Abstract
HDL-associated paraoxonase 1 (PON1) activity has been consistently associated with cardiovascular and other diseases. Vitamins C and E intake have previously been positively associated with PON1 in a subset of the Carotid Lesion Epidemiology and Risk (CLEAR) cohort. The goal of this study was to replicate these findings and determine whether other nutrient intake affected PON1 activity. To predict nutrient and mineral intake values, 1,402 subjects completed a standardized food frequency survey of their dietary habits over the past year. Stepwise regression was used to evaluate dietary and covariate effects on PON1 arylesterase activity. Five dietary components, cholesterol (P < 2.0 × 10−16), alcohol (P = 8.51 × 10−8), vitamin C (P = 7.97 × 10−5), iron (P = 0.0026), and folic acid (0.037) were independently predictive of PON1 activity. Dietary cholesterol was positively associated and predicted 5.5% of PON1 activity, second in variance explained. This study presents a novel finding of dietary cholesterol, iron, and folic acid predicting PON1 activity in humans and confirms prior reported associations, including that with vitamin C. Identifying and understanding environmental factors that affect PON1 activity is necessary to understand its role and that of HDL in human disease.
Keywords: cholesterol/dietary, diet, dietary lipids, folate, LDL/oxidation/antioxidants, nutrition, iron, vitamin C
High density lipoprotein (HDL) levels have long believed to be cardioprotective (1) through a variety of processes, including reverse cholesterol transport in which excess cholesterol is taken to the liver for excretion (2). However, recent studies have highlighted the need to better understand the cardioprotective role of HDL. For example, two recent Mendelian randomization studies found that single nucleotide polymorphisms (SNP) that predict plasma HDL cholesterol (HDL-C) variance were not associated with myocardial infarction (MI) (3, 4). Moreover, a recent drug trial with torcetrapib, a cholesterol ester transport protein inhibitor, found that although torcetrapib increased levels of HDL and lowered deleterious low density lipoprotein (LDL) levels, it did not affect atherosclerotic measures and paradoxically led to an increase in adverse cardiovascular events (5). In addition, a recent large randomized clinical trial did not show a benefit of niacin in preventing adverse cardiovascular outcomes, despite improved levels of HDL-C and triglycerides in the treatment group (6). These results refocus attention on the many aspects of HDL biology that are not captured by serum measurements of HDL, such as its paraoxonase 1 (PON1) activity, which itself is inversely associated with cardiovascular and other human diseases.
The activity of PON1, a liver-produced glycoprotein enzyme bound to the surface of HDL, is consistently correlated with atherosclerotic vascular disease and end-organ damage (7–9). PON1 is at least partially responsible for the cardioprotective inhibitory effects of HDL on LDL peroxidation (10–12), and it also has been demonstrated to hydrolyze oxidized lipid or lipid hydroperoxides in LDL (13). Inactivation of PON1 reduced the ability of HDL to inhibit both the oxidation of LDL and the interaction between macrophages and endothelium (12), both likely key factors in the inflammatory changes underlying atherogenesis. PON1-deficient mice cannot neutralize the oxidized LDL lipids and have an increased susceptibility to organophosphate toxicity and coronary heart disease (CHD) (14, 15). Finally, PON1 activity appears to plays a role in maintaining the endothelial-atheroprotective effects of HDL (16).
PON1 has broad substrate specificity and is protective against exposure to toxic organophosphorus insecticides (17). For biological purposes, PON1 activity is generally measured with regard to the rate of hydrolysis of paraoxon, diazoxon, and phenylacetate (arylesterase activity) (18, 19). These are termed POase, DZOase, and AREase activities, respectively. AREase activity is unaffected by the functional PON1Q192R polymorphism, thus making it the best reflection of the levels of PON1 protein (20).
PON1 enzyme activity is influenced by both genetic and environmental factors. There are four well-established functional PON1 mutations (21): two missense mutations [PON1Q192R (rs662) and PON1M55L (rs854560)] and two 5′ regulatory [PON1-108C/T (rs705379) and PON1-162A/G (rs705381)]. PON1-108C/T has the largest effect on activity, altering expression likely due to modification of an Sp1 binding site (22). Recent findings within this Carotid Lesion Epidemiology and Risk (CLEAR) cohort attribute approximately 21% of PON1 AREase activity to the four functional PON1 mutations and six additional common variants within the PON gene cluster (including PON2 and PON3) in 1,328 European males (23). Rare deleterious variants have also been identified (24).
Environmental factors that influence PON1 enzyme activity include tobacco use, which has been reported to depress PON1 enzyme activity and concentration (25). Moderate alcohol consumption (∼40 g per day) has been reported to increase PON1 activity (26–28), whereas heavy alcohol drinking (>80 g per day) has the opposite effect (28, 29). With regard to dietary intake, fatty meals rich in oxidized lipids have been reported to decrease (30), whereas diets rich in olive oil (31) and monounsaturated fats (32) increase, postprandial PON1 activity. PON1 activity is affected by drugs: statin use (33) and anti-diabetic drugs, such as sulphonylureas (34) and rosiglitazone (35), have been reported to increase PON1 enzyme activity. As well, PON1 has been reported to be an antidiabetic enzyme (36) that increases insulin release from pancreatic β cells (37).
In 2002, Jarvik et al. first reported the associations of the antioxidant vitamins C (ascorbic acid) and E (e.g., α-tocopherol) with an increase in PON1 POase and DZOase activities in an overlapping, but much smaller, subset of the CLEAR cohort (n = 189 versus 1,402 in the current study) (38). Experiments in quail demonstrated that vitamin C reversed the decrease in PON1 enzyme activity induced by heat stress, especially when given in conjunction with folic acid (39). In addition, a study of healthy humans showed that vitamin E supplementation prevented an exercise-induced decrease in PON1 activity (40).
However, the results of Jarvik et al. have not been restudied in humans, nor have other dietary environmental factors, such as fat and cholesterol intake or other vitamin use, been investigated with regard to effects on PON1 activity. Thus, the goal of the present study was to examine the effects of common nutrients on PON1 activity as measured by AREase enzyme activity, to confirm previous dietary relationships with PON1 and to determine whether any novel associations exist within this CLEAR study cohort.
METHODS
Ethics statement
Institutional review boards at the University of Washington, Virginia Mason Medical Center, and Veterans Affairs Puget Sound Health Care approved this study. Written, informed consent was obtained from all participants.
Sample
The study population for this analysis consisted of 1,402 samples from the previously described Carotid Lesion Epidemiology and Risk (CLEAR) study (8, 9, 41). The cohort includes 402 subjects with carotid artery disease (CAAD) and 857 controls, and 143 subjects of other phenotypes, including moderate carotid artery obstruction (15–49% by ultrasound), as well as coronary artery and peripheral artery disease. Descriptive statistics are presented in Table 1. Current smoking status and reported ancestry were obtained by self-reporting. Medication use, including statins and insulin injection, were ascertained from the Veterans Affairs Puget Sound Health Care medical record review. Exclusion criteria included familial hypercholesterolemia, total fasting cholesterol greater than 400 mg/dl, hypocoagulable state and/or the use of anticoagulant medication, postorgan transplant, or the inability to consent.
TABLE 1.
Baseline characteristics of the CLEAR cohort
| Baseline Characteristics | CLEAR Cohort (n = 1,402) |
| Gender, n (%) | |
| Female | 444 (31.7%) |
| Male | 958 (68.3%) |
| Ethnicity, n (%) | |
| Asian/Pacific Islander, Native American, or other ancestry | 134 (9.6%) |
| African ancestry, not Hispanic | 113 (8.1%) |
| European ancestry, not Hispanic | 1126 (80.3%) |
| Hispanic ancestry | 29 (2.0%) |
| Age, mean ± SD, years | 65.84 ± 9.31 |
| Current smoking status, n (%) | 156 (11.1%) |
| Diabetes status, n (%) | 195 (13.9%) |
| Medication use | |
| Statin use, n (%) | 508 (36.2%) |
| Oral hypoglycemic use, n (%) | 164 (11.7%) |
| Insulin use, n (%) | 67 (4.8%) |
| Ln(dietary cholesterol) intake, mean ± SD, mg/day | 5.24 ± 0.21 |
| Ln(vitamin C) intake, mean ± SD, mg/day | 5.42 ± 1.01 |
| Ln(vitamin E) intake, mean ± SD, mg/day | 3.43 ± 1.38 |
| Folate intake, mean ± SD, µg/day | 338.9 ± 291.3 |
| Iron intake, mean ± SD, mg/day | 20.4 ± 16.5 |
| Alcohol intake | |
| 0 = 0 g/day, n (%) | 592 (42.2%) |
| 1 = 0–12 g/day, n (%) | 485 (34.6%) |
| 2 = 12–24 g/day, n (%) | 170 (12.1%) |
| 3 = 24–60 g/day, n (%) | 130 (9.3%) |
| 4 = > 60 g/day, n (%) | 25 (1.8%) |
| Plasma lipid measurementsa | |
| ApoA1, mean ± SD, mg/dl | 148.92 ± 29.35 |
| Plasma cholesterol, mean ± SD, mg/dl | 192.74 ± 38.71 |
| HDL-C, mean ± SD, mg/dl | 52.93 ± 16.85 |
| LDL-C, mean ± SD, mg/dl | 112.80 ± 33.24 |
| VLDL-C, mean ± SD, mg/dl | 26.71 ± 16.51 |
| AREase activity, mean ± SD, IU | 150.7 ± 51.18 |
| AREase/HDL-C ratio, mean ± SDb | 3.03 ± 1.19 |
Plasma lipid measurements taken from a subset of the CLEAR cohort (n = 1,388). Although raw values are shown in the table, analyses considered natural log-transformed lipid measures.
AREase/HDL-C ratio calculated using a subset of the CLEAR cohort (n = 1,388).
Survey methods
Subjects were asked to complete the standardized Harvard food frequency questionnaire developed by the Health Professionals Follow-Up Study (41a). The survey asked about i) the average frequency of intake over the previous year of specified portions of 131 foods and ii) the use of vitamins and mineral supplements, including the dose and duration of use. Questions regarding brand of multivitamins and cereal used were asked to clarify the quantities of specific vitamin supplementation. Subjects were excluded from analyses if i) their caloric intake was not between 800 and 4,200 kcal/day or ii) their surveys had more than 70 blank items of a total of 131 questions. All vitamin usage was energy-adjusted to 2,000 kcal/day. This food frequency survey has been validated against two 1-week diet records taken approximately 6 months apart (42).
Genotyping and PON1 phenotypes
The four known functional PON1 polymorphisms with largest effects on activity, PON1Q192R, PON1M55L, PON1-108C/T, and PON1-162A/G, were genotyped using previously described methods (22, 43). PON1 arylesterase activity measured by degradation of phenylacetate (AREase) was utilized as the primary measured outcome of PON gene cluster variation, due to its closer correlation with protein levels. The PON1 AREase were measured by a continuous spectrophotometric assay with lithium heparin plasma as previously described (43). AREase activity was measured in triplicate and averaged.
Plasma lipid measurements
Lipid measurements were performed on fasting whole plasma. Standard enzymatic methods were utilized to determine the levels of total cholesterol, triglycerides, very low-density lipoprotein (VLDL), HDL, and apolipoprotein A1 (apoA1) (44–46). LDL was calculated using the Friedwald equation (47) or directly measured if triglycerides were elevated.
Analysis
Of the estimated nutrient intakes from the Health Professionals Food Survey, 47 variables had complete and nonredundant data across the cohort. Natural log transformation was performed for covariates that displayed a skewed distribution, including dietary cholesterol, vitamins C and E, and all of the plasma lipid measures. Extreme observations were Winsorized at 3 standard deviations from the mean (48). Due to the distribution of subjects across the alcohol intake variable, a previously reported semi-quantitative measure was adopted (49, 50), with 0 = no alcohol consumption in the past year, 1 = 0–12 g/day, 2 = 12–24 g/day, 3 = 24–60 g/day, and 4 = >60 g/day.
Analyses were done in R (http://www.r-project.org/) using the standard regression tools available. Genotypes were coded using an additive model. Stepwise linear regression was performed with 47 dietary covariates entering the model. Model comparison was done using Akaike's information criterion (AIC) to examine the fit of each model, beginning with a base model that included age, sex, current smoking status, insulin use, race dummy covariates (with European ancestry as the baseline, as it represented the majority of the cohort), and the genotypes for the four functional PON1 SNPs as covariates (8, 9, 51). Dietary covariates that were included in the final model increased the ability of the model to predict PON1 AREase activity. A secondary analysis to test the hypothesis that ln(dietary cholesterol) predicted variation in AREase/HDL ratio was performed using linear regression, with age, sex, current smoking status, insulin use, race dummy covariates, and genotypes for the four functional PON1 SNPs as covariates.
Statin drug use can influence PON1 expression, and this appears to be influenced by PON1-108 genotype (52). However, statin drug use could not be included as a covariate due to confounding with CAAD status; the preferential use of statins in cases in the CLEAR cohort could lead to an erroneous estimation of statin effects on PON1 activity.
RESULTS
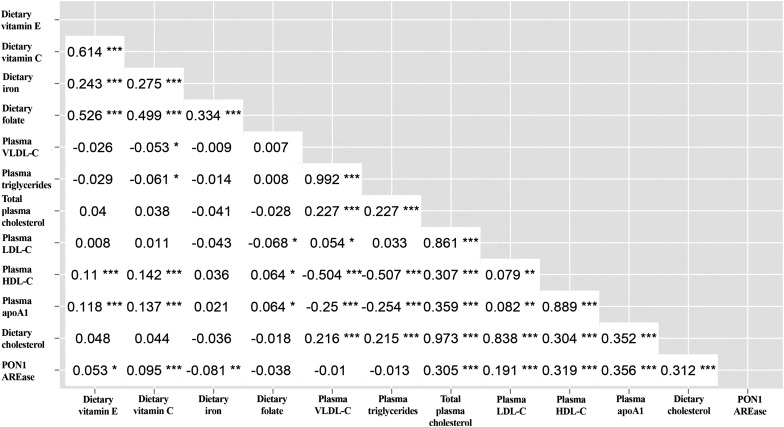
Demographic, clinical, dietary, and plasma lipid measures for the cohort are presented in Table 1. Of the dietary and plasma lipid measures, vitamin E was correlated with folic acid (r = 0.53) and vitamin C (r = 0.61), and vitamin C was also associated with folic acid (r = 0.50), with P < 0.001 for all three correlations. Dietary cholesterol intake was strongly correlated with plasma total cholesterol (r = 0.97) and LDL (r = 0.84) levels (both P < 0.001). In addition, apoA1 was highly associated with HDL-C levels (r = 0.89, P < 0.001). Plasma total cholesterol was associated with HDL-C (r = 0.31, P < 0.001) and apoA1 (r = 0.36, P < 0.001) levels. Although AREase activity was correlated with P < 0.001 with dietary cholesterol (r = 0.31), plasma cholesterol (r = 0.31), apoA1 (r = 0.36), and LDL levels (r = 0.19), AREase was not significantly correlated with VLDL levels (r = −0.01, P = 0.70). Pairwise correlations between selected phenotypes are summarized in Fig. 1.
Fig. 1.
Correlation matrix between AREase and selected dietary and plasma lipid, vitamin, and mineral covariates. Value inside each box represents r, the pairwise correlation coefficient, unadjusted for covariates. *P < 0.05, **P < 0.01, and ***P < 0.001.
A baseline regression model containing the four functional PON1 mutations (PON1Q192R, PON1M55L, PON1−108C/T, and PON1−162A/G), age, sex, current smoking status, insulin use, and the four race dummy variables explained 26.5% of PON1 AREase enzyme activity. We then examined a best-fit model utilizing stepwise linear regression including the aforementioned variables plus 47 dietary covariates. As a result, only dietary intake of nutrients or minerals that improved the predictive power of the best-fit model using an AIC criterion were retained in the full model.
In addition to the base model of the four functional PON1 mutations, age, sex, and current smoking status, an additional five dietary variables were retained in the best-fit model (Table 2). Together, the base model and these five dietary variables explained 34.67% of PON1 AREase activity. Addition of these dietary predictors (cholesterol intake, alcohol category, vitamin C, iron, and folic acid) explained 5.45%, 1.59%, 0.33%, 0.58%, and 0.21% of AREase activity, respectively (see Fig. 2). Dietary cholesterol, alcohol use, and vitamin C were associated with an increase in AREase activity. Iron and folic acid were associated with a decrease in AREase activity. Sensitivity analyses repeating the analyses when stratifying by sex, CAAD status, or statin use did not reveal significant differences in coefficient sizes or direction of effects in stratified subgroups, suggesting that these factors do not influence the association of the dietary factors and paraoxonase.
TABLE 2.
Best-fit model from stepwise regression predicting PON1 AREase activity utilizing diet nutritional intake variables
| Variable | Coefficient (± SE) | % AREase Variation | Pa |
| (Intercept) | −101.00 (± 36.40) | — | 0.0058 |
| PON1C-108T | −27.00 (± 2.01) | 12.62% | < 2 × 10−16 |
| PON1G-162A | −2.33 (± 2.25) | 0.093% | 0.302 |
| PON1Q192R | −13.20 (± 2.09) | 1.86% | 3.70 × 10−10 |
| PON1M55L | −6.89 (± 2.18) | 1.03% | 0.0016 |
| Age | −0.88 (± 0.13) | 3.78% | 6.22 × 10−12 |
| Sex | 15.90 (± 2.56) | 3.65% | 5.98 × 10−10 |
| Current smoker | −13.30 (± 3.67) | 1.46% | 3.18 × 10−4 |
| Insulin use | −16.40 (± 5.38) | 0.54% | 0.0024 |
| African ancestry | −7.04 (± 8.89) | 0.80% | 0.428 |
| Asian/NA ancestry | 4.89 (± 8.92) | 0.04% | 0.584 |
| Hispanic ancestry | 8.02 (± 11.0) | 0.003% | 0.466 |
| Ln(dietary cholesterol)b | 59.70 (± 6.17) | 5.45% | <2 × 10−16 |
| Alcohol category | 6.03 (± 1.12) | 1.59% | 8.51 × 10−8 |
| Ln(vitamin C)c | 5.22 (± 1.32) | 0.33% | 7.97 × 10−5 |
| Iron | −0.22 (± 0.07) | 0.58% | 0.0026 |
| Folate | −0.0096 (± 0.0045) | 0.21% | 0.037 |
NA, Native American.
P values were calculated from the coefficients (from all subjects) and standard errors within the best-fit multivariate model by the lm function in R.
When plasma cholesterol replaced dietary cholesterol as a covariate in the stepwise regression model, it had a P < 2 × 10−16, a coefficient of 59.75 ± 6.04, and explained 5.35% of AREase variance (n = 1388).
When ln(vitamin C) was replaced in the model with ln(vitamin E), ln(vitamin E) was significant (P = 0.048) with a coefficient = 1.91 ± 0.96 and predicted 0.15% of PON1 AREase activity.
Fig. 2.
Percentage of PON1 AREase variation explained by the covariates considered. Refer to Table 2 for complete stepwise model information.
Vitamin E did not enter the AREase prediction model. However, as it was highly correlated with both vitamin C (r = 0.60) and folic acid (r = 0.53), we sought to determine whether it would be significant if vitamin C were excluded from the best-fit model. In this stepwise regression, ln(vitamin E) was significant (P = 0.048) with a coefficient = 1.91 ± 0.96 and predicted 0.15% of AREase activity.
Secondarily, we removed the 189 subjects that were previously considered by Jarvik et al. (38) to examine the effects of vitamin C in independent data from the prior report (n = 1,213). Vitamin C remained significant (P = 0.0013) with a coefficient = 4.27 ± 1.32 and predicted 0.47% of AREase activity, replicating the original finding of dietary vitamin C predicting paraoxonase activity in independent data.
The majority of the predictive power for AREase from the new dietary covariates was attributed to dietary cholesterol intake (5.45% of 8.20%), which was positively associated with AREase activity in the best-fit model. However, dietary cholesterol is associated with an increase in atherosclerotic plaque (53), whereas PON1 enzyme activity is inversely correlated with atherosclerosis. To explore this paradox, we created a best-fit model for AREase in a subset of the cohort (n = 1,388) in which dietary cholesterol was replaced by plasma cholesterol to see whether the effect was the same. Similar to dietary cholesterol intake, plasma cholesterol was the most significant covariate to join the model (P < 2 × 10−16), explained 5.35% of AREase variation, and had a coefficient (59.75 ± 6.04) with the same direction and similar effect size as dietary cholesterol.
To examine whether the effects of dietary cholesterol were still significant when plasma lipid measures were added to the model, we created a second best-fit model for AREase in a subset of the cohort (n = 1,388), including the base model, the five dietary factors, and five natural log transformed lipid and lipoprotein plasma measures: apoA1, HDL, LDL, total cholesterol, and VLDL. In addition to the base model, all five of the dietary covariates and apoA1 were retained in the stepwise regression model (see Table 3), together explaining 39.39% of PON1 activity, with 13.8% attributable to new variables in the model. In this model, apoA1 was the second most significant predictor of AREase after PON1-108, explaining 8.74% of the variance in PON1 enzyme activity. However, dietary cholesterol remained a significant (P = 7.27 × 10−13) predictor of AREase activity (2.49%). Although the dietary cholesterol effect size was decreased (β coefficient = 59.1 in the diet-only model, 44.0 in the plasma measures and diet model), it remained strongly associated with AREase activity. Again, when adding plasma cholesterol to the model, a similar effect size (42.1) and level of significance (P = 8.54 × 10−12) were observed. However, plasma cholesterol was not a significant predictor of AREase when dietary cholesterol is included in the predictive model. Finally, we determined that ln(dietary cholesterol) was a significant predictor of AREase/HDL-C ratio (P = 0.001), with a β coefficient and standard deviation equal to 0.50 and 0.15, respectively, through linear regression adjusting for age, sex, current smoking status, insulin use, race dummy covariates, and the four PON1 functional mutations.
TABLE 3.
Best-fit model from stepwise regression predicting PON1 AREase activity utilizing dietary and plasma measures
| Variable | Coefficient (± SE) | % AREase Variation | Pa |
| (Intercept) | −317.70 (±41.10) | — | 2.06 × 10−14 |
| PON1C-108T | −27.07 (±1.92) | 12.89% | < 2 × 10−16 |
| PON1G-162A | −4.30 (±2.16) | 0.049% | 0.046 |
| PON1Q192R | −14.73 (±2.01) | 2.12% | 3.39 × 10−13 |
| PON1M55L | −9.52 (±2.10) | 1.35% | 6.29 × 10−6 |
| Age | −1.01(±0.12) | 3.99% | 2.41 × 10−16 |
| Sex | 4.61(±2.66) | 2.98% | 0.083 |
| Current smoker | −10.72 (±3.55) | 1.45% | 0.0026 |
| Insulin use | −15.89 (±5.15) | 0.42% | 0.0021 |
| African Ancestry | −18.18 (± 8.61) | 0.806% | 0.135 |
| Asian/NA Ancestry | −3.34 (± 8.64) | 0.0098% | 0.698 |
| Hispanic Ancestry | 5.42 (± 10.69) | 0.0053% | 0.612 |
| Ln(apoA1) | 65.59 (±6.74) | 8.74% | < 2 × 10−16 |
| Ln(dietary cholesterol)b | 43.97 (±6.07) | 2.49% | 7.27 × 10−13 |
| Ln(vitamin C) | 4.78 (±1.26) | 0.48% | 1.5 × 10−4 |
| Iron | −0.20 (±0.07) | 0.38% | 0.0057 |
| Folate | −0.01(±0.001) | 0.23% | 0.019 |
| Alcohol category | 2.37 (±1.10) | 0.25% | 0.031 |
P values were calculated from the coefficients (from all subjects) and standard errors within the best-fit multivariate model by the lm function in R.
When plasma cholesterol replaced dietary cholesterol as a covariate in the stepwise regression model, it had a P = 8.54 × 10−12, a coefficient of 42.14 ± 6.12, a t-statistic of 6.89, and explained 2.10% of AREase variance.
DISCUSSION
PON1 has broad substrate specificity, not only metabolizing pesticides but also statins (54) and the quorum-sensing factor of pseudomonas aeruginosa (55, 56). PON1 has an equally broad influence on human health, having been linked to vascular disease, Parkinson's disease (57–59), systemic lupus erythematosus (60–62), breast cancer (63, 64), age-related macular degeneration (65–71), and diabetes (36, 37) among other disorders. A fuller understanding of the determinants of PON1 activity should include the effects of diet.
In the current study, we utilized a cohort 7.42 times larger than the sample first used by Jarvik et al. (38) to examine the effects of dietary nutrient and mineral intake on PON1 activity. We believe that the associations of dietary cholesterol, iron in nonanemic patients, and folate with PON1 activity in humans have not been previously reported. We also replicate past findings of vitamin C being associated with PON1 activity in an independent group of subjects. Vitamin E does not remain in the predictive model due to its correlation with vitamin C, but it does predict AREase if vitamin C is not considered in the model. Finally, we confirm prior reports of alcohol intake associating with AREase activity.
Dietary cholesterol predicted 5.45% of AREase activity (of a total 8.2% related to diet) in a best-fit model utilizing only dietary covariates. It was positively correlated with AREase activity. This may be contrary to expectations, as PON1 activity is considered atheroprotective, whereas cholesterol intake has been linked with increased risk of CHD (53). However, this same direction of effect and similar effect size were seen when replacing plasma cholesterol measures with dietary cholesterol intake in the stepwise regression. Dietary cholesterol has been described as increasing PON1 in baboons undergoing dietary interventions by Rainwater et al. (72). Rainwater et al. hypothesized that the reason for the positive association between dietary cholesterol and PON1 activity in baboons was that dietary cholesterol acted as a surrogate for HDL, which is where PON1 is localized. However, in our analyses, although dietary cholesterol was correlated with HDL (r = 0.30) and its major lipoprotein component, apoA1 (r = 0.35), dietary cholesterol remained significant in a model that included the significant effects of apoA1, indicating that this hypothesis does not fully explain the relationship between dietary cholesterol and AREase in our study. This is consistent with the result that dietary cholesterol positively predicted variation in the AREase/HDL-C ratio, indicating additional AREase activity exceeds any increase in HDL-C.
Alternative hypotheses for the mechanism of increased AREase activity and AREase/HDL-C ratio associated with dietary cholesterol include that hepatic PON1 expression is upregulated or that enzymatic activity is directly increased by feedback mechanisms initiated by, or downstream of, increased cholesterol intake. PON1’s transcription regulation includes lipid regulatory elements. The PON1 5′ region has two sterol responsive element (SRE)-like regions, and sterol regulatory element binding protein 2 (SREBP2) has been shown to bind to the PON1 promoter and increase expression (52). SREBP2 also upregulates LDLR; thus, it is possible that the hepatocytes respond to the increased cholesterol load or a correlated elevation, such as that of insulin (73), by activating SREBP2, which then activates PON1 expression. The putative antiatherogenic dietary flavonoid quercetin has been shown to increase both PON1 (74) and LDLR (75) expression via the SRE pathway. Another alternative hypothesis for the increased AREase activity associated with dietary cholesterol is the role of oxidized LDL in increasing the AREase/HDL ratio. Increased LDL has been reported to be associated with increased oxidized LDL (76). Elevated oxidized LDL has been reported to be increased along with an increased AREase/HDL ratio in subjects at risk of end stage renal disease (77). Further studies, including functional analyses, will be necessary to elucidate the etiology of this paradoxical relationship between dietary cholesterol and plasma PON1 AREase activity.
Jarvik et al. first reported the association of the antioxidants vitamin C and E with PON1 POase and DZOase activity utilizing a smaller, overlapping portion of the cohort (n = 189) that was composed entirely of European males (38). In this study, we have utilized a larger cohort with mixed ethnicity and sex to replicate the association and direction of effect for vitamin C while also discovering that folic acid intake was significant in the CLEAR cohort, similar to previous reports in quail (39). While we do not find prior reports of folate decreasing PON1 activity in humans, B12 treatment in subjects with B12 deficiency increased AREase (78). When we removed the 189 subjects previously considered by Jarvik et al., vitamin C remained significant and with the same positive direction of effect. Thus, we can confirm that the effects of vitamin C replicated in a nonoverlapping and larger cohort. In addition, our results suggest that vitamin E failing to improve prediction of AREase is due to the high correlation between it and vitamin C.
With regard to the finding of iron being negatively associated with AREase, Aslan et al. previously observed an association between iron-deficiency anemia (IDA) and decreased levels of PON1 (79). However, other studies since have not yet replicated this finding (78, 80). Our result that dietary iron supplementation is associated with decreased PON1 levels in human subjects with normal iron homeostasis is not consistent with the Aslan result. This difference can potentially be attributed to the following factors. First, the selection criterion for the cohorts are fundamentally different, as CLEAR was primarily for CAAD, and these studies selected for IDA. The physiological effects of iron observed in our cohort could potentially only be observed in subjects of normal iron homeostasis. Moreover, the Aslan report of association between PON1 and iron did not adjust for the potential confounder of impaired hemoglobin synthesis. In a more recent study, it was found that depleted iron stores by itself was not associated with PON1, whereas impaired erythropoiesis resulting from IDA was (80). Finally, our study was much larger (n = 1,402 versus 25 cases and 22 controls in Aslan et al.), with greater power to detect the potentially small change in AREase activity attributed to iron intake. Our result is consistent with prior reports of various metals (81–83), including iron (84, 85), inhibiting PON1 activity in vitro.
The strong positive correlation of apoA1 and AREase activity supports past reports (72, 86). ApoA1 is a major component of HDL and is largely responsible for reverse cholesterol transport in which excess cholesterol is transferred to the liver for excretion (2). Oral administration of exogenous apoA1 peptides alone is sufficient to decrease atherosclerosis in mice, independent of changes in total or HDL cholesterol (87). As PON1 is localized to HDL and apoA1 is a major component of HDL, it is unsurprising that they are strongly correlated.
The positive association of alcohol intake and PON1 activity is consistent with past findings (26–28). It has been suggested that alcohol's effect on protein kinase C (PKC) may phosphorylate Sp1 and regulate the binding of Sp1 to the promoter region of PON1 (88), potentially explaining the positive effect of alcohol on PON1 expressions levels. Although Sp1 levels are associated with PON1 expression, overexpression of PKC results in downregulation of PON1 (28); thus, heavy alcohol consumption leads to a decrease in PON1.
Although the positive association of dietary cholesterol and PON1 activity appears paradoxical given their opposing relationships with vascular disease, the other predictors of PON1 activity have PON1 effects consistent with their known relationship to vascular disease. Dietary vitamin C is inversely associated with vascular disease (89), and dietary iron from red meat is associated with increased vascular risk (89). As reviewed elsewhere (90), moderate alcohol consumption is consistently associated with reduced vascular risk. Folate supplementation has not been found to be cardioprotective (91).
Some limitations of this study must be considered. First, this cohort is composed primarily of older Europeans collected for the presence or absence of CAAD. This could limit the generalizations drawn from these findings. However, stratifying by CAAD status or statin use did not impact our results. Second, the dietary covariates that were analyzed are limited to those from the Health Professionals Follow-Up Study. As a result, some dietary intakes that have been reported to be associated with PON1, such as polyphenols found in pomegranate (17, 92), were not analyzed in this study.
In conclusion, our analysis of dietary intake data in the CLEAR cohort has identified a novel finding in humans of dietary cholesterol being associated with PON1 AREase activity. We also replicate past findings of vitamin C intake predicting PON1 activity and report the effects of alcohol intake, folic acid, iron, and insulin use on AREase. The dietary variables alone accounted for an additional 8.2% of PON1 AREase activity, for a total of 34.67% of variance explained. When considering plasma lipid measures, apoA1 also entered the best-fit model, which now explained 39.39% of PON1 AREase activity. Given the importance of PON1 in the pathogenesis of numerous human diseases, additional work into rare genetic variation, epistasis, and gene-environment interactions will be needed to further elucidate the determinations of PON1 enzymatic activity.
Acknowledgments
The authors thank the study participants. The authors also thank the following people for their technical assistance: Tamara Bacus, Edward Boyko, Laura McKinstry, and Martha Horike-Pyne.
Footnotes
Abbreviations:
- AREase
- PON1 arylesterase enzyme activity
- AIC
- Akaike' s information criterion
- CAAD
- carotid artery disease
- CHD
- coronary heart disease
- CLEAR
- Carotid Lesion Epidemiology and Risk cohort
- DZOase
- PON1 diazoxon hydrolysis
- POase
- PON1 paraoxon hydrolysis
- PON
- paraoxonase, SNP, single nucleotide polymorphism
This work was supported by National Institutes of Health Grant RO1-HL-67406. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. This work was also supported by a State of Washington Life Sciences Discovery Award to the Northwest Institute of Genetic Medicine. DSK is supported by a Sarnoff Cardiovascular Research Fellowship for Medical Students award. This work utilized resources of SeattleSNPs; NHLBI Program for Genomic Applications, SeattleSNPs, Seattle, WA (http://pga.gs.washington.edu). Past work in this cohort was supported in part by VA Puget Sound Health Care System, Seattle, Washington, including the Veterans Affairs Epidemiology Research and Information Center Program (award CSP-701S).
REFERENCES
- 1.Gotto A. M., Jr, Brinton E. A. 2004. Assessing low levels of high-density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report and update. J. Am. Coll. Cardiol. 43: 717–724 [DOI] [PubMed] [Google Scholar]
- 2.Stein O., Dabach Y., Hollander G., Ben-Naim M., Halperin G., Breslow J. L., Stein Y. 1997. Delayed loss of cholesterol from a localized lipoprotein depot in apolipoprotein A-I-deficient mice. Proc. Natl. Acad. Sci. USA. 94: 9820–9824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haase C. L., Tybjaerg-Hansen A., Qayyum A. A., Schou J., Nordestgaard B. G., Frikke-Schmidt R. 2012. LCAT, HDL cholesterol and ischemic cardiovascular disease: a Mendelian randomization study of HDL cholesterol in 54,500 individuals. J. Clin. Endocrinol. Metab. 97: E248–E256 [DOI] [PubMed] [Google Scholar]
- 4.Voight B. F., Peloso G. M., Orho-Melander M., Frikke-Schmidt R., Barbalic M., Jensen M. K., Hindy G., Holm H., Ding E. L., Johnson T., et al. 2012. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 380: 472–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkowski R. S., Frishman W. H. 2008. HDL metabolism and CETP inhibition. Cardiol. Rev. 16: 154–162 [DOI] [PubMed] [Google Scholar]
- 6.AIM-HIGH Investigators, Boden W. E., Probstfield J. L., Anderson T., Chaitman B. R., Desvignes-Nickens P., Koprowicz K., McBride R., Teo K., Weintraub W. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267 [DOI] [PubMed] [Google Scholar]
- 7.Mackness M. I., Mackness B., Durrington P. N. 2002. Paraoxonase and coronary heart disease. Atheroscler. Suppl. 3: 49–55 [DOI] [PubMed] [Google Scholar]
- 8.Jarvik G. P., Rozek L. S., Brophy V. H., Hatsukami T. S., Richter R. J., Schellenberg G. D., Furlong C. E. 2000. Paraoxonase (PON1) phenotype is a better predictor of vascular disease than is PON1192 or PON155 genotype. Arterioscler. Thromb. Vasc. Biol. 20: 2441–2447 [DOI] [PubMed] [Google Scholar]
- 9.Jarvik G. P., Hatsukami T. S., Carlson C., Richter R. J., Jampsa R., Brophy V. H., Margolin S., Rieder M., Nickerson D., Schellenberg G. D., et al. 2003. Paraoxonase activity, but not haplotype utilizing the linkage disequilibrium structure, predicts vascular disease. Arterioscler. Thromb. Vasc. Biol. 23: 1465–1471 [DOI] [PubMed] [Google Scholar]
- 10.Mackness M. I., Arrol S., Abbott C., Durrington P. N. 1993. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis. 104: 129–135 [DOI] [PubMed] [Google Scholar]
- 11.Graham A., Hassall D. G., Rafique S., Owen J. S. 1997. Evidence for a paraoxonase-independent inhibition of low-density lipoprotein oxidation by high-density lipoprotein. Atherosclerosis. 135: 193–204 [DOI] [PubMed] [Google Scholar]
- 12.Watson A. D., Berliner J. A., Hama S. Y., La Du B. N., Faull K. F., Fogelman A. M., Navab M. 1995. Protective effect of high-density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Invest. 96: 2882–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao H., Girard-Globa A., Berthezene F., Moulin P. 1999. Paraoxonase protection of LDL against peroxidation is independent of its esterase activity towards paraoxon and is unaffected by the Q→R genetic polymorphism. J. Lipid Res. 40: 133–139 [PubMed] [Google Scholar]
- 14.Shih D. M., Gu L., Xia Y-R., Navab M., Li W-F., Hama S., Castellani L. W., Furlong C. E., Costa L. G., Fogelman A. M., et al. 1998. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 394: 284–287 [DOI] [PubMed] [Google Scholar]
- 15.Shih D. M., Xia Y-R., Wang X-P., Miller E., Castellani L. W., Subbanagounder G., Cheroutre H., Faull K. F., Berliner J. A., Witztum J. L., et al. 2000. Combined serum paraoxonase knockout/apolipoprotein E knockout mice exhibit increased lipoprotein oxidation and atherosclerosis. J. Biol. Chem. 275: 17527–17535 [DOI] [PubMed] [Google Scholar]
- 16.Besler C., Heinrich K., Rohrer L., Doerries C., Riwanto M., Shih D. M., Chroni A., Yonekawa K., Stein S., Schaefer N., et al. 2011. Mechanisms underlying adverse effects of HDL on eNOS-activating pathways in patients with coronary artery disease. J. Clin. Invest. 121: 2693–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa L. G., McDonald B. E., Murphy S. D., Omenn G. S., Richter R. J., Motulsky A. G., Furlong C. E. 1990. Serum paraoxonase and its influence on paraoxon and chlorpyrifos-oxon toxicity in rats. Toxicol. Appl. Pharmacol. 103: 66–76 [DOI] [PubMed] [Google Scholar]
- 18.Richter R. J., Jarvik G. P., Furlong C. E. 2008. Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ. Cardiovasc. Genet. 1: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richter R. J., Jarvik G. P., Furlong C. E. 2009. Paraoxonase 1 (PON1) status and substrate hydrolysis. Toxicol. Appl. Pharmacol. 235: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furlong C. E., Holland N., Richter R. J., Bradman A., Ho A., Eskenazi B. 2006. PON1 status of farmworker mothers and children as a predictor of organophosphate sensitivity. Pharmacogenet. Genomics. 16: 183–190 [DOI] [PubMed] [Google Scholar]
- 21.Richter R. J., Jarvik G. P., Furlong C. E. 2010. Paraoxonase 1 status as a risk factor for disease or exposure. Adv. Exp. Med. Biol. 660: 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brophy V. H., Hastings M., Clendenning J. B., Richter R. J., Jarvik G. P., Furlong C. E. 2001. Polymorphisms in the human paraoxonase (PON1) promoter. Pharmacogenetics. 11: 77–84 [DOI] [PubMed] [Google Scholar]
- 23.Kim D. S., Burt A. A., Ranchalis J. E., Richter R. J., Marshall J., Eintracht J. F., Rosenthal E. A., Furlong C. E., Jarvik G. P. 2012. Additional common polymorphisms in the PON gene cluster predict PON1 activity but not vascular disease. J. Lipids. 2012: 476316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvik G. P., Jampsa R., Richter R. J., Carlson C., Rieder M., Nickerson D., Furlong C. E. 2003. Novel paraoxonase (PON1) nonsense and missense mutations predicted by functional genomic assay of PON1 status. Pharmacogenetics. 13: 291–295 [DOI] [PubMed] [Google Scholar]
- 25.James R. W., Leviev I., Righetti A. 2000. Smoking is associated with reduced serum paraoxonase activity and concentration in patients with coronary artery disease. Circulation. 101: 2252–2257 [DOI] [PubMed] [Google Scholar]
- 26.van der Gaag M. S., van Tol A., Scheek L. M., James R. W., Urgert R., Schaafsma G., Hendriks H. F. J. 1999. Daily moderate alcohol consumption increases serum paraoxonase activity; a diet-controlled, randomised intervention study in middle-aged men. Atherosclerosis. 147: 405–410 [DOI] [PubMed] [Google Scholar]
- 27.Sierksma A., van der Gaag M. S., van Tol A., James R. W., Hendriks H. F. 2002. Kinetics of HDL cholesterol and paraoxonase activity in moderate alcohol consumers. Alcohol. Clin. Exp. Res. 26: 1430–1435 [DOI] [PubMed] [Google Scholar]
- 28.Rao M. N., Marmillot P., Gong M., Palmer D. A., Seeff L. B., Strader D. B., Lakshman M. R. 2003. Light, but not heavy alcohol drinking, stimulates paraoxonase by upregulating liver mRNA in rats and humans. Metabolism. 52: 1287–1294 [DOI] [PubMed] [Google Scholar]
- 29.Marsillach J., Ferre N., Vila M. C., Lligona A., Mackness B., Mackness M., Deulofeu R., Sola R., Pares A., Pedro-Botet J., et al. 2007. Serum paraoxonase-1 in chronic alcoholics: relationship with liver disease. Clin. Biochem. 40: 645–650 [DOI] [PubMed] [Google Scholar]
- 30.Sutherland W. H. F., Walker R. J., de Jong S. A., van Rij A. M., Phillips V., Walker H. L. 1999. Reduced postprandial serum paraoxonase activity after a meal rich in used cooking fat. Arterioscler. Thromb. Vasc. Biol. 19: 1340–1347 [DOI] [PubMed] [Google Scholar]
- 31.Wallace A. J., Sutherland W. H., Mann J. I., Williams S. M. 2001. The effect of meals rich in thermally stressed olive and safflower oils on postprandial serum paraoxonase activity in patients with diabetes. Eur. J. Clin. Nutr. 55: 951–958 [DOI] [PubMed] [Google Scholar]
- 32.Tomás M., Senti M., Elosua R., Vila J., Sala J., Masia R., Marrugat J. 2001. Interaction between the Gln Arg 192 variants of the paraoxonase gene and oleic acid intake as a determinant of high-density lipoprotein cholesterol and paraoxonase activity. Eur. J. Pharmacol. 432: 121–128 [DOI] [PubMed] [Google Scholar]
- 33.Tomás M., Senti M., Garcia-Faria F., Vila J., Torrents A., Covas M., Marrugat J. 2000. Effect of simvastatin therapy on paraoxonase activity and related lipoproteins in familial hypercholesterolemic patients. Arterioscler. Thromb. Vasc. Biol. 20: 2113–2119 [DOI] [PubMed] [Google Scholar]
- 34.van Wijk J., Coll B., Cabezas M. C., Koning E., Camps J., Rabelink T., Mackness B., Joven J. 2006. Rosiglitazone modulates fasting and post-prandial paraoxonase 1 activity in type 2 diabetic patients. Clin. Exp. Pharmacol. Physiol. 33: 1134–1137 [DOI] [PubMed] [Google Scholar]
- 35.Wójcicka G., Jamroz-Wisniewska A., Marciniak A., Lowicka E., Beltowski J. 2010. The differentiating effect of glimepiride and glibenclamide on paraoxonase 1 and platelet-activating factor acetylohydrolase activity. Life Sci. 87: 126–132 [DOI] [PubMed] [Google Scholar]
- 36.Mackness B., Mackness M. I., Arrol S., Turkie W., Julier K., Abuasha B., Miller J. E., Boulton A. J. M., Durrington P. N. 1998. Serum paraoxonase (PON1) 55 and 192 polymorphism and paraoxonase activity and concentration in non-insulin dependent diabetes mellitus. Atherosclerosis. 139: 341–349 [DOI] [PubMed] [Google Scholar]
- 37.Koren-Gluzer M., Aviram M., Meilin E., Hayek T. 2011. The antioxidant HDL-associated paraoxonase-1 (PON1) attenuates diabetes development and stimulates B-cell insulin release. Atherosclerosis. 219: 510–518 [DOI] [PubMed] [Google Scholar]
- 38.Jarvik G. P., Tsai N. T., McKinstry L. A., Wani R., Brophy V. H., Richter R. J., Schellenberg G. D., Heagerty P. J., Hatsukami T. S., Furlong C. E. 2002. Vitamin C and E intake is associated with increased paraoxonase activity. Arterioscler. Thromb. Vasc. Biol. 22: 1329–1333 [DOI] [PubMed] [Google Scholar]
- 39.Gursu M. F., Onderci M., Gulcu F., Sahin K. 2004. Effect of vitamin C and folic acid supplementation on serum paraoxonase activity and metabolites induced by heat stress in vivo. Nutr. Res. 24: 157–164 [Google Scholar]
- 40.Tsakiris S., Karikas G. A., Parthimos T., Tsakiris T., Bakogiannis C., Schulpis K. H. 2009. Alpha-tocopherol supplementation prevents the exercise-induced reduction of serum paraoxonase 1/arylesterase activities in healthy individuals. Eur. J. Clin. Nutr. 63: 215–221 [DOI] [PubMed] [Google Scholar]
- 41.Ronald J., Rajagopalan R., Ranchalis J., Marshall J., Hatsukami T., Heagerty P., Jarvik G. 2009. Analysis of recently identified dyslipidemia alleles reveals two loci that contribute to risk for carotid artery disease. Lipids Health Dis. 8: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41a.Rimm E. B., Stampfer M. J., Colditz G. A., Giovannucci E., Willett W. C. 1990. Effectiveness of various mailing strategies among nonrespondents in a prospective cohort study. Am. J. Epidemiol. 131: 1068–1071 [DOI] [PubMed] [Google Scholar]
- 42.Rimm E. B., Giovannucci E. L., Stampfer M. J., Colditz G. A., Litin L. B., Willett W. C. 1992. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am. J. Epidemiol. 135: 1114–1126, discussion 1127–1136 [DOI] [PubMed] [Google Scholar]
- 43.Brophy V. H., Jampsa R. L., Clendenning J. B., McKinstry L. A., Jarvik G. P., Furlong C. E. 2001. Effects of 5′ regulatory-region polymorphisms on paraoxonase-gene (PON1) expression. Am. J. Hum. Genet. 68: 1428–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bachorik P. S., Albers J. J. 1986. Precipitation methods for quantification of lipoproteins. Methods Enzymol. 129: 78–100 [DOI] [PubMed] [Google Scholar]
- 45.Warnick G. R. 1986. Enzymatic methods for quantification of lipoprotein lipids. Methods Enzymol. 129: 101–123 [DOI] [PubMed] [Google Scholar]
- 46.Warnick G. R., Benderson J., Albers J. J. 1982. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin. Chem. 28: 1379–1388 [PubMed] [Google Scholar]
- 47.Friedewald W. T., Levy R. I., Fredrickson D. S. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18: 499–502 [PubMed] [Google Scholar]
- 48.Dixon W. J., Tukey J. W. 1968. Approximate behavior of the distribution of Winsorized t (Trimming/Winsorization 2). Technometrics. 10: 83–98 [Google Scholar]
- 49.Djoussé L., Ellison R. C., Beiser A., Scaramucci A., D'Agostino R. B., Wolf P. A. 2002. Alcohol consumption and risk of ischemic stroke: The Framingham Study. Stroke. 33: 907–912 [DOI] [PubMed] [Google Scholar]
- 50.Djoussé L., Dorgan J. F., Zhang Y., Schatzkin A., Hood M., D'Agostino R. B., Copenhafer D. L., Kreger B. E., Ellison R. C. 2002. Alcohol consumption and risk of lung cancer: The Framingham Study. J. Natl. Cancer Inst. 94: 1877–1882 [DOI] [PubMed] [Google Scholar]
- 51.Carlson C. S., Heagerty P. J., Hatsukami T. S., Richter R. J., Ranchalis J., Lewis J., Bacus T. J., McKinstry L. A., Schellenberg G. D., Rieder M., et al. 2006. TagSNP analyses of the PON gene cluster: effects on PON1 activity, LDL oxidative susceptibility, and vascular disease. J. Lipid Res. 47: 1014–1024 [DOI] [PubMed] [Google Scholar]
- 52.Deakin S., Leviev I., Guernier S., James R. W. 2003. Simvastatin modulates expression of the pon1 gene and increases serum paraoxonase. Arterioscler. Thromb. Vasc. Biol. 23: 2083–2089 [DOI] [PubMed] [Google Scholar]
- 53.Manolio T. A., Pearson T. A., Wenger N. K., Barrett-Connor E., Payne G. H., Harlan W. R. 1992. Cholesterol and heart disease in older persons and women review of an NHLBI workshop. Ann. Epidemiol. 2: 161–176 [DOI] [PubMed] [Google Scholar]
- 54.Riedmaier S., Klein K., Winter S., Hofmann U., Schwab M., Zanger U. 2011. Paraoxonase (PON1 and PON3) polymorphisms: impact on liver expression and atorvastatin-lactone hydrolysis. Front. Pharmacol. 2: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoltz D. A., Ozer E. A., Taft P. J., Barry M., Liu L., Kiss P. J., Moninger T. O., Parsek M. R., Zabner J. 2008. Drosophila are protected from Pseudomonas aeruginosa lethality by transgenic expression of paraoxonase-1. J. Clin. Invest. 118: 3123–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozer E. A., Pezzulo A., Shih D. M., Chun C., Furlong C. E., Lusis A. J., Greenberg E. P., Zabner J. 2005. Human and murine paraoxonase 1 are host modulators of Pseudomonas aeruginosa quorum-sensing. FEMS Microbiol. Lett. 253: 29–37 [DOI] [PubMed] [Google Scholar]
- 57.Carmine A., Buervenich S., Sydow O., Anvret M., Olson L. 2002. Further evidence for an association of the paraoxonase 1 (PON1) Met-54 allele with Parkinson's disease. Mov. Disord. 17: 764–766 [DOI] [PubMed] [Google Scholar]
- 58.Kondo I., Yamamoto M. 1998. Genetic polymorphism of paraoxonase 1 (PON1) and susceptibility to Parkinson's disease. Brain Res. 806: 271–273 [DOI] [PubMed] [Google Scholar]
- 59.Akhmedova S. N., Yakimovsky A. K., Schwartz E. I. 2001. Paraoxonase 1 Met-Leu 54 polymorphism is associated with Parkinson's disease. J. Neurol. Sci. 184: 179–182 [DOI] [PubMed] [Google Scholar]
- 60.Dasgupta S., Demirci F. Y., Dressen A., Kao A., Rhew E., Ramsey-Goldman R., Manzi S., Kammerer C., Kamboh M. I. 2011. Association analysis of PON2 genetic variants with serum paraoxonase activity and systemic lupus erythematosus. BMC Med. Genet. 12: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiss E., Seres I., Tarr T., Kocsis Z., Szegedi G., Paragh G. 2007. Reduced paraoxonase1 activity is a risk for atherosclerosis in patients with systemic lupus erythematosus. Ann. N. Y. Acad. Sci. 1108: 83–91 [DOI] [PubMed] [Google Scholar]
- 62.Tripi L. M., Manzi S., Chen Q., Kenney M., Shaw P., Kao A., Bontempo F., Kammerer C., Kamboh M. I. 2006. Relationship of serum paraoxonase 1 activity and paraoxonase 1 genotype to risk of systemic lupus erythematosus. Arthritis Rheum. 54: 1928–1939 [DOI] [PubMed] [Google Scholar]
- 63.Saadat M. 2011. Paraoxonase 1 genetic polymorphisms and susceptibility to breast cancer: a meta-analysis. Cancer Epidemiol. 36: e101–e103. [DOI] [PubMed] [Google Scholar]
- 64.Liu C., Liu L. 2011. Polymorphisms in three obesity-related genes (LEP, LEPR, and PON1) and breast cancer risk: a meta-analysis. Tumour Biol. 32: 1233–1240 [DOI] [PubMed] [Google Scholar]
- 65.Ates O., Azizi S., Alp H. H., Kiziltunc A., Beydemir S., Cinici E., Kocer I., Baykal O. 2009. Decreased serum paraoxonase 1 activity and increased serum homocysteine and malondialdehyde levels in age-related macular degeneration. Tohoku J. Exp. Med. 217: 17–22 [DOI] [PubMed] [Google Scholar]
- 66.Baird P. N., Chu D., Guida E., Vu H. T. V., Guymer R. 2004. Association of the M55L and Q192R paraoxonase gene polymorphisms with age-related macular degeneration. Am. J. Ophthalmol. 138: 665–666 [DOI] [PubMed] [Google Scholar]
- 67.Baskol G., Karakucuk S., Oner A., Baskol M., Kocer D., Mirza E., Saraymen R., Ustdal M. 2006. Serum paraoxonase 1 activity and lipid peroxidation levels in patients with age-related macular degeneration. Ophthalmologica. 220: 12–16 [DOI] [PubMed] [Google Scholar]
- 68.Brión M., Sanchez-Salorio M., Cortón M., de la Fuente M., Pazos B., Othman M., Swaroop A., Abecasis G., Sobrino B., Carracedo A., et al. 2011. Genetic association study of age-related macular degeneration in the Spanish population. Acta Ophthalmol. 89: e12–e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Esfandiary H., Chakravarthy U., Patterson C., Young I., Hughes A. 2005. Association study of detoxification genes in age related macular degeneration. Br. J. Ophthalmol. 89: 470–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Javadzadeh A., Ghorbanihaghjo A., Bahreini E., Rashtchizadeh N., Argani H., Alizadeh S. 2011. Serum paraoxonase phenotype distribution in exudative age-related macular degeneration and its relationship to homocysteine and oxidized low-density lipoprotein. Retina. 32: 658–666. [DOI] [PubMed] [Google Scholar]
- 71.Pauer G. J. T., Sturgill G. M., Peachey N. S., Hagstrom S. A. 2010. Protective effect of paraoxonase 1 gene variant Gln192Arg in age-related macular degeneration. Am. J. Ophthalmol. 149: 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rainwater D. L., Mahaney M. C., Wang X. L., Rogers J., Cox L. A., VandeBerg J. L. 2005. Determinants of variation in serum paraoxonase enzyme activity in baboons. J. Lipid Res. 46: 1450–1456 [DOI] [PubMed] [Google Scholar]
- 73.Galgani J. E., Uauy R. D., Aguirre C. A., Diaz E. O. 2008. Effect of the dietary fat quality on insulin sensitivity. Br. J. Nutr. 100: 471–479 [DOI] [PubMed] [Google Scholar]
- 74.Garige M., Gong M., Varatharajalu R., Lakshman M. R. 2010. Quercetin up-regulates paraoxonase 1 gene expression via sterol regulatory element binding protein 2 that translocates from the endoplasmic reticulum to the nucleus where it specifically interacts with sterol responsive element-like sequence in paraoxonase 1 promoter in HuH7 liver cells. Metabolism. 59: 1372–1378 [DOI] [PubMed] [Google Scholar]
- 75.Moon J., Lee S. M., Do H. J., Cho Y., Chung J. H., Shin M. J. Quercetin up-regulates LDL receptor expression in HepG2 cells. Phytother. Res. Epub ahead of print. March 3, 2012; doi:10.1002/ptr.4646. [DOI] [PubMed]
- 76.Augusti P. R., Ruviaro A. R., Quatrin A., Somacal S., Conterato G. M., Vicentini J. T., Duarte M. M., Emanuelli T. 2012. Imbalance in superoxide dismutase/thioredoxin reductase activities in hypercholesterolemic subjects: relationship with low density lipoprotein oxidation. Lipids Health Dis. 11: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahrooz A., Zargari M., Sedighi O., Shaygani H., Gohari G. 2012. Increased oxidized-LDL levels and arylesterase activity/HDL ratio in ESRD patients treated with hemodialysis. Clin. Invest. Med. 35: E144–E151 [DOI] [PubMed] [Google Scholar]
- 78.Koc A., Cengiz M., Ozdemir Z. C., Celik H. 2012. Paraoxonase and arylesterase activities in children with iron deficiency anemia and vitamin B12 deficiency anemia. Pediatr. Hematol. Oncol. 29: 345–353 [DOI] [PubMed] [Google Scholar]
- 79.Aslan M., Kosecik M., Horoz M., Selek S., Celik H., Erel O. 2007. Assessment of paraoxonase and arylesterase activities in patients with iron deficiency anemia. Atherosclerosis. 191: 397–402 [DOI] [PubMed] [Google Scholar]
- 80.Martinović J., Kotur-Stevuljevic J., Dopsaj V., Dopsaj M., Stefanovic A., Kasum G. 2010. Paraoxonase activity in athletes with depleted iron stores and iron-deficient erythropoiesis. Clin. Biochem. 43: 1225–1229 [DOI] [PubMed] [Google Scholar]
- 81.Pla A., Rodrigo L., Hernandez A. F., Gil F., Lopez O. 2007. Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem. Biol. Interact. 167: 63–70 [DOI] [PubMed] [Google Scholar]
- 82.Gonzalvo M. C., Gil F., Hernandez A. F., Villanueva E., Pla A. 1997. Inhibition of paraoxonase activity in human liver microsomes by exposure to EDTA, metals and mercurials. Chem. Biol. Interact. 105: 169–179 [DOI] [PubMed] [Google Scholar]
- 83.Gençer N., Arslan O. 2009. Purification human PON1Q192 and PON1R192 isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 877: 134–140 [DOI] [PubMed] [Google Scholar]
- 84.Ekinci D., Beydemir S. 2010. Purification of PON1 from human serum and assessment of enzyme kinetics against metal toxicity. Biol. Trace Elem. Res. 135: 112–120 [DOI] [PubMed] [Google Scholar]
- 85.Nguyen S. D., Hung N. D., Cheon-Ho P., Ree K. M., Dai-Eun S. 2009. Oxidative inactivation of lactonase activity of purified human paraoxonase 1 (PON1). Biochim. Biophys. Acta. 1790: 155–160 [DOI] [PubMed] [Google Scholar]
- 86.Ayub A., Mackness M. I., Arrol S., Mackness B., Patel J., Durrington P. N. 1999. Serum paraoxonase after myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 19: 330–335 [DOI] [PubMed] [Google Scholar]
- 87.Navab M., Anantharamaiah G. M., Hama S., Garber D. W., Chaddha M., Hough G., Lallone R., Fogelman A. M. 2002. Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 105: 290–292 [DOI] [PubMed] [Google Scholar]
- 88.Osaki F., Ikeda Y., Suehiro T., Ota K., Tsuzura S., Arii K., Kumon Y., Hashimoto K. 2004. Roles of Sp1 and protein kinase C in regulation of human serum paraoxonase 1 (PON1) gene transcription in HepG2 cells. Atherosclerosis. 176: 279–287 [DOI] [PubMed] [Google Scholar]
- 89.de Oliveira Otto M. C., Alonso A., Lee D. H., Delclos G. L., Bertoni A. G., Jiang R., Lima J. A., Symanski E., Jacobs D. R., Jr, Nettleton J. A. 2012. Dietary intakes of zinc and heme iron from red meat, but not from other sources, are associated with greater risk of metabolic syndrome and cardiovascular disease. J. Nutr. 142: 526–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flesch M., Rosenkranz S., Erdmann E., Bohm M. 2001. Alcohol and the risk of myocardial infarction. Basic Res. Cardiol. 96: 128–135 [DOI] [PubMed] [Google Scholar]
- 91.Miller E. R., 3rd, Juraschek S., Pastor-Barriuso R., Bazzano L. A., Appel L. J., Guallar E. 2010. Meta-analysis of folic acid supplementation trials on risk of cardiovascular disease and risk interaction with baseline homocysteine levels. Am. J. Cardiol. 106: 517–527 [DOI] [PubMed] [Google Scholar]
- 92.Rock W., Rosenblat M., Miller-Lotan R., Levy A. P., Elias M., Aviram M. 2008. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 56: 8704–8713 [DOI] [PubMed] [Google Scholar]