Abstract
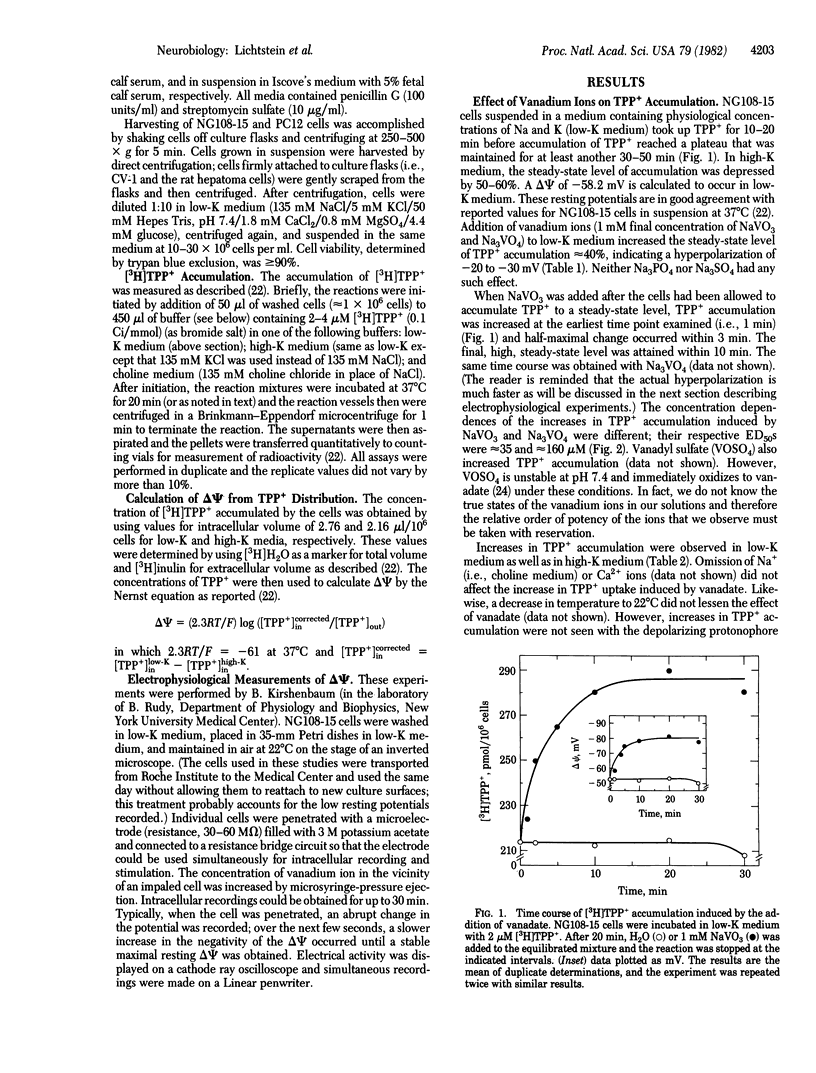
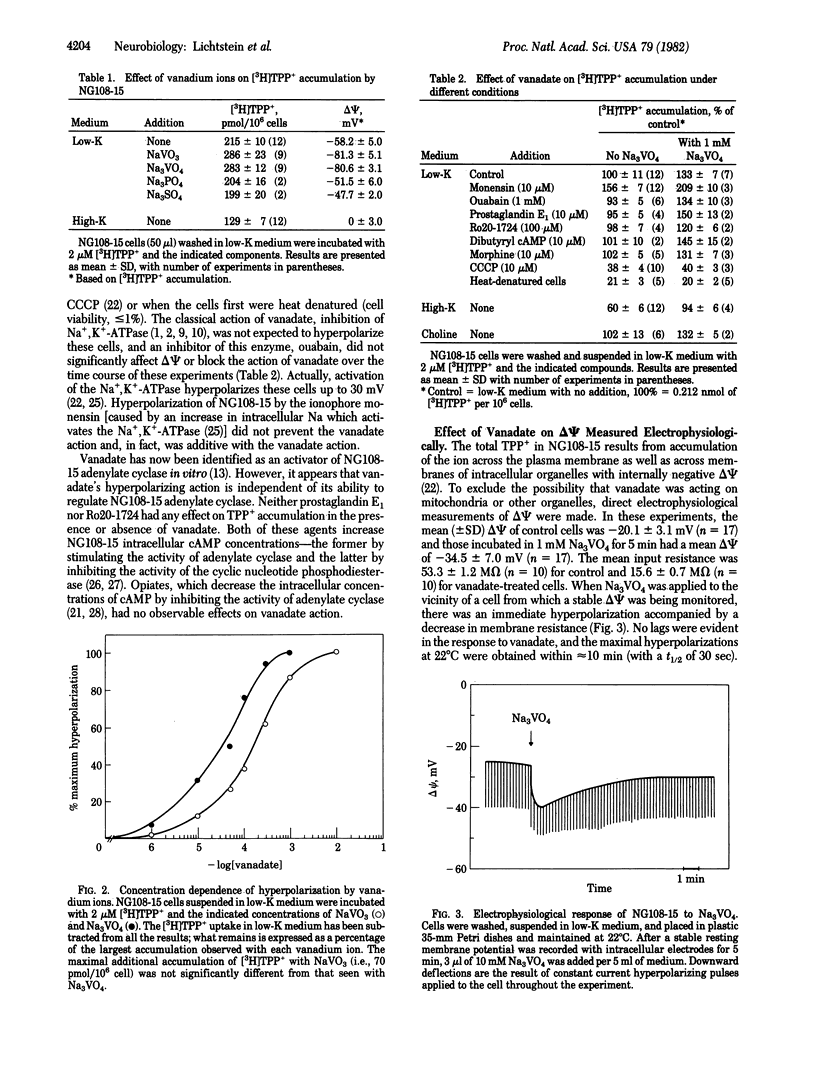
Vanadate hyperpolarizes mouse neuroblastoma-glioma hybrid NG108-15 by 20-30 mV. These changes in membrane potential (delta psi) are observed by monitoring the equilibrium distribution (intracellular/extracellular) of the lipophilic cation [3H]tetraphenylphosphonium (TTP+) and by directly measuring delta psi with intracellular microelectrodes. In physiological media (i.e., 135 mM NaCl/5 mM KCl), the half-maximal effective concentrations of sodium orthovanadate (Na3VO4) and sodium metavanadate (NaVO3) are 35 and 160 microM, respectively. The maximal effects for both these ions are quantitatively indistinguishable. The hyperpolarizing responses to vanadate occur without any observable lag, have t1/2 less than or equal to 30 sec, and are always accompanied by simultaneous decreases in membrane resistance. Neither ouabain nor media containing high K (i.e., 120 mM) devoid of Na and K (isotonicity maintained by choline) prevent the change in delta psi induced by vanadate. Therefore, vanadate produces a unique hyperpolarization which does not depend upon Na, K, or the Na/K pump. Furthermore, the accompanying decreases in membrane resistance indicate that vanadate must increase the permeability of the membrane to some ion. Our data are consistent with it being an anion, such as chloride or vanadate itself. Finally, vanadate hyperpolarizes many different types of cultured cells, only some of which are of neuronal origin. This indicates that a hyperpolarization of delta psi must be considered in any assessment of the physiological actions of the vanadates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adám-Vizi V., Váradi G., Simon P. Reduction of Vanadate by ascorbic acid and noradrenaline in synaptosomes. J Neurochem. 1981 May;36(5):1616–1620. doi: 10.1111/j.1471-4159.1981.tb00410.x. [DOI] [PubMed] [Google Scholar]
- Balfour W. E., Grantham J. J., Glynn I. M. Vanadate-stimulated natriuresis. Nature. 1978 Oct 26;275(5682):768–768. doi: 10.1038/275768a0. [DOI] [PubMed] [Google Scholar]
- Barrabin H., Garrahan P. J., Rega A. F. Vanadate inhibition of the Ca2+-ATPase from human red cell membranes. Biochim Biophys Acta. 1980 Aug 14;600(3):796–804. doi: 10.1016/0005-2736(80)90482-4. [DOI] [PubMed] [Google Scholar]
- Beaugé L. A., Glynn I. M. Commercial ATP containing traces of vanadate alters the response of (Na+ + K+) ATPase to external potassium. Nature. 1978 Apr 6;272(5653):551–552. doi: 10.1038/272551a0. [DOI] [PubMed] [Google Scholar]
- Blume A. J., Chen C., Foster C. J. Muscarinic regulation of cAMP in mouse neuroblastoma. J Neurochem. 1977 Oct;29(4):625–632. doi: 10.1111/j.1471-4159.1977.tb07778.x. [DOI] [PubMed] [Google Scholar]
- Bond G. H., Hudgins P. M. Inhibition of red cell Ca2+-ATPase by vanadate. Biochim Biophys Acta. 1980 Aug 14;600(3):781–790. doi: 10.1016/0005-2736(80)90480-0. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. The nature of the membrane sites controlling anion permeability of human red blood cells as determined by studies with disulfonic stilbene derivatives. J Membr Biol. 1972 Dec 29;10(3):311–330. doi: 10.1007/BF01867863. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Caroni P., Carafoli E. The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification. J Biol Chem. 1981 Apr 10;256(7):3263–3270. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- DeMaster E. G., Mitchell A. A comparison of arsenate and vanadate as inhibitors or uncouplers of mitochondrial and glycolytic energy metabolism. Biochemistry. 1973 Sep 11;12(19):3616–3621. doi: 10.1021/bi00743a007. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Properties and structural basis of simple diffusion pathways in the erythrocyte membrane. Rev Physiol Biochem Pharmacol. 1977;78:1–97. doi: 10.1007/BFb0027721. [DOI] [PubMed] [Google Scholar]
- Greengard P., McAfee D. A., Kebabian J. W. On the mechanism of action of cyclic AMP and its role in synaptic transmission. Adv Cyclic Nucleotide Res. 1972;1:337–355. [PubMed] [Google Scholar]
- Grupp G., Grupp I., Johnson C. L., Wallick E. T., Schwartz A. Effect of vanadate on cardiac contraction and adenylate cyclase. Biochem Biophys Res Commun. 1979 May 28;88(2):440–447. doi: 10.1016/0006-291x(79)92068-0. [DOI] [PubMed] [Google Scholar]
- Hackbarth I., Schmitz W., Scholz H., Erdmann E., Krawietz W., Philipp G. Positive inotropism of vanadate in cat papillary muscle. Nature. 1978 Sep 7;275(5675):67–67. doi: 10.1038/275067a0. [DOI] [PubMed] [Google Scholar]
- Klee W. A., Nirenberg M. A neuroblastoma times glioma hybrid cell line with morphine receptors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3474–3477. doi: 10.1073/pnas.71.9.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtshtein D., Boone G., Blume A. J. A physiological requirement of Na+ for the regulation of cAMP levels in intact NG108-15 cells. Life Sci. 1979 Sep 11;25(11):985–991. doi: 10.1016/0024-3205(79)90504-6. [DOI] [PubMed] [Google Scholar]
- Lichtshtein D., Dunlop K., Kaback H. R., Blume A. J. Mechanism of monensin-induced hyperpolarization of neuroblastoma-glioma hybrid NG108-15. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2580–2584. doi: 10.1073/pnas.76.6.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtstein D., Mullikin-Kilpatrick D., Blume A. J. Modification of neuroblastoma x glioma hybrid NG108-15 adenylate cyclase by vanadium ions. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1157–1165. doi: 10.1016/0006-291x(82)91091-9. [DOI] [PubMed] [Google Scholar]
- Lindquist R. N., Lynn J. L., Jr, Lienhard G. E. Possible transition-state analogs for ribonuclease. The complexes of uridine with oxovanadium(IV) ion and vanadium(V) ion. J Am Chem Soc. 1973 Dec 26;95(26):8762–8768. doi: 10.1021/ja00807a043. [DOI] [PubMed] [Google Scholar]
- Lopez V., Stevens T., Lindquist R. N. Vanadium ion inhibition of alkaline phosphatase-catalyzed phosphate ester hydrolysis. Arch Biochem Biophys. 1976 Jul;175(1):31–38. doi: 10.1016/0003-9861(76)90482-3. [DOI] [PubMed] [Google Scholar]
- MacDermot J., Higashida H., Wilson S. P., Matsuzawa H., Minna J., Nirenberg M. Adenylate cyclase and acetylcholine release regulated by separate serotonin receptors of somatic cell hybrids. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1135–1139. doi: 10.1073/pnas.76.3.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal S. G., Rhoads D. B., Racker E. Vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase and other ATPases. Biochem Biophys Res Commun. 1979 Aug 13;89(3):845–850. doi: 10.1016/0006-291x(79)91855-2. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Puchstein C., Hannemann H., Söchtig E. Activation of adenylate cyclase by vanadate. Nature. 1979 Jan 11;277(5692):143–145. doi: 10.1038/277143a0. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanEtten R. L., Waymack P. P., Rehkop D. M. Letter: Transition metal ion inhibition of enzyme-catalyzed phosphate ester displacement reactions. J Am Chem Soc. 1974 Oct 16;96(21):6782–6785. doi: 10.1021/ja00828a053. [DOI] [PubMed] [Google Scholar]
- Wang T., Tsai L. I., Solaro R. J., Grassi de Gende A. O., Schwartz A. Effects of potassium on vanadate inhibition of sarcoplasmic reticulum Ca2+-ATPase from dog cardiac and rabbit skeletal muscle. Biochem Biophys Res Commun. 1979 Nov 14;91(1):356–361. doi: 10.1016/0006-291x(79)90626-0. [DOI] [PubMed] [Google Scholar]



