–139 [DOI] [PubMed] [Google Scholar]
- 14.Hirasawa A., Tsumaya K., Awaji T., Katsuma S., Adachi T., Yamada M., Sugimoto Y., Miyazaki S., Tsujimoto G. 2005. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat. Med. 11: 90–94 [DOI] [PubMed] [Google Scholar]
- 15.Baggio L. L., Drucker D. J. 2007. Biology of incretins: GLP-1 and GIP. Gastroenterology. 132: 2131–2157 [DOI] [PubMed] [Google Scholar]
- 16.Shin Y. K., Martin B., Golden E., Dotson C. D., Maudsley S., Kim W., Jang H. J., Mattson M. P., Drucker D. J., Egan J. M., et al. 2008. Modulation of taste sensitivity by GLP-1 signaling. J. Neurochem. 106: 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Febbraio M., Abumrad N. A., Hajjar D. P., Sharma K., Cheng W., Pearce S. F., Silverstein R. L. 1999. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J. Biol. Chem. 274: 19055–19062 [DOI] [PubMed] [Google Scholar]
- 18.Scrocchi L. A., Brown T. J., MaClusky N., Brubaker P. L., Auerbach A. B., Joyner A. L., Drucker D. J. 1996. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 2: 1254–1258 [DOI] [PubMed] [Google Scholar]
- 19.Condreay J. P., Witherspoon S. M., Clay W. C., Kost T. A. 1999. Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. Proc. Natl. Acad. Sci. USA. 96: 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 25: 402–408 [DOI] [PubMed] [Google Scholar]
- 21.Jang H. J., Kokrashvili Z., Theodorakis M. J., Carlson O. D., Kim B. J., Zhou J., Kim H. H., Xu X., Chan S. L., Juhaszova M., et al. 2007. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. USA. 104: 15069–15074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng X. H., Liu X. M., Zhou L. H., Wang J., Liu G. D. 2008. Expression of glucagon-like peptide-1 in the taste buds of rat circumvallate papillae. Acta Histochem. 110: 151–154 [DOI] [PubMed] [Google Scholar]
- 23.Oh D. Y., Talukdar S., Bae E. J., Imamura T., Morinaga H., Fan W., Li P., Lu W. J., Watkins S. M., Olefsky J. M. 2010. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 142: 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittman D. W., Labban C. E., Anderson A. A., O'Connor H. E. 2006. Linoleic and oleic acids alter the licking responses to sweet, salt, sour, and bitter tastants in rats. Chem. Senses. 31: 835–843 [DOI] [PubMed] [Google Scholar]
- 25.Yoneda T., Saitou K., Asano H., Mizushige T., Matsumura S., Eguchi A., Manabe Y., Tsuzuki S., Inoue K., Fushiki T. 2009. Assessing palatability of long-chain fatty acids from the licking behavior of BALB/c mice. Physiol. Behav. 96: 735–741 [DOI] [PubMed] [Google Scholar]
- 26.Matsumura S., Eguchi A., Mizushige T., Kitabayashi N., Tsuzuki S., Inoue K., Fushiki T. 2009. Colocalization of GPR120 with phospholipase-Cbeta2 and alpha-gustducin in the taste bud cells in mice. Neurosci. Lett. 450: 186–190 [DOI] [PubMed] [Google Scholar]
- 27.Damak S., Rong M., Yasumatsu K., Kokrashvili Z., Varadarajan V., Zou S., Jiang P., Ninomiya Y., Margolskee R. F. 2003. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 301: 850–853 [DOI] [PubMed] [Google Scholar]
- 28.Kokrashvili Z., Mosinger B., Margolskee R. F. 2009. T1R3 and alpha gustducin in gut regulate secretion of glucagon-like peptide 1. Ann. N. Y. Acad. Sci. 1170: 91–94 [DOI] [PubMed] [Google Scholar]
- 29.Gilbertson T. A., Liu L., York D. A., Bray G. A. 1998. Dietary fat preferences are inversely correlated with peripheral gustatory fatty acid sensitivity. Ann. N. Y. Acad. Sci. 855: 165–168 [DOI] [PubMed] [Google Scholar]
- 30.Stewart J. E., Feinle-Bisset C., Golding M., Delahunty C., Clifton P. M., Keast R. S. 2010. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br. J. Nutr. 104: 145–152 [DOI] [PubMed] [Google Scholar]
- 31.Gilbertson T. A., Fontenot D. T., Liu L., Zhang H., Monroe W. T. 1997. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am. J. Physiol. 272: C1203–C1210 [DOI] [PubMed] [Google Scholar]