Abstract
Periodontitis is a prevalent oral chronic inflammatory disease which, in severe forms, may exert a major impact on systemic health. Clinical and histological observations, as well as experimental animal studies, suggest involvement of the complement system in periodontitis. However, the precise roles of the various complement components and pathways in periodontitis have only recently started to be elucidated. In this paper, we review recent progress in the field and discuss the potential of complement-targeted therapeutics in the treatment of periodontitis.
1 Introduction
Periodontal disease is a prevalent chronic condition that causes inflammatory destruction of the tooth-supporting tissues (Pihlstrom et al., 2005). Severe periodontitis exerts a systemic impact on health and the patients run increased risk for systemic diseases, such as atherosclerosis, diabetes, aspiration pneumonia, averse pregnancy outcomes, and perhaps rheumatoid arthritis (Tonetti et al., 2007, de Pablo et al., 2009, Kebschull et al., 2010, Pihlstrom et al., 2005, Scannapieco et al., 2010, Genco and Van Dyke, 2010). Treated periodontal patients often develop recurrent disease for reasons that are not clear, thus necessitating better understanding of the underlying immunopathology (Armitage, 2002, Hajishengallis, 2009b). The annual cost of periodontal therapy in the U.S. exceeds $14 billion (Brown et al., 2002) and the suspected association of periodontitis with systemic conditions underscores the importance of implementing new and effective treatment options.
Although a group of tooth-associated subgingival anaerobic bacteria is strongly associated with periodontitis (Socransky et al., 1998), it is the host inflammatory response to uncontrolled bacterial challenge, rather than direct bacterial toxic effects, that primarily mediates periodontal tissue destruction(Gaffen and Hajishengallis, 2008, Graves, 2008). In this context, periodontal health represents a dynamic state where proinflammatory and antimicrobial activities are optimally regulated to prevent unwarranted host reactions (Gaffen and Hajishengallis, 2008). This homeostatic balance may be disrupted, however, either by genetic immunoregulatory defects or by pathogens that subvert the host response, thereby leading to non-protective and non-resolving chronic inflammation (Gaffen and Hajishengallis, 2008, Kinane et al., 2006, Kumpf and Schumann, 2008). Available evidence implicates the periodontal pathogen Porphyromonas gingivalis as a master of immune subversion (Hajishengallis, 2009a) (Figure 1). Indeed, P. gingivalis inhibits critical antimicrobial responses that could eliminate it, while on the other hand stimulates local inflammation, which may facilitate nutrient acquisition (e.g., gingival crevicular fluid-derived hemin) and additionally cause collateral tissue damage (Hajishengallis et al., 2008, Coats et al., 2009, Wang et al., 2007, Burns et al., 2006, Hajishengallis et al., 2007, Slaney and Curtis, 2008, Potempa and Pike, 2009, Krauss et al., 2010). Recently, P. gingivalis was shown to act as a keystone pathogen which promotes the survival and virulence of the entire microbial community (Hajishengallis et al., 2011).
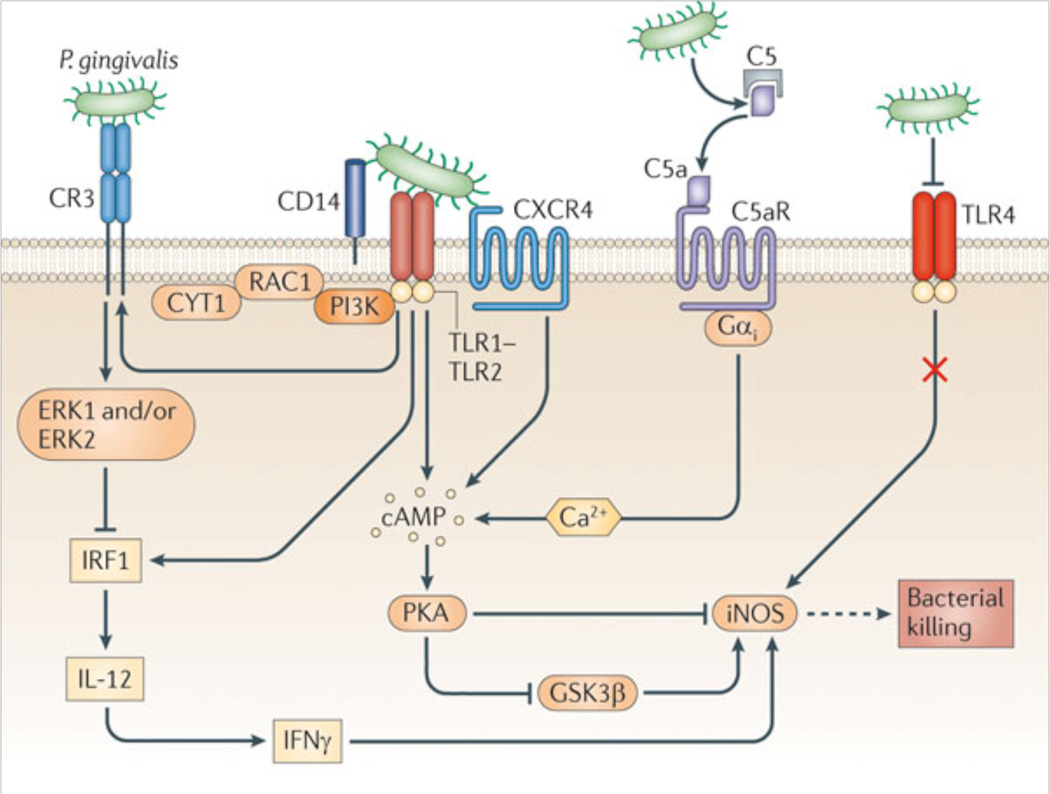
Figure 1. Exploitation of C5aR and other innate immune receptors by P. gingivalis to undermine host immunity.
P. gingivalis has surface structures that interact with Toll-like receptor (TLR)-2 (specifically with the CD14–TLR2–TLR1 signaling complex) and with TLR4. The activation of TLR4, however, is blocked by the bacterium’s atypical lipopolysaccharide which acts as an antagonist; therefore, TLR4 is unlikely to induce protective responses. The TLR2 response is proactively modified through crosstalk with other receptors that are under P. gingivalis control. P. gingivalis regulates C5a receptor (C5aR) by virtue of Arg-specific cysteine proteinases, which attack C5 and release biologically active C5a. C5a stimulates intracellular Ca2+ signaling which synergistically enhances the otherwise weak cAMP responses induced by TLR2 activation alone. Maximal cAMP induction requires the participation of CXC-chemokine receptor 4 (CXCR4), which is activated directly by the bacterium’s fimbriae. The resulting activation of the cAMP-dependent protein kinase A (PKA) inactivates glycogen synthase kinase-3β (GSK3β) and inhibits the inducible nitric oxide synthase (iNOS)-dependent killing of the pathogen in macrophages. An additional pathway induced downstream of TLR2 is an inside-out signaling pathway, mediated by RAC1, phosphatidylinositol-3 kinase (PI3K) and cytohesin 1 (CYT1), which transactivates complement receptor-3 (CR3). Activated CR3 binds P. gingivalis and induces extracellular signal-regulated kinase-1/ERK2 signaling, which in turn selectively downregulates IL-12 p35 and p40 mRNA expression through suppression of interferon regulatory factor 1 (IRF1). Inhibition of bioactive IL-12, and secondarily IFNγ, leads to impaired immune clearance of P. gingivalis.
From Hajishengallis and Lambris, 2011, Nature Reviews Immunology, 11, 187–200 (used by permission).
At least in principle, periodontitis could be inhibited by interventions aiming to control inflammation and counteract microbial subversion of the host response. This concept is discussed here in the context of the complement system, which is now recognized as a central network that orchestrates the host response (Ricklin et al., 2010). Specifically, besides its classic antimicrobial functions (see below), complement crosstalks with and regulates other signaling systems, including Toll-like receptor (TLR) pathways (Hajishengallis and Lambris, 2010). Despite its potentially host-protective role, however, complement forms a major link between infection and inflammatory pathology if overactivated or deregulated (Ricklin and Lambris, 2007, Wagner and Frank, 2010, Holers, 2008, Ricklin et al., 2010).
2 Complement and periodontal disease
Besides the characteristic group of serum proteins (C1-9), the integrated complement system includes pattern-recognition molecules, convertases and other proteases, regulators, and receptors for interactions with immune mediators (Ricklin et al., 2010). The complement cascade can be triggered via distinct pathways (classical, lectin, or alternative) which converge at the third complement component (C3). The activation of the classical pathway is initiated by antigen-antibody complexes recognized by the C1q subunit of C1. The lectin pathway is triggered through interaction of the mannose-binding lectin, a secreted pattern-recognition molecule, with specific carbohydrate groups on microbial surfaces. Both the classical and lectin pathways proceed through C4 and C2 cleavage for the generation of the classical/lectin C3 convertase. The alternative pathway is initiated by low-level, spontaneous hydrolysis of C3 to C3[H2O], which forms the initial alternative pathway C3 convertase in the presence of factors B (fB) and D (fD). As long as there is no sufficient negative regulation (as in the case of bacteria or other nonself surfaces), this initiation is followed by rapid propagation of the alternative pathway involving an amplification loop (Dunkelberger and Song, 2010, Ricklin et al., 2010). The alternative pathway can also be triggered by lipopolysaccharide and lipooligosacharide in a way dependent upon the plasma protein properdin attached to bacterial surfaces (Spitzer et al., 2007, Kimura et al., 2008), and may potentially contribute to ≥80% of total complement activation (Harboe and Mollnes, 2008). C3 activation by pathway-specific C3 convertases leads to the generation of effector molecules involved in (a) the recruitment and activation of inflammatory cells (e.g., the C3a and C5a anaphylatoxins that activate specific G-protein–coupled receptors, C3aR and C5aR [CD88], respectively); (b) microbial opsonization and phagocytosis (e.g., through the C3b opsonin); and (c) direct lysis of targeted pathogens (by means of the C5b-9 membrane attack complex [MAC]) (Ricklin et al., 2010). An alternative receptor for C5a is the C5a-like receptor 2 (C5L2; GPR77), which has been assigned both regulatory and proinflammatory roles (Ward, 2009, Hajishengallis and Lambris, 2010, Zhang et al., 2010, Bamberg et al., 2010).
At least in principle, local complement activation could promote periodontal inflammation through multiple pathways, including C5a-induced vasodilation, increased vascular permeability, and chemotactic recruitment of inflammatory cells, including neutrophils. In addition to their role in acute inflammation, neutrophils have been implicated in chronic inflammatory diseases, e.g., rheumatoid arthritis, inflammatory bowel disease, and chronic obstructive pulmonary disease (Kasama et al., 2005, Kanazawa and Furukawa, 2007, Simpson et al., 2009, Kitsis and Weissmann, 1991). Neutrophils are also key effectors of inflammatory tissue injury in periodontitis (Serhan et al., 2008, Nussbaum and Shapira, 2011) and can be found in great numbers in the gingival crevice (≥95% of total leukocytes) (Delima and Van Dyke, 2003). Although gingival crevicular neutrophils form what looks like a “defense wall” against the periodontal bacteria, they largely fail to control the bacteria despite maintaining viability and capacity to elicit inflammatory responses (Delima and Van Dyke, 2003, Lange and Schroeder, 1971, Newman, 1980, Schroeder and Listgarten, 1997, Vitkov et al.). The underlying reasons are largely unexplored.
Clinical and histological observations suggest that complement is indeed involved in periodontitis (Hajishengallis, 2010). Chronically inflamed gingiva or gingival crevicular fluid from periodontitis patients display increased levels of activated complement fragments relative to control samples from healthy individuals (Patters et al., 1989, Nikolopoulou-Papaconstantinou et al., 1987, Rautemaa and Meri, 1996, Beikler et al., 2008, Courts et al., 1977, Schenkein and Genco, 1977, Niekrash and Patters, 1986). Importantly, induction of experimental gingival inflammation in human volunteers causes progressive elevation of complement cleavage products correlating with increased clinical indices of inflammation (Patters et al., 1989).
Interestingly, a single nucleotide polymorphism of C5 (rs17611), which is associated with increased serum C5 levels and susceptibility to liver fibrosis and rheumatoid arthritis (Hillebrandt et al., 2005, Chang et al., 2008), was shown to be more prevalent in periodontitis patients than in healthy controls (Chai et al., 2010). Moreover, an immunohistochemical study showed weaker expression of CD59 in the gingiva of periodontitis patients compared to healthy controls, suggesting reduced protection of diseased tissues against autologous MAC-mediated tissue damage (Rautemaa and Meri, 1996). A case of aggressive periodontitis accompanied by severe gingival angioedema was linked to dysregulated complement function, specifically C1INH deficiency (Roberts et al., 2003).
These studies suggest complement involvement in periodontal inflammation and pathogenesis. However, their correlative nature does not allow reliable conclusions as to the precise role(s) of the various complement pathways, nor do these observations necessarily imply that all complement pathways mediate destructive inflammation. In this regard, partial C4 gene deficiencies are significantly more frequent in periodontal patients relative to healthy controls (Seppanen et al., 2007), therefore suggesting involvement of the classical and/or lectin pathway in a protective function. For instance, C3b generation via the C4-dependent classical and/or lectin pathways could promote opsonophagocytosis of periodontal bacteria, secondarily contributing to control of infection-induced inflammation. In conclusion, it has been uncertain which specific complement pathways need to be blocked to attenuate inflammatory pathology or kept intact to promote host defense. However, considerable insights have been gained by studies in preclinical models. At this point, there is sufficient evidence to implicate the C5a-C5aR axis in the pathogenesis of periodontitis (below).
3 Involvement of the C5a-C5aR pathway in periodontitis
The C5a anaphylatoxin is perhaps the most powerful effector molecule of the complement cascade, as it mediates chemotactic recruitment and activation of neutrophils and other inflammatory cells and is involved in synergistic complement interactions with Toll-like receptors (Guo and Ward, 2005, Zhang et al., 2007). These immunostimulatory effects of C5a can potentially protect the host against microbial pathogens. In this regard, a major medical pathogen, Staphylococcus aureus, has evolved a strategy that allows it to block C5a binding and C5aR activation, via a secreted chemotaxis inhibitory protein (de Haas et al., 2004). Nevertheless, C5aR signaling can contribute to the pathogenesis of a number of acute or chronic inflammatory diseases, such as sepsis, acute lung injury, ischemia-reperfusion injury, and rheumatoid arthritis (Guo and Ward, 2005, Okroj et al., 2007).
Intriguingly, in contrast to the S. aureus strategy, P. gingivalis is proactively involved in C5aR activation. Specifically, P. gingivalis employs its Arg-specific gingpains to generate biologically active C5a through limited degradation of C5, whereas the C5b remnant is proteolytically destroyed, ostensibly to prevent activation of the terminal complement pathway (Wingrove et al., 1992, Popadiak et al., 2007, Liang et al., 2011, Wang et al., 2010). P. gingivalis in fact can generate high levels of C5a (> 30 nM) after a 30-min incubation in heat-inactivated human serum (Wang et al., 2010). This activity may appear to be counterproductive for the pathogen, given the important role of C5a in host defense. Strikingly, however, P. gingivalis was shown to exploit C5a to impair the killing function of macrophages via manipulation of specific signaling events in the absence of generalized immune suppression (Wang et al., 2010).
The mechanism involves synergistic production of high and sustained cAMP levels, which inhibit nitric oxide-dependent killing of P. gingivalis (Wang et al., 2010). This synergism requires a crosstalk between C5a-activated C5aR and P. gingivalis-activated TLR2, whereas downstream players include cAMP-dependent protein kinase A and glycogen synthase kinase-3β, the interplay of which inhibits the inducible nitric oxide synthase (Wang et al., 2010) (Figure 1).
Moreover, the P. gingivalis-induced C5aR-TLR2 crosstalk regulates cytokine production that favors the pathogen (Liang et al., 2011). In this regard, the C5aR-TLR2 crosstalk inhibits TLR2-induced interleukin (IL)-12p70 which promotes immune clearance of P. gingivalis (Liang et al., 2011). In contrast, the same C5aR-TLR2 crosstalk upregulates inflammatory and bone-resorptive cytokines (IL-1β, IL-6, and TNF-α) which do not seem to harm P. gingivalis. These effects collectively lead to enhanced persistence of P. gingivalis in the host and set the stage for inflammatory tissue damage. This notion is supported by experimental periodontitis studies in the mouse model: Mice deficient in either C5aR or TLR2 were protected against P. gingivalis-induced inflammatory periodontal bone loss, whereas wild-type controls developed inflammation and suffer serious bone loss (Liang et al., 2011). In this inflammatory context, the proactive release of C5a by P. gingivalis could contribute to stimulation of inflammatory exudate for acquisition of nutrients like hemin and tissue breakdown products (peptides) which are essential for P. gingivalis and other asaccharolytic periodontal bacteria (Krauss et al., 2010). On the other hand, an isogenic mutant of P. gingivalis, which is deficient in all gingipain genes (KDP128), failed to persist in vivo and could not cause periodontitis (Liang et al., 2011, Hajishengallis et al., 2011). This difference in survival capacity may be related, at least in part, to the inability of KDP128 to generate C5a (Liang et al., 2011). These studies collectively indicate that the C5a-C5aR axis exerts a destructive role in periodontitis through a dual mechanism: a) It is exploited by P. gingivalis to escape host defense and b) it mediates inflammatory periodontal bone loss.
4 C5aR-targeted intervention in periodontitis
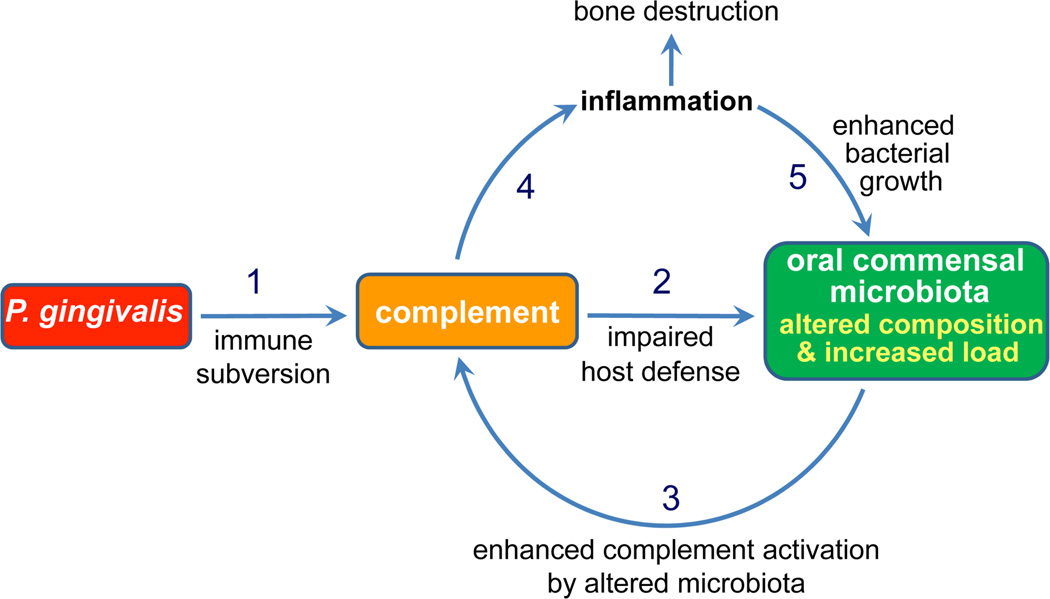
The above discussed findings provided a solid basis for rational C5aR-targeted intervention against P. gingivalis and periodontitis. This notion is supported by experimental evidence. Indeed, blockade of C5aR with a specific antagonist (Ac-F[OP(D)Cha-WR]; also known as PMX-53) abrogated the C5a-dependent subversive strategy of P. gingivalis and facilitated its immune clearance in vitro and in vivo (Wang et al., 2010, Liang et al., 2011). This effect may not necessarily imply protection against periodontal infection and inflammation in general, given the polymicrobial nature of periodontitis. However, it was recently shown that complement subversion by P. gingivalis can additionally benefit bystander periodontal bacteria in the same biofilm, which thereby displays quantitative and qualitative changes that lead to complement-dependent periodontitis at least in a mouse model (Hajishengallis et al., 2011) (Figure 2).
Figure 2. P. gingivalis subverts complement leading to alterations in the oral microbiota and development of periodontitis.
P. gingivalis impairs innate immunity through complement subversion leading to quantitative and qualitative changes to the oral commensal microbiota. Through complement, the altered microbiota causes inflammatory bone loss, whereas tissue breakdown products may further stimulate oral bacterial growth.
From Hajishengallis et al, 2011, Cell Host and Microbe, 10, 497–506 (used by permission).
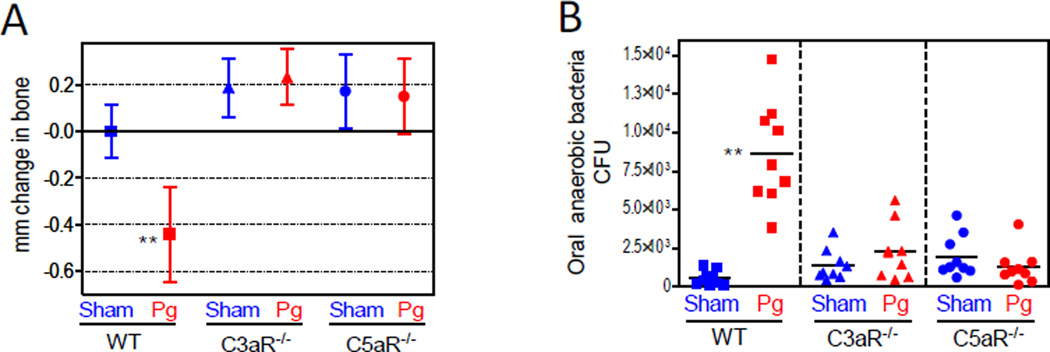
Specifically, oral inoculation of mice with P. gingivalis exerts growth-enhancing effects and compositional changes in the oral microbiota, despite the very low colonization levels of P. gingivalis (<0.01% of the total bacterial counts) (Hajishengallis et al., 2011). Collectively, these actions lead to destructive inflammatory disease that requires the presence of the commensal microbiota and intact complement pathways, since P. gingivalis fails to cause periodontitis in germ-free mice or conventionally raised mice deficient in C3aR or C5aR (Hajishengallis et al., 2011) (Figure 3). Moreover, P. gingivalis fails to cause changes to the oral commensal microbiota of C3aR−/− or C5aR−/− mice, in contrast to normal specific-pathogen-free (SPF) mice (Figure 3).
Figure 3. Complement-dependent elevation of the oral bacterial load and induction of bone loss.
BALB/c mice, either wild-type (WT) or deficient in C3aR (C3aR−/−) or C5aR (C5aR−/−), were orally inoculated with P. gingivalis (Pg) or vehicle only (Sham) and assessed for bone loss (A) and levels of oral anaerobic bacteria (B). Negative values indicate bone loss relative to bone levels of the indicated controls (zero baseline), whereas positive values indicate increased bone levels. CFU counts are shown for each individual mouse with horizontal lines denoting mean values. **p <0.01 versus corresponding control.
From Hajishengallis et al, 2011, Cell Host and Microbe, 10, 497–506 (used by permission).
The inability of P. gingivalis to alter the oral microbiota in C5aR−/− mice can be explained by the lack of C5aR, which is required by P. gingivalis to inhibit the killing capacity of leukocytes (Wang et al., 2010, Liang et al., 2011). P. gingivalis-affected leukocytes with impaired killing capacity would likely allow uncontrolled growth of other bacterial species in the same biofilm, accounting for the elevation of the total bacterial numbers. Consistent with the importance of gingipains in C5aR-dependent subversion of leukocytes (Liang et al., 2011), a gingipain-deficient isogenic mutant (KDP128) of P. gingivalis failed to elevate the oral bacterial load even in normal SPF mice. The C3aR requirement for the P. gingivalis effect on the oral microbiota and bone loss may be related to its synergistic interactions with C5aR that include reciprocal augmentation of receptor expression (Ricklin et al., 2010). In this regard, P. gingivalis-inoculated C3aR−/− mice displayed significantly reduced expression of C5aR (Hajishengallis et al., 2011).
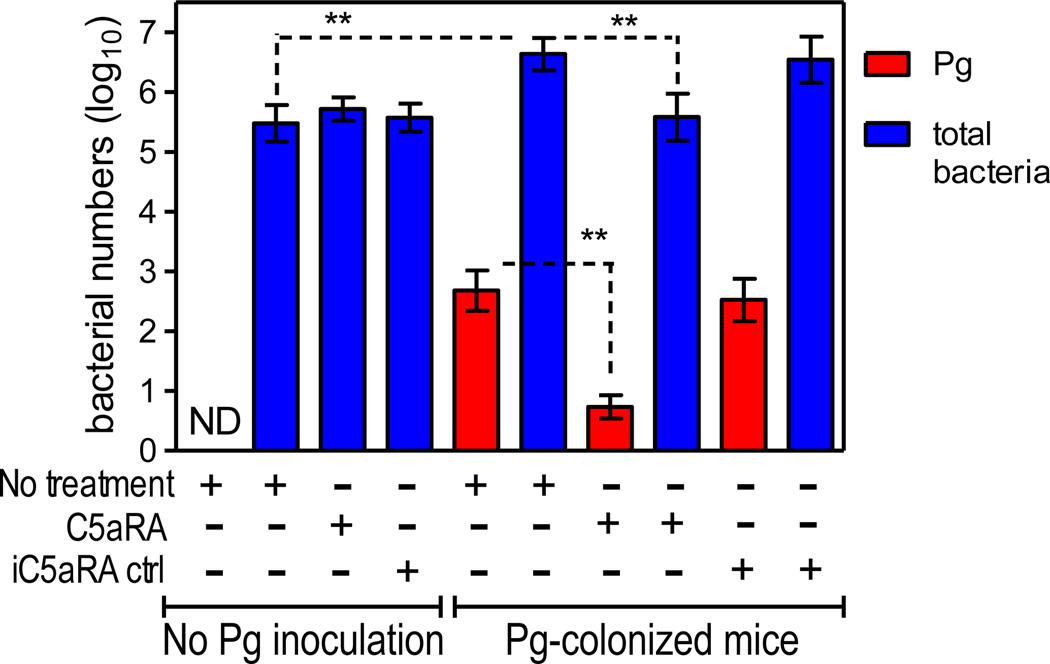
Since C5aR is required for the in vivo survival of P. gingivalis (Wang et al., 2010, Liang et al., 2011), local administration of a C5aR antagonist (PMX-53) could block the persistence of P. gingivalis, leading to its removal from the periodontal tissue and perhaps adversely affecting the total microbiota. Indeed, local administration of PMX-53 resulted in almost complete elimination of P. gingivalis, accompanied by a >10-fold reduction in the total numbers of oral anaerobic bacteria, which returned to their original lower levels (Hajishengallis et al., 2011) (Figure 4). This reduction in the total microbiota was not a direct effect on the commensal bacteria by C5aRA since the antagonist failed to reduce the total oral bacterial numbers in mice not colonized with P. gingivalis (Figure 4). These data clearly indicated that the experimental removal of P. gingivalis from the periodontal tissue, similar to its introduction, exerts a major influence on the oral microbiota. By analogy to the ‘keystone species’ concept in macro-ecology, i.e., low-abundance species with a major supporting role for an entire ecological community (Power et al., 1996, Brown et al., 2001, Ebenman and Jonsson, 2005), P. gingivalis may be regarded as such a species by fulfilling the criteria of low abundance and major influence on the microbial community. In fact, P. gingivalis could be characterized as a keystone pathogen, defined as ‘a keystone species which supports and shapes a microbial community in ways that also promote disease pathogenesis’ (Hajishengallis et al., 2011).
Figure 4. Effects of C5aR antagonist (C5aRA), or inactive analog control (iC5aRA ctrl), on the numbers of P. gingivalis or total bacteria in the periodontal tissue of mice with or without previous inoculation with P. gingivalis.
Groups of mice were orally inoculated or not with P. gingivalis (as described above) and 7 days later were injected with C5aRA, or iC5aRA control, into the palatal gingiva, on the mesial of the first molar and in the papillae between first and second and third molars on both sides of the maxilla (1 µl of 1 µg per site; total of six sites). Two days later (day 9), the mice were sacrificed and maxillary periodontal tissue was harvested to determine the levels of P. gingivalis colonization and the number of total oral bacteria, using quantitative real-time PCR of the ISPg1 gene (P. gingivalis) or the 16S rRNA gene (total oral bacteria). Two groups of mice, one of which was inoculated with P. gingivalis, were not treated with C5aRA or iC5aRA at day 7 and were sacrificed the same day for determining the levels of P. gingivalis and of total oral bacteria prior to the C5aRA or iC5aRA interventions. ND, Pg not detected. **p <0.01 between indicated groups.
From Hajishengallis et al, 2011, Cell Host and Microbe, 10, 497–506 (used by permission).
5 Conclusions and future directions
Despite being a very minor constituent of the total oral microbiota, P. gingivalis can alter the numbers and community organization of the commensal bacteria, the presence of which is essential for inflammatory periodontal bone loss. Although PMX-53 has not been shown yet to inhibit periodontal bone loss after local administration, its capacity to block P. gingivalis persistence in the periodontal tissue and to inhibit the overgrowth of the oral microbiota strongly suggests that it is a promising therapeutic against periodontitis. Moreover, C5aR inhibitors may have important therapeutic implications also in other infections or inflammatory diseases where P. gingivalis is thought to be implicated, such as oral aspiration pneumonia and atherosclerosis (Gibson et al., 2006, Okuda et al., 2005).
Despite this progress, additional systematic approaches are required to comprehensively identify the precise roles of the various complement pathways in the context of periodontal pathogenesis. Such information could reveal which specific pathways need to be blocked to reverse inflammatory pathology or, conversely, be enhanced (or left intact) to promote host defense. This would greatly facilitate complement-targeted therapeutic intervention against periodontitis.
Importantly, a number of complement-specific drugs are already in clinical trials for other inflammatory diseases (Ricklin and Lambris, 2007, Wagner and Frank, 2010). Among them, PMX-53, which blocks both mouse and human C5aR, has a good safety record when given orally to humans (Ricklin and Lambris, 2007, Wagner and Frank, 2010). This, and potentially other complement-targeted drugs, may prove effective as adjunctive treatments for periodontitis in the near future.
Acknowledgments
The authors acknowledge support by U.S. Public Health Service Grants DE015254, DE018292, DE021685 (GH) and AI068730, AI030040, AI072106, and GM062134 (JDL).
References
- Armitage GC. Classifying periodontal diseases--a long-standing dilemma. Periodontol. 2000. 2002;30:9–23. doi: 10.1034/j.1600-0757.2002.03002.x. [DOI] [PubMed] [Google Scholar]
- Bamberg CE, Mackay CR, Lee H, Zahra D, Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C, Gerard NP. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. J. Biol. Chem. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beikler T, Peters U, Prior K, Eisenacher M, Flemmig TF. Gene expression in periodontal tissues following treatment. BMC Med. Genomics. 2008;1:30. doi: 10.1186/1755-8794-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JH, Whitham TG, Morgan Ernest SK, Gehring CA. Complex species interactions and the dynamics of ecological systems: long-term experiments. Science. 2001;293:643–650. doi: 10.1126/science.293.5530.643. [DOI] [PubMed] [Google Scholar]
- Brown LJ, Johns BA, Wall TP. The economics of periodontal diseases. Periodontol. 2000. 2002;29:223–234. doi: 10.1034/j.1600-0757.2002.290111.x. [DOI] [PubMed] [Google Scholar]
- Burns E, Bachrach G, Shapira L, Nussbaum G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: Activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 2006;177:8296–8300. doi: 10.4049/jimmunol.177.12.8296. [DOI] [PubMed] [Google Scholar]
- Chai L, Song Y-Q, Zee K-Y, Leung WK. Single nucleotide polymorphisms of complement component 5 and periodontitis. J. Periodont. Res. 2010;45:301–308. doi: 10.1111/j.1600-0765.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- Chang M, Rowland CM, Garcia VE, Schrodi SJ, Catanese JJ, van der Helm-van Mil AH, Ardlie KG, Amos CI, Criswell LA, Kastner DL, Gregersen PK, Kurreeman FA, Toes RE, Huizinga TW, Seldin MF, Begovich AB. A large-scale rheumatoid arthritis genetic study identifies association at chromosome 9q33.2. PLoS Genet. 2008;4:e1000107. doi: 10.1371/journal.pgen.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coats SR, Jones JW, Do CT, Braham PH, Bainbridge BW, To TT, Goodlett DR, Ernst RK, Darveau RP. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4'- phosphatase activities. Cell Microbiol. 2009;11:1587–1599. doi: 10.1111/j.1462-5822.2009.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courts FJ, Boackle RJ, Fudenberg HH, Silverman MS. Detection of functional complement components in gingival crevicular fluid from humans with periodontal diseases. J. Dent. Res. 1977;56:327–331. doi: 10.1177/00220345770560032001. [DOI] [PubMed] [Google Scholar]
- de Haas CJC, Veldkamp KE, Peschel A, Weerkamp F, Van Wamel WJB, Heezius ECJM, Poppelier MJJG, Van Kessel KPM, van Strijp JAG. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 2004;199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat. Rev. Rheumatol. 2009;5:218–224. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- Delima AJ, Van Dyke TE. Origin and function of the cellular components in gingival crevice fluid. Periodontol 2000. 2003;31:55–76. doi: 10.1034/j.1600-0757.2003.03105.x. [DOI] [PubMed] [Google Scholar]
- Dunkelberger JR, Song WC. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- Ebenman B, Jonsson T. Using community viability analysis to identify fragile systems and keystone species. Trends Ecol. Evol. 2005;20:568–575. doi: 10.1016/j.tree.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J. Dent. Res. 2008;87:817–828. doi: 10.1177/154405910808700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco RJ, Van Dyke TE. Prevention: Reducing the risk of CVD in patients with periodontitis. Nat Rev Cardiol. 2010;7:479–480. doi: 10.1038/nrcardio.2010.120. [DOI] [PubMed] [Google Scholar]
- Gibson FC, 3rd, Yumoto H, Takahashi Y, Chou HH, Genco CA. Innate immune signaling and Porphyromonas gingivalis-accelerated atherosclerosis. J. Dent. Res. 2006;85:106–121. doi: 10.1177/154405910608500202. [DOI] [PubMed] [Google Scholar]
- Graves D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Porphyromonas gingivalis-host interactions: open war or intelligent guerilla tactics? Microbes Infect. 2009a;11:637–645. doi: 10.1016/j.micinf.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Toll gates to periodontal host modulation and vaccine therapy. Periodontol. 2000. 2009b;51:181–207. doi: 10.1111/j.1600-0757.2009.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. Complement and periodontitis. Biochem. Pharmacol. 2010;80:1992–2001. doi: 10.1016/j.bcp.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Liang S, Payne MA, Hashim A, Jotwani R, Eskan MA, McIntosh ML, Alsam A, Kirkwood KL, Lambris JD, Darveau RP, Curtis MA. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506. doi: 10.1016/j.chom.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Shakhatreh M-AK, Wang M, Liang S. Complement receptor 3 blockade promotes IL-12-mediated clearance of Porphyromonas gingivalis and negates its virulence in vivo. J. Immunol. 2007;179:2359–2367. doi: 10.4049/jimmunol.179.4.2359. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc. Natl. Acad. Sci. U S A. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M, Mollnes TE. The alternative complement pathway revisited. J Cell. Mol. Med. 2008;12:1074–1084. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrandt S, Wasmuth HE, Weiskirchen R, Hellerbrand C, Keppeler H, Werth A, Schirin-Sokhan R, Wilkens G, Geier A, Lorenzen J, Kohl J, Gressner AM, Matern S, Lammert F. Complement factor 5 is a quantitative trait gene that modifies liver fibrogenesis in mice and humans. Nat. Genet. 2005;37:835–843. doi: 10.1038/ng1599. [DOI] [PubMed] [Google Scholar]
- Holers VM. The spectrum of complement alternative pathway-mediated diseases. Immunol. Rev. 2008;223:300–316. doi: 10.1111/j.1600-065X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- Kanazawa N, Furukawa F. Autoinflammatory syndromes with a dermatological perspective. J. Dermatol. 2007;34:601–618. doi: 10.1111/j.1346-8138.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr. Drug Targets Inflamm. Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- Kebschull M, Demmer RT, Papapanou PN. "Gum bug leave my heart alone"--Epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J. Dent. Res. 2010;89:879–902. doi: 10.1177/0022034510375281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Miwa T, Zhou L, Song W-C. Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood. 2008;111:732–740. doi: 10.1182/blood-2007-05-089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinane DF, Peterson M, Stathopoulou PG. Environmental and other modifying factors of the periodontal diseases. Periodontol 2000. 2006;40:107–119. doi: 10.1111/j.1600-0757.2005.00136.x. [DOI] [PubMed] [Google Scholar]
- Kitsis E, Weissmann G. The role of the neutrophil in rheumatoid arthritis. Clin. Orthop. Relat. Res. 1991:63–72. [PubMed] [Google Scholar]
- Krauss JL, Potempa J, Lambris JD, Hajishengallis G. Complementary Tolls in the periodontium: how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol. 2000. 2010;52:141–162. doi: 10.1111/j.1600-0757.2009.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumpf O, Schumann RR. Genetic influence on bloodstream infections and sepsis. Int. J. Antimicrob. Agents. 2008;32(Suppl 1):S44–S50. doi: 10.1016/j.ijantimicag.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Lange D, Schroeder HE. Cytochemistry and ultrastructure of gingival sulcus cells. Helv Odontol Acta. 1971;15:65–86. [PubMed] [Google Scholar]
- Liang S, Krauss JL, Domon H, McIntosh ML, Hosur KB, Qu H, Li F, Tzekou A, Lambris JD, Hajishengallis G. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J. Immunol. 2011;186:869–877. doi: 10.4049/jimmunol.1003252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman HN. Neutrophils and IgG at the host-plaque interface on children's teeth. J. Periodontol. 1980;51:642–651. doi: 10.1902/jop.1980.51.11.642. [DOI] [PubMed] [Google Scholar]
- Niekrash CE, Patters MR. Assessment of complement cleavage in gingival fluid in humans with and without periodontal disease. J. Periodont. Res. 1986;21:233–242. doi: 10.1111/j.1600-0765.1986.tb01455.x. [DOI] [PubMed] [Google Scholar]
- Nikolopoulou-Papaconstantinou AA, Johannessen AC, Kristoffersen T. Deposits of immunoglobulins, complement, and immune complexes in inflamed human gingiva. Acta Odontol. Scand. 1987;45:187–193. doi: 10.3109/00016358709098858. [DOI] [PubMed] [Google Scholar]
- Nussbaum G, Shapira L. How has neutrophil research improved our understanding of periodontal pathogenesis? J. Clin. Periodontol. 2011;38:49–59. doi: 10.1111/j.1600-051X.2010.01678.x. [DOI] [PubMed] [Google Scholar]
- Okroj M, Heinegard D, Holmdahl R, Blom AM. Rheumatoid arthritis and the complement system. Ann. Med. 2007;39:517–530. doi: 10.1080/07853890701477546. [DOI] [PubMed] [Google Scholar]
- Okuda K, Kimizuka R, Abe S, Kato T, Ishihara K. Involvement of periodontopathic anaerobes in aspiration pneumonia. J. Periodontol. 2005;76:2154–2160. doi: 10.1902/jop.2005.76.11-S.2154. [DOI] [PubMed] [Google Scholar]
- Patters MR, Niekrash CE, Lang NP. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. J. Clin. Periodontol. 1989;16:33–37. doi: 10.1111/j.1600-051x.1989.tb01609.x. [DOI] [PubMed] [Google Scholar]
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J. Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- Potempa J, Pike RN. Corruption of innate immunity by bacterial proteases. J. Innate Immun. 2009;1:70–87. doi: 10.1159/000181144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power ME, Tilman D, Estes JA, Menge BA, Bond WJ, Mills LS, Daily G, Castilla JC, Lubchenco J, Paine RT. Challenges in the quest for keystones. BioScience. 1996;46:609–620. [Google Scholar]
- Rautemaa R, Meri S. Protection of gingival epithelium against complement-mediated damage by strong expression of the membrane attack complex inhibitor protectin (CD59) J. Dent. Res. 1996;75:568–574. doi: 10.1177/00220345960750010901. [DOI] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement-targeted therapeutics. Nat. Biotechnol. 2007;25:1265–1275. doi: 10.1038/nbt1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Shah M, Chapple IL. C-1 esterase inhibitor dysfunction localised to the periodontal tissues: clues to the role of stress in the pathogenesis of chronic periodontitis? J. Clin. Periodontol. 2003;30:271–277. doi: 10.1034/j.1600-051x.2003.01266.x. [DOI] [PubMed] [Google Scholar]
- Scannapieco FA, Dasanayake AP, Chhun N. "Does periodontal therapy reduce the risk for systemic diseases?". Dent. Clin. North Am. 2010;54:163–181. doi: 10.1016/j.cden.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. J. Periodontol. 1977;48:778–784. doi: 10.1902/jop.1977.48.12.778. [DOI] [PubMed] [Google Scholar]
- Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontol. 2000. 1997;13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Seppanen M, Lokki ML, Notkola IL, Mattila K, Valtonen V, Nieminen A, Vesanen M, Asikainen S, Meri S. Complement and c4 null alleles in severe chronic adult periodontitis. Scand. J. Immunol. 2007;65:176–181. doi: 10.1111/j.1365-3083.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JL, Phipps S, Gibson PG. Inflammatory mechanisms and treatment of obstructive airway diseases with neutrophilic bronchitis. Pharmacol Ther. 2009;124:86–95. doi: 10.1016/j.pharmthera.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Slaney JM, Curtis MA. Mechanisms of evasion of complement by Porphyromonas gingivalis. Front. Biosci. 2008;13:188–196. doi: 10.2741/2669. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Spitzer D, Mitchell LM, Atkinson JP, Hourcade DE. Properdin can initiate complement activation by binding specific target surfaces and providing a platform for de novo convertase assembly. J. Immunol. 2007;179:2600–2608. doi: 10.4049/jimmunol.179.4.2600. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N. Eng.l J. Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Vitkov L, Klappacher M, Hannig M, Krautgartner WD. Neutrophil fate in gingival crevicular fluid. Ultrastruct. Pathol. 2010;34:25–30. doi: 10.3109/01913120903419989. [DOI] [PubMed] [Google Scholar]
- Wagner E, Frank MM. Therapeutic potential of complement modulation. Nat. Rev. Drug Discov. 2010;9:43–56. doi: 10.1038/nrd3011. [DOI] [PubMed] [Google Scholar]
- Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. Microbial hijacking of complement-toll-like receptor crosstalk. Sci. Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Shakhatreh M-AK, James D, Liang S, Nishiyama S-i, Yoshimura F, Demuth DR, Hajishengallis G. Fimbrial proteins of Porphyromonas gingivalis mediate in vivo virulence and exploit TLR2 and complement receptor 3 to persist in macrophages. J. Immunol. 2007;179:2349–2358. doi: 10.4049/jimmunol.179.4.2349. [DOI] [PubMed] [Google Scholar]
- Ward PA. Functions of C5a receptors. J. Mol. Med. 2009;87:375–378. doi: 10.1007/s00109-009-0442-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove JA, DiScipio RG, Chen Z, Potempa J, Travis J, Hugli TE. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J. Biol. Chem. 1992;267:18902–18907. [PubMed] [Google Scholar]
- Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Schmudde I, Laumonnier Y, Pandey MK, Clark JR, Konig P, Gerard NP, Gerard C, Wills-Karp M, Kohl J. A critical role for C5L2 in the pathogenesis of experimental allergic asthma. J. Immunol. 2010;185:6741–6752. doi: 10.4049/jimmunol.1000892. [DOI] [PubMed] [Google Scholar]