Abstract
Lipoic acid (LA) is a naturally occurring compound with beneficial effects on obesity. The aim of this study was to evaluate its effects on lipolysis in 3T3-L1 adipocytes and the mechanisms involved. Our results revealed that LA induced a dose- and time-dependent lipolytic action, which was reversed by pretreatment with the c-Jun N-terminal kinase inhibitor SP600125, the PKA inhibitor H89, and the AMP-activated protein kinase activator AICAR. In contrast, the PI3K/Akt inhibitor LY294002 and the PDE3B antagonist cilostamide enhanced LA-induced lipolysis. LA treatment for 1 h did not modify total protein content of hormone-sensitive lipase (HSL) but significantly increased the phosphorylation of HSL at Ser563 and at Ser660, which was reversed by H89. LA treatment also induced a marked increase in PKA-mediated perilipin phosphorylation. LA did not significantly modify the protein levels of adipose triglyceride lipase or its activator comparative gene identification 58 (CGI-58) and inhibitor G(0)/G(1) switch gene 2 (G0S2). Furthermore, LA caused a significant inhibition of adipose-specific phospholipase A2 (AdPLA) protein and mRNA levels in parallel with a decrease in the amount of prostaglandin E2 released and an increase in cAMP content. Together, these data suggest that the lipolytic actions of LA are mainly mediated by phosphorylation of HSL through cAMP-mediated activation of protein kinase A probably through the inhibition of AdPLA and prostaglandin E2.
Keywords: hormone-sensitive lipase, adipose triglyceride lipase, adipose-specific phospholipase A2, prostaglandin E2
Lipoic acid (LA), or 1,2-dithiolane-3-pentaenoic acid, is a naturally occurring compound that contains two thiol groups with diverse beneficial effects on health. The biological effects of LA were primarily associated with its antioxidant properties. In fact, LA is able to directly scavenge reactive oxygen species (ROS) and regenerate endogenous antioxidants, such as glutathione and vitamins E and C (1, 2). Moreover, several studies have described potential beneficial effects of LA on obesity and its associated comorbidities, such as insulin resistance, type 2 diabetes, or fatty liver diseases. Thus, in rodents LA has been shown to cause profound weight loss by reducing food intake and enhancing energy expenditure (3) as well as by inducing a reduction on intestinal sugar absorption (4). More recently, two clinical trials in humans reported that LA caused significant reductions of body weight, body mass index, blood pressure, and abdominal circumference in obese subjects (5, 6). LA also improved insulin sensitivity and plasma lipid profile possibly through amelioration of oxidative stress and chronic inflammatory status in obese patients with impaired glucose tolerance (7). Previous studies have provided strong evidence that LA is able to deeply affect adipose tissue development and function by the inhibition of adipogenesis (8), the regulation of the secretion of several adipokines such as leptin (9) and apelin (10), and by the promotion of mitochondrial biogenesis (11).
In this context, previous studies suggested that LA seems to stimulate the lipolytic response in an in vitro model of broiler chicken adipocytes (12). However, the molecular mechanisms that mediate these effects remain unclear. Lipolysis is a complex process that is highly regulated and involves the coordinated participation of several lipases and lipid droplet (LD) proteins (13). Thus, the lipolytic process occurs through the consecutive action of three lipases: adipose triglyceride lipase (ATGL/desnutrin), hormone-sensitive lipase (HSL), and monoacylglycerol lipase (14). ATGL exhibits high substrate specificity for triacyl glycerol (TAG) (15). Lipase activity of ATGL largely depends on its coactivation by comparative gene identification 58 (CGI-58), whereas G(0)/G(1) switch gene 2 (G0S2) acts as an inhibitor of ATGL activity and ATGL-mediated lipolysis (16). Recently it has been shown that ATGL is phosphorylated by AMPK at Ser406, increasing TAG hydrolase activity (17).
The activity of HSL is well known to be regulated post-transcriptionally by reversible phosphorylation. In murine adipocytes, PKA phosphorylates HSL at several serine residues (563, 659, and 660), resulting in increased translocation of HSL to the lipid droplet surface and increased lipolytic activity (18). Furthermore, AMP-activated protein kinase (AMPK) phosphorylates HSL at Ser565, which prevents phosphorylation induced by PKA (19, 20). Activation of phosphodiesterase 3B (PDE3B) via the Akt-mediated phosphorylation of Ser273 attenuates PKA activity and thereby HSL activation and lipolysis (21, 22). In addition to the PKA-mediated phosphorylation, HSL may be phosphorylated by other kinases, such as extracellular signal-regulated kinase (ERK1/2), which activates HSL by phosphorylation on Ser600 (23). It has been suggested that c-Jun N-terminal kinase (JNK) could play a role in the regulation of lipolysis based on the fact that silencing of Jnk1 and Jnk2 accelerates basal lipolysis in mouse adipocytes (24).
Protein trafficking and specific protein-protein interactions at the surface of lipid droplets are also key factors in the regulation of lipolysis. Perilipin A is a lipid droplet scaffold protein that plays a central role in orchestrating interactions among lipolytic effector proteins (25). Under basal conditions, perilipin restricts the access of cytosolic lipases to LD, thereby maintaining a low rate of basal lipolysis. However, the phosphorylation of perilipin by PKA results in perilipin conformational changes that expose LD stores and facilitates the translocation of phosphorylated HSL to the LD, thereby increasing the lipolytic process (26).
Recently, a novel intracellular adipose-specific phospholipase A2 (AdPLA) has been identified (27). It was suggested that AdPLA could be another mediator in the regulation of lipolysis by generating arachidonic acid for the production of prostaglandins (28). In fact, AdPLA-null mice exhibited reduced adipose tissue prostaglandin E2 (PGE2) production and augmented HSL-phosphorylation leading to increased lipolysis, supporting that AdPLA is a major regulator of adipocyte lipolysis by regulating PGE2 abundance (28).
Previous studies have demonstrated the ability of LA to modulate ERK, JNK, and Akt signaling pathways (8, 9, 29) as well as AMPK activity (30, 31) in different cell types. Moreover, LA stimulates cAMP production in purified human NK cells (32) and modulates the production of PGE2 in osteoblasts (33).
Based on these previous findings, we hypothesized that LA could be a key regulator of lipolysis in mammals through modulation of lipases and lipid droplet proteins activities. Therefore, the aim of this study was to characterize the lipolytic action of LA in cultured adipocytes and to elucidate the molecular mechanisms and signaling pathways involved.
MATERIALS AND METHODS
Cell culture and differentiation of 3T3-L1 cells
Murine 3T3-L1 cells (American Type Culture Collection, Rockville, MD) were cultured in DMEM containing 25 mM glucose, 10% calf bovine serum (Invitrogen, Carlsbad, CA), and 1% penicillin and streptomycin (Gibco, Invitrogen Corp.) and were maintained in an incubator set up to 37°C and 5% of CO2. At confluence, preadipocytes were induced to differentiate into adipocytes by culturing them for 48 h in DMEM containing 10% FBS (Invitrogen) and antibiotics and supplemented with dexamethasone (1 mM; Sigma, St. Louis, MO), isobutylmethylxantine (0.5 mM; Sigma), and insulin (10 mg/ml; Sigma). Then, cells were cultured with 10% FBS and insulin for 48 h. After that, media was replaced with 10% FBS in DMEM and antibiotics but without insulin, and this media was changed every 2 days up to day 8 after confluence, when cells were completely differentiated to adipocytes (34, 35).
Treatments
LA (α-lipoic acid; Sigma) was dissolved in ethanol. The inhibitors SP600125 (SP) (Calbiochem, San Diego, CA), PD98059 (PD) (Sigma), H89 (Santa Cruz Biotechnology, Santa Cruz, CA), LY294002 (LY) (Sigma), Cilostamide (CILO) (Sigma), and L798106 (Tocris, Ellisville, MO) were dissolved in DMSO. The AMPK activator AICAR (Sigma) was dissolved in ultrapurified water. All compounds were prepared as 1,000× stock solutions and added to the culture medium. Control cells were treated with the same amount of the corresponding vehicle.
Before the addition of the appropriate treatments, fully differentiated 3T3-L1 adipocytes were serum starved for at least 4 h by switching to DMEM containing 2–2.5% fatty acid free-BSA or to DMEM with 1% FBS and then treated with or without LA (1–500 µM) during different time intervals (30 min to 24 h). To analyze the signaling pathways involved in LA actions, adipocytes were preincubated for 1 h with the selective inhibitors or activators (20 µM SP, 50 µM PD, 1 µM H89, 50 µM LY, 2 µM CILO, 10 µM L798106, and 2 mmol/l AICAR).
Lipolysis measurement
Lipolysis was evaluated by measuring the amount of glycerol and free fatty acids (FFAs) released to the media. Glycerol was determined after 1 to 24 h of LA treatment using an autoanalyser following the manufacturer instructions (Cobas-Mira; Roche Diagnostics, Basel, Switzerland). Free fatty acids were quantified after 3 h of LA treatment by using the Lipolysis Assay KIT for Free Fatty Acids Detection (Zen-Bio Inc, Research Triangle Park, NC) according to the manufacturer's instructions.
Analysis of mRNA levels
Total RNA was extracted from 3T3-L1 cells using TRIzol® reagent (Invitrogen) according to the manufacturer's instructions. RNA concentrations and quality were measured by Nanodrop Spectrophotometer ND1000 (Thermo Scientific, Wilminton, DE). RNA was then incubated with the RNase-free kit DNase (Ambion, Austin, TX) for 30 min at 37°C. RNA (2 µg) was reverse-transcribed to cDNA using Moloney Murine Leukaemia Virus reverse transcriptase (Invitrogen). For the real-time quantitative PCR analysis, 4.5 µl of 1/100 or 1/50 dilution of cDNA per reaction were used in a final reaction volume of 10 µl.
ATGL, HSL, perilipin, AdPLA, PPARγ, CCAAT/enhancer-binding protein alpha (C/EBPα), and CCAAT/enhancer-binding protein beta (C/EBPβ) mRNA levels were determined using predesigned Taqman® Assays-on-Demand (Applied Biosystems, Foster City, CA). Taqman Universal Master Mix was also provided by Applied Biosystems. The reaction conditions were followed according to the manufacturer's instructions.
Amplification and detection of specific products were performed using the ABI PRISM 7900HT Fast System Sequence Detection System (Applied Biosystems).
All mRNA levels were normalized by the housekeeping gene β-actin obtained from Applied Biosystems. Samples were analyzed in duplicate. Ct values (the cycle where the emitted fluorescence signal is significantly above background levels and is inversely proportional to the initial template copy number) were generated by the ABI software. Finally, the relative expression level of each gene was calculated as 2−ΔΔCt (36).
Western blot analysis
Western blot analyses were performed in adipocytes 8 days after differentiation. Cells were incubated in serum-free DMEM overnight and then with or without the appropriate treatment. Lysates were obtained by the addition of a buffer containing 2 mM Tris HCl (pH 8), 137 mM NaCl, 2 mM EDTA, 1% protease inhibitor cocktail 1 (Sigma), 1 mM sodium orthovanadate, and 1 mM PMSF. Protein extracts were collected after sample centrifugation. Proteins were quantified with the BCA method according to the supplier's instructions (Pierce-Thermo Scientific, Rockford, IL). Total proteins were resolved in SDS-PAGE minigels and electroblotted onto polyvinylidene difluoride membranes (GE Healthcare Europe GmbH, Barcelona, Spain). The membranes were blocked and incubated with specific antibodies against ATGL, HSL, HSL phospho Ser565, HSL phospho Ser563, HSL phospho Ser660, perilipin, phospho (ser/thr) PKA substrate (p-perilipin), AMPK, AMPK phospho Thr172, AKT, AKT phospho Ser473, MAPK (ERK1/2), ERK1/2 phospho Thr202/204, JNK, and JNK phospho Thr183/Tyr185 (Cell Signaling Technologies, Beverly, MA); AdPLA (Cayman Chemical, Ann Arbor, MI); and CGI-58, G0S2 (Santa Cruz), and Actin (from Sigma). Secondary antibody was HRP goat anti-rabbit IgG-HRP (Bio Rad Laboratories). The immunoreactive proteins were detected with enhanced chemiluminescence (Pierce Biotechnology, Rockford, IL). Band intensities were quantified using a GS-800 calibrated densitometer (Bio Rad Laboratories).
Fatty acid oxidation determination
Fatty acid oxidation to acid-soluble metabolites was measured with radiolabeled 14C- palmitate (Perkin Elmer, Boston, MA) in mature 3T3-L1 adipocytes as previously described (37). Acid-soluble metabolites were extracted by addition of 1 ml cold 1 M HClO4 (Panreac, Barcelona, Spain). After centrifugation (10 min, 1,800 g), radioactivity in the supernatant was measured by scintillation counting by using a Wallac 1409 liquid scintillation counter (EG and G Co., Turku, Finland). Protein content in parallel cultures of vehicle- and LA-treated cells was analyzed using a BCA method.
ELISA assays
PGE2 concentration in the media was determined after 4, 8, and 24 h of LA (250 µM) treatment by using a PGE2 Enzyme Immunoassay kit (Arbor Assays, Ann Arbor, MI). The amount of intracellular cAMP was quantified after 1 and 24 h of LA (250 µM) treatment by using the cAMP Direct EIA kit (Arbor Assays).
Data analysis
Data are expressed as mean ± standard error (SE). Differences were set up as statistically significant at P < 0.05. Comparisons between the values for different variables were analyzed by one-way ANOVA followed by Bonferroni post hoc tests or by Student's t-test or Mann-Whitney U test once the normality with the Kolmogorov-Smirnoff and Shapiro-Wilk tests was screened. SPSS 19.0 version for Windows (SPSS, Chicago, IL) and GraphPad Prism 5.0 (Graph-Pad Software Inc. San Diego, CA) were used for statistical analysis.
RESULTS
Effects of LA on lipolysis in 3T3-L1 adipocytes
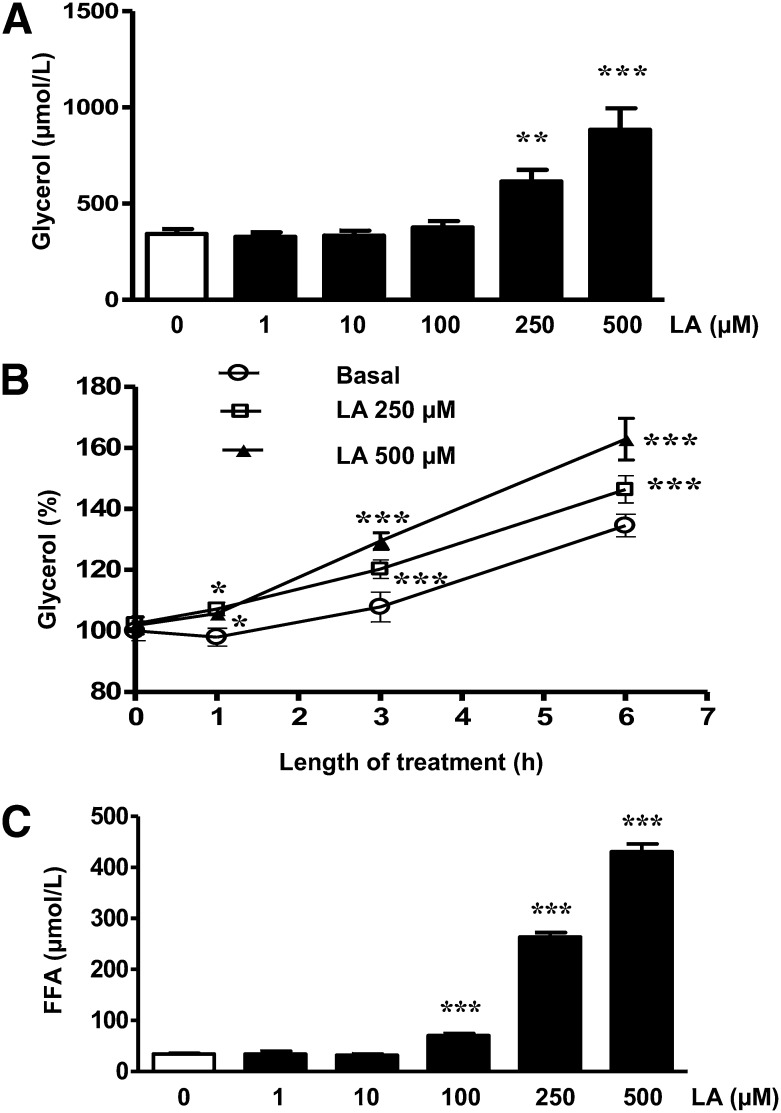
A dose-dependent significant increase in the amount of glycerol released into the media was observed in those adipocytes treated with LA (250–500 µM; P < 0.01 and P < 0.001) for 24 h (Fig. 1A). Moreover, the lipolytic effect of LA was time dependent. Thus, the significant increase in glycerol release was observed after 1 h of treatment (P < 0.05), and it became more prominent after 3 and 6 h of treatment (250–500 µM; P < 0.001) (Fig. 1B). Furthermore, LA induced a concentration-dependent increase in the amount of FFAs released after 3 h treatment (100–500 µM; P < 0.001) (Fig. 1C). We also tested the effects of LA on isoproterenol-induced lipolysis and the data revealed that LA did not have any additional effect on the lipolytic effect of isoproterenol (supplementary Fig. I). To rule out if the lipolytic effect of LA was caused by a global down-regulation of adipocyte differentiation markers, PPARγ, C/EBPα, and C/EBPβ gene expression levels were analyzed after 24 h of LA (250 µM) treatment, and no differences were observed when compared with control cells (supplementary Fig. II). Moreover, to test if the lipolytic actions of LA were shared by other molecules with antioxidant properties, the effects of vitamin C, resveratrol, N-acetyl cysteine (NAC), and butylhydroxyanisole (BHA) on glycerol release were evaluated. The data showed that, at the concentration tested, resveratrol and BHA, but not NAC or vitamin C, were able to stimulate lipolysis in 3T3-L1 adipocytes (supplementary Fig. III).
Fig. 1.
LA stimulates lipolysis in 3T3-L1 adipocytes. Mature 3T3-L1 adipocytes were treated with LA (0–500 μM) for the indicated times (1, 3, 6, or 24 h). A: Lipolysis was assessed by the amount of glycerol released into media in adipocytes treated for 24 h. B: Time-dependent effects of LA (250 and 500 μM) on glycerol release. C: Concentration-dependent effects of LA on FFA release in adipocytes treated for 3 h. Data are expressed as mean ± SE of six independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. control (vehicle-treated cells).
Signaling pathways involved in the lipolytic actions of LA
To evaluate the ability of LA to modify some signaling pathways involved in the regulation of lipolysis, the phosphorylation levels of JNK, ERK1/2, AMPK, and PI3K/AKT were analyzed after short-term (30 min to 1 h) and long-term treatment (24 h).
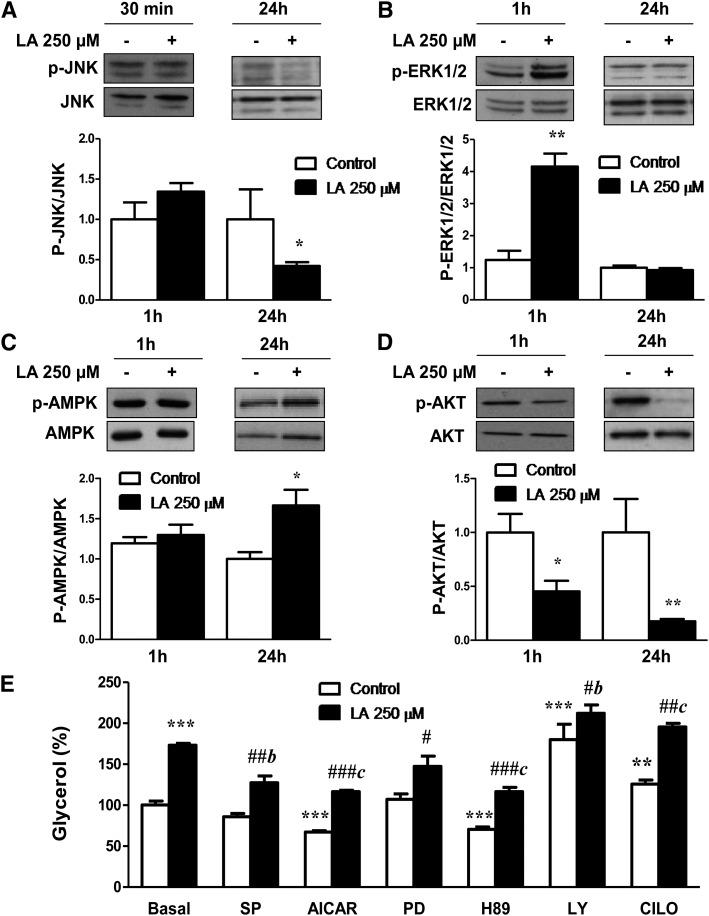
No effects were observed in JNK Thr183/Tyr185 phosphorylation after 30 min of treatment with LA (250 µM), but a significant (P < 0.05) reduction of JNK phosphorylation was observed after 24 h of treatment (Fig. 2A). In contrast, the significant increase on ERK1/2 Thr202/Tyr204 phosphorylation (P < 0.01) induced by LA (250 µM) after 1 h was reversed to basal levels after 24 h of treatment (Fig. 2B). Moreover, the stimulatory effect (P < 0.05) of LA on AMPK Thr172 phosphorylation was only observed in long-term treated (24 h) adipocytes (Fig. 2C). Regarding the PI3K/AKT signaling pathway, LA (250 µM) treatment caused a significant inhibition of AKT Ser473 phosphorylation after short-term (P < 0.05) and long-term (P < 0.01) treatments (Fig. 2D).
Fig. 2.
Signaling pathways involved in the lipolytic effects of LA. A–D: Effects of LA on the phosphorylation of JNK (A), ERK1/2 (B), AMPK (C), and PI3K/AKT (D). Band intensities for each phosphorylated species were normalized to their respective total fractions. E: Effects of LA treatment for 24 h on glycerol release in the presence or absence of the JNK inhibitor SP600125 (SP), the AMPK activator AICAR, the ERK1/2 inhibitor PD98059 (PD), the PKA inhibitor H89, the PI3K/AKT inhibitor LY294002 (LY), and the PDE3B inhibitor Cilostamide. Data are expressed as mean ± SE of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. basal control (vehicle-treated cells). #P < 0.05, ##P <0 .01, and ###P < 0.001 vs. respective control. bP < 0.01 and cP < 0.001 vs. basal LA-treated adipocytes.
For a better understanding of the potential signaling pathways involved in the lipolytic action of LA, the effects of specific kinase inhibitors or activators on LA-induced glycerol release were studied. Basal lipolysis was significantly enhanced by the PI3K/Akt inhibitor LY294002 (P < 0.001) and the PDE3B antagonist Cilostamide (P < 0.01) and decreased by the PKA inhibitor H89 and the AMPK activator AICAR (P < 0.001). Our data revealed that the lipolytic actions of LA were reversed by pretreatment with the JNK inhibitor SP600125 (P < 0.01), the PKA inhibitor H89, and the AMPK activator AICAR (P < 0.001). The stimulatory effects of LA on lipolysis were significantly enhanced (P < 0.01 and P < 0.001) in adipocytes treated with the PI3K/AKT inhibitor LY294002 and the PDE3B antagonist Cilostamide (Fig. 2E).
Effects of LA treatment on HSL, ATGL, Perilipin, CGI-58, and G0S2 levels
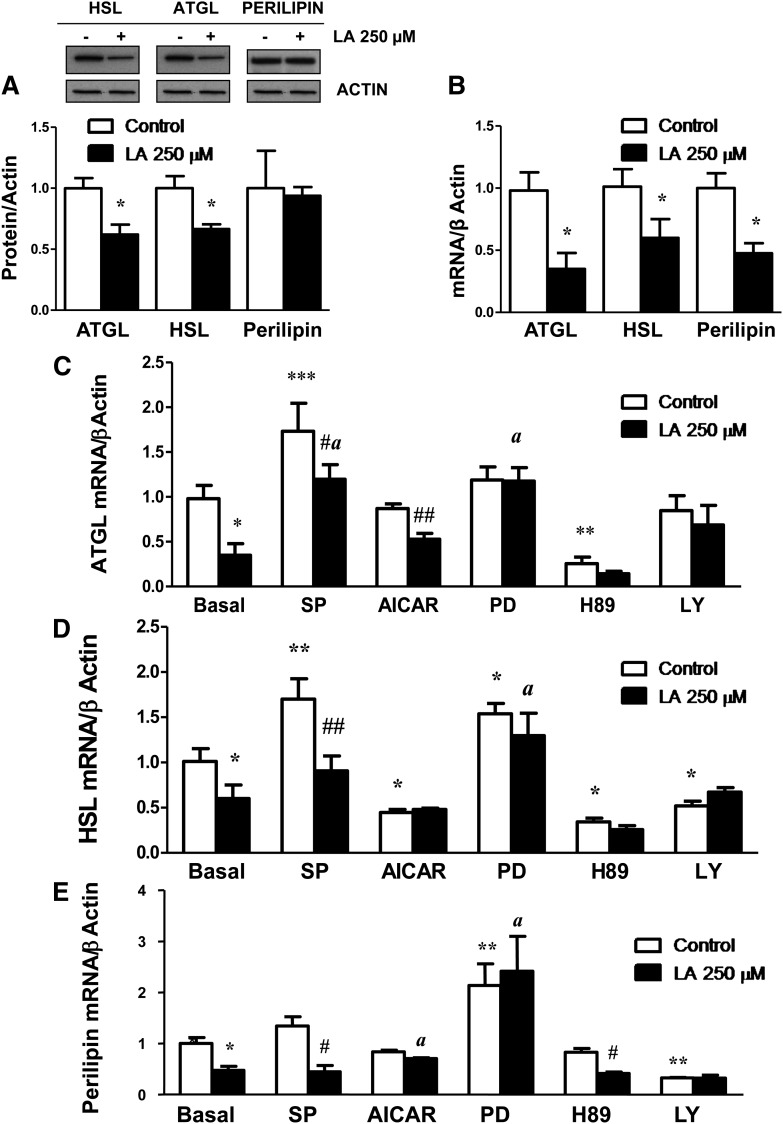
In contrast to the LA lipolytic actions, a significant (P < 0.05) decrease on total protein content of the two main lipases, ATGL and HSL, was observed in LA-treated (250 µM for 24 h) adipocytes (Fig. 3A). Accordingly, gene expression levels of ATGL and HSL were also significantly down-regulated (P < 0.05) by LA treatment for 24 h (Fig. 3B). Perilipin mRNA levels were also reduced in LA-treated adipocytes, whereas no changes in perilipin protein content were observed (Fig. 3A, B).
Fig. 3.
Long-term LA treatment down-regulates total HSL, ATGL, and perilipin transcripts. The effects of LA (250 μM) on total ATGL, HSL, and perilipin protein (A) and mRNA (B) levels were assessed in 3T3-L1 adipocytes after 24 h of treatment. C–E: Effects of the JNK inhibitor SP600125 (SP), the ERK1/2 inhibitor PD98059 (PD), the PKA inhibitor H89, the AMPK activator AICAR, and the PI3K/AKT inhibitor LY294002 (LY) on ATGL (C), HSL (D), and Perilipin mRNA (E) levels in control and LA-treated 3T3-L1 adipocytes. Data are expressed as mean ± SE of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.01 vs. basal control (vehicle-treated cells). #P < 0.05 and ##P < 0.01 vs. respective control. aP < 0.05 vs. basal LA-treated adipocytes.
The inhibitory actions of LA treatment on ATGL gene expression were not observed in the presence of the JNK inhibitor SP600125 and the ERK1/2 inhibitor PD98059 (P < 0.05) (Fig. 3C). Similarly, the inhibition of the ERK1/2 signaling pathway was able to reverse the down-regulation of HSL and perilipin gene expression observed after LA treatment (P < 0.05) (Fig. 3D, E).
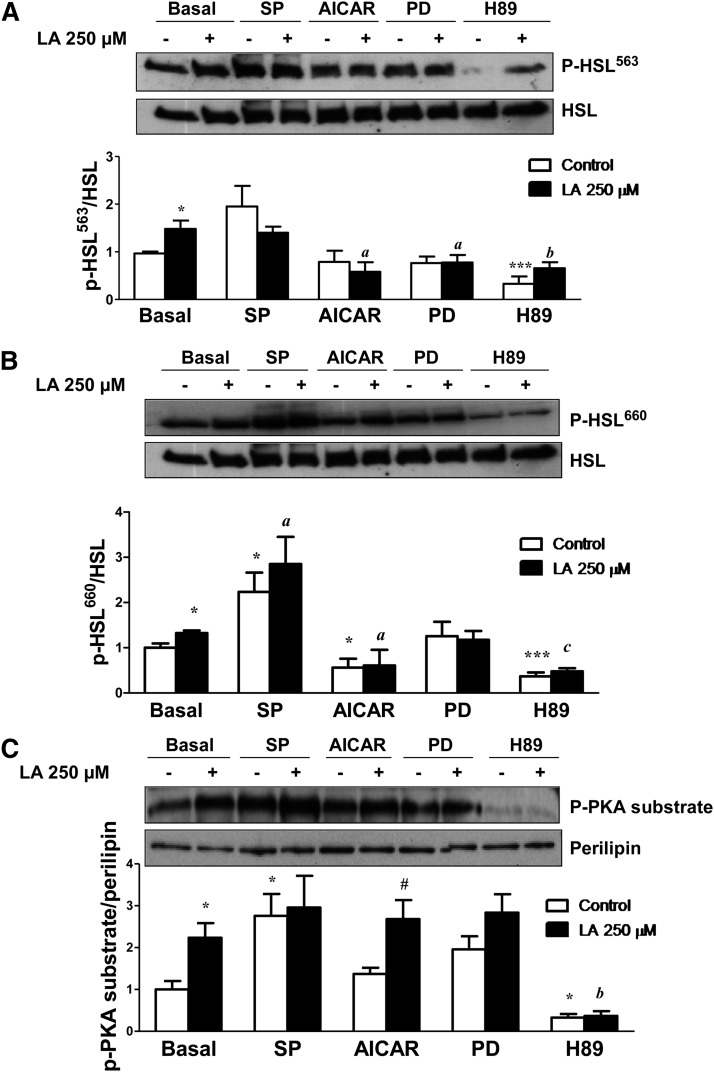
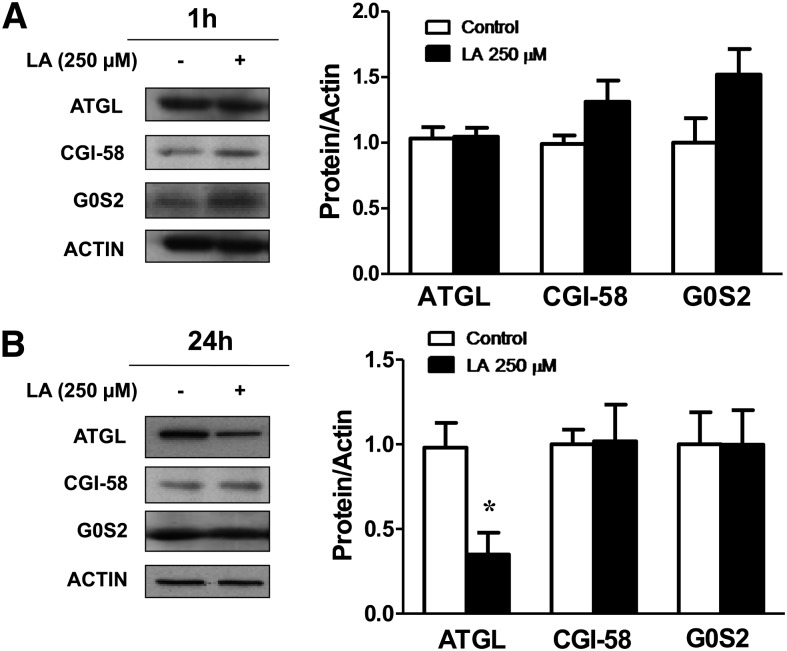
HSL activity is regulated by reversible phosphorylation in serine residues. PKA phosphorylates HSL at Ser563 and Ser660, which stimulates HSL activity. Thus, to better elucidate the mechanisms underlying the lipolytic actions of LA, we investigated the effects of LA on HSL phosphorylation in Ser563 and Ser660. LA treatment (250 µM) during 1 h did not modify total protein content of HSL but significantly increased (P < 0.05) the phosphorylation of HSL at Ser563 (Fig. 4A) and at Ser660 (Fig. 4B). However, LA did not modify the AMPK-induced phosphorylation of HSL at Ser565 (supplementary Fig. IV). These data suggest that LA stimulates lipolysis by increasing PKA activity. Perilipin phosphorylation is also PKA dependent. Using a perilipin-specific antibody and a phospho-PKA-motif-specific substrate antibody, we found that LA treatment induced a marked increase (P < 0.01) in PKA-mediated perilipin phosphorylation (Fig. 4C). In fact, the LA-induced phosphorylation of HSL at Ser563 and Ser660 as well as of perilipin was dramatically blunted in the presence of the PKA inhibitor H89. We also found that AMPK activation disrupted the LA-induced phosphorylation of HSL at Ser563 and Ser660 (Fig. 4A, B) without modifying the p-PKA substrate/perilipin ratio (Fig. 4C). The inhibition of the JNK pathway induced a significant increase in the phosphorylation of HSL at Ser660 in the absence and presence of LA and in PKA-mediated perilipin phosphorylation (Fig. 4B, C). Moreover, the ERK1/2 inhibitor PD98059 prevented the LA-induced phosphorylation of HSL at Ser563 without modifying LA effects on Ser660 and the p-PKA substrate/perilipin ratio. All these data suggest that LA stimulates lipolysis mainly through the PKA-mediated phosphorylation of perilipin and HSL. However, LA treatment for 1 h did not significantly modify the protein levels of ATGL. Neither CGI-58 nor G0S2, the activator and inhibitor of ATGL activity, respectively, were significantly altered after 1 or 24 h of LA treatment (Fig. 5A and B, respectively).
Fig. 4.
LA stimulates PKA-mediated phosporylation of HSL and perilipin. A and B: Representative Western blots for Ser563-phosphorylated HSL (A) and Ser660-phosphorylated HSL (B) in differentiated 3T3-L1 adipocytes treated with LA (250 μM) for 1 h in the presence or absence of the JNK inhibitor SP600125 (SP), the AMPK activator AICAR, the ERK1/2 inhibitor PD98059 (PD), and the PKA inhibitor H89. Band intensities were normalized to total HSL. C: Adipocyte lysates were immunoblotted using a phospho-PKA-motif-specific antibody, and the blots were stripped and reprobed with antiperilipin antibodies to detect native perilipins. The density of the protein bands was quantified, and the data (mean ± SE) were expressed as p-PKA substrate/perilipin ratio (n ≥ 3 independent experiments). *P < 0.05 and ***P < 0.001 vs. basal control (vehicle-treated cells). #P < 0.05 vs. respective control. aP < 0.05, bP < 0.01, and cP < 0.001 vs. basal LA-treated cells.
Fig. 5.
LA does not modify the levels of the ATGL coactivator CGI-58 or the ATGL inhibitor G0S2. A and B: Lysates from 3T3-L1 adipocytes treated with LA (250 μM) for 1 h (A) and 24 h (B) were immunoblotted for ATGL, CGI-58, G0S2, and actin antibody. Band intensities for ATGL, CGI-58, and G0S2 were normalized to actin. Data are expressed as mean ± SE of at least five independent experiments. *P < 0.05 vs. control (vehicle-treated cells).
Effects of LA on AdPLA levels and on PGE2 and cAMP production
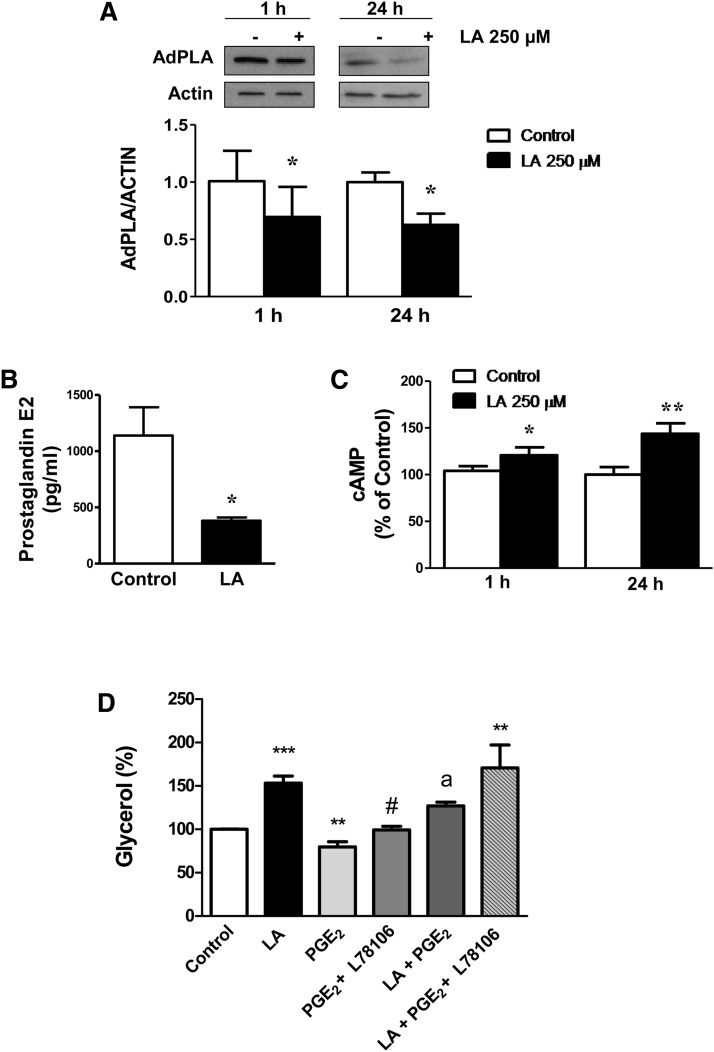
AdPLA has been described as the major phospholipase A2 in adipose tissue with a key role in the regulation of lipolysis through the modulation of PGE2 levels. As shown in Fig. 6A, LA treatment for 1 and 24 h (250 µM) caused a significant inhibition (P < 0.05) of AdPLA protein content as well as on mRNA levels (data not shown).
Fig. 6.
LA reduces AdPLA levels and PGE2 secretion and increases intracellular cAMP levels in 3T3-L1 adipocytes. A: AdPLA protein levels at 1 and 24 h of treatment with LA (250 μM). B: PGE2 released to the media in 3T3-L1 adipocytes treated with LA (250 µM) for 24 h. C: Intracellular cAMP levels at 1 and 24 h of treatment with LA (250 μM). D: Effects of PGE2 (0.5 ng/ml) on the lipolytic action of LA (250 μM) in the presence or absence of the EP3 antagonist L78106 (10 μM). Data are expressed as mean ± SE of at least three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.01 vs. control (vehicle-treated cells). #P < 0.05 vs. PGE2-treated cells. aP < 0.05 vs. basal LA-treated cells.
We next evaluated the effects of LA on the major AdPLA product, PGE2, which binds the Gαi-coupled receptor EP3 and down-regulates lipolysis by inhibiting cAMP production. Our data showed that the amount of PGE2 released to the media was significantly reduced in LA-treated adipocytes at 24 h of treatment (P < 0.05) (Fig. 6B) and also at shorter (4 and 8 h) periods of treatment (data not shown). In parallel, a significant increase in cAMP levels was found in LA-treated adipocytes for 1 and 24 h (Fig. 6C). Moreover, the lipolytic effect of LA was partially reversed by cotreatment with PGE2, an effect that was not observed in the presence of the EP3-receptor antagonist L798106 (Fig. 6D).
DISCUSSION
Our current data demonstrate the lipolytic action of LA in cultured adipocytes in a concentration- and time-dependent manner. The doses able to induce lipolysis were similar to those that inhibited adipogenesis in 3T3-L1 preadipocytes (8), and no toxicity was observed. Previous studies in broiler chickens also support the lipolytic action of LA in vitro and in vivo (12, 38). However, the mechanisms of action remain uncertain. In the present study, we tested if the lipolytic effects of LA were shared by other compounds with antioxidant properties. Our data revealed that resveratrol and BHA, but not vitamin C or NAC, were able to stimulate lipolysis, suggesting that the lipolytic actions are independent of the antioxidant capacities.
Our data showed that despite the stimulatory effects of LA on lipolysis, HSL and ATGL gene expression and protein levels were inhibited after 24 h of LA treatment, together with a decrease in perilipin mRNA levels. These effects of LA on HSL, ATGL, and perilipin were reversed by the presence of the ERK1/2 inhibitor PD98059 in the media. A down-regulation of ATGL, HSL, and perilipin gene expression together with increased lipolysis has also been described after TNF-α treatment in adipocytes (39–41). Moreover, it was observed that the administration of Trecadrine, a β-3 adrenergic agonist that stimulates lipolysis (42), induced a decrease in HSL mRNA levels in abdominal white adipose tissue, whereas an increase in HSL activity was observed (43). Furthermore, a recent study reported that serum amyloid A stimulated lipolysis in parallel with a reduced HSL protein content. However, serum amyloid A caused a significant increase of PKA-mediated HSL phosphorylation (44), suggesting opposite trends in HSL expression and activity. In this context, the mechanisms controlling HSL activity have been thoroughly studied, showing that reversible phosphorylation at several serine sites is a hallmark of HSL regulation. Indeed, HSL is activated by PKA-induced phosphorylation at Ser563 and Ser660. Moreover, the lipid droplet protein perilipin is also phosphorylated by PKA. Upon phosphorylation, perilipin shifts to the cytoplasm, accessibility of HSL to the lipid surface is promoted, and lipolysis is enhanced (45–47). The results of our study suggest a key role of PKA-induced lipolysis in the lipolytic actions of LA because i) LA increased HSL phosphorylation at Ser563 and Ser660, ii) PKA-induced perilipin phosphorylation was increased by LA treatment, and iii) the PKA inhibitor H89 completely blunted the lipolytic action of LA as well as the LA-induced phosphorylation of phospho-PKA substrates. Taken together, these data suggest an important role of PKA-mediated phosphorylation of perilipin and HSL in the lipolytic effect of LA.
ATGL plays a governing role in basal and adrenergically stimulated TAG breakdown in adipocytes (14). However, our data suggest that ATGL activation is not importantly involved in the lipolytic action of LA, as concluded from the findings that no significant changes were observed on the levels of the ATGL coactivator protein CGI-58 or on the inhibitory protein G0S2 (16, 48).
PI3K/AKT is a major player of insulin action, and its activation increases PDE3B activity and hydrolysis of cAMP, leading to a net dephosphorylation of HSL and inhibition of lipolysis (49). In our experimental cell model, LA inhibited AKT phosphorylation both at 30 min and 24 h of treatment, and the PI3K/AKT inhibitor LY294002 and the PDE3B antagonist Cilostamide potentiated the stimulatory effects of LA on basal lipolysis. Therefore, the present results suggest that the lipolytic effects of LA could be mediated by decreasing AKT activation, which might increase cAMP, and that lipolysis could be mediated by HSL and perilipin activation.
Mitogen-activated protein (MAP) kinases are serine/threonine-specific protein kinases that regulate various cellular activities, including lipolysis. Regarding the role of JNK activation in the regulation of lipolysis, it was described that JNK1/JNK2 deficiency drastically enhanced basal lipolysis (24). In this context, our data show that incubation with the JNK inhibitor SP600125 (2 h) stimulates the phosphorylation of HSL at Ser563 and Ser660 as well as phospho-PKA substrate/perilipin ratio, supporting the idea that JNK inhibition leads to increased lipolysis. However, our current data and previous studies show that the amount of glycerol released into the media is not modified or even reduced by longer-term incubation with SP600125 (41, 50), suggesting that the effects of JNK inhibition on lipolysis might be time dependent. Our results demonstrated that LA induced a time-dependent inhibition of JNK phosphorylation, which might suggest the involvement of this pathway in the lipolytic actions of LA. Thus, preincubation with SP600125 for 1 h potentiated the phosphorylation of HSL at Ser660 observed after 1 h of treatment with LA. However, coincubation with the JNK inhibitor SP600125 partially reversed the stimulatory effect on lipolysis and the inhibition induced by LA on ATGL gene expression after 24 h of treatment, suggesting that the involvement of JNK on LA-induced lipolysis is complex and seems to be time dependent. On the other hand, the fact that pretreatment with the ERK1/2 inhibitor PD98059 reversed the down-regulation of ATGL, HSL, and perilipin gene expression induced by LA treatment during 24 h might suggest the involvement of this pathway in LA-induced lipolysis. However, our data show that ERK1/2 phosphorylation is not affected by LA after 24 h of treatment and that pretreatment with PD98059 was not able to reverse the lipolytic action of LA, arguing against the involvement of this pathway.
AMPK has been also involved in the regulation of lipolysis (51, 52). Thus, it has been reported that phosphorylation of HSL at Ser565 by AMPK prevents activation by PKA, inhibiting lipolysis (19, 53, 54). Moreover, the negative regulation of AMPK activity by PKA has been shown to be important for converting a lipolytic signal into an effective lipolytic response (55). However, it has been recently reported that ATGL is phosphorylated and activated by AMPK to increase lipolysis (17). Thus, the effects described for AMPK activators on lipolysis are controversial, showing inhibition (56, 57) and activation of lipolysis (17, 58), and it has been suggested that the effects of AMPK activation on lipolysis might be time dependent (59). Our present data show that LA treatment stimulates AMPK phosphorylation and promotes lipolysis. However, the lipolytic effects of LA were already observed after 1 h of treatment when AMPK phosphorylation was not induced, suggesting that AMPK is not involved in the short-term lipolytic effects of LA. On the contrary, the presence of the AMPK activator AICAR inhibited LA-stimulated lipolysis at 24 h of treatment, according with the remarkable increase of AMPK phosphorylation observed at this time. Taken together, these data suggest that the lipolytic action of LA is not mediated by the activation of AMPK in the first stages but could contribute to the regulation of the long-term lipolytic effects of LA.
A new adipocyte phospholipase A2, called AdPLA, has recently been described and functionally characterized (27). It has been demonstrated that AdPLA ablation increased lipolysis by reducing PGE2 levels and thereby stimulating cAMP and phosphorylation of HSL through cAMP-mediated activation of PKA (28). Our results showed for the first time that AdPLA expression and PGE2 levels were down-regulated by LA treatment, accompanied by an increase in cAMP levels, which could also contribute to the increased phosphorylation of HSL at Ser563 and Ser660 and thereby to the lipolytic effects of LA. In support of this, our data revealed that coincubation with PGE2 was able to partially reverse the stimulatory effect of LA on lipolysis, whereas this effect of PGE2 was not observed in the presence of an EP3 antagonist.
All these data suggest that the ability of LA to stimulate lipolysis in adipocytes could also contribute to its antiobesity properties. Increased lipolysis and FFA release from adipose tissue has been associated with the development of insulin resistance (60). However, recent findings have demonstrated that increasing lipolysis in adipose tissue does not necessarily increase serum FFA levels because increasing lipolysis in adipose tissue causes a shift within adipocytes toward increased FA utilization and energy expenditure and thus protects against obesity. Therefore, it has been suggested that an activation of lipolysis may be a promising therapeutic target for the treatment of obesity (13, 61). In this context, we and others have demonstrated that dietary supplementation with LA reduces weight loss and fat mass without increasing circulating FFA and improves insulin resistance in rodents (10, 62, 63) and in humans (7), and, as previously suggested, this could be associated with LA-induced fatty acid oxidation. In this context, our experimental data support the notion about the ability of LA to promote fatty acid oxidation in 3T3-L1 adipocytes (supplementary Fig. V). A recent study have also shown that LA increased AMPK and ACC phosphorylation, leading to increased palmitate β-oxidation in myotubes (64). Moreover, studies of our group have shown that LA supplementation prevents the down-regulation of genes involved in mitochondrial and peroxisomal β-oxidation in liver of high fat-induced obese rats (65).
In summary, the present data demonstrate the ability of LA to stimulate lipolysis in 3T3-L1 adipocytes and suggest that these lipolytic actions of LA are mainly mediated by the phosphorylation of HSL through cAMP-mediated activation of PKA, probably through the inhibition of AdPLA and PGE2.
Supplementary Material
Acknowledgments
The authors thank María Zabala-Navó for excellent technical assistance. This manuscript was improved by comments from anonymous reviewers.
Footnotes
Abbreviations:
- AdPLA
- adipose-specific phospholipase A2
- Akt
- serine-threonine protein kinase Akt
- AMPK
- AMP-activated protein kinase
- ATGL
- adipose triglyceride lipase
- BHA
- butylated hydroxyanisole
- C/EBPα
- CCAAT/enhancer-binding protein alpha;
- C/EBPβ
- CCAAT/enhancer-binding protein β
- CGI-58
- comparative gene identification 58
- CILO
- cilostamide
- ERK1/2
- extracellular signal-regulated kinase
- FFA
- free fatty acid
- G0S2
- G(0)/G(1) switch gene 2
- HSL
- hormone-sensitive lipase
- JNK
- c-Jun N-terminal kinase
- LA
- lipoic acid
- LD
- lipid droplet
- LY
- LY294002
- MAPK
- mitogen-activated protein kinase
- NAC
- n-acetyl cysteine
- PD
- PD98059
- PDE3B
- phosphodiesterase 3B
- PGE2
- prostaglandin E2
- PI3K
- phosphatidylinositol 3 kinase
- PKA
- protein kinase A
- PPARγ
- peroxixome proliferator-activated receptor γ
- ROS
- reactive oxygen species
- SP
- SP600125
- TAG
- triacyl glycerol
- TNF-α
- tumor necrosis factor alpha
This work was supported, in part, by Ministerio de Ciencia e Innovación of Spain (AGL2009-10873/ALI and AGL2006-04716/ALI), and Línea Especial de Investigación “Nutrición, Obesidad y Salud” (University of Navarra LE/97), by a predoctoral grant from “Asociación de Amigos of the University of Navarra” and by a scholarship from Department of Education of Navarra Government (M. F.-G.); also CIBER and RETICS networks are gratefully acknowledged.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
REFERENCES
- 1.Scholich H., Murphy M. E., Sies H. 1989. Antioxidant activity of dihydrolipoate against microsomal lipid peroxidation and its dependence on alpha-tocopherol. Biochim. Biophys. Acta. 1001: 256–261 [DOI] [PubMed] [Google Scholar]
- 2.Han D., Tritschler H. J., Packer L. 1995. Alpha-lipoic acid increases intracellular glutathione in a human T-lymphocyte Jurkat cell line. Biochem. Biophys. Res. Commun. 207: 258–264 [DOI] [PubMed] [Google Scholar]
- 3.Kim M. S., Park J. Y., Namkoong C., Jang P. G., Ryu J. W., Song H. S., Yun J. Y., Namgoong I. S., Ha J., Park I. S., et al. 2004. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat. Med. 10: 727–733 [DOI] [PubMed] [Google Scholar]
- 4.Prieto-Hontoria P. L., Perez-Matute P., Fernandez-Galilea M., Barber A., Martinez J. A., Moreno-Aliaga M. J. 2009. Lipoic acid prevents body weight gain induced by a high fat diet in rats: effects on intestinal sugar transport. J. Physiol. Biochem. 65: 43–50 [DOI] [PubMed] [Google Scholar]
- 5.Carbonelli M. G., Di Renzo L., Bigioni M., Di Daniele N., De Lorenzo A., Fusco M. A. 2010. Alpha-lipoic acid supplementation: a tool for obesity therapy? Curr. Pharm. Des. 16: 840–846 [DOI] [PubMed] [Google Scholar]
- 6.Koh E. H., Lee W. J., Lee S. A., Kim E. H., Cho E. H., Jeong E., Kim D. W., Kim M. S., Park J. Y., Park K. G., Lee H. J., Lee I. K., Lim S., Jang H. C., Lee K. H., Lee K. U. 2011. Effects of alpha-lipoic acid on body weight in obese subjects. Am. J. Med. 124: 85e1–8 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Han P., Wu N., He B., Lu Y., Li S., Liu Y., Zhao S., Liu L., Li Y. 2011. Amelioration of lipid abnormalities by alpha-lipoic acid through antioxidative and anti-inflammatory effects. Obesity (Silver Spring). 19: 1647–1653 [DOI] [PubMed] [Google Scholar]
- 8.Cho K. J., Moon H. E., Moini H., Packer L., Yoon D. Y., Chung A. S. 2003. Alpha-lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways. J. Biol. Chem. 278: 34823–34833 [DOI] [PubMed] [Google Scholar]
- 9.Prieto-Hontoria P. L., Perez-Matute P., Fernandez-Galilea M., Martinez J. A., Moreno-Aliaga M. J. 2011. Lipoic acid inhibits leptin secretion and Sp1 activity in adipocytes. Mol. Nutr. Food Res. 55: 1059–1069 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Galilea M., Perez-Matute P., Prieto-Hontoria P., Martinez J. A., Moreno-Aliaga M. J. 2011. Effects of lipoic acid on apelin in 3T3–L1 adipocytes and in high-fat fed rats. J. Physiol. Biochem. 67: 479–486 [DOI] [PubMed] [Google Scholar]
- 11.Shen W., Hao J., Feng Z., Tian C., Chen W., Packer L., Shi X., Zang W., Liu J. 2011. Lipoamide or lipoic acid stimulates mitochondrial biogenesis in 3T3–L1 adipocytes via the endothelial NO synthase-cGMP-protein kinase G signalling pathway. Br. J. Pharmacol. 162: 1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamano Y. 2006. Effects of dietary lipoic acid on plasma lipid, in vivo insulin sensitivity, metabolic response to corticosterone and in vitro lipolysis in broiler chickens. Br. J. Nutr. 95: 1094–1101 [DOI] [PubMed] [Google Scholar]
- 13.Ahmadian M., Duncan R. E., Sul H. S. 2009. The skinny on fat: lipolysis and fatty acid utilization in adipocytes. Trends Endocrinol. Metab. 20: 424–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lass A., Zimmermann R., Oberer M., Zechner R. 2011. Lipolysis: a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 50: 14–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. 2004. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 279: 47066–47075 [DOI] [PubMed] [Google Scholar]
- 16.Lu X., Yang X., Liu J. 2010. Differential control of ATGL-mediated lipid droplet degradation by CGI-58 and G0S2. Cell Cycle. 9: 2719–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmadian M., Abbott M. J., Tang T., Hudak C. S., Kim Y., Bruss M., Hellerstein M. K., Lee H. Y., Samuel V. T., Shulman G. I., et al. 2011. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13: 739–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watt M. J., Holmes A. G., Pinnamaneni S. K., Garnham A. P., Steinberg G. R., Kemp B. E., Febbraio M. A. 2006. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am. J. Physiol. Endocrinol. Metab. 290: E500–E508 [DOI] [PubMed] [Google Scholar]
- 19.Anthony N. M., Gaidhu M. P., Ceddia R. B. 2009. Regulation of visceral and subcutaneous adipocyte lipolysis by acute AICAR-induced AMPK activation. Obesity (Silver Spring). 17: 1312–1317 [DOI] [PubMed] [Google Scholar]
- 20.Gaidhu M. P., Anthony N. M., Patel P., Hawke T. J., Ceddia R. B. 2010. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am. J. Physiol. Cell Physiol. 298: C961–C971 [DOI] [PubMed] [Google Scholar]
- 21.Degerman E., Landstrom T. R., Wijkander J., Holst L. S., Ahmad F., Belfrage P., Manganiello V. 1998. Phosphorylation and activation of hormone-sensitive adipocyte phosphodiesterase type 3B. Methods. 14: 43–53 [DOI] [PubMed] [Google Scholar]
- 22.Kitamura T., Kitamura Y., Kuroda S., Hino Y., Ando M., Kotani K., Konishi H., Matsuzaki H., Kikkawa U., Ogawa W., et al. 1999. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol. Cell. Biol. 19: 6286–6296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg A. S., Shen W. J., Muliro K., Patel S., Souza S. C., Roth R. A., Kraemer F. B. 2001. Stimulation of lipolysis and hormone-sensitive lipase via the extracellular signal-regulated kinase pathway. J. Biol. Chem. 276: 45456–45461 [DOI] [PubMed] [Google Scholar]
- 24.Rozo A. V., Vijayvargia R., Weiss H. R., Ruan H. 2008. Silencing Jnk1 and Jnk2 accelerates basal lipolysis and promotes fatty acid re-esterification in mouse adipocytes. Diabetologia. 51: 1493–1504 [DOI] [PubMed] [Google Scholar]
- 25.Granneman J. G., Moore H. P., Krishnamoorthy R., Rathod M. 2009. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J. Biol. Chem. 284: 34538–34544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyoshi H., Perfield J. W., 2nd, Souza S. C., Shen W. J., Zhang H. H., Stancheva Z. S., Kraemer F. B., Obin M. S., Greenberg A. S. 2007. Control of adipose triglyceride lipase action by serine 517 of perilipin A globally regulates protein kinase A-stimulated lipolysis in adipocytes. J. Biol. Chem. 282: 996–1002 [DOI] [PubMed] [Google Scholar]
- 27.Duncan R. E., Sarkadi-Nagy E., Jaworski K., Ahmadian M., Sul H. S. 2008. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA). J. Biol. Chem. 283: 25428–25436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaworski K., Ahmadian M., Duncan R. E., Sarkadi-Nagy E., Varady K. A., Hellerstein M. K., Lee H. Y., Samuel V. T., Shulman G. I., Kim K. H., et al. 2009. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat. Med. 15: 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min A. K., Kim M. K., Seo H. Y., Kim H. S., Jang B. K., Hwang J. S., Choi H. S., Lee K. U., Park K. G., Lee I. K. 2010. Alpha-lipoic acid inhibits hepatic PAI-1 expression and fibrosis by inhibiting the TGF-beta signaling pathway. Biochem. Biophys. Res. Commun. 393: 536–541 [DOI] [PubMed] [Google Scholar]
- 30.Packer L., Cadenas E. 2011. Lipoic acid: energy metabolism and redox regulation of transcription and cell signaling. J. Clin. Biochem. Nutr. 48: 26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng P. Y., Lee Y. M., Yen M. H., Peng J. C., Lam K. K. 2011. Reciprocal effects of alpha-lipoic acid on adenosine monophosphate-activated protein kinase activity in obesity induced by ovariectomy in rats. Menopause. 18: 1010–1017 [DOI] [PubMed] [Google Scholar]
- 32.Salinthone S., Schillace R. V., Tsang C., Regan J. W., Bourdette D. N., Carr D. W. 2011. Lipoic acid stimulates cAMP production via G protein-coupled receptor-dependent and -independent mechanisms. J. Nutr. Biochem. 22: 681–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ha H., Lee J. H., Kim H. N., Kim H. M., Kwak H. B., Lee S., Kim H. H., Lee Z. H. 2006. Alpha-lipoic acid inhibits inflammatory bone resorption by suppressing prostaglandin E2 synthesis. J. Immunol. 176: 111–117 [DOI] [PubMed] [Google Scholar]
- 34.Lorente-Cebrian S., Bustos M., Marti A., Martinez J. A., Moreno-Aliaga M. J. 2009. Eicosapentaenoic acid stimulates AMP-activated protein kinase and increases visfatin secretion in cultured murine adipocytes. Clin. Sci. (Lond.). 117: 243–249 [DOI] [PubMed] [Google Scholar]
- 35.Lorente-Cebrian S., Bustos M., Marti A., Martinez J. A., Moreno-Aliaga M. J. 2010. Eicosapentaenoic acid up-regulates apelin secretion and gene expression in 3T3–L1 adipocytes. Mol. Nutr. Food Res. 54: S104–S111 [DOI] [PubMed] [Google Scholar]
- 36.Perez-Matute P., Neville M. J., Tan G. D., Frayn K. N., Karpe F. 2009. Transcriptional control of human adipose tissue blood flow. Obesity (Silver Spring). 17: 681–688 [DOI] [PubMed] [Google Scholar]
- 37.Mercader J., Palou A., Bonet M. L. 2011. Resveratrol enhances fatty acid oxidation capacity and reduces resistin and Retinol-Binding Protein 4 expression in white adipocytes. J. Nutr. Biochem. 22: 828–834 [DOI] [PubMed] [Google Scholar]
- 38.Hamano Y. 2002. Influence of lipoic acid on lipid metabolism and beta-adrenergic response to intravenous or oral administration of clenbuterol in broiler chickens. Reprod. Nutr. Dev. 42: 307–316 [DOI] [PubMed] [Google Scholar]
- 39.Kim J. Y., Tillison K., Lee J. H., Rearick D. A., Smas C. M. 2006. The adipose tissue triglyceride lipase ATGL/PNPLA2 is downregulated by insulin and TNF-alpha in 3T3–L1 adipocytes and is a target for transactivation by PPARgamma. Am. J. Physiol. Endocrinol. Metab. 291: E115–E127 [DOI] [PubMed] [Google Scholar]
- 40.Kralisch S., Sommer G., Stangl V., Kohler U., Kratzsch J., Stepan H., Faber R., Schubert A., Lossner U., Vietzke A., et al. 2008. Secretory products from human adipocytes impair endothelial function via nuclear factor kappaB. Atherosclerosis. 196: 523–531 [DOI] [PubMed] [Google Scholar]
- 41.Ryden M., Arvidsson E., Blomqvist L., Perbeck L., Dicker A., Arner P. 2004. Targets for TNF-alpha-induced lipolysis in human adipocytes. Biochem. Biophys. Res. Commun. 318: 168–175 [DOI] [PubMed] [Google Scholar]
- 42.Moreno-Aliaga M. J., Martinez J. A., Stanhope K. L., Fernandez-Otero M. P., Havel P. J. 2002. Effects of Trecadrine, a beta3-adrenergic agonist, on leptin secretion, glucose and lipid metabolism in isolated rat adipocytes. Int. J. Obes. Relat. Metab. Disord. 26: 912–919 [DOI] [PubMed] [Google Scholar]
- 43.Berraondo B., Martinez J. A. 2000. Free fatty acids are involved in the inverse relationship between hormone-sensitive lipase (HSL) activity and expression in adipose tissue after high-fat feeding or beta3-adrenergic stimulation. Obes. Res. 8: 255–261 [DOI] [PubMed] [Google Scholar]
- 44.Liu L. R., Lin S. P., Chen C. C., Chen Y. J., Tai C. C., Chang S. C., Juang R. H., Tseng Y. W., Liu B. H., Mersmann H. J., et al. 2011. Serum amyloid A induces lipolysis by downregulating perilipin through ERK1/2 and PKA signaling pathways. Obesity (Silver Spring). 19: 2301–2309 [DOI] [PubMed] [Google Scholar]
- 45.Holm C. 2003. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 31: 1120–1124 [DOI] [PubMed] [Google Scholar]
- 46.Shen W. J., Patel S., Miyoshi H., Greenberg A. S., Kraemer F. B. 2009. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J. Lipid Res. 50: 2306–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu C., He J., Jiang H., Zu L., Zhai W., Pu S., Xu G. 2009. Direct effect of glucocorticoids on lipolysis in adipocytes. Mol. Endocrinol. 23: 1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang X., Lu X., Lombes M., Rha G. B., Chi Y. I., Guerin T. M., Smart E. J., Liu J. 2010. The G(0)/G(1) switch gene 2 regulates adipose lipolysis through association with adipose triglyceride lipase. Cell Metab. 11: 194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridderstrale M. 2005. Signaling mechanism for the insulin-like effects of growth hormone–another example of a classical hormonal negative feedback loop. Curr. Drug Targets Immune Endocr. Metabol. Disord. 5: 79–92 [DOI] [PubMed] [Google Scholar]
- 50.Deng J., Liu S., Zou L., Xu C., Geng B., Xu G. 2012. Lipolysis response to endoplasmic reticulum stress in adipose cells. J. Biol. Chem. 287: 6240–6249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hardie D. G. 2008. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 582: 81–89 [DOI] [PubMed] [Google Scholar]
- 52.McGee S. L., Hargreaves M. 2010. AMPK-mediated regulation of transcription in skeletal muscle. Clin. Sci. (Lond.). 118: 507–518 [DOI] [PubMed] [Google Scholar]
- 53.Dagon Y., Avraham Y., Berry E. M. 2006. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2alpha in adipocytes. Biochem. Biophys. Res. Commun. 340: 43–47 [DOI] [PubMed] [Google Scholar]
- 54.Boon H., Bosselaar M., Praet S. F., Blaak E. E., Saris W. H., Wagenmakers A. J., McGee S. L., Tack C. J., Smits P., Hargreaves M., et al. 2008. Intravenous AICAR administration reduces hepatic glucose output and inhibits whole body lipolysis in type 2 diabetic patients. Diabetologia. 51: 1893–1900 [DOI] [PubMed] [Google Scholar]
- 55.Djouder N., Tuerk R. D., Suter M., Salvioni P., Thali R. F., Scholz R., Vaahtomeri K., Auchli Y., Rechsteiner H., Brunisholz R. A., et al. 2010. PKA phosphorylates and inactivates AMPKalpha to promote efficient lipolysis. EMBO J. 29: 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bourron O., Daval M., Hainault I., Hajduch E., Servant J. M., Gautier J. F., Ferre P., Foufelle F. 2010. Biguanides and thiazolidinediones inhibit stimulated lipolysis in human adipocytes through activation of AMP-activated protein kinase. Diabetologia. 53: 768–778 [DOI] [PubMed] [Google Scholar]
- 57.Lorente-Cebrian S., Bustos M., Marti A., Fernandez-Galilea M., Martinez J. A., Moreno-Aliaga M. J. 2012. Eicosapentaenoic acid inhibits tumour necrosis factor-alpha-induced lipolysis in murine cultured adipocytes. J. Nutr. Biochem. 23: 218–227 [DOI] [PubMed] [Google Scholar]
- 58.Gaidhu M. P., Fediuc S., Anthony N. M., So M., Mirpourian M., Perry R. L., Ceddia R. B. 2009. Prolonged AICAR-induced AMP-kinase activation promotes energy dissipation in white adipocytes: novel mechanisms integrating HSL and ATGL. J. Lipid Res. 50: 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yin W., Mu J., Birnbaum M. J. 2003. Role of AMP-activated protein kinase in cyclic AMP-dependent lipolysis In 3T3–L1 adipocytes. J. Biol. Chem. 278: 43074–43080 [DOI] [PubMed] [Google Scholar]
- 60.Ormseth M. J., Swift L. L., Fazio S., Linton M. F., Chung C. P., Raggi P., Rho Y. H., Solus J., Oeser A., Bian A., et al. 2011. Free fatty acids are associated with insulin resistance but not coronary artery atherosclerosis in rheumatoid arthritis. Atherosclerosis. 219: 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ahmadian M., Wang Y., Sul H. S. 2010. Lipolysis in adipocytes. Int. J. Biochem. Cell Biol. 42: 555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park K. G., Min A. K., Koh E. H., Kim H. S., Kim M. O., Park H. S., Kim Y. D., Yoon T. S., Jang B. K., Hwang J. S., et al. 2008. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology. 48: 1477–1486 [DOI] [PubMed] [Google Scholar]
- 63.El Midaoui A., Lungu C., Wang H., Wu L., Robillard C., Deblois D., Couture R. 2011. Impact of alpha-lipoic acid on liver peroxisome proliferator-activated receptor-alpha, vascular remodeling, and oxidative stress in insulin-resistant rats. Can. J. Physiol. Pharmacol. 89: 743–751 [DOI] [PubMed] [Google Scholar]
- 64.Chen W. L., Kang C. H., Wang S. G., Lee H. M. 2012. Alpha-lipoic acid regulates lipid metabolism through induction of sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase. Diabetologia. 55: 1824–1835 [DOI] [PubMed] [Google Scholar]
- 65.Valdecantos M. P., Perez-Matute P., Gonzalez-Muniesa P., Prieto-Hontoria P., Moreno-Aliaga M., Martinez J. 2012. Lipoic acid improves mitochondrial function in non-alcoholic steatosis through the stimulation of sirtuin 1 and sirtuin 3. Obesity (Silver Spring). In press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.