Abstract
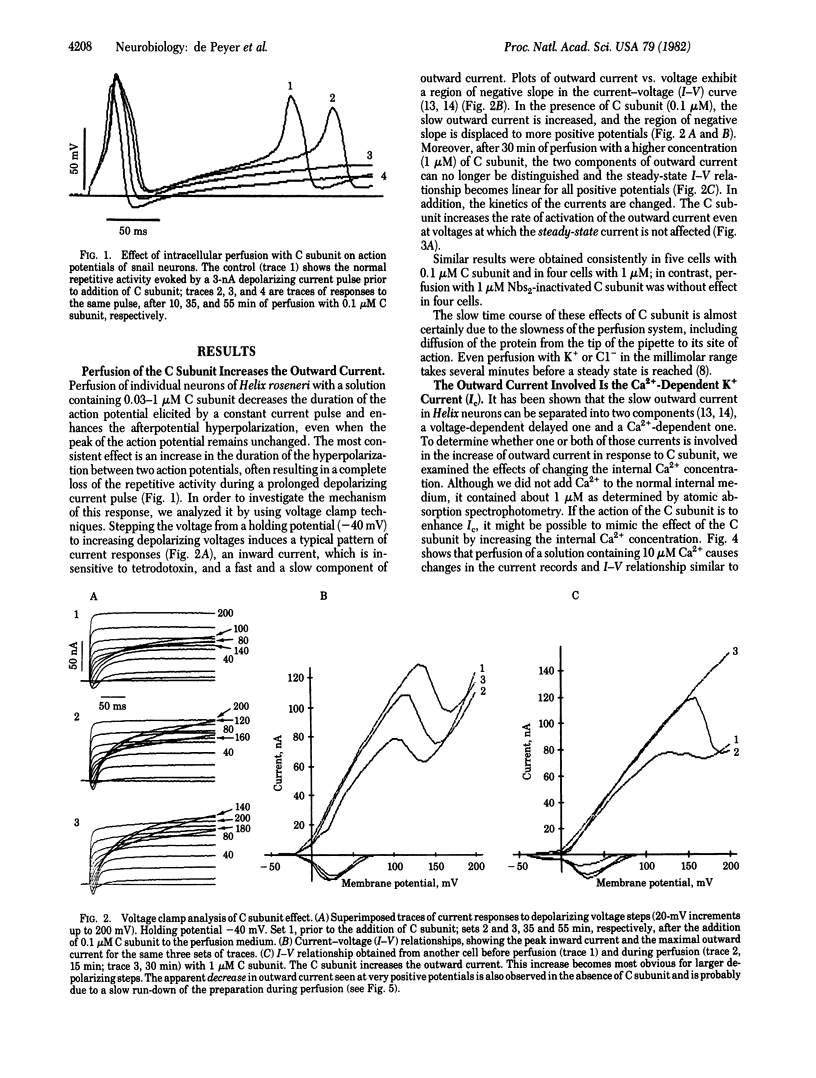
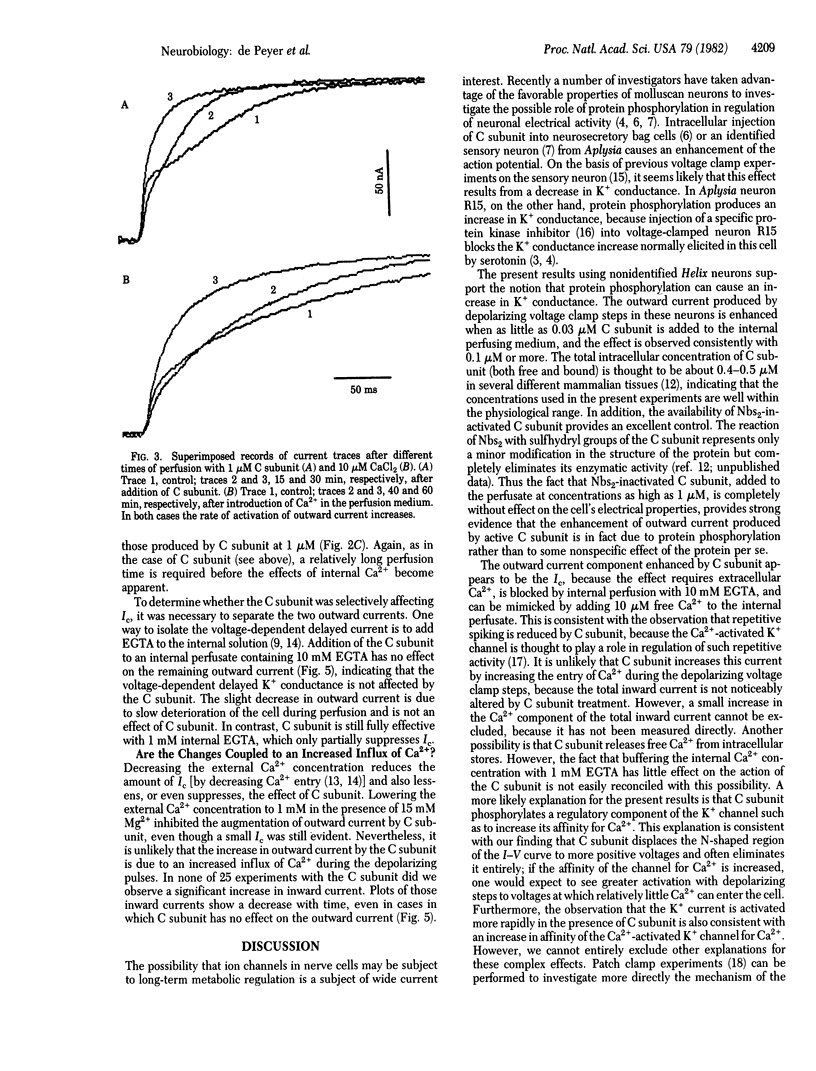
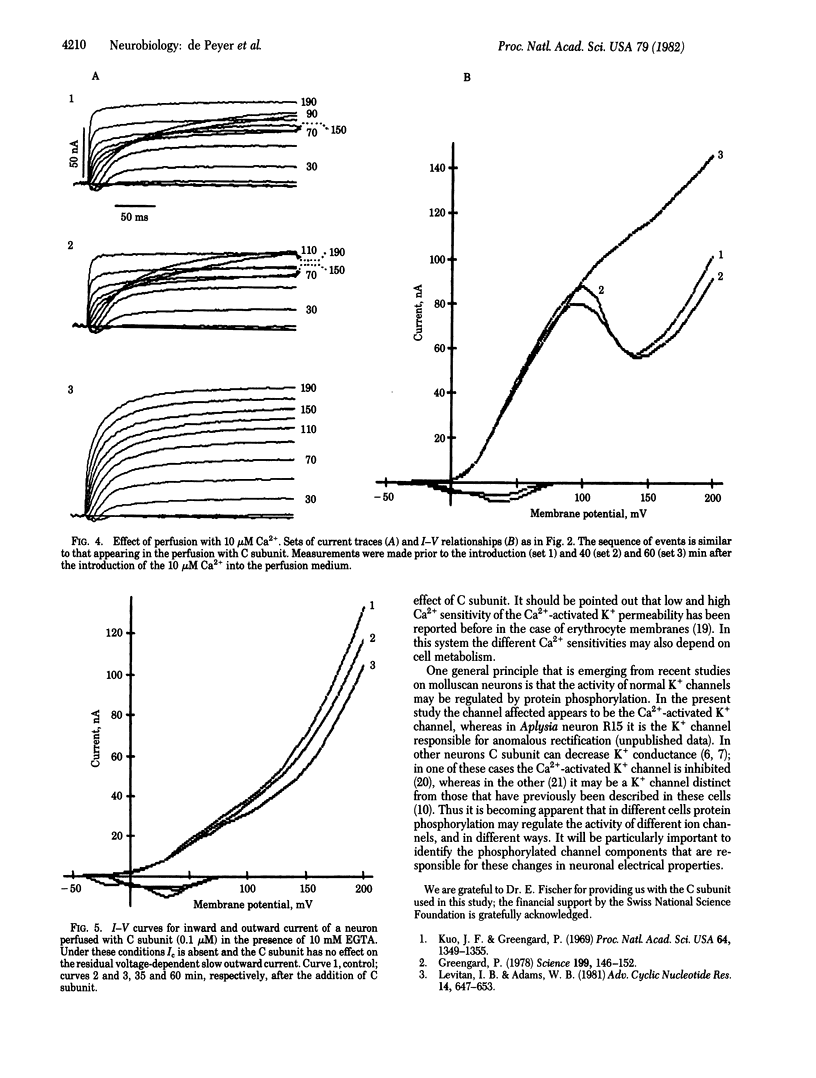
Depolarizing voltage steps induce inward and outward currents in voltage-clamped, internally perfused neurons from the snail Helix roseneri. Addition of the catalytic subunit of cyclic AMP-dependent protein kinase (ATP:protein phosphotransferase, EC 2.7.1.37) to the internal perfusing medium results in an increase in the net outward current, with no apparent effect on the inward current. Catalytic subunit inactivated by 5,5'-dithiobis(2-nitrobenzoic acid) is without effect, indicating that the increase in net outward current results from protein phosphorylation rather than an unspecific effect of protein perfusion. Decreasing the external Ca2+ concentration from 10 to 1 mM eliminates the effect of catalytic subunit, suggesting that Ca2+ plays an important role in this response. This suggestion is supported by the fact that the stimulation by catalytic subunit can be mimicked by increasing the Ca2+ concentration in the internal perfusion medium and can be prevented by intracellular perfusion with 10 mM EGTA. The results are consistent with the hypothesis that cyclic AMP-dependent protein phosphorylation regulates the Ca2+-activated K+ conductance in these cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Smith S. J., Thompson S. H. Ionic currents in molluscan soma. Annu Rev Neurosci. 1980;3:141–167. doi: 10.1146/annurev.ne.03.030180.001041. [DOI] [PubMed] [Google Scholar]
- Adams W. B., Levitan I. B. Intracellular injection of protein kinase inhibitor blocks the serotonin-induced increase in K+ conductance in Aplysia neuron R15. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3877–3880. doi: 10.1073/pnas.79.12.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby C. D., Walsh D. A. Characterization of the interaction of a protein inhibitor with adenosine 3',5'-monophosphate-dependent protein kinases. I. Interaction with the catalytic subunit of the protein kinase. J Biol Chem. 1972 Oct 25;247(20):6637–6642. [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R., Schwartz J. H., Wilson F. D., Nairn A. C., Greengard P. Intracellular injection of t he catalytic subunit of cyclic AMP-dependent protein kinase simulates facilitation of transmitter release underlying behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7492–7496. doi: 10.1073/pnas.77.12.7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. H., Benson J. A., Levitan I. B. Serotonin-induced hyperpolarization of an indentified Aplysia neuron is mediated by cyclic AMP. Proc Natl Acad Sci U S A. 1980 Aug;77(8):5013–5017. doi: 10.1073/pnas.77.8.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L. K., Jennings K. R., Strumwasser F., Nairn A. C., Walter U., Wilson F. D., Greengard P. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7487–7491. doi: 10.1073/pnas.77.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. IV. Widespread occurrence of adenosine 3',5'-monophosphate-dependent protein kinase in various tissues and phyla of the animal kingdom. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1349–1355. doi: 10.1073/pnas.64.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan I. B., Adams W. B. Cyclic AMP modulation of a specific ion channel in an identified nerve cell: possible role for protein phosphorylation. Adv Cyclic Nucleotide Res. 1981;14:647–653. [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K. A., Demaille J. G., Fischer E. H. Adenosine 3':5'-monophosphate dependent protein kinase from bovine heart. Characterization of the catalytic subunit. Biochemistry. 1977 Dec 27;16(26):5691–5697. doi: 10.1021/bi00645a007. [DOI] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F. Ion conductance and ion selectivity of potassium channels in snail neurones. J Membr Biol. 1980 Dec 15;57(2):103–118. doi: 10.1007/BF01868997. [DOI] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]



