Abstract
Purpose.
Toxoplasma gondii, the parasite responsible for ocular toxoplasmosis, accesses the retina from the bloodstream. We investigated the dendritic cell as a potential taxi for T. gondii tachyzoites moving across the human retinal endothelium, and examined the participation of adhesion molecules and chemokines in this process.
Methods.
CD14-positive monocytes were isolated from human peripheral blood by antibody-mediated cell enrichment, and cultured in granulocyte-macrophage colony-stimulating factor and interleukin-4 to generate dendritic cells. Transmigration assays were performed over 18 hours in transwells seeded with human retinal endothelial cells and using dendritic cells exposed to laboratory or natural strains of T. gondii tachyzoites. Parasites were tagged with yellow fluorescent protein to verify infection. In some experiments, endothelial monolayers were preincubated with antibody directed against adhesion molecules, or chemokine was added to lower chambers of transwells.
Results.
Human monocyte–derived dendritic cell preparations infected with laboratory or natural strain T. gondii tachyzoites transmigrated in larger numbers across simulated human retinal endothelium than uninfected dendritic cells (P ≤ 0.0004 in 5 of 6 experiments). Antibody blockade of intercellular adhesion molecule (ICAM)–1, vascular cell adhesion molecule (VCAM)–1, and activated leukocyte cell adhesion molecule (ALCAM) inhibited transmigration (P ≤ 0.007), and CCL21 or CXCL10 increased transmigration (P ≤ 0.031).
Conclusions.
Transmigration of human dendritic cells across retinal endothelium is increased following infection with T. gondii. Movement may be impacted by locally produced chemokines and is mediated in part by ICAM-1, VCAM-1, and ALCAM. These findings have implications for development of novel therapeutics aimed at preventing retinal infection by T. gondii.
Transmigration of human dendritic cells across retinal endothelium is increased following infection with T. gondii. Movement may be impacted by locally produced chemokines and is mediated in part by ICAM-1, VCAM-1, and ALCAM.
Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite that promiscuously infects nucleated mammalian and avian cells.1 The seroprevalence of human toxoplasmosis varies according to geographical area, but it has been estimated that as many as one in three individuals across the globe are infected with the parasite.2 Retinitis with secondary choroiditis is the most common clinical disease caused by infection with T. gondii, and this condition may affect immunocompetent individuals as well as immunocompromised patients.3 In a majority of cases, the parasite is ingested in bradyzoite or sporozoite form following consumption of undercooked meat containing tissue cysts, or water or food contaminated with oocysts, respectively.2 In the small intestine, these forms convert to the fast-replicating tachyzoite.4 T. gondii tachyzoites disseminate from the gut to target organs, including the retina, via the circulation.5 Examination of peripheral blood taken from patients who have been acutely or chronically infected with T. gondii has demonstrated tachyzoites circulating both as free forms or within peripheral blood mononuclear cells.6 However, the route by which T. gondii moves across the retinal vascular endothelium from the blood stream into the human retina is poorly understood.
Recently we reported that free T. gondii tachyzoites had the ability to transmigrate a simulated human retinal endothelium monolayer.7 On the other hand, Lambert et al.8 observed a significantly higher parasite load in the brain of mice following adoptive transfer of tachyzoite-infected dendritic cells than after inoculation with free tachyzoites. They also noted significantly higher speed, and mean and maximum migration distances after human monocyte-derived dendritic cells were infected with tachyzoites.8 Working independently, while also using an adoptive transfer mouse model of toxoplasmic encephalitis, Courret et al.9 tracked fluorescently labeled tachyzoite-infected CD11c-positive or CD11b-positive leukocytes from blood to brain. Interestingly, simple infectivity assays have showed that human dendritic cells and monocytes are more permissive to infection with T. gondii tachyzoites than neutrophils or lymphocytes.10 Taken together, these observations suggest that in the human, dendritic cells may provide an additional mechanism by which T. gondii gain access to the retina following systemic infection.
We investigated the ability of human monocyte–derived dendritic cells to transmigrate human retinal vascular endothelium following infection with T. gondii tachyzoites, using transwell migration assays and with fluorescently tagged tachyzoites. In addition, we examined the participation in the migration of key endothelial adhesion molecules (i.e., intercellular adhesion molecule [ICAM]–1, vascular cell adhesion molecule [VCAM]–1, and activated leukocyte cell adhesion molecule [ALCAM]), as well as chemokines implicated in toxoplasmic inflammation (i.e., CCL21/secondary lymphoid tissue chemokine [SLC] and CXCL10/interferon gamma-induced protein 10 [IP-10]).
Methods
Parasites
Yellow fluorescent protein (YFP)–expressing RH strain (RH-YFP; clonal isolate in haplogroup 1; gift of Boris Striepen, PhD, University of Georgia, Athens, GA)11 and GPHT strain (natural isolate in haplogroup 6; gift of L. David Sibley, PhD, Washington University, St. Louis, MO)12 T. gondii were used in these experiments. Tachyzoites were maintained by serial passage in human neonatal foreskin fibroblasts (Cascade Biologics, Portland, OR) in Dulbecco's modified Eagle's medium (DMEM; catalog number: 12100; Invitrogen-Gibco, Grand Island, NY) supplemented with 44 mM sodium bicarbonate and 1% heat-inactivated fetal bovine serum (FBS; HyClone Laboratories, Logan, UT) at 37°C and at 5% CO2. For every experiment, plaque assays were performed using a fibroblast monolayer; the criterion for inclusion was parasite viability of at least 35% for the clonal isolate and at least 15% for the natural isolate, consistent with published measurements.13
Production of Yellow Fluorescent Protein (YFP)–Expressing GPHT Strain T. gondii (GPHT-YFP)
GPHT strain T. gondii was transduced with a 9289-bp plasmid expressing a tandem-repeat YFP element under control of the alpha-tubulin promoter and a chloramphenicol resistance element under control of the SAG promoter (ptubYFP-YFPsagCAT, gift of Boris Striepen, PhD). Plasmid was isolated using a commercial kit (GenElute HP Endotoxin-Free Plasmid MaxiPrep Kit; Sigma-Aldrich, St. Louis, MO), precipitated with 100% isopropanol and dissolved at 1 mg/mL in transfection reagent (120 mM potassium chloride, 0.15 mM calcium chloride, 10 mM dipotassium hydrogen phosphate/potassium dihydrogen phosphate, 25 mM HEPES, 2 mM ethylenediaminetetraacetic acid, and 5 mM magnesium chloride) supplemented with 2 mM adenosine 5′-triphosphate and 5 mM glutathione (recipe shared by Boris Striepen, PhD). Fifty million freshly egressed GPHT strain T. gondii tachyzoites were suspended in 700 μL of supplemented transfection reagent, to which 100 μL of plasmid solution was added, and subsequently subjected to electroporation (ECM 630 Electro Cell Manipulator; Harvard Apparatus-BTX, Holliston, MA) set at 2000 V, 50 μF, and 25 Ω. After electroporation, tachyzoites were passaged in human fibroblasts in modified DMEM supplemented with 1% FBS, and after the first passage, 2 μM chloramphenicol. Following at least 10 passages in the presence of chloramphenicol, freshly egressed ptubYFP-YFPsagCAT-bearing GPHT T. gondii clones were selected by fluorescence-activated cell sorting (Turbo DiVa Vantage; Becton Dickinson, Franklin Lakes, NJ), and subsequently expanded for use in experiments.
Generation and Flow Cytometric Characterization of Human Dendritic Cells
Dendritic cells were generated from monocytes in the peripheral blood of healthy individuals, obtained under approval of the Oregon Health and Science University Institutional Review Board, and following the methods described by Lambert et al.8 Monocytes were isolated from blood (RosetteSep Human Monocyte Enrichment Cocktail; STEMCELL Technologies, Inc., Vancouver, BC, Canada), according to the manufacturer's instructions, and finally suspended in RPMI 1640 medium supplemented with 10% FBS and 200 mM l-glutamine. Cells were plated in 10-cm-diameter dishes at 20 × 106 cells/dish in modified RPMI medium with 10% FBS, interleukin (IL)-4 at 12.5 ng/mL (R&D Systems, Minneapolis, MN), and granulocyte-macrophage colony-stimulating factor (GM-CSF) at 100 ng/mL (PeproTech, Rocky Hill, NJ). The medium was changed after 24 hours, followed by every 72 hours, and dendritic cells were harvested after 7 days. Expression of cell surface molecules was determined by labeling the cells with mouse anti-human monoclonal antibodies diluted in phosphate-buffered saline with 1% FBS and 0.1% sodium azide for 30 minutes on ice: Pacific Blue–tagged anti-CD14 antibody (2 μg/mL, clone M5E2, isotype IgG2aκ); PE-tagged anti-CD11c antibody (25 μg/mL, clone B-ly6, isotype IgG1κ); PE-Cy5.5–tagged anti-CD80 antibody (0.75 μg/mL, clone L307.4, isotype IgG1κ); biotin-tagged anti-CD86 antibody (1.5 μg/mL, clone 2331 [FUN-1], isotype IgG1κ); and FITC-tagged anti-HLA-DR antibody (25 μg/mL, clone G46-6, isotype IgG2aκ) (all obtained from BD Pharmingen, San Jose, CA). After washing with buffer, cells were treated with streptavidin-conjugated APC-Cy7 (2μg/mL, BD Pharmingen) for 15 minutes, washed again, and fixed in 4% paraformaldehyde. Data were acquired on a flow cytometer (BD LSR II; BD Biosciences, San Jose, CA) and analyzed using flow cytometry data analysis software (FCS Express; De Novo Software, Los Angeles, CA). All viable cells, as determined by size, were included in the gates.
Adhesion-Blocking Antibodies and Recombinant Chemokines
Mouse anti-human ICAM-1 (50 μg/mL, clone BBIG-I1, isotype IgG1), VCAM-1 (30 μg/mL, clone BBIG-V1, isotype IgG1), ALCAM (30 μg/mL, clone 105901, isotype IgG1), and control (identical concentration, clone 11711, isotype IgG1) monoclonal antibodies (all obtained from R&D Systems) were used in adhesion blockade experiments. Recombinant human CCL21 (100 ng/mL) and CXCL10 (20 ng/mL) (both obtained from R&D Systems) were used in chemotaxis induction experiments.
Human Retinal Endothelial Transmigration Assay
Polyethylene terephthalate transwell membranes (0.3 cm2 diameter with 3-μm pores; BD Labware, Franklin Lakes, NJ) were inserted into wells of 24-well plates. Membranes were coated with 50 μg/mL bovine type I collagen (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours at 37°C, and then seeded with 30,000 human retinal endothelial cells, isolated and immortalized as previously described,7 suspended in MCDB-131 medium with 10% FBS and endothelial growth factors (EGM-2 SingleQuots, omitting gentamicin, hydrocortisone, and FBS; Lonza-Clonetics, Walkersville, MD). Transwells were incubated at 37°C and 5% CO2 for 4 to 5 days. A portion of the dendritic cell preparation, suspended in phenol red-free endothelial basal medium (EBM; Lonza-Clonetics) supplemented with up to 10% FBS and endothelial growth factors, was exposed to RH-YFP or GPHT-YFP T. gondii tachyzoites, suspended in modified DMEM with 1% FBS, at a multiplicity of infection of 3:1 for 6 hours at 37 °C and 5% CO2. Cells were then separated from any extracellular parasites by 3 rounds of centrifugation at 80g for 10 minutes. The T. gondii–exposed dendritic cell isolates, subsequently referred to as “infected dendritic cells,” were photographed (mean of 8 random fields/isolate) at ×630 magnification under epifluorescence and a YPF filter, using a digital camera (Leica DM IRB; Leica Microsystems, Deerfield, IL; and Image-Pro Plus; Media Cybernetics, Bethesda, MD). Percentage of infected cells was calculated from the photographs.
Infected and/or uninfected dendritic cells were suspended in modified EBM with up to 10% FBS, and applied to upper chambers of transwells (5 × 105 cells/well). Lower chambers were filled with the same medium, and transwells were incubated for 18 hours at 37°C and 5% CO2 (n = 4–8 wells/condition). After the incubation, dendritic cells in lower chambers were counted by hematocytometer. In certain experiments, endothelial monolayers were incubated with adhesion molecule blocking or control antibody for 1 hour ahead of the addition of the dendritic cells. Antibody was removed immediately prior to addition of cells. In other experiments, chemokine was added to lower chambers simultaneously with the addition of dendritic cells to upper chambers. Retinal endothelial monolayer integrity was verified for each experiment by measuring a significant difference in permeability of membranes coated with collagen I alone versus membranes coated with collagen I plus endothelial cells (n = 4 to 6 wells/condition) to 1 mg/mL Texas Red–conjugated 70,000 molecular weight dextran (Molecular Probes/Invitrogen, Eugene, OR), according to the method of Harhaj et al.14
Statistical Analyses
For statistical analyses, the Student's t-test, two-tailed, was used to compare results obtained under control versus test conditions. In these comparisons, a value of P < 0.05 was taken to indicate a statistically significant difference.
Results
Dendritic Cells Infected with T. gondii Tachyzoites Migrate Readily across Simulated Human Retinal Endothelium
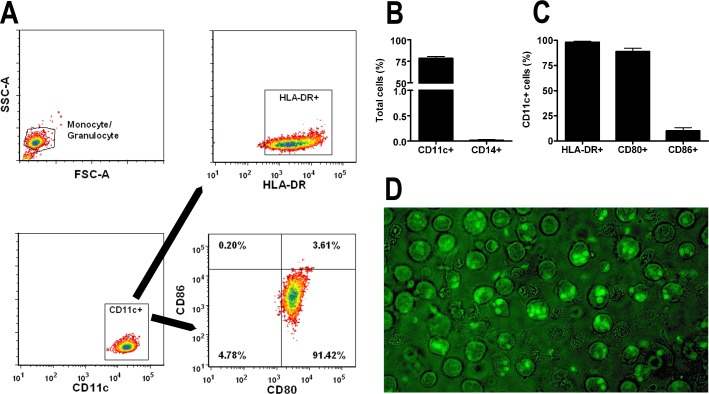
To examine the ability of human dendritic cells to transmigrate human retinal endothelium, we generated dendritic cells from human peripheral blood CD14-positive monocytes. Following an established protocol, in which the critical element was culture in the presence of IL-4 and GM-CSF, 76.4% to 82.6% (mean = 78.5%) of cells expressed CD11c and ≤0.03% of cells expressed CD14; a percentage of CD11c-positive cells expressed HLA-DR (≥98%), CD80 (84.5%–95.3%, mean = 88.9%), and CD86 (3.9%–14.1%, mean = 10.2%) (n = 3 experiments), according to flow cytometric analysis (Figs. 1A–C). The dendritic cells were exposed to recombinant T. gondii tachyzoites that constitutively expressed YFP, belonging to laboratory RH strain or natural GPHT strain. After a 6-hour infection period, 55.9% to 89.6% (mean = 71.1%, n = 13 experiments) of cells incubated with RH-YFP were infected, and 72.1% to 80.8% (mean = 76.1%, n = 3 experiments) of cells incubated with GPHT-YFP were infected, as observed under epifluorescence (Fig. 1D). Both single and multiple tachyzoites were observed intracellularly within different dendritic cells. Transmigration of human retinal endothelium by infected versus uninfected dendritic cells was determined using the Boyden chamber assay. Both infected and uninfected cells transmigrated over an 18-hour period. Although variation in the absolute number of migrating cells was noted between experiments, infected cells reached lower chambers in significantly greater numbers than uninfected cells in 3 of 3 experiments performed with RH-YFP (P ≤ 0.0004; representative experiment shown as Fig. 2A), and in 2 of 3 experiments performed with GPHT-YFP (P < 0.0001; representative experiment shown as Fig. 2B).
Figure 1. .
Characterization of dendritic cells and T. gondii tachyzoite infection. (A) Flow cytometry plots and (B, C) graphs describing dendritic cells generated from human peripheral blood monocytes following 7-day culture in GM-CSF and IL-4. Dendritic cells were stained for selected surface markers (i.e., CD14, CD11c, HLA-DR, CD80, and CD86). Cells were first gated based on a monocyte/granulocyte gate, and HLA-DR, CD80, and CD86 were further gated on the CD11c-positive population. Plots show results that are representative of those obtained in 3 independent experiments. Graphs include results from 3 independent experiments. Bars: mean; error bars: SEM. (D) Image of dendritic cells infected with RH-YFP tachyzoites after the 6-hour infection period (original magnification: ×630).
Figure 2. .
T. gondii tachyzoite–infected dendritic cells transmigrate simulated human retinal vascular endothelium in larger numbers than uninfected dendritic cells. Graphs showing the number of dendritic cells recovered from lower chambers of transwells divided by human retinal endothelial cell monolayers 18 hours after upper chambers were loaded with 5 × 105 dendritic cells that were previously exposed to (A) RH-YFP or (B) GPHT-YFP T. gondii tachyzoites, or were not infected. Bars: mean; error bars: SEM; n = 4 to 7 wells; P values by Student's t-test, two-tailed. Graph presented in (A) shows results that are representative of those obtained in 3 independent experiments. Graph presented in (B) shows results that are representative of those obtained in 2 of 3 independent experiments.
Human Retinal Endothelial Transmigration of T. gondii Tachyzoite–Infected Dendritic Cells Is Reduced by Antibodies Directed against ICAM-1, VCAM-1, or ALCAM Antibodies
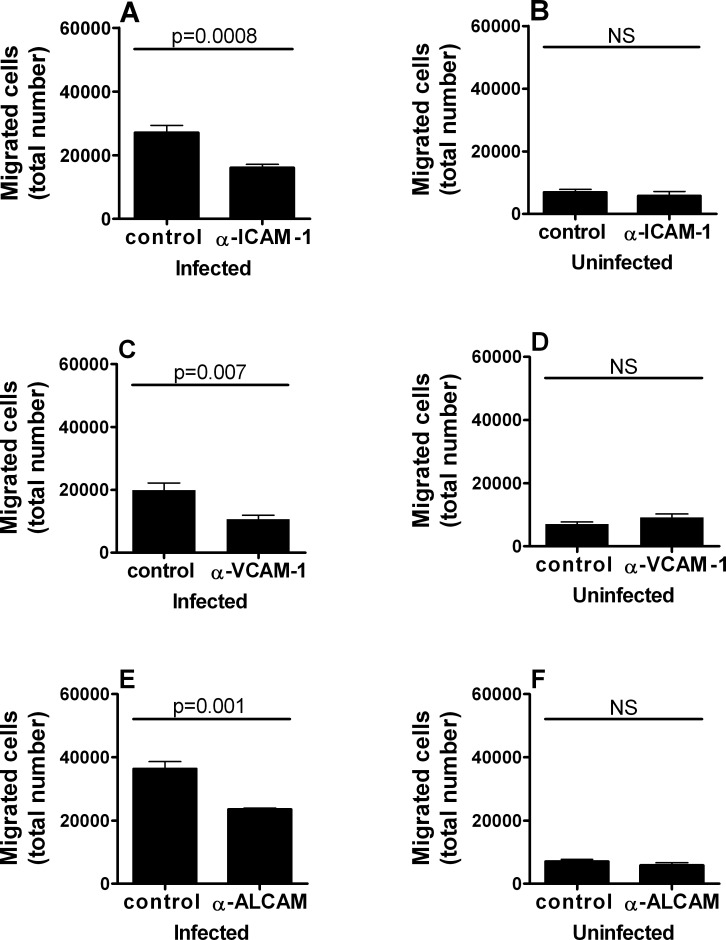
Having demonstrated transmigration of human retinal endothelium, we sought to establish endothelial cell adhesion molecules involved in this process. We focused on three IgG superfamily members that were previously implicated in dendritic cell homing, or leukocyte homing to the central nervous system (i.e., ICAM-1, VCAM-1, and ALCAM). Retinal endothelial monolayers were incubated with antibody for 1 hour prior to the addition of RH-YFP T. gondii tachyzoite–infected dendritic cells into upper chambers of transwells. In two independent experiments for each antibody, the number of migrating cells recovered from lower chambers after 18 hours was significantly reduced in comparison with control IgG by specific antibody directed against human ICAM-1 (P ≤ 0.0008; representative experiment shown as Fig. 3A), VCAM-1 (P ≤ 0.007; representative experiment shown as Fig. 3C), or ALCAM (P ≤ 0.001; representative experiment shown as Fig. 3E). In contrast, there was no significant impact on migration, when the studies were performed using uninfected dendritic cells in place of infected cells (representative experiments shown as Figs. 3B, 3D, 3F).
Figure 3. .
ICAM-1, VCAM-1, or ALCAM blockade inhibits transmigration of T. gondii tachyzoite–infected human dendritic cells across simulated human retinal vascular endothelium. Graphs showing the number of dendritic cells recovered from lower chambers of transwells divided by human retinal endothelial cell monolayers preincubated with anti-human (A, B) ICAM-1, (C, D) VCAM-1, or (E, F) ALCAM antibody, or control mouse IgG1. Prior to use in assays, dendritic cells (A, C, E) were exposed to RH strain T. gondii tachyzoites or (B, D, F) remained unexposed. Bars: mean; error bars: SEM; n = 4 to 8 wells; P values by Student's t-test, two-tailed; NS, no significant difference. Each graph shows results that are representative of those obtained in 2 independent experiments.
Human Retinal Endothelial Transmigration of T. gondii Tachyzoite–Infected Dendritic Cells Is Augmented by CCL21 or CXCL10
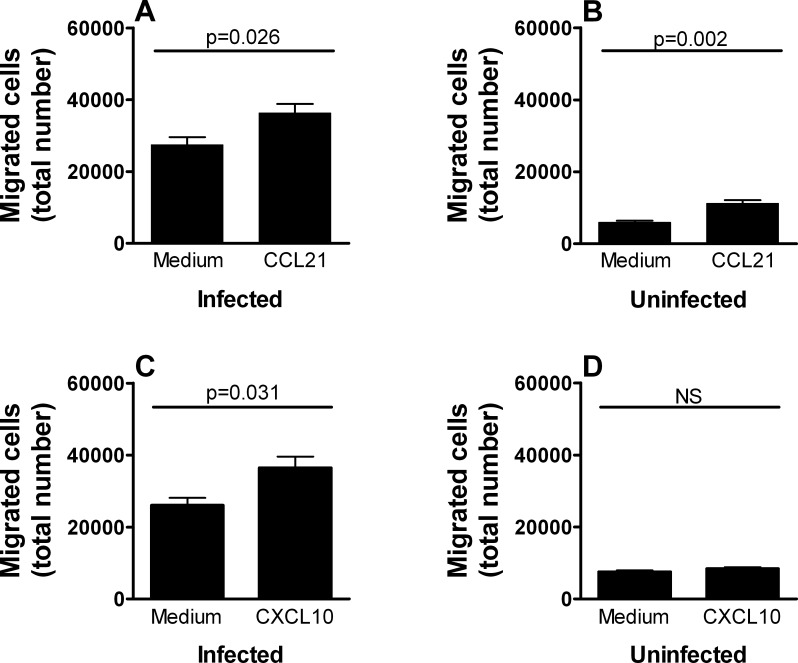
We next investigated the possibility that chemokines might impact the capacity of infected dendritic cells to transmigrate human retinal endothelium. We selected two chemokines with documented chemotactic activity for dendritic cells in other systems that were likely to be expressed at the site of toxoplasmic infection (i.e., CCL21 and CXCL10). In two independent assays for each chemokine, a significantly higher number of RH-YFP T. gondii tachyzoite–infected dendritic cells moved through the transwell over 18 hours when CCL21 was applied to lower chambers (P ≤ 0.026; representative experiment shown as Fig. 4A) and when CXCL10 was applied to lower chambers (P ≤ 0.031; representative experiment shown as Fig. 4C) in comparison with medium alone. CCL21 induced a significantly higher number of uninfected dendritic cells to migrate in two of four donors (P ≤ 0.001; representative experiment shown as Fig. 4B), but CXCL10 did not promote migration of uninfected cells (representative experiment shown as Fig. 4D).
Figure 4. .
CCL21 or CXCL10 increases transmigration of T. gondii tachyzoite–infected human dendritic cells across simulated human retinal vascular endothelium. Graphs showing the number of dendritic cells recovered from lower chambers of transwells divided by human retinal endothelial cell monolayers with (A, B) CCL21 or (C, D) CXCL10, or no chemokine added to lower chambers at commencement of the experiment. Prior to use in assays, dendritic cells were (A, C) exposed to RH strain T. gondii tachyzoites or (B, D) remained unexposed. Bars: mean; error bars: SEM; n = 4 to 8 wells; P values by Student's t-test, two-tailed; NS, no significant difference. Graphs presented in (A), (C), and (D) show results that are representative of those obtained in 2 to 3 independent experiments. Graph presented in (B) shows results that are representative of those obtained in 2 of 4 independent experiments.
Discussion
Until recently, the route used by T. gondii to cross the retinal vascular endothelium from the circulation to the retina proper in the human host had not been investigated. In 2012, we reported that free tachyzoites were capable of transmigrating human retinal endothelium in vitro, and that this interaction had a molecular basis since anti-ICAM-1 antibody reduced the movement by approximately 50%.7 Here we demonstrate another potential route that the parasite may use to enter the retina. We show that human dendritic cells infected with T. gondii tachyzoites cross-simulated human retinal vascular endothelium. We further show that cell adhesion molecules, ICAM-1, VCAM-1, and ALCAM, participate in this transmigration, and that chemokines, CCL21 and CXCL10, promote the process. Dendritic cells were generated from human peripheral blood monocytes, and the vast majority expressed CD11c, but not CD14, indicating a pure population; high levels of HLA-DR and CD80 confirmed a mature phenotype. Recombinant expression of YFP by the two T. gondii strains used in our studies allowed us to confirm the consistent infection of more than 50% of the dendritic cells in preparations used in the experiments.
Our observation that human dendritic cells may taxi T. gondii tachyzoites across a human retinal vascular endothelial monolayer is consistent with independent reports from three groups that implicate dendritic cells in T. gondii trafficking to the brain in a mouse model of toxoplasmic encephalitis.8,9,15 Working in a human-based experimental system is an important complement to mouse studies, because mouse and human interactions with T. gondii differ at multiple cellular and molecular levels.16 Interestingly, the infection of dendritic cells resulted in the migration of significantly higher numbers of cells across the simulated retinal endothelium. This “state of hypermobility” was previously described by Lambert et al.8 in relation to migration of infected dendritic cells across umbilical vein and aortic endothelial monolayers. In using human retinal endothelial cells, our observations have immediate clinical relevance, given that retinitis is the common presentation of human toxoplasmosis.3 The majority of our experiments used the RH strain of T. gondii, which is the most commonly used in research in this field. However, RH was first isolated in 1938 and has developed traits as a result of being maintained in vitro for multiple decades, including extended survival outside the host cell, rapid growth, and failure to differentiate into the bradyzoite form.13,17 Thus we confirmed the effect of tachyzoite infection on dendritic cell migration by replacing RH with GPHT, which is a strain that was relatively recently introduced into the laboratory.12
By incubating the endothelial monolayer with blocking antibodies ahead of the retinal endothelial transmigration assay, we identified a role for IgG superfamily members (i.e., ICAM-1, VCAM-1, and ALCAM) in mediating the migration of infected dendritic cells. These cell adhesion molecules were not previously investigated in relation to experimental models of ocular toxoplasmosis, but each is known to participate in the arrest, firm adhesion, and/or diapedesis steps of the leukocyte adhesion cascade.18 Different leukocyte subpopulations use different combinations of adhesion molecules to cross the endothelium, but both ICAM-1 and VCAM-1 have been implicated in the migration of dendritic cells in the setting of lymph node trafficking via afferent lymphatics.19 Activated leukocyte cell adhesion molecule was recognized to influence leukocyte transmigration relatively recently and its roles remain relatively uncharacterized. However, detailed studies conducted with mouse and human in vitro models have identified ALCAM as a major molecular mediator of leukocyte trafficking into the central nervous system specifically.20 Silva et al.21 have reported that ICAM-1, VCAM-1, and ALCAM transcripts are upregulated in the brain of mice following infection with T. gondii. Previous work in this model by another group has indirectly implicated ICAM-1 in trafficking of T. gondii tachyzoite–infected dendritic cells into the mouse brain; antibody blockade of CD11a, which is a subunit of the ICAM-1 receptor, lymphocyte function-associated antigen (LFA)–1, inhibits entry of dendritic cells into chronically infected mice.15 Similar blockade of very late antigen (VLA)–4, a VCAM-1 receptor, does not influence entry. The apparent discrepancy between our results and these findings may represent a species-specific difference or be the result of vascular endothelial cell heterogeneity; our previous work indicates that VCAM-1 is highly expressed by human retinal endothelial cells.22
Chemokines control leukocyte movement through the endothelium.18 In particular, CCL21 and CXCL10, which are chemotactic for dendritic cells in other settings,23,24 are produced in the context of experimental toxoplasmic infections in mice. John et al.15 demonstrated elevated expression of CCL21 in infected mouse brain by immunohistochemistry, although dendritic cells from mice genetically deficient in receptor, CCR7, accessed the brain in equivalent numbers as those from wild type animals. Norose et al.25 have described an elegant mouse model of chronic ocular toxoplasmosis induced by intraperitoneal injection of tissue cysts, in which, in addition to T lymphocytes, CD11c-positive cells are present within retina by 35 days postinjection. In this model, CXCL10 transcript is induced approximately 800-fold in the retina at 5 weeks. The retinal vascular endothelium is one potential source of the chemokine.22 We observed an increase in the number of infected dendritic cells transmigrating human retinal endothelium in the presence of either CCL21 or CXCL10. In the model of Norose et al.,25 antibody neutralization of CXCL10 results in increased retinal destruction and inflammatory cell infiltration, and increased parasite load, coincident with decreased CD4+ T-cell presence. Thus, in the acute infection, CXCL10 may promote the entry of T. gondii into the retina, whereas in the chronic phase, it may maintain the immune response against the parasite.
In summary, we demonstrate that dendritic cells infected with either clonal or natural strain T. gondii tachyzoites transmigrate human retinal endothelium in vitro. This transmigration is mediated in part by IgG superfamily adhesion molecules and may be augmented by chemokines produced in the course of infection. Taken together with our previous work,7 these results indicate that T. gondii tachyzoites are able to reach retina both within transmigrating dendritic cells and unassisted, to initiate ocular toxoplasmosis. The ability to access the retina by more than one route is likely to contribute to the success of T. gondii as a human parasite. This conclusion highlights the difficulty in developing therapeutic strategies that aim to prevent parasite entry into the retina.
Acknowledgments
The authors thank Boris Striepen, PhD (University of Georgia, Athens, GA), for providing ptubYFP-YFPsagCAT and RH-YFP T. gondii; L. David Sibley, PhD (Washington University, St. Louis, MO), for providing GPHT T. gondii; and L. David Sibley, PhD, and Antonio Barragan, PhD (Karolinska Institutet, Stockholm, Sweden), for expert advice.
Footnotes
Supported in part by grants from the National Eye Institute/National Institutes of Health (R21 EY019550 and R01 EY019875), Knights Templar Eye Foundation, and Research to Prevent Blindness (unrestricted grant to Casey Eye Institute), and the Casey Eye Institute VISION 2020 International Fellowship Program (JMF).
Disclosure: J.M. Furtado, None; A.S. Bharadwaj, None; L.M. Ashander, None; A. Olivas, None; J.R. Smith, None
References
- 1.Black MW, Boothroyd JC. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev. 2000;64:607–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–1976 [DOI] [PubMed] [Google Scholar]
- 3.Holland GN. Ocular toxoplasmosis: a global reassessment. Part II: disease manifestations and management. Am J Ophthalmol. 2004;137:1–17 [PubMed] [Google Scholar]
- 4.Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev. 1998;11:267–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montoya JG, Remington JS. Toxoplasmosis of the central nervous system. In: Peterson PK, Remington JS.eds Defense of the Brain: Current Concepts in the Immunopathogenesis and Clinical Aspects of CNS Infections. Boston: Blackwell Scientific; 1997:163–188 [Google Scholar]
- 6.Silveira C, Vallochi AL, Rodrigues da Silva U, et al. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br J Ophthalmol. 2011;95:396–400 [DOI] [PubMed] [Google Scholar]
- 7.Furtado JM, Bharadwaj AS, Chipps TJ, Pan Y, Ashander LM, Smith JR. Toxoplasma gondii tachyzoites cross retinal endothelium assisted by intercellular adhesion molecule-1 in vitro. Immunol Cell Biol. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert H, Hitziger N, Dellacasa I, Svensson M, Barragan A. Induction of dendritic cell migration upon Toxoplasma gondii infection potentiates parasite dissemination. Cell Microbiol. 2006;8:1611–1623 [DOI] [PubMed] [Google Scholar]
- 9.Courret N, Darche S, Sonigo P, Milon G, Buzoni-Gatel D, Tardieux I. CD11c- and CD11b-expressing mouse leukocytes transport single Toxoplasma gondii tachyzoites to the brain. Blood. 2006;107:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Channon JY, Seguin RM, Kasper LH. Differential infectivity and division of Toxoplasma gondii in human peripheral blood leukocytes. Infect Immun. 2000;68:4822–4826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gubbels MJ, Li C, Striepen B. High-throughput growth assay for Toxoplasma gondii using yellow fluorescent protein. Antimicrob Agents Chemother. 2003;47:309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan A, Fux B, Su C, et al. Recent transcontinental sweep of Toxoplasma gondii driven by a single monomorphic chromosome. Proc Natl Acad Sci USA. 2007;104:14872–14877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan A, Behnke MS, Dunay IR, White MW, Sibley LD. Phenotypic and gene expression changes among clonal type I strains of Toxoplasma gondii. Eukaryot Cell. 2009;8:1828–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, Antonetti DA. VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci. 2006;47:5106–5115 [DOI] [PubMed] [Google Scholar]
- 15.John B, Ricart B, Tait Wojno ED, et al. Analysis of behavior and trafficking of dendritic cells within the brain during toxoplasmic encephalitis. PLoS Pathog. 2011;7:e1002246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Konen-Waisman S, Howard JC. Cell-autonomous immunity to Toxoplasma gondii in mouse and man. Microbes Infect. 2007;9:1652–1661 [DOI] [PubMed] [Google Scholar]
- 17.Dubey JP. The history of Toxoplasma gondii---the first 100 years. J Eukaryot Microbiol. 2008;55:467–475 [DOI] [PubMed] [Google Scholar]
- 18.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689 [DOI] [PubMed] [Google Scholar]
- 19.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. 2006;203:2763–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cayrol R, Wosik K, Berard JL, et al. Activated leukocyte cell adhesion molecule promotes leukocyte trafficking into the central nervous system. Nat Immunol. 2008;9:137–145 [DOI] [PubMed] [Google Scholar]
- 21.Silva NM, Manzan RM, Carneiro WP, et al. Toxoplasma gondii: the severity of toxoplasmic encephalitis in C57BL/6 mice is associated with increased ALCAM and VCAM-1 expression in the central nervous system and higher blood-brain barrier permeability. Exp Parasitol. 2010;126:167–177 [DOI] [PubMed] [Google Scholar]
- 22.Smith JR, Choi D, Chipps TJ, et al. Unique gene expression profiles of donor-matched human retinal and choroidal vascular endothelial cells. Invest Ophthalmol Vis Sci. 2007;48:2676–2684 [DOI] [PubMed] [Google Scholar]
- 23.Tal O, Lim HY, Gurevich I, et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med. 2011;208:2141–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum Immunol. 2002;63:1164–1171 [DOI] [PubMed] [Google Scholar]
- 25.Norose K, Kikumura A, Luster AD, Hunter CA, Harris TH. CXCL10 is required to maintain T-cell populations and to control parasite replication during chronic ocular toxoplasmosis. Invest Ophthalmol Vis Sci. 2011;52:389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]