Abstract
An injection of 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) to mice lacking Niemann Pick type C (NPC) protein results in delayed neurodegeneration, decreased inflammation, and prolonged lifespan. Changes in sterol balance observed in Npc1−/− mice 24 h after HP-β-CD administration suggest that HP-β-CD facilitates the release of accumulated lysosomal cholesterol, the molecular hallmark of this genetic disorder. Current studies were performed to evaluate the time course of HP-β-CD effects. Within 3 h after HP-β-CD injection, decreases in cholesterol synthesis rates and increases in cholesteryl ester levels were detected in tissues of Npc1−/− mice. The levels of RNAs for target genes of sterol-sensing transcription factors were altered by 6 h in liver, spleen, and ileum. Despite the cholesterol-binding capacity of HP-β-CD, there was no evidence of increased cholesterol in plasma or urine of treated Npc1−/− mice, suggesting that HP-β-CD does not carry sterol from the lysosome into the bloodstream for ultimate urinary excretion. Similar changes in sterol balance were observed in cultured cells from Npc1−/− mice using HP-β-CD and sulfobutylether-β-CD, a variant that can interact with sterol but not facilitate its solubilization. Taken together, our results demonstrate that HP-β-CD works in cells of Npc1−/− mice by rapidly liberating lysosomal cholesterol for normal sterol processing within the cytosolic compartment.
Keywords: Niemann-Pick type C, cholesterol balance, lipoprotein profiles, liver, spleen, macrophage, hippocampal neurons, lysosome, inflammation
Cyclodextrins (CDs) are cyclic oligosaccharides composed of 6, 7, or 8 glucopyranosides, which are called α, β, or γ-CD, respectively. CDs have a distinctive barrel configuration with a hydrophilic exterior promoting water solubility and a hydrophobic interior that accommodates small lipophilic molecules. Both of these properties make CDs ideal vehicles to increase the solubility of hydrophobic drugs; thus CDs have been approved by the US Food and Drug Administration (FDA) as delivery agents (1–4). In addition to natural CDs that are products of starch, several modified forms of β-CD have been synthesized, including 2-hydroxypropyl (HP-β-CD) and sulfobutylether (SBE-β-CD), which have less toxic effects in vivo. These CDs were considered to possess no therapeutic potential and were thought to simply serve as benign carriers of lipophilic drugs, which could be released into the bloodstream allowing CDs to be excreted intact separately through the kidneys (3). That is, until 2009, when it was reported that a single injection of HP-β-CD led to dramatic improvements in cholesterol homeostasis, inflammation, and lifespan in the mouse model for the lysosomal storage disease Niemann Pick Type C (NPC) (5).
NPC disease is a rare autosomal recessive disorder in which inactivating mutations in either NPC1 or NPC2 genes result in aberrant intracellular transport of unesterified cholesterol from the late endosome/lysosome (LE/L) and massive lipid accumulation within this compartment in all cells (6, 7). NPC disease presents as hepatic dysfunction, splenomegaly, pulmonary disease, and neurodegeneration, resulting in premature death, which typically occurs during adolescence in humans (8) and at around 85 days in the Npc1−/− mouse (9). As there are no FDA-approved treatments to extend lifespan for NPC patients, the Npc1−/− mouse has been used as a model to test potential therapies. Therefore when HP-β-CD was shown to extend the lifespan of Npc1−/− mice to 118 days with a single injection to 7-day-old pups (5), it quickly became a major focus of the NPC research community.
Since the initial studies in mice, HP-β-CD therapy has been shown to be effective in the Npc1−/− cat model (10), and a NIH-sponsored clinical trial to test HP-β-CD efficacy in humans is being organized (http://nctt.nih.gov/2754421). Additionally, much research has been undertaken to further understand how CD is working in Npc1−/− animal models. Notably, serial injections of HP-β-CD were shown to double the lifespan of the Npc1−/− mouse (11, 12), and continuous intrathecal administration was found to dramatically rescue Purkinje cell loss within the cerebellum (13). HP-β-CD does not require a functional NPC1 or NPC2 protein to work as suggested by studies using cell culture models (14–16) and confirmed in mice when HP-β-CD was shown to significantly decrease hepatic cholesterol synthesis rates in Npc1−/−, Npc2−/−, and rare Npc1−/−Npc2−/− double-knockout mice (17). Studies performed within a variety of tissues harvested 24 h after a single HP-β-CD injection to 7- or 49-day-old Npc1−/− mice have shown dramatic changes in sterol dynamics, including a decrease in cholesterol synthesis rates, an increase in the ratio of esterified/unesterified cholesterol, downregulation of the sterol regulatory element-binding protein 2 (SREBP2) and its target genes, and increased expression of liver X receptor (LXR) target genes (5, 18). These measurable shifts in sterol homeostasis serve as surrogate markers to indicate that the cell is responding to a state of cytosolic cholesterol excess. Because these changes were observed after HP-β-CD administration to Npc1−/− mice only and not seen for wild-type mice, they further suggest that the excess unesterified cholesterol is being released from the LE/L compartment in Npc1−/− mice. Overall, these data suggest that HP-β-CD is able to facilitate the release of the lysosomal pool of unesterified cholesterol from NPC-deficient cells within 24 h.
Although virtually all studies thus far have been performed 24 h after HP-β-CD, a single time course experiment using [14C]radiolabeled HP-β-CD in 49-day-old mice demonstrated that it is cleared from plasma within 3 h and from the whole body within 6 h after a subcutaneous dose of 4,000 mg/kg body weight (mpk) (18). Therefore, we performed a series of studies in Npc1−/− mice to evaluate the time course of changes elicited by HP-β-CD from 1 to 12 h after administration to further understand its mechanism of action. The experiments were designed to i) measure the rate of change in lipid balance and corresponding mRNA levels; ii) determine whether a CD/cholesterol complex is evident at any time in plasma or urine; iii) evaluate how rapidly inflammation is reduced; and iv) assess the direct effects of HP-β-CD and SBE-β-CD on primary cells (neurons and macrophages) in culture. Together, these studies provide novel insights into the mechanism by which HP-β-CD frees trapped unesterified cholesterol from the LE/L of cells lacking NPC1.
MATERIALS AND METHODS
Animals and treatments
Heterozygous (Npc1+/-) mice on a BALB/c background were bred to generate wild-type (Npc1+/+) and homozygous-null (Npc1−/−) littermates (19). Mice were group-housed in plastic cages containing wood-chip bedding in an animal facility with temperature-controlled rooms (23 ± 1°C) and a maintained light cycle (12 h light on/12 h light off). The mice were allowed ad libitum access to water and a standard rodent diet containing 0.02% w/w cholesterol (7001; Harlan Teklad, Madison, WI). Mice were genotyped upon weaning between 19 and 21 days of age (19). For all in vivo studies, 49-day-old mice were given a single subcutaneous injection of HP-β-CD (H107, Sigma) at 4,000 mpk or vehicle [saline at equivalent volume of 20 μl/g body weight (bw)]. When mice were used as a source of cells for culture, macrophages were obtained from 2-month-old mice, and hippocampal neurons were harvested from 1-day-old pups. As no differences in response to HP-β-CD were evident between male and female mice in our previous reports (5, 9, 12, 13, 17, 18), these studies utilized either equal numbers of males and females per group (Figs. 1,4D, 5–7) or only females (Figs. 2, 3, 4A–CFigs. 2, 3, 4A–C). All animal research was conducted in conformity with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, and all experiments were performed with prior approval from the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
Fig. 1.
Time course of HP-β-CD's effects on tissue cholesterol content and sterol synthesis rates in Npc1−/−mice. Forty-nine-day-old Npc1−/− mice were injected at time 0 with a 4,000 mpk bolus of HP-β-CD, and tissues were harvested after 0, 3, 6, 9, or 12 h. Npc1+/+ controls were studied 24 h after an injection of HP-β-CD. Organ weights were measured and expressed relative to the total body weight for liver (A) and spleen (B). Liver and spleen tissues were saponified to measure the total concentration of cholesterol (C, D) by GC and the rates of cholesterol synthesis (E, F) by the [3H]water method. Each bar represents the mean ± SEM for 4–6 mice. Statistically significant differences (P < 0.05) between the Npc1+/+ and the Npc1−/− (0 h) groups are represented by a double dagger (‡), whereas differences occurring in Npc1−/− mice following injection with HP-β-CD are indicated by asterisks (*P < 0.05 to ***P < 0.0005) versus the 0 h group.
Fig. 4.
Cholesterol distribution in plasma and urine of Npc1−/−mice after HP-β-CD injection. Plasma samples were collected from 49-day-old Npc1+/+ and Npc1−/− mice 0, 1, 2, 3, 6, and 12 h after an injection with HP-β-CD (4,000 mpk, sc) or equivalent volume of vehicle (saline). Plasmas were pooled (n = 2 pooled samples/group), and lipoprotein profiles were generated by FPLC. Lipoprotein profiles were plotted to allow comparison between (A) saline-treated Npc1+/+ and Npc1−/− mice; (B) the very early time points, 0–3 h, after HP-β-CD injection of Npc1−/− mice; and (C) later time points, 6–12 h, after HP-β-CD injection of Npc1−/− mice. Urine samples were collected from 49-day-old Npc1+/+ and Npc1−/− mice over a 48 h period after a HP-β-CD (4,000 mpk, sc) or vehicle (saline) injection. The total urinary cholesterol (D) values depict the mean ± SEM for 6 mice/group. A significant difference (P< 0.05) between the Npc1+/+ and Npc1−/− groups is represented by a double dagger (‡).
Fig. 5.
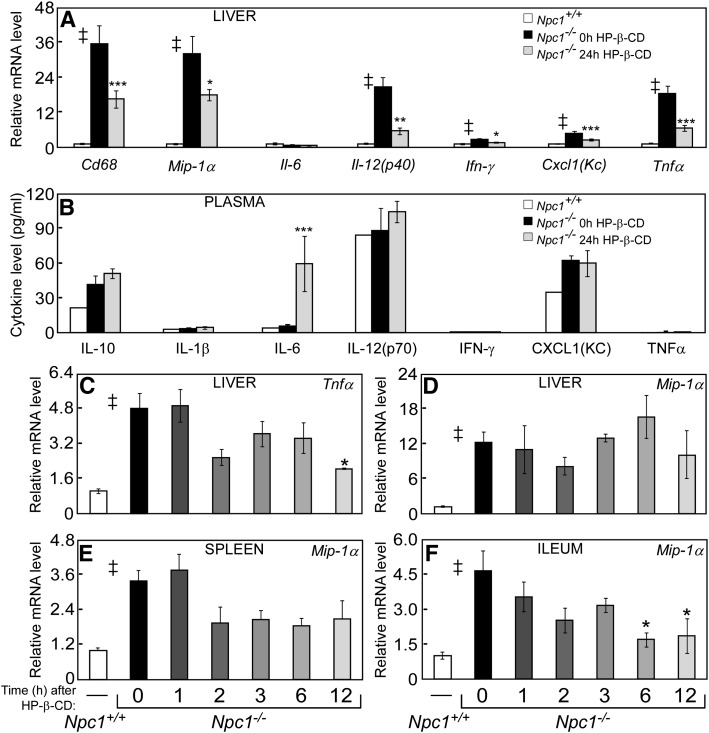
Markers of inflammation after HP-β-CD treatment. Livers were collected from 49-day-old Npc1+/+ and Npc1−/− mice 24 h after a saline or HP-β-CD injection (4,000 mpk, sc). Hepatic RNA was harvested and used to survey changes in mRNA levels of cytokines and other inflammatory markers after HP-β-CD (A). Blood was collected from the tail vein of 49-day-old Npc1−/− mice at 0, 3, 6, 12, and 24 h after a HP-β-CD injection (4,000 mpk, sc). The same mice were used for all time points. Plasma was used to measure the protein levels of cytokines (B). Liver, spleen, and ileum were collected from Npc1−/− mice 0, 1, 2, 3, 6, and 12 h after HP-β-CD injections (see Figs. 2 and 3 for details) and processed for RNA. The relative mRNA level of cytokines and other inflammatory markers (C–F) were measured. Each bar represents the mean ± SEM for 4–6 mice. Statistically significant differences (P < 0.05) between the Npc1+/+ and the 0 h Npc1−/− groups are represented by a double dagger (‡), whereas differences after HP-β-CD treatment in Npc1−/− mice compared with the 0 h group are represented by asterisks (*P < 0.05 to ***P < 0.0005).
Fig. 6.
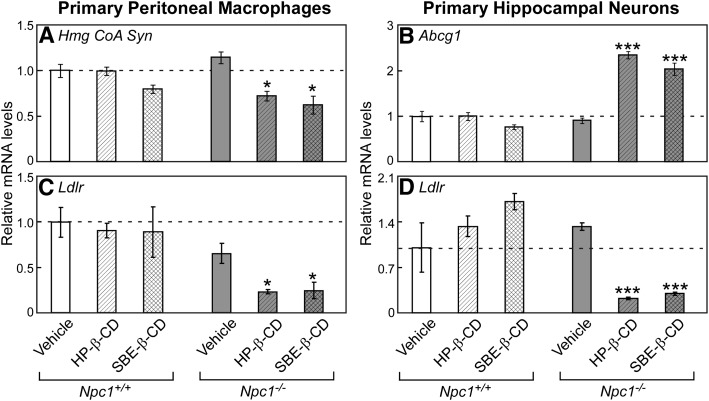
Acute effects of HP-β-CD in cultured cells from Npc1−/−mice. Thioglycollate-elicited peritoneal macrophages (from 49-day-old mice) and hippocampal neurons (from neonatal mouse pups) were obtained from Npc1+/+ and Npc1−/− mice. These cells were treated in FBS-containing culture media with increasing amounts of HP-β-CD (0.3 mM to 1.5 mM) or an equivalent volume of vehicle (saline). After 4 h, the cells were harvested for RNA isolation, and the relative mRNA levels of LXR target genes, Idol (A) and Abcg1 (B), and SREBP2 target genes Hmg CoA Syn (C) and Ldlr (D) were determined by qPCR. Each bar represents the mean ± SEM of triplicate wells. A statistically significant difference (P < 0.05) between saline-treated Npc1+/+ and Npc1−/− cells is represented by a double dagger (‡), and differences in RNA levels resulting from HP-β-CD treatment within cells of a given genotype are represented by asterisks (*P < 0.05 to ***P < 0.0005).
Fig. 7.
Effects of HP-β-CD compared to SBE-β-CD in cultured cells from Npc1−/−mice. Thioglycollate-elicited peritoneal (from 49-day-old mice) and hippocampal neurons (from neonatal mouse pups) were harvested from Npc1+/+ and Npc1−/− mice. These cells were treated in FBS-containing culture media with vehicle (saline), 0.3 mM HP-β-CD, or 0.3 mM SBE-β-CD. After 16 h, the cells were harvested for RNA, and the relative mRNA levels of SREBP2 target genes Hmg CoA Syn (A) and Ldlr (C, D) and the LXR target gene Abcg1 (B) were measured by qPCR. Each bar represents the mean ± SEM of triplicate wells. Statistically significant differences by CD treatment for cells of a given genotype compared with the vehicle-treated group are represented by asterisks (*P < 0.05 to ***P < 0.0005).
Fig. 2.
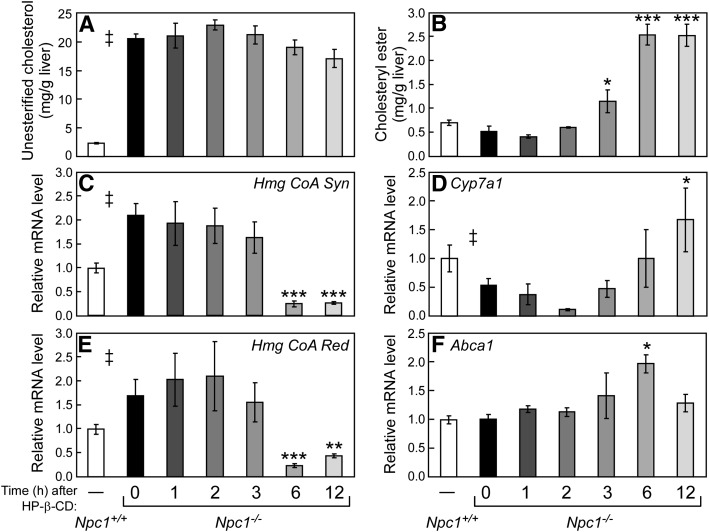
Time course of HP-β-CD's effects on hepatic esterified and unesterified cholesterol concentrations and sterol-regulated mRNA levels in Npc1−/−mice. Forty-nine-day-old Npc1−/− mice were injected at time 0 with a 4,000 mpk bolus of HP-β-CD, and tissues were harvested after 0, 1, 2, 3, 6, or 12 h. Injection times were staggered so that all tissues were harvested at the same point during the light cycle (ZT 9). Concentrations of hepatic unesterified cholesterol (A) and cholesteryl ester (B) were determined at all-time points. Relative mRNA levels in liver for SREBP2 target genes Hmg CoA Syn (C) and Hmg CoA Red (E) and for LXR target genes Cyp7a1 (D) and Abca1 (F) were measured by qPCR using cyclophilin as the invariant housekeeping gene. Values represent the mean ± SEM for 4–6 mice per group. Statistically significant differences (P < 0.05) between the Npc1+/+ and the Npc1−/− (0 h) groups are represented by a double dagger (‡), whereas differences occurring in Npc1−/− mice following injection with HP-β-CD are indicated by asterisks (*P < 0.05 to ***P < 0.0005) versus the 0 h group.
Fig. 3.
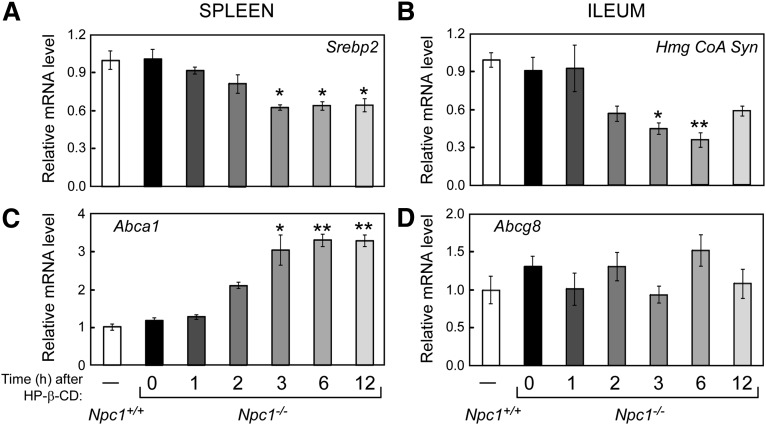
Time course of HP-β-CD's effects on spleen and ileum mRNA levels. Refer to the legend of Fig. 2 for experimental details and statistical analyses. Spleen and ileum (defined as the scraped mucosae from the distal third of small intestine) were harvested and processed for RNA. The relative mRNA levels of SREBP2 (A) and its target gene Hmg CoA Syn (B) and LXR target genes Abca1 (C) and Abcg8 (D) were measured by qPCR using cyclophilin as the invariant housekeeping gene.
Measures of cholesterol balance
Npc1+/+ and Npc1−/− mice (n = 4–6 mice/group) were given a subcutaneous injection of HP-β-CD or saline. At time points ranging from 0 to 12 h after the injection, the mice were weighed and euthanized (exsanguinated under deep anesthesia) to harvest tissues for the analyses described below.
Sterol synthesis rate.
Exactly 1 h prior to euthanasia and tissue harvest, one cohort of mice was given an intraperitoneal injection of 1.76 mCi of [3H] water/g bw. Aliquots of liver and spleen were quickly removed, weighed, and saponified in alcoholic KOH. Organ contents of [3H]labeled digitonin-precipitable sterols (DPS) were determined as previously described (20). The rate of sterol synthesis is reported as the nanomoles of [3H] water incorporated into DPS per hour per gram of tissue.
Tissue cholesterol concentration.
The aliquots of liver and spleen in KOH used for the synthesis determination were also used to measure total cholesterol content. Briefly, the cholesterol was extracted and quantified by gas chromatography (GC) with a stigmastanol internal standard as previously described (21). The total cholesterol concentration of each organ is expressed as the milligrams of cholesterol per gram of tissue.
Unesterified/esterified cholesterol determination.
From a different cohort of mice, livers were flash frozen and were crushed into a powder, which was weighed and solubilized in a 2:1 chloroform to methanol solution for lipid extraction. Free and esterified cholesterol were separated using a silica column (Sep-Pak Vac RC; Waters Corp., Milford, MA) and were quantified by GC as previously described (22). The cholesterol concentration of each organ is reported as the milligrams of unesterified cholesterol or cholesteryl ester per gram of tissue.
Plasma lipoprotein profile.
Blood samples were collected using EDTA-coated syringes from the ascending vena cava of anesthetized mice. Injection times for HP-β-CD were staggered so that all animals were exsanguinated at the same point during the light cycle [9 h after lights on or Zeitgeber time (ZT) 9]. Equal volumes of plasma/mouse were pooled for similarly treated animals (n = 2/group) and samples were provided to a core facility where lipoprotein profiles were obtained using fast protein liquid chromatography (FPLC) as described previously (23). Total cholesterol concentrations in eluted fractions were measured using both an enzyme-based method (Infinity Cholesterol Reagent, Sigma) and by solvent extraction followed with measurement by GC (see above). No differences were found between the two methods, so only values from the enzyme-based method, expressed as micrograms of total cholesterol per fraction, are reported.
Urine cholesterol concentration.
After receiving an injection of saline or HP-β-CD before lights out (ZT 10), a separate cohort of mice were individually housed in metabolic chambers in a maintained animal facility to collect urine for the following 48 h. While in the chamber, mice were allowed ad libitum access to food and water. Urine collections were spun at 1 × 104 g for 5 min at 4°C, and the supernantants were stored at 4°C. Later, the samples were dried down and then saponified in alcoholic KOH. Total cholesterol was extracted and measured by GC as described above. The cholesterol content of each urine sample is reported as the micrograms of total cholesterol per 24 h.
Relative mRNA levels
Tissues.
Npc1+/+ or Npc1−/− mice (n = 4 mice/group) were given a single subcutaneous injection of HP-β-CD (4,000 mpk) or saline and were euthanized 0 to 12 h later. Injection times were staggered so that all tissues were harvested at the same point during the light cycle (ZT 9). Mice were exsanguinated; then liver, spleen, and ileum (defined as the mucosae from the distal third of the small intestine) were collected and flash-frozen in liquid nitrogen. Tissues were stored at −80°C until total RNA was isolated using RNA STAT-60 (Tel-Test, Inc.). RNA concentrations were determined by absorbance at 260 nm with a Thermo Scientific Nanodrop 100 Spectrophotometer. RNA was treated with RNase-free DNase (Roche) and converted to cDNA using SuperScript II reverse transcriptase (Invitrogen) as previously described (23, 24). Quantitative real-time PCR (qPCR) was performed using an Applied Biosystems 7900HT sequence detection system. Each qPCR was analyzed in duplicate and contained in a final volume of 10 µl: 25 ng of cDNA, each primer at 150 nM, and 5 µl of 2× SYBR Green PCR Master Mix (Applied Biosystems). The nucleotide sequences of the primers used in these analyses are listed in supplementary Table I. Results were evaluated by the comparative cycle number at threshold (CT) method (25) using cyclophilin as the invariant housekeeping gene (26, 27), and values were arithmetically adjusted to yield a unit of 1 for the control group.
Primary macrophages.
Npc1+/+ or Npc1−/− mice (n = 4–6 mice/group) received a 1 ml intraperitoneal injection of 3% thioglycollate (autoclaved and aged for 3 months; Becton Dickinson) to elicit macrophages. Three days later, mice were euthanized, and macrophages were obtained from the peritoneal cavity by sterile lavage using ice-cold PBS. Collections from mice of the same genotype were pooled, and cells were collected by centrifugation for 5 min at 150 g at 4°C. Cells were resuspended in media [high-glucose DMEM (Invitrogen) containing 10% heat-inactivated FBS (Atlanta Biologicals) and 1% Pen/Strep (Invitrogen)], counted and plated at 1 × 105 cells/cm2. Once plated, cells were maintained in a humid incubator at 37°C with 5% CO2. After 6 h, cells were washed with PBS and exposed to fresh media containing either vehicle (PBS) or treatment. For the CD dose-response experiments, cells were treated for 4 h with varying amounts of a 250 mM solution of HP-β-CD (H107; Sigma) made in PBS to yield final concentrations of 0.3, 0.75, and 1.5 mM. For experiments to compare HP-β-CD and SBE-β-CD (Captisol; CYDEX Pharmaceuticals, Inc.), cells were treated for 16 h with 0.3 mM of either compound from a 250 mM stock solution made in PBS. After treatment, culture medium was removed from each well, and RNA-STAT60 was used to lyse cells and obtain RNA for analysis by qPCR (as described above for tissues).
Primary hippocampal neurons.
At birth, pups from Npc1+/− breeding pairs were genotyped and sacrificed one hour later by decapitation. Under a dissecting scope, the hippocampus was removed, minced, and incubated at 37°C in 10% trypsin (Sigma) for 10 min. Cells were collected by centrifugation for 5 min at 300 g, and the pellet was resuspended in media [high-glucose DMEM (Invitrogen) containing 10% heat-inactivated FBS (Atlanta Biologicals) and 1% neurobasal B27 supplement (Invitrogen)]. The primary hippocampal cells were counted and plated at 2.6 × 105 cells/cm2 on poly-L-lysine-coated plates. Once plated, the cells were maintained in a humid incubator at 37°C with 5% CO2. The next day, cells were exposed to 5 µM Ara-C (to reduce astrocyte contamination; Sigma) in DMEM supplemented with 2 mM L-glutamine. On culture day 8, cells were provided fresh media containing vehicle or treatment and then harvested for RNA using the experimental designs described above for macrophage cultures.
Measurement of plasma cytokine concentrations
For the 0 h time point, 60 µl of blood was harvested from the tail vein of Npc1+/+ or Npc1−/− mice. The mice were allowed to recover for 1 day, and then mice were injected with HP-β-CD or saline. Then 3, 6, 12, and 24 h later, another 60 µl aliquot of blood was harvested from the tail. Each tail-blood isolation was collected into a tube with 3 µl of 0.5 M EDTA on ice. Blood was spun down at 1 × 104 g for 10 min at 10°C, and plasma was moved to a fresh tube, flash frozen in liquid nitrogen, and stored at −80°C until analyses for cytokine levels were performed. Ten microliters of each sample was analyzed in duplicate, alongside standard curves, on a 7-plex ultrasensitive proinflammatory mouse cytokine plate (#K11012C-1; MesoScale Discovery, Gaithersburg, MD) according to the manufacturer's instructions. The plate was read using a SECTOR® Imager 6000 instrument (MesoScale Discovery). Concentrations for IFNγ, interleukin (IL)-1β, IL-6, IL-10, IL-12p70, KC/GRO, and tumor necrosis factor (TNF)α are reported as picogram (pg) of protein per milliliter of plasma.
Data analysis
All data are presented as the mean ± SEM. Statistically significant differences (P < 0.05) between the Npc1+/+ controls and the 0 h CD or saline-treated Npc1−/− group were tested using an unpaired Student t-test and are represented by a double dagger (‡). Significant differences between Npc1−/− mice studied 0 h after CD treatment and those studied at later time points were determined using a one-way ANOVA followed by Dunnett's posthoc analysis and are represented by asterisks (*P < 0.05 to ***P < 0.0005). All statistical tests were performed using GraphPad Prism5 software (GraphPad Software, Inc. San Diego, CA).
RESULTS
De novo cholesterol synthesis rates are reduced in Npc1−/− mice 3 h after HP-β-CD injection
As HP-β-CD is cleared within 3 to 6 h from the plasma and total body of 49-day-old mice (18), it is likely that HP-β-CD exerts its effect(s) on Npc1−/− mice during this timeframe. To test this, 49-day-old Npc1−/− mice were given a single dose of HP-β-CD, and indices of sterol balance were characterized 0 to 12 h later. Significant increases in organ weights, tissue total cholesterol content, and cholesterol synthesis rates in Npc1−/− mice compared with their wild-type littermates were evident at the outset (0 h) of this study (Fig. 1), which are consistent with previous reports (28). Over the course of these 12 h studies, the single dose of HP-β-CD caused no differences in organ weights or total cholesterol concentrations in liver and spleen of Npc1−/− mice (Fig. 1A–D). However, a significant decrease in cholesterol synthesis rates in these tissues was evident within 3 h of the HP-β-CD injection (Fig. 1E, F). By 6 h after HP-β-CD administration, hepatic cholesterol synthesis rates in Npc1−/− mice leveled off at a rate of 197 nmol/g/h and then remained steady for the duration of the 12 h study (Fig. 1E). This was a reduction of ∼80% compared with the initial rate (Npc1−/− 0 h, 1,010 nmol/g/h) in liver and was even less than that measured in Npc1+/+ mice (580 nmol/g/h). A similar timeframe of change was observed in sterol synthesis rates from spleen of Npc1−/− mice after HP-β-CD administration, although the absolute rates of cholesterol synthesis were much lower compared with liver (Fig. 1F).
Hepatic cholesteryl ester content is increased in Npc1−/− mice 3 h after HP-β-CD injection
Although there was no measurable change in total cholesterol content within the first 12 h after HP-β-CD injection in Npc1−/− mice (Fig. 1C), the relative quantities of unesterified cholesterol (largely representing cholesterol sequestered within the LE/L in the Npc1−/− model) and cholesteryl ester were measured in liver. A modest, albeit not statistically significant, decrease in hepatic unesterified cholesterol from 20.5 mg/g at 0 h to 17.1 mg/g (Fig. 2A) was observed 12 h after HP-β-CD injection in Npc1−/− mice. In contrast, hepatic cholesteryl ester concentrations in Npc1−/− mice were significantly increased at 3 h after HP-β-CD injection and reached a plateau by 6 h (Fig. 2B). The rate of change observed in cholesteryl ester content after HP-β-CD administration is consistent with that observed for cholesterol synthesis (Fig. 1E, F).
Significant changes in mRNA levels are observed 6 h after HP-β-CD injection
To identify molecular mechanisms that could account for the changes in cholesterol balance measured in Npc1−/− mice just 3 h after HP-β-CD administration, mRNA levels for cholesterol-related genes were measured. Previously, significant increases in the expression of target genes for the oxysterol-responsive nuclear hormone receptor LXR and decreases in the mRNA levels of genes regulated by the sterol-sensing transcription factor SREBP2 were reported 24 h after HP-β-CD treatment in Npc1−/− mice (5). These observed mRNA changes are consistent with an increase of intracellular sterol, which would reduce de novo cholesterol synthesis via SREBP2 and enhance cholesterol efflux and catabolism via LXR. As shown in Fig. 2, these changes are observed in the livers of Npc1−/− mice as early as 6 h after HP-β-CD treatment. The mRNA levels of the SREBP2 target genes, Hmg CoA Synthase (Hmg CoA Syn) and Hmg CoA Reductase (Hmg CoA Red), are significantly reduced 6 h after HP-β-CD by >85% compared with Npc1−/− mice at 0 h to levels even lower than those measured in Npc1+/+ mice (Fig. 2C, E). A decrease in the mRNA levels of Srebp2 and its target genes is also seen in spleen and ileum as early as 3 h after HP-β-CD treatment in Npc1−/− mice (Fig. 3A, B), although the reduction observed in these tissues was not as profound as in liver. It is noteworthy that initial changes in sterol synthesis rates (Fig. 1) occurred at or before the changes in mRNA levels of the rate-limiting enzyme Hmg CoA Red (Fig. 2), which is consistent with rapid sterol-sensing mechanisms involving posttranslational modifications that affect HMG-CoA Red protein stability and activity (reviewed in Ref. 29).
Cholesterol 7α-hydroxylase (Cyp7a1), the LXR target gene in mice, is a rate-limiting enzyme for conversion of cholesterol to bile acids in liver. It should be noted that hepatic Cyp7a1 mRNA levels exhibit a pronounced circadian rhythm (30); thus, our study design utilized staggered HP-β-CD injection times to limit tissue harvesting to a single time of day (ZT 9). Cyp7a1 mRNA levels were reduced by 50% in Npc1−/− mice compared with Npc1+/+ littermates. In Npc1−/− mice, HP-β-CD administration resulted in increased Cyp7a1 mRNA levels with a trend observed as early as 6 h and a significant increase (3-fold) evident at 12 h (Fig. 2D). The mRNA level of ATP-binding cassette transporter a1 (Abca1), another LXR target gene, was found to be only transiently increased in livers of Npc1−/− mice at 6 h after HP-β-CD (Fig. 2F). These results from liver are in contrast to Abca1 mRNA levels in spleen (Fig. 3C), where Abca1 expression was dramatically increased by 3 h (2.8-fold) after HP-β-CD in Npc1−/− mice and remained at this level throughout the 12 h study. This Abca1 expression difference between tissues can likely be explained by the fact that Abca1 is not an LXR target gene in hepatocytes, where a distinct ABCA1 promoter is utilized (31). Thus, the increase in Abca1 mRNA observed in liver is likely to result from LXR activation of other cells types, such as macrophages or resident Kupffer cells, which have very high levels of ABCA1. The decrease measured at 12 h in liver ABCA1 could be accounted for by a diminished number of macrophages. Within the first 12 h following HP-β-CD treatment, no changes in mRNA levels were observed for the LXR target gene Abcg8 in either liver (data not shown) or ileum (Fig. 3D).
No change in plasma lipoprotein cholesterol levels or evidence of a CD/cholesterol complex in plasma or urine was observed within 12 h after HP-β-CD in Npc1−/− mice
As cholesterol balance changes in Npc1−/− mice occur as early as 3 h after HP-β-CD administration during the 6 h timeframe when radiolabeled CD was shown to clear from mature mice (18), one might hypothesize that HP-β-CD binds and transports the excess cholesterol from Npc1−/− cells out of the body. An alternative hypothesis is that HP-β-CD facilitates egress of cholesterol from the LE/L of Npc1−/− cells to allow sterol to be processed by normal cellular mechanisms. To test these hypotheses, the levels of cholesterol in the plasma and urine were measured following HP-β-CD injection of Npc1−/− mice at various times consistent with the renal clearance rates of intact HP-β-CD (3).
To measure total plasma cholesterol, FPLC gel filtration for fractionation of lipoproteins was performed on pooled plasma samples (Fig. 4). First, lipoprotein profiles of Npc1+/+ and Npc1−/− mice were compared, and it was observed that plasma HDL cholesterol content (represented by fractions 23–35 in Fig. 4A) is significantly greater in Npc1−/− mice, consistent with a previous report (32). During the first 3 h after injection when HP-β-CD is still present in the plasma, there were no apparent changes in the total plasma lipoprotein cholesterol levels of Npc1−/− mice (Fig. 4B). Likewise, at time points (6 and 12 h) when changes in sterol balance are underway and HP-β-CD is no longer detectable in the whole animal, there was no evidence of altered total cholesterol content of any lipoprotein fractions in plasma from Npc1−/− mice (Fig. 4C). Notably, there was no shift in sterol within any other fractions or in the eluate collected 20 min before and after fractionation (data not shown); therefore, no increase in the level of total cholesterol was measurable in the bloodstream at any time to suggest the presence of a CD/cholesterol complex.
HP-β-CD is excreted intact into urine (3), thus urine was collected for 48 h after HP-β-CD administration to determine whether cholesterol was present as a complex with excreted CD (Fig. 4D). A significantly higher quantity of cholesterol was excreted by Npc1−/− mice (11 μg/day) compared with Npc1+/+ mice (6 μg/day). This difference was not accounted for by differences in body weight, as Npc1−/− mice excreted urinary cholesterol at a rate of 0.72 μg/day/g bw while this rate in wild-type littermates was 0.33 μg/day/g bw. This observation may be a reflection of the increased levels of cellular unesterified cholesterol in Npc1−/− mice. Trace amounts of cell lysate from epithelial cells that were shed from the urinary tract wall may have contributed to the increased amount of sterol observed in urine. Importantly however, the injection of HP-β-CD did not result in a change in urinary cholesterol excretion in any mice tested. It should be noted that the amount of cholesterol excreted in urine of mice is insignificant compared with the amount of sterol secreted from the body by the feces, which was 126 μg/day/g bw in Npc1+/+ mice and 199 μg/day/g bw in Npc1−/− mice (18).
Minimal changes were observed in the expression of cytokines within 12 h of HP-β-CD injection
Molecular markers of the proinflammatory state have been shown to be elevated in virtually all tissues of Npc1−/− mice and to increase with disease progression (28, 33, 34). These same markers are reduced when Npc1−/− mice are provided with a therapy, such as HP-β-CD, that relieves lysosomal lipid accumulation and extends lifespan (5, 12, 34, 35). In liver, the mRNA levels of Cd68 (macrosialin, a cell surface marker of monocytes and macrophages) and cytokines Mip1α, Il-12p40, Ifn-γ, Cxcl1, and Tnfα were dramatically higher in Npc1−/− mice compared with Npc1+/+ mice (Fig. 5A), and similar elevations were observed for selected cytokines in other organs (spleen, Fig. 5E; and ileum, Fig. 5F). Twenty-four hours after a single injection of HP-β-CD, the mRNA levels of elevated inflammation markers in Npc1−/− liver were significantly reduced (Fig. 5A), and comparable changes were observed for the hepatic protein levels of IFN-γ, CXCL1, and TNFα (data not shown). These findings were extended to determine whether plasma levels of these cytokines were similarly affected in Npc1−/− mice and whether they could potentially serve as biomarkers to follow NPC disease progression and/or efficacy of novel therapies. While the mRNA levels of Il-12p40, Ifn-γ, Cxcl1, and Tnfα were much higher in the livers of Npc1−/− mice and reduced with HP-β-CD treatment (Fig. 5A), these changes were not mirrored by the circulating protein levels of these cytokines in mouse plasma (Fig. 5B). A significant increase was only observed for plasma IL-6 levels 24 h after HP-β-CD injection of Npc1−/− mice; however, this increase was also evident in Npc1+/+ injected with saline (HP-β-CD) and Npc1−/− injected with saline (vehicle) (supplementary Fig. I).
As nearly all cytokine mRNA levels were reduced by 24 h after HP-β-CD administration to Npc1−/− mice (Fig. 5A), the time course of this HP-β-CD effect was evaluated in several tissues, for which a representative set of mRNA levels is provided (Fig. 5C–F). There was a trend toward decreased mRNA levels of Tnfα in liver following HP-β-CD administration, which reached significance by 12 h (Fig. 5C). There was no change in Mip1α mRNA levels in liver or spleen by 12 h (Fig. 5D, E); however, a significant decrease in Mip1α mRNA in ileum was observed as early as 6 h (Fig. 5F). In summary, changes in cytokine expression in tissues of Npc1−/− mice were absent or modest during the first 12 h after HP-β-CD despite dramatic reductions in mRNA and protein levels in liver by 24 h, suggesting that the anti-inflammatory effects of HP-β-CD are secondary to changes in sterol homeostasis.
Significant changes in mRNA levels were observed in Npc1−/− cells treated with HP-β-CD and SBE-β-CD in culture
In the adult mouse, a single subcutaneous injection of HP-β-CD at 4,000 mpk is estimated to provide a maximal plasma concentration of 1.3 mM (18). To confirm the acute changes observed for sterol balance and sterol-associated gene expression in vivo, a relevant concentration range of HP-β-CD (0.3–1.5 mM) was applied in vitro to primary neurons and macrophages cultured from Npc1+/+ and Npc1−/− mice (Fig. 6). As observed in whole animals, administration of HP-β-CD had virtually no effect in wild-type cultured cells. However, after only 4 h of exposure, gene expression changes consistent with an increase in intracellular sterol were observed in Npc1−/− cells. Representative LXR target genes, Idol in macrophages (Fig. 6A) and Abcg1 in neurons (Fig. 6B), exhibited a dose-dependent increase in mRNA level; whereas the mRNA levels of selected SREBP2 target genes, Hmg CoA Syn (Fig. 6C) and low density lipoprotein receptor (Ldlr) (Fig. 6D), were maximally reduced with the minimal dose of 0.3 mM HP-β-CD. Although the reduction in Hmg CoA Syn mRNA levels did not reach significance in macrophage, the decrease in Ldlr was statistically significant between the vehicle and HP-β-CD-treated Npc1−/− neurons.
The ability of various cyclodextrins to interact, adsorb, and ultimately extract unesterified cholesterol from lipid membranes into an aqueous environment has been extensively studied (2, 16, 36–40). Single β-CDs can accommodate an unesterified cholesterol in their hydrophobic pocket, but about one-third of the sterol molecule is expected to protrude from the CD (36, 40). In this 1:1 configuration, all β-CDs appear capable of facilitating the translocation of free cholesterol between apposing membranes (or between donor and acceptor lipid vesicles), thus behaving like a “shuttle.” To fully extract unesterified cholesterol from lipid membranes, β-CDs must form a 2:1 complex to allow for solubilization of the sterol into an aqueous environment (2), thus acting as a “sink.” HP-β-CD can form such a 2:1 complex, whereas sulfobutylether-β-CD (SBE-β-CD), which harbors chemical modifications that sterically hinder it from dimerization (2, 41), cannot form the requisite 2:1 complex with cholesterol and fails to solubilize cholesterol. To determine whether a sink/solubilizing function of HP-β-CD is essential to its mechanism of action in Npc1−/− cells, the effects of HP-β-CD and SBE-β-CD were compared in vitro, using the concentration of 0.3 mM and the readout of altered gene expression established in our previous studies (Figs. 2, 3, and 6Figs. 2, 3, and 6). Both forms of β-CD led to nearly equivalent reductions in Hmg CoA Syn and Ldlr mRNA levels in macrophages (Fig. 7A, C). In addition, SBE-β-CD and HP-β-CD exhibited comparable efficacy in inducing the LXR target gene Abcg1 and decreasing Ldlr mRNA in neurons (Fig. 7B, D). Neither HP-β-CD nor SBE-β-CD treatment had any effect of Npc1+/+ cells. Thus, these cell-based studies demonstrate a cell-autonomous activity of β-CDs and further support a mechanism of action that does not rely on the ability of cyclodextrins to solubilize cholesterol into an aqueous environment.
DISCUSSION
Niemann-Pick type C disease is a rather unique lysosomal storage disorder because the protein products of mutated genes, NPC1 or NPC2, do not harbor enzymatic activity. Rather, NPC1, a large integral protein of the lysosomal membrane, and NPC2, a small soluble protein localized to the lumen of the LE/L, work in tandem to translocate LDL-derived cholesterol from the lyosomal compartment into the cytosol of cells (42, 43). Thus, in the absence of NPC1 and/or NPC2 function, unesterified cholesterol, as well as other lipids, accumulates in the LE/L of cells, where it is inaccessible to the sterol-sensing machinery of the cytosol/nucleus. Therefore, while there is an excess of cholesterol trapped in the LE/L in NPC-diseased cells, these cells are in a state of cholesterol deprivation and therefore upregulate de novo cholesterol synthesis.
While enzyme replacement therapies are being attempted for many other lysosomal storage disorders (reviewed in Refs. 44, 45), this approach is not amenable to NPC disease caused by the loss of NPC1 function (∼95% of cases). A limited number of small-molecule therapies have been reported to extend the lifespan of Npc1−/− mice (34, 35, 46–48), but none have been shown to extend lifespan more than 10–15% without undesirable side effects. Thus, the initial report (5) demonstrating that a single injection of HP-β-CD extended the lifespan of young Npc1−/− mice by greater than 35% and subsequent studies (11, 12, 18) showing that repeated injections increased longevity by nearly 2-fold pointed to this agent as a novel therapy for NPC patients.
CDs are membrane-impermeant oligosaccharides that have been extensively used by cell biologists to manipulate cholesterol content and trafficking in cultured cells (38). In elegant studies by Rothblat and colleagues (36, 37), addition of HP-β-CD at concentrations exceeding 1 mM to the media of cultured cells acted as a cholesterol “sink,” resulting in the rapid efflux of cholesterol from these cells. The kinetics of this sterol extraction strongly suggested that HP-β-CD was desorbing cholesterol from the plasma membrane of cells into the aqueous media by the formation of a 2:1 complex (2). This mechanism has now been further studied at atomic resolution using molecular dynamics simulations (40). Notably, β-CD variants unable to form this 2:1 complex, such as tetradecasulfated-β-cyclodextrin, were unable to extract sterol under these conditions. When provided at concentrations <1 mM in the presence of added phospholipid vesicles, virtually all β-CDs could serve as a “shuttle” to transfer sterol down a concentration gradient from membrane to vesicle acceptor. Thus, the behaviors of CDs as “sinks” and “shuttles” to affect plasma membrane cholesterol have been quite thoroughly characterized.
More recent studies utilizing a methyl-β-cyclodextrin modified by the addition of a fluorescein tag demonstrated that this CD is, in fact, taken up from media into cultured cells by a clathrin-dependent pathway (49). This fluorescent CD appeared in endosomal structures of HeLa and BHK cells within minutes in a dose- and time-dependent manner. It should be noted that the cells used for these studies have functional NPC proteins, so confirmation of this behavior in NPC-deficient cells is warranted. However, the directed delivery of CD as a conjugate of dextran to the lysosomal compartment of human NPC-mutant fibroblasts results in the loss of cholesterol, as revealed by diminished filipin staining of this cellular compartment (15).
Accumulating evidence suggests that β-CD molecules can enter cells by endocytosis, and in the case of NPC-deficient cells, promote the egress of lysosomal cholesterol. But do CDs serve as a “sink” (which binds cholesterol at a 2:1 ratio to fully solubilize it) or a “shuttle” (which binds cholesterol at a 1:1 ratio) in their capacity to move sterol from this intracellular compartment, and what are the fates of CD and liberated cholesterol? The studies presented in this work addressed these questions using a complement of in vitro and in vivo models.
Using cell culture studies of neurons and macrophages isolated from wild-type and Npc1−/−mice, a 4 h exposure to increasing concentrations of HP-β-CD (ranging 0–1.5 mM) had virtually no effect on wild-type cells but caused altered gene expression consistent with sterol release from the LE/L into the cytosol of cells lacking NPC1 function (Fig. 6). A CD-facilitated egress of lysosomal sterol would be predicted to reduce processing of SREBP2 and decrease expression of its target genes, including Hmg CoA Syn and Ldlr. In addition, this flux of sterol from lysosome to cytosol/nucleus would increase the expression of LXR target genes, such as Idol and Abcg1. These findings are consistent with the recent report of Peake and Vance (50) demonstrating the reduction of lysosomal sterol and increase of cellular cholesteryl ester in cultured Npc1−/− neurons, astrocytes, and microglia treated with HP-β-CD. The cytotoxic effects they observed for high-dose HP-β-CD, which were not observed in our studies, were likely the result of longer incubation times and the absence of serum in their culture conditions.
In our subsequent comparison of HP-β-CD (which can act as “shuttle” or “sink”) against SBE-β-CD (which can function only as “shuttle”), similar changes in sterol-responsive gene expression were obtained for these two CDs, but only in Npc1−/− cells (Fig. 7). Thus, it can be concluded that under these culture conditions and CD concentrations, β-CD is not acting as an extracellular “sink” to extract/solubilize plasma membrane cholesterol, a mechanism that should affect cholesterol dynamics of both control and mutant cells. In addition, the similar behaviors of HP-β-CD and SBE-β-CD to elicit changes in intracellular sterol revealed by altered gene expression suggest that only the “shuttle” capacity of CD is required. This scenario is consistent with proposals that CD is promoting the translocation of unesterfied cholesterol from internal membranes of the LE/L across the limiting lysosomal membrane (16, 17) for egress into the cytosolic compartment of Npc1−/− cells.
A subcutaneous injection of HP-β-CD at 4,000 mpk to mice is estimated to result in maximal plasma levels of ∼1.3 mM (18) with a systemic half-life of ∼1.6 h (13). Within 4 h, greater than 90% of a CD dose is eliminated intact by urinary excretion from rats (51). On the basis of these observations, we elected to evaluate the temporal changes in sterol homeostasis and the fates of CD and sterol in Npc1−/− mice injected with HP-β-CD (summarized in supplementary Fig. II). The first significant changes observed were a pronounced decrease in sterol synthesis rates in liver and other organs (Fig. 1) and an increase in hepatic cholesteryl ester (Fig. 2B). By 3 h after injection, sterol synthesis rates were reduced by half and reached a plateau at 6 h that was maintained for the duration of these 12 h studies (at a level ∼20% of 0 h Npc1−/− and below that of wild-type mice). At 6 and 12 h after injection, changes in the expression of genes associated with altered cellular sterol balance were evident (Figs. 2 and 3). This sequence of changes is consistent with CD-mediated lysosomal cholesterol release, first to affect posttranslation mechanisms reducing sterol synthesis rates (via regulation of HMG-CoA Red stability) and increasing formation of cholesteryl ester via ACAT activity, and second to modulate transcriptional processes regulated by SREBP2 and LXR.
During this timeframe, there was no evidence of increased cellular efflux of sterol either to alter the cholesterol content of circulating lipoproteins (Fig. 4B, C) or as a CD-cholesterol complex in plasma or urine (Fig. 4B–D). Our methods were able to accurately detect significant but subtle increases (in the microgram range) in the level of cholesterol within urine and plasma in Npc1−/− mice compared with Npc1+/+ mice. Therefore, if a change had occurred after HP-β-CD to alter the level of cholesterol in plasma or urine, it would have been easily ascertained through our analyses. Thus, the breadth of these studies suggest that HP-β-CD acts within Npc1−/− cells as a shuttle to facilitate the egress of lysosomal unesterfied cholesterol to intracellular membrane or protein acceptors, without serving as a sink to facilitate cholesterol transport and elimination via plasma and/or urine.
Finally, all agents that have been identified to extend the lifespan of Npc1−/− mice have decreased expression of proinflammatory cytokines in virtually every tissue measured (5, 12, 34, 35, 47). Therefore, we included this readout in our time course studies of HP-β-CD. Twenty-four hours after injection of HP-β-CD, mRNA levels (Fig. 5) for Tnfα, Mip-1α, and other markers of increased macrophage number and activation were significantly reduced in tissues of Npc1−/− mice. However, these changes were for the most part not observed within 12 h of HP-β-CD administration, suggesting that they occur after changes in sterol homeostasis. And importantly, although tissue RNAs and proteins were altered, circulating cytokine levels were neither elevated in the Npc1−/− mice nor affected to a significant degree by CD treatment (Fig. 5 and supplementary Fig. I).
In conclusion, comprehensive studies have been done to monitor the acute effects of HP-β-CD in Npc1−/− mice. Overall, these data suggest that HP-β-CD is not acting as a “sink” to bind and carry cholesterol out of cells into plasma and/or out of the body but that it is liberating the trapped unesterified cholesterol from the lysosomes of Npc1−/− cells very rapidly by facilitating the egress of this cholesterol to intracellular sites for normal sterol processing.
Supplementary Material
Acknowledgments
The authors thank Scott Clark for processing the plasma lipoprotein profiles, and John Dietschy and Stephen Turley for their collaboration and support. The authors especially thank Brock McNeal, Lilja Kjalarsdóttir, Ryan D. Jones, and Ernest Tong for assistance and discussion.
Footnotes
Abbreviations:
- bw
- body weight
- CD
- cyclodextrin
- Cyp7a1
- cholesterol 7α-hydroxylase
- DPS
- digitonin-precipitable sterol
- FDA
- US Food and Drug Administration
- FPLC
- fast protein liquid chromatography
- HP-β-CD
- 2-hydroxypropyl-β-cyclodextrin
- IL
- interleukin
- Ldlr
- low density lipoprotein receptor
- LE/L
- late endosome/lysosome
- LXR
- liver X receptor
- mpk
- mg/kg body weight
- NPC
- Niemann-Pick type C
- SBE-β-CD
- sulfobutylether-β-cyclodextrin with 7 substitutions (trade name Captisol)
- SREBP2
- sterol regulatory element-binding protein 2
- TNF
- tumor necrosis factor
- ZT
- Zeitgeber time
This work was supported by Ara Parseghian Medical Research Foundation grants (J.J.R. and B.L.), and National Institutes of Health Grants T32-GM-007062 (A.T.) and TL1-DK-081181 (Y.M.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and one table.
REFERENCES
- 1.Irie T., Fukunaga K., Garwood M. K., Carpenter T. O., Pitha J., Pitha J. 1992. Hydroxypropylcyclodextrins in parenteral use. II: Effects on transport and disposition of lipids in rabbit and humans. J. Pharm. Sci. 81: 524–528 [DOI] [PubMed] [Google Scholar]
- 2.Thompson D. O. 1997. Cyclodextrins--enabling excipients: their present and future use in pharmaceuticals. Crit. Rev. Ther. Drug Carrier Syst. 14: 1–104 [PubMed] [Google Scholar]
- 3.Stella V. J., He Q. 2008. Cyclodextrins. Toxicol. Pathol. 36: 30–42 [DOI] [PubMed] [Google Scholar]
- 4.Gould S., Scott R. C. 2005. 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): a toxicology review. Food Chem. Toxicol. 43: 1451–1459 [DOI] [PubMed] [Google Scholar]
- 5.Liu B., Turley S. D., Burns D. K., Miller A. M., Repa J. J., Dietschy J. M. 2009. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc. Natl. Acad. Sci. USA. 106: 2377–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson M. C. 2003. A riddle wrapped in a mystery: understanding Niemann-Pick disease, type C. Neurologist. 9: 301–310 [DOI] [PubMed] [Google Scholar]
- 7.Patterson M. C., Vanier M. T., Suzuki K., Morris J. A., Carstea E. D., Neufeld E. B., Blanchette-Mackie J. E., Pentchev P. G.2001. Niemann-Pick Disease Type C: a lipid trafficking disorder. In The Metabolic and Molecular Bases of Inherited Disease. C. R. Scriver, editor. McGraw-Hill, New York. 3611–3633.
- 8.Vanier M. T. 2010. Niemann-Pick disease type C. Orphanet J. Rare Dis. 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B., Li H., Repa J. J., Turley S. D., Dietschy J. M. 2008. Genetic variations and treatments that affect the lifespan of the NPC1 mouse. J. Lipid Res. 49: 663–669 [DOI] [PubMed] [Google Scholar]
- 10.Ward S., O'Donnell P., Fernandez S., Vite C. H. 2010. 2-Hydroxypropyl-β-cyclodextrin raises hearing threshold in normal cats and in cats with Niemann-Pick type C disease. Pediatr. Res. 68: 52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson C. D., Ali N. F., Micsenyi M. C., Stephney G., Renault S., Dobrenis K., Ory D. S., Vanier M. T., Walkley S. U. 2009. Chronic cyclodextrin treatment of murine Niemann-Pick C disease ameliorates neuornal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE. 4: e6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramirez C. M., Liu B., Taylor A. M., Repa J. J., Burns D. K., Weinberg A. G., Turley S. D., Dietschy J. M. 2010. Weekly cyclodextrin administration normalizes cholesterol metabolism in nearly every organ of the Niemann-Pick type C1 mouse and markedly prolongs life. Pediatr. Res. 68: 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aqul A., Liu B., Ramirez C. M., Pieper A. A., Estill S. J., Burns D. K., Liu B., Repa J. J., Turley S. D., Dietschy J. M. 2011. Unesterified cholesterol accumulation in late endosomes/lysosomes causes neurodegeneration and is prevented by driving cholesterol export from this compartment. J. Neurosci. 31: 9404–9413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abi-Mosleh L., Infante R. E., Radhakrishnan A., Goldstein J. L., Brown M. S. 2009. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc. Natl. Acad. Sci. USA. 106: 19316–19321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum A. I., Zhang G., Warren J. D., Maxfield F. R. 2010. Endocytosis of beta-cyclodextrins is responsible for cholesterol reduction in Niemann-Pick type C mutant cells. Proc. Natl. Acad. Sci. USA. 107: 5477–5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCauliff L. A., Xu Z., Storch J. 2011. Sterol transfer between cyclodextrin and membranes: similar but not identical mechanism to NPC2-mediated cholesterol transfer. Biochemistry. 50: 7341–7349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramirez C. M., Liu B., Aqul A., Taylor A. M., Repa J. J., Turley S. D., Dietschy J. M. 2011. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J. Lipid Res. 52: 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu B., Ramirez C. M., Miller A. M., Repa J. J., Turley S. D., Dietschy J. M. 2010. Cyclodextrin overcomes the transport defect in nearly every organ of NPC1 mice leading to excretion of sequestered cholesterol as bile acid. J. Lipid Res. 51: 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loftus S. K., Morris J. A., Carstea E. D., Gu J. Z., Cummings C., Brown A., Ellison J., Ohno K., Rosenfeld M. A., Tagle D. A., et al. 1997. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 277: 232–235 [DOI] [PubMed] [Google Scholar]
- 20.Dietschy J. M., Spady D. K. 1984. Measurement of rates of cholesterol synthesis using tritiated water. J. Lipid Res. 25: 1469–1476 [PubMed] [Google Scholar]
- 21.Turley S. D., Herndon M. W., Dietschy J. M. 1994. Reevaluation and application of the dual-isotope plasma ratio method for the measurement of intestinal cholesterol absorption in the hamster. J. Lipid Res. 35: 328–339 [PubMed] [Google Scholar]
- 22.Beltroy E. P., Liu B., Dietschy J. M., Turley S. D. 2007. Lysosomal unesterifed cholesterol content correlates with liver cell death in murine Niemann-Pick type C disease. J. Lipid Res. 48: 869–881 [DOI] [PubMed] [Google Scholar]
- 23.Valasek M. A., Weng J., Shaul P. W., Anderson R. G. W., Repa J. J. 2005. Caveolin-1 is not required for murine intestinal cholesterol transport. J. Biol. Chem. 280: 28103–28109 [DOI] [PubMed] [Google Scholar]
- 24.Kurrasch D. M., Huang J., Wilkie T. M., Repa J. J. 2004. Quantitative real-time polymerase chain reaction measurement of regulators of G-protein signaling mRNA levels in mouse tissues. Methods Enzymol. 389: 3–15 [DOI] [PubMed] [Google Scholar]
- 25.Schmittgen T. D., Livak K. J. 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3: 1101–1108 [DOI] [PubMed] [Google Scholar]
- 26.Dheda K., Huggett J. F., Bustin S. A., Johnson M. A., Rook G., Zumla A. 2004. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 37: 112–114, 116, 118–119. [DOI] [PubMed] [Google Scholar]
- 27.Kosir R., Acimovic J., Golicnik M., Perse M., Majdic G., Fink M., Rozman D. 2010. Determination of reference genes for circadian studies in different tissues and mouse strains. BMC Mol. Biol. 11: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Repa J. J., Valasek M. A., Beltroy E. P., Turley S. D., German D. C., Dietschy J. M. 2005. Molecular, anatomical, and biochemical events associated with neurodegeneration in mice with Niemann-Pick Type C disease. J. Neuropathol. Exp. Neurol. 64: 323–333 [DOI] [PubMed] [Google Scholar]
- 29.Jo Y., DeBose-Boyd R. A. 2010. Control of cholesterol synthesis through regulated ER-associated degradation of HMG CoA reductase. Crit. Rev. Biochem. Mol. Biol. 45: 185–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panda S., Antoch M. P., Miller B. H., Su A. I., Schook A. B., Straume M., Schultz P. G., Kay S. A., Takahashi J. S., Hogenesch J. B. 2002. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 109: 307–320 [DOI] [PubMed] [Google Scholar]
- 31.Singaraja R. R., James E. R., Crim J., Visscher H., Chatterjee A., Hayden M. R. 2005. Alternate transcripts expressed in response to diet reflect tissue-specific regulation of ABCA1. J. Lipid Res. 46: 2061–2071 [DOI] [PubMed] [Google Scholar]
- 32.Xie C., Burns D. K., Turley S. D., Dietschy J. M. 2000. Cholesterol is sequestered in the brains of mice with Niemann-Pick Type C disease but turnover is increased. J. Neuropathol. Exp. Neurol. 59: 1106–1117 [DOI] [PubMed] [Google Scholar]
- 33.Wu Y-P., Mizukami H., Matsuda J., Saito Y., Proia R. L., Suzuki K. 2005. Apoptosis accompanied by up-regulation of TNF-α death pathway genes in the brain of Niemann-Pick type C disease. Mol. Genet. Metab. 84: 9–17 [DOI] [PubMed] [Google Scholar]
- 34.Langmade S. J., Gale S. E., Frolov A., Mohri I., Suzuki K., Mellon S. H., Walkley S. U., Covey D. F., Schaffer J. E., Ory D. S. 2006. Pregnane X receptor (PXR) activation: a mechanism for neuroprotection in a mouse model of Niemann-Pick C disease. Proc. Natl. Acad. Sci. USA. 103: 13807–13812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Repa J. J., Li H., Frank-Cannon T. C., Valasek M. A., Turley S. D., Tansey M. G., Dietschy J. M. 2007. Liver X receptor activation enhances cholesterol loss from the brain, decreases neuroinflammation, and increases survival of the NPC1 mouse. J. Neurosci. 27: 14470–14480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yancey P. G., Rodriguez W. V., Kilsdonk E. P. C., Stoudt G. W., Johnson W. J., Phillips M. C., Rothblat G. H. 1996. Cellular cholesterol efflux mediated by cyclodextrins. Demonstration of kinetic pools and mechanism of efflux. J. Biol. Chem. 271: 16026–16034 [DOI] [PubMed] [Google Scholar]
- 37.Atger V. M., de la Llera-Moya M., Stoudt G. W., Rodrigueza W. V., Phillips M. C., Rothblat G. H. 1997. Cyclodextrins as catalysts for the removal of cholesterol from macrophage foam cells. J. Clin. Invest. 99: 773–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zidovetzki R., Levitan I. 2007. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta. 1768: 1311–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puglisi G., Fresta M., Ventura C. A. 1996. Interaction of natural and modified β-cyclodextrins with a biological membrane model of dipalmitoylphosphatidylcholine. J. Colloid Interface Sci. 180: 542–547 [Google Scholar]
- 40.López C. A., de Vries A. H., Marrink S. J. 2011. Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLOS Comput. Biol. 7: e1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajewski R. A., Traiger G., Bresnahan J., Jaberaboansari P., Stella V. J., Thompson D. O. 1995. Preliminary safety evaluation of parenterally administered sulfoalkyl ether β-cyclodextrin derivatives. J. Pharm. Sci. 84: 927–932 [DOI] [PubMed] [Google Scholar]
- 42.Kwon H. J., Abi-Mosleh L., Wang M. L., Deisenhofer J., Goldstein J. L., Brown M. S., Infante R. E. 2009. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 137: 1213–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deffieu M. S., Pfeffer S. R. 2011. Niemann-Pick type C 1 function requires lumenal domain residues that mediate cholesterol-dependent NPC2 binding. Proc. Natl. Acad. Sci. USA. 108: 18932–18936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lachmann R. 2010. Treatments for lysosomal storage disorders. Biochem. Soc. Trans. 38: 1465–1468 [DOI] [PubMed] [Google Scholar]
- 45.Urbanelli L., Magini A., Polchi A., Polidoro M., Emiliani C. 2011. Recent developments in therapeutic approaches for lysosomal storage diseases. Recent Pat. CNS Drug Discov. 6: 1–19 [DOI] [PubMed] [Google Scholar]
- 46.Alvarez A. R., Klein A., Castro J., Cancino G. I., Amigo J., Mosqueira M., Vargas L. M., Yevenes L. F., Bronfman F. C., Zanlungo S. 2008. Imatinib therapy blocks cerebellar apoptosis and improves neurological symptoms in a mouse model of Niemann-Pick type C disease. FASEB J. 22: 3617–3627 [DOI] [PubMed] [Google Scholar]
- 47.Smith D., Wallom K-L., Williams I. M., Jeyakumar M., Platt F. M. 2009. Beneficial effects of anti-inflammatory therapy in a mouse model of Niemann-Pick disease type C1. Neurobiol. Dis. 36: 242–251 [DOI] [PubMed] [Google Scholar]
- 48.Zervas M., Somers K. L., Thrall M. A., Walkley S. U. 2001. Critical role for glycosphingolipids in Niemann-Pick disease type C. Curr. Biol. 11: 1283–1287 [DOI] [PubMed] [Google Scholar]
- 49.Plazzo A. P., Höfer C. T., Jicinszky L., Fenyvesi E., Szente L., Schiller J., Herrmann A., Muller P. 2012. Uptake of a fluorescent methyl-β-cyclodextrin via clathrin-dependent endocytosis. Chem. Phys. Lipids. 165: 505–511 [DOI] [PubMed] [Google Scholar]
- 50.Peake K. B., Vance J. E. 2012. Normalization of cholesterol homeostasis by 2-hydroxypropyl-β-cyclodextrin in neurons and glia from Niemann-Pick C1 (NPC1)-deficient mice. J. Biol. Chem. 287: 9290–9298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitha J., Gerloczy A., Olivi A. 1994. Parenteral hydroxypropyl cyclodextrins: intravenous and intracerebral administration of lipophiles. J. Pharm. Sci. 83: 833–837 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.