Abstract
Dermal species of Leishmania have a relatively broad temperature range for optimal growth in vitro, with temperature differences accompanied by a form change. This suggests that when the host is living in moderate temperatures (22°C), infection may proceed at temperatures lower than those that occur in tropical regions (32°C), and a different clinical expression of the disease due to a different parasitic form may result. The aim of this study was to investigate the effect of environmental temperature on the clinical expression of the disease. BALB/C mice infected with Leishmania mexicana were housed at 32°±2°C or 22°±1°C, and assessed for the development of inflammation and the presence of parasites in organs using PCR and immunohistology. The clinical expression of leishmaniasis at 32°C included inflammation at the site of inoculation with swelling of the nose and tail, whereas at 22°C, up to 50% of the infected mice developed dry exfoliative dermatitis with alopecia on the dorsum. In both cases, parasite colonization was confirmed in the skin, with parasites at more external locations at 22°C. Parasite visceralization was confirmed in all internal organs and glands in both cases based on PCR and immunohistology. In conclusion, the clinical expression of diffuse leishmaniasis by Leishmania mexicana in laboratory mice is modified by temperature, from nodular inflammation at 32°C, to dry exfoliative dermatitis and alopecia at 22°C, with parasite visceralization in both cases.
Key Words: Alopecia, Ambient temperature, Dermatitis, Leishmania
Introduction
Leishmania is a dimorphic parasite, alternating between promastigotes, the flagellated form seen in the blood-feeding insect vector where temperatures vary around 22°C, and amastigotes, the non-flagellated form observed on the skin where the temperature is approximately 32°C for dermal species, or 37°C in the liver or spleen for visceral species (Akiyama and Taylor 1970; Wonde and Honigberg 1971; Berman and Neva 1981). Few human pathogenic microorganisms undergo thermodimorphic changes, although fungi, such as Histoplasma capsulatum (Maresca et al. 1981), Cryptococcus neoformans (Vallim et al. 2005), Paracoccidioides brasiliensis (Goldman et al. 2003), and Candida albicans (Rooney and Klein 2002), have been known to do so. Dimorphism is defined as an environmentally controlled reversible interconversion of morphology. Chemical and environmental parameters that are known to cause dimorphism include temperature, pH, glucose levels, nitrogen source, carbon dioxide levels, transition metals, chelating agents, and inoculum size or initial cell density (Nickerson et al. 2006).
Dimorphism confers virulence to fungi, as is documented for C. albicans (Young 2006), P. brasiliensis, H. capsulatum, and Blastomyces dermatitidis, which are not virulent when unable to transform into yeast (Rooney and Klein 2002). For Leishmania, virulence is also partly associated with parasite transformation, as impairing the ability to transform into an amastigote due to a flagellum biogenesis defect results in attenuated virulence in an animal model. This phenotype is associated with increased temperature sensitivity of the parasite, as is the case when there is a lack of lysosomal cysteine proteases (Williams et al. 2006), a component of endosome-lysosome function; ATG4, a component of the autophagosome assembly pathway (Besteiro et al. 2006); or adaptor protein-1 complex (Vince et al. 2008). Flagellum biogenesis is a critical feature for shape presentation in Leishmania, and is temperature-dependent (Vince et al. 2008). It requires β-tubulin gene transcription (Shapira et al. 1988; Kidane et al. 1989), and synthesis (Fong et al. 1981). However, flagellum biogenesis failure has little effect on parasite growth in vitro (Vince et al. 2008).
Thus, there is evidence that temperature, through regulating flagellum biogenesis, affects parasite form, motility, and infectivity in Leishmania; nevertheless, there is little information about how ambient temperature affects the clinical expression of dermal leishmaniasis, during which the parasite has the opportunity to be affected by the ambient temperature due to the proximity of the skin to the environmental air. Information on how ambient temperature affects leishmaniasis is focused on the ability of Leishmania to survive inside a vector, which confers an advantage to the parasite during global warming (Sutherst 2004), when changes in climate may expand the geographic range and abundance of both vectors and reservoir hosts (Greer et al. 2008). However, the alternative of climatic change toward a lower temperature, as is the case for an infected host moving from a tropical area to an area with cooler ambient temperature, is poorly studied. There are a large number of travelers from industrialized countries who visit endemic areas and enjoy doing outdoor activities in those areas, putting them at considerable risk of contracting cutaneous leishmaniasis. Most physicians in industrialized countries have little experience with cutaneous leishmaniasis in returning travelers. This often leads to delayed diagnosis and inappropriate management. Evidence-based data on travelers are limited to a few studies, anecdotal reports, and investigations among military personnel deployed in endemic areas (Blum et al. 2004). Moreover, the appearance of the lesions is quite variable, as this presentation depends on the species of parasite involved, and on the genetic and immunological background of the host; however, the effect of cold ambient temperature has been ignored to date. A mouse model of diffuse leishmaniasis has been developed to address the question of how a cold ambient temperature can influence the clinical presentation of leishmaniasis. Notably, the experiments were performed in the most severe disease model available (Barral et al. 1983; Hill et al. 1983; Roberts et al. 1989) in order to maximize the expression of the lesions and favor observation.
Materials and Methods
Parasites
Leishmania mexicana MNYC/BZ/62/M379 was subcultured axenically at room temperature by passaging every 7 days in Dulbecco's modified Eagle's medium (DMEM) containing L-glutamine and glucose (4500 mg/L), without sodium bicarbonate (Gibco, Grand Island, NY), supplemented with 10% fetal calf serum (Gibco). This medium provides good support for the growth of promastigotes at 22°C, and allows parasite transformation to amastigotes at 32°C. Amastigotes were used for inoculation, with an infectivity rate of 100% in BALB/c mice for 1×106 parasites loaded in 10 μL of phosphate-buffered saline (PBS).
Mouse model of diffuse leishmaniasis
Four-week-old male BALB/c mice were purchased from a certified animal care facility (Bioterio México, México City, México). The maintenance and care of animals was conducted in compliance with international guidelines for the use of laboratory animals. Experiments in the tropical environmental conditions at 32°±2°C were performed in the facilities of Universidad de Tabasco. Ambient temperature in Tabasco may reach 38–40°C from April to May, after which standard air conditioning equipment was used to keep the ambient temperature of the experimental animals close to 32°C. Experiments in mild weather conditions at 22°±1°C were performed in the facilities of Instituto Nacional de Perinatologia in México City, where the ambient temperature was controlled throughout the year. Batches of approximately 12 mice (4 weeks old) were inoculated subcutaneously in the right hind footpad with 10 μL of a preparation containing 1×106 amastigote-like forms in mid log-phase; the infection rate for this dose was always 100%. The amount of parasite required to reach diffuse leishmaniasis (DL) was chosen after testing doses of 1×100 (n=6/6, males), 1×103 (n=6/6, males), and 1×106 (n=6/6, males) parasites, and we found that 1×100 parasites did not cause lesions, 1×103 caused localized lesions, and 1×106 caused DL. The mice were examined twice a week for signs of leishmaniasis, and progression of the disease was monitored by measuring the thickness of the footpad using a caliper. Diffuse leishmaniasis was diagnosed when the mice presented with nodules on the tail and snout, with parasite recovery in cultures from the tail nodules, snouts, and footpads. The animals were sacrificed no later than 10 months post-infection. The mice were euthanized by asphyxiation in a CO2 chamber. Animal care and maintenance followed the recommendations of the International Animal Care and Use Committee. Tissue sections of the site of parasite injection, the contralateral footpad, and any other tissue seen to be compromised during autopsy were immediately immersed in buffered formalin, and retained for immunohistopathological analysis. For PCR, the tissue was immersed in PBS and frozen immediately at −70°C until studied.
Parasite culture from lesions
Aspirated fluids from lesions were deposited into 15-mL tubes containing modified Novy-MacNeal-Nicolle (NNN) medium (Difco, Detroit, MI) with 15% defibrinated rabbit blood, incubated at room temperature, and reviewed every second day over 2 weeks.
Immunohistology of tissues from infected mice
Sections from selected organs were stained with hematoxylin and eosin (H&E), Giemsa, or immunohistological labeling of formalin-fixed tissues following standard protocols. Briefly, after paraffin removal, the sections (5 μm thick) were rehydrated in PBS for 30 min at room temperature. Then the sections were preincubated with 5% bovine serum albumin for 60 min, and then incubated overnight at 4°C in a humidified chamber with a 1:500 dilution of patient serum from a 55-year-old man who had suffered from DL for 16 years (Galindo-Sevilla et al. 2007). It was determined by Western blot analysis of promastigote or amastigote lysates that the serum of this patient had an antibody titer greater than 1:10,000. Serum from a young man with no history of living in or visiting endemic areas of leishmaniasis was used as control. The sections were washed three times with PBS and incubated with a 1:50 dilution of FITC-conjugated anti-human antibody (Bio-Rad Laboratories, Hercules, CA). Unbound antibody was removed by three gentle washes with PBS for 5 min each. Double-stranded nucleic acid was stained with 5 μL of 50 μg/mL propidium iodide (Invitrogen, Camarillo, CA) for 5 min at room temperature, washed, and then counterstained with 0.3% Erichrome Black T (Sigma-Aldrich, St. Louis, MO) to eliminate possible autofluorescence (Hangai et al. 1998), and the excess fluid was removed by shaking. The slides were then carefully wiped, prepared for microscopy with mounting buffer, and examined microscopically.
Tissue detection of Leishmania by PCR
Sections of organs from infected or uninfected mice (controls), stored separately at −70°C, were disrupted by using MagNA Lyser green 1.4-mm ceramic beads (MagNA Lyser; Roche Diagnostics, Mannheim, Germany) for 30 sec at 6500 rpm, followed by DNA purification of the pellet using the illustra™ blood genomicPrep Mini Spin Kit (GE Healthcare, Hertsfordshire, U.K.) according to the manufacturer's instructions. DNA was amplified using primers JW11 and JW12 for the Leishmania genus (Nicolas et al. 2000) in a standard PCR reaction mix: 50 mM MgCl2, 1 U Taq polymerase, 1 ng primers, dNTPs, buffer, and 1 ng DNA. Amplification was performed with an initial denaturation step for 4 min at 94°C, 40 cycles of denaturation for 1 min at 94°C, annealing for 30 sec at 58°C, and elongation for 30 sec at 72°C, and a final extension for 10 min at 72°C. The products of PCR were analyzed by agarose gel electrophoresis. Negative controls in which the DNA was replaced with 2 μL of water or DNA extracted from tissues of a non-infected animal were included in each run.
Results
Murine leishmaniasis at 32°C or 22°C
Using a model of high parasitism to emphasize changes that might be otherwise undetectable, at least five batches with 20 mice each were infected for each condition. The presentation of leishmaniasis required 4–6 months to develop and was not resolved in any case. Infected mice housed at a temperature of 32°±2°C developed typical localized nodules of granulation tissue at the inoculation site, with an increase in the thickness of the infected footpads 4 weeks after infection. The lesion size progressed rapidly to ulceration; after week 20 multiple secondary disseminated acral lesions were positioned contralaterally on the back or front footpads, the eyelids, the nostrils, and the ears, with further nodules in the tail. The lesions were at sites without hair, and fur was always fine, without visible lesions (Fig. 1A and 1C). The histologic pattern of infection was consistent during the 5 years of experimentation and was similar to the previously-described patterns (Barral et al. 1983), with parasitized macrophages spreading in the subcutaneous tissue and muscle. The lesions became dominated by large vacuolated and heavily parasitized macrophages, with focal areas of necrosis. Only occasionally were lymphocytes present.
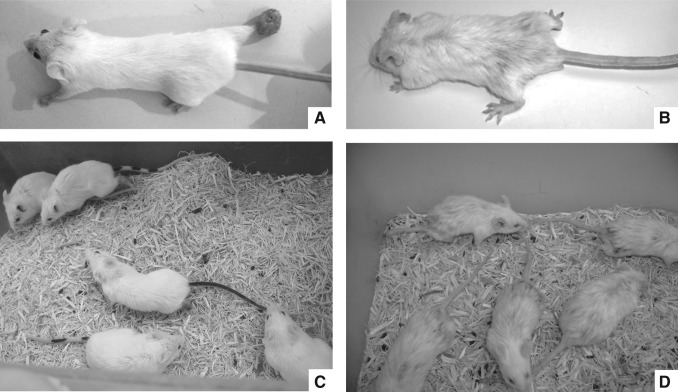
FIG. 1.
Cutaneous changes in an animal model of Leishmania mexicana induced by ambient temperature. Lesions observed 6 months after infection of BALB/C mice with 1×106 parasites inoculated into the right footpad. (A and C) In the tropical climate, the expression revealed typical footpad inflammation. (B and D) In the mild climate, up to 50% of infected animals exhibited hair loss on the dorsum with minimal footpad inflammation.
Conversely, when experimental infection was carried out at a milder temperature of 22°±1°C, the presentation of the disease was unusual. Unexpectedly, up to 50% of the infected mice presented severe skin damage, although footpad and tail lesions also appeared at a later point in the disease. Mice preferentially presented generalized dermatosis (a total of 46/89 in five experiments; Table 1), mainly in the dorsum and posterior extremities, characterized by fur changes involving diffuse alopecia, dry exfoliative dermatitis, and brittle hair and crust (Fig. 1B and 1D).
Table 1.
Temperature Influence on the Type of Lesion Development
| Temperature (°C) | Typical lesiona | Atypical lesionb | Fisher's exact test (p) | RR (95% confidence interval) | |
|---|---|---|---|---|---|
| Exp 1 | 32 | 20/20 | 0/20 | 0.000 | 3.3 (1.7–6.5) |
| 22 | 6/20 | 14/20 | |||
| Exp 2 | 32 | 22/22 | 0/22 | 0.000 | 2.1 (1.3–3.4) |
| 22 | 9/19 | 10/19 | |||
| Exp 3 | 32 | 19/19 | 0/19 | 0.006 | 1.5 (1.1–2.0) |
| 22 | 14/21 | 7/21 | |||
| Exp 4 | 32 | 20/20 | 0/20 | 0.001 | 1.9 (1.2–2.9) |
| 22 | 9/17 | 8/17 | |||
| Exp 5 | 32 | 18/18 | 0/18 | 0.000 | 2.4 (1.2–4.7) |
| 22 | 5/12 | 7/12 | |||
| Total | 32 | 99/99 | 0/99 | 0.000 | 2.1 (1.7–2.6) |
| 22 | 43/89 | 46/89 |
Typical lesion: localized nodules of granulation tissue at the inoculation site.
Atypical lesion: generalized dermatosis in the dorsum and posterior extremities.
Parasite culture, and parasite detection by PCR, histology, or immunohistology were prepared in 1–2 mice from each experiment.
Parasites were recovered in culture from footpad lesions or tail nodules, but could not be recovered from organs or skin, probably because parasite density was lower in those sites, and the antibiotic-antimycotic added to minimize microorganism contamination from the environment did not allow them to grow.
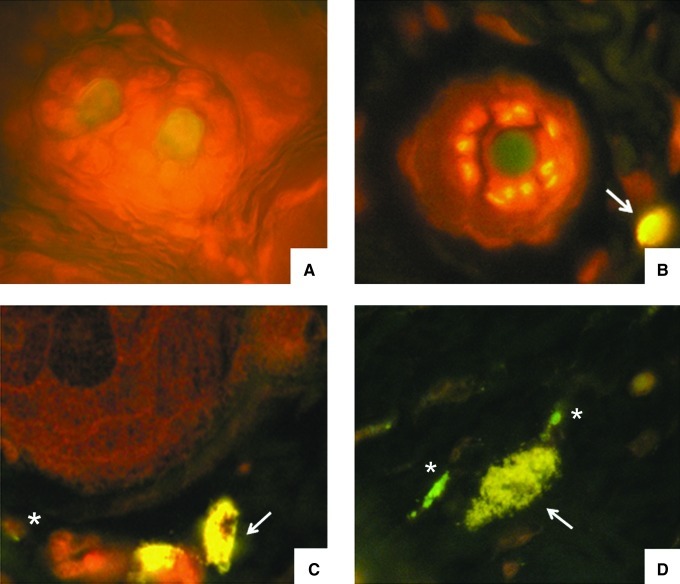
Parasites embedded within scales of the hair cortex rather than internalized in macrophages were observed in skin smears stained with immunofluorescence using serum from a patient with diffuse leishmaniasis as the primary antibody (Fig. 2).
FIG. 2.
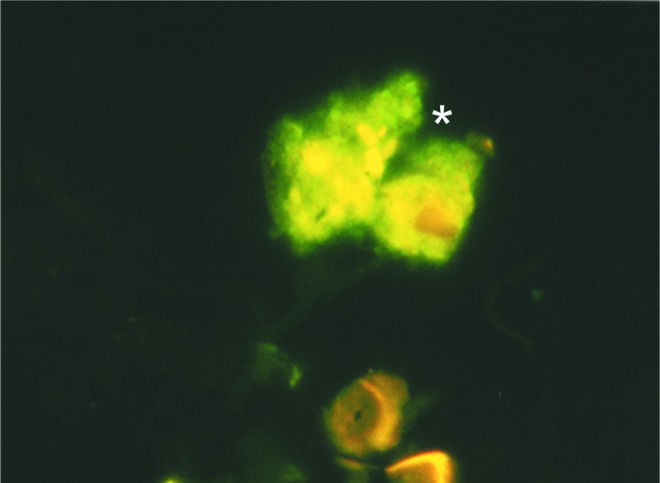
Smear from skin on the dorsum of a mouse after 8 months of Leishmania infection. Indirect immunofluorescence stain prepared with the serum from a mouse with diffuse leishmaniasis shows a corneocyte. Parasites are denoted with an asterisk (magnification 1000×).
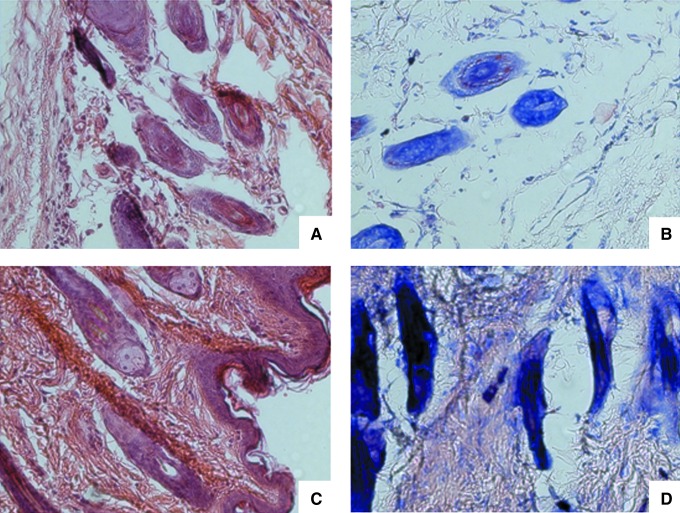
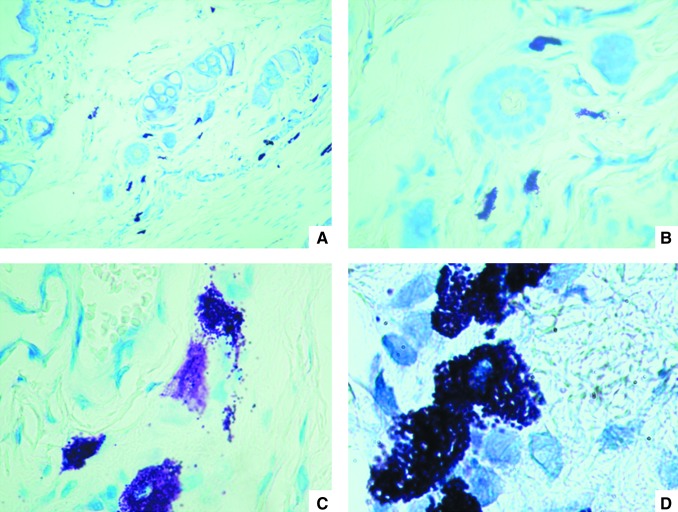
Histology revealed a thin epidermis with some acanthotic foci with corneal plugs. Small exocytic foci were seen. The superficial and intermediate dermis showed moderate inflammatory infiltrates with perianexial predominance, consisting of lymphocytes, monocytes, scanty eosinophils, and sporadic giant multinucleate cells with nuclear rings. Hair follicles were fragmented with abnormal architecture; there was also loss of follicle sheaths, and the hair follicle cells showed degenerative changes in their nuclei. Blood vessels were congested and expanded; collagen fibers were disorganized, fragmented, and separated (Fig. 3). Abundant mast cells around the hair follicles were stained dark purple with Giemsa stain (Fig. 4) or green-yellow when stained with normal or DL patient serum and FITC-labeled anti-human antibodies (Fig. 5); the green-yellow color seen in Figure 5 probably originated from free FITC in the FITC-labeled second antibody. Some parasites could be seen close to mast cells (Fig. 5D), but only when skin from infected mice was stained with infected human serum, and these parasites were not observed when normal skin (Fig. 5A), or control secondary antibody in skin from infected mice (Fig. 5B) was used. Hairs acquired a green-yellow color in the control preparations of normal skin stained with DL patient serum (Fig. 5A), and in the skin from an infected mouse stained with secondary antibody as a control (Fig. 5B), as was described above for mast cells.
FIG. 3.
Histological analysis of the skin of Leishmania mexicana-infected mice in a mild climate. Hair changes from control mice (A and C) compared to infected mice (B and D; A and B, transverse plane; C and D, longitudinal plane; hematoxylin and eosin stain, magnification 1000×).
FIG. 4.
Giemsa stain of the skin from an infected mouse (A, 40×; B, 100×; C, 400×; D, 1000×). Mast cells are purple.
FIG. 5.
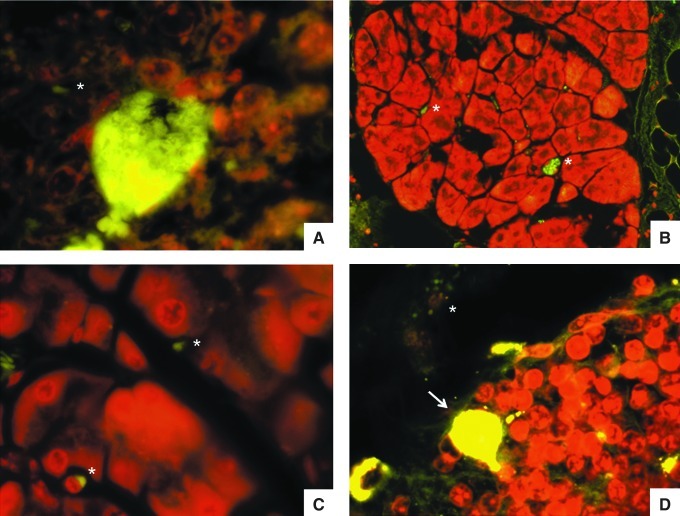
Indirect immunofluorescence stain of the same serum as in Figure 2 from a patient with diffuse leishmaniasis. (A) Normal skin from a non-infected mouse. (B) Infected skin stained only with secondary FITC-labeled anti-human antibody. Arrow indicates a mast cell. (C and D) Skin from a Leishmania mexicana-infected mouse. Arrows indicate mast cells close to hair follicles, and the asterisks indicate parasites (perifollicular cuts, 1000×).
Internal organs were analyzed using PCR and immunohistology. At both temperatures, parasitism was detected in the spleen and liver. Furthermore, parasites were present in almost every gland and organ analyzed with PCR amplification (Fig. 6), and histology (Fig. 7). Intense PCR bands were amplified from footpad lesions, skin, blood, kidneys, colon, esophagus, trachea, mesenteric nodules, pancreas, thyroid, and salivary gland samples. The one exception was the brain. Histology of all organs at both temperatures demonstrated low chronic inflammatory infiltration, with the presence of mast cells (Fig. 7) and parasites.
FIG. 6.
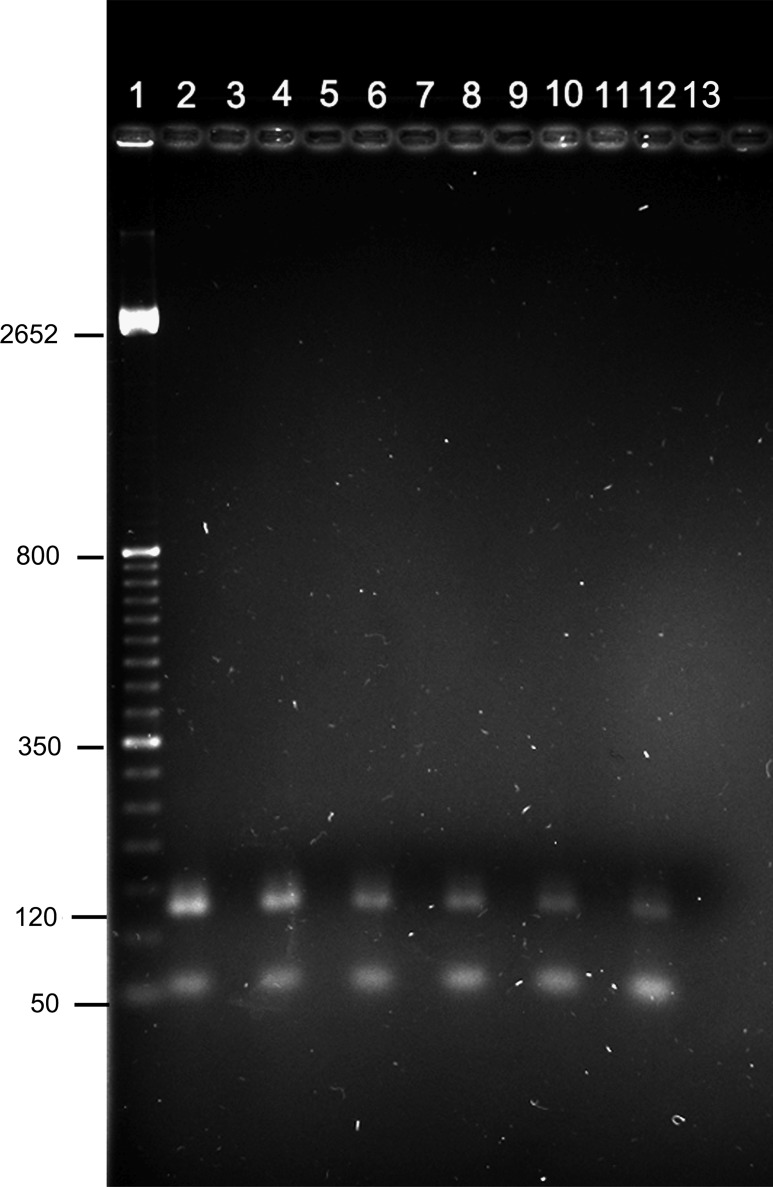
PCR to assess parasite load in internal organs of infected mice in a mild climate. Amplicons were resolved in 2.0% agarose gels. Even numbers correspond to infected mice harboring the M379 strain after 6 months of evolution. Odd numbers correspond to healthy mice of the same age (1, molecular weight; 2, positive control M379 axenic culture; 3, tissue culture negative control; 4 and 5, footpad; 6 and 7, liver; 8 and 9, spleen; 10 and 11, pancreas; 12 and 13, kidney). The experiments were performed in triplicate. More than 10 infected mice were analyzed with similar results.
FIG. 7.
Histological analysis of organs from mice infected with Leishmania mexicana in a mild climate. Indirect immunofluorescence staining as in Figure 2 (A, salivary gland 1000×; B, pancreas 400×; C, pancreas 1000×; D, liver 400×). Mice after 8 months of Leishmania infection. Arrows indicate mast cells, and asterisks show parasites.
Discussion
When the M379 strain of L. mexicana is exposed to 25°C, it is able to transform from intracellular amastigotes to its promastigote form (Martinez-Rojano et al. 2008), and thereby acquires the motility necessary to leave the macrophage and multiply. Transformation of dermal Leishmania parasites requires only temperature control, while visceral Leishmania proceeds under temperature- and pH-control (Zilberstein and Shapira 1994). This means there is no impairment of in vivo parasite transformation if the temperature favors it, an event that may happen on the surface of the skin of a host when subjected to temperatures other than tropical levels when traveling to cold places.
Different dermal expression of leishmaniasis was seen between the two temperatures. While at 32°C the lesions were typical, like those expressed by hosts in the tropics, with inflammation at the inoculation site and the characteristic intracellular amastigotes seen after scraping lesions, which were mainly non-ulcerated. Parasitic infection of the other foot, tail, or nose was suspected by inflammation or granuloma formation and confirmed by parasite isolation by culture and/or PCR. Fur was fine without any hair loss or dermatitis. In contrast, in cold and dry conditions, even when inflammation at the inoculation site was seen, a dry exfoliative squamous dermatitis with hair loss until alopecia was noted, starting at the inguen and spreading mainly to the back of the mice until it reached the face. This type of dermatitis has been reported for dogs (Binhazim et al. 1992; Brachelente et al. 2005; Melo et al. 2008; Travi et al. 2009), and felines (Solano-Gallego et al. 2007) infected with L. infantum. In humans, this may be similar to the post-kala-azar dermal leishmaniasis caused by L. infantum (Zijlstra et al. 1995) or L. donovani after treatment (Das et al. 2009). Occasionally in dermal species, dermatitis is associated with L. amazonensis (Barral et al. 1991), but it is unusual to seek dermal Leishmania parasites as a cause of dry exfoliative dermatitis with alopecia. The dermatitis could be explained by a change in parasite morphology that enables parasites to transform into the flagellated promastigote form, which is capable of leaving the intracellular milieu of the macrophage and reaching the stratum corneum of the skin.
The presence of abundant cells morphologically compatible with mast cells in the skin, trachea, and several glands, is of particular interest because mast cell activities, including degranulation, could contribute to the infection-associated inflammatory events that help control the disease (Maurer et al. 2006), or could contribute to contact hypersensitivity (Gonzalo et al. 2007); these possibilities provide an explanation for the broad inflammatory response to the infection reported in dogs.
Promastigote forms on the skin surface could preclude the detection of Leishmania because this parasite form is not expected by physicians or laboratory workers, and is not easily detected because it is embedded in squamous epithelium. In fact, doubt has recently emerged as to whether the amastigote is the only form of Leishmania present in cutaneous leishmaniasis (Daboul 2008), as was seen during a review of slides prepared from cutaneous lesions, where in 22/42 cases promastigote forms were observed, which is consistent with our findings. In fact, parasite forms are not easily distinguished because they are often incorporated into scales or bound to hair, where immunofluorescence is important to visualize them.
PCR from skin biopsies could help in the identification of Leishmania in dry squamous dermatitis if universal primers or a selection of several primers covering several species are used. The amount of parasite in the sample could be an important factor in achieving the required sensitivity of the technique. In this study, low parasite load was not a problem due to the use of a model of severe infection. For the model presented here, low inocula did not cause lesion development; a localized lesion developed after medium inocula, and diffuse leishmaniasis was a consequence of high inocula. The results are consistent with the experimental quantification of L. major delivered into mouse ears by individual flies, with the mice developing large lesions with high-dose inocula and only minor pathology at low doses (Kimblin et al. 2008). Moreover, the parasite load influences disease presentation (Doherty and Coffman 1996; Menon and Bretscher 1998; Lira et al. 2000; Aguilar Torrentera et al. 2002; Lang et al. 2003; Kimblin et al. 2008). The model we chose, with high inocula, revealed that parasites are not restricted to lesions, which is consistent with several studies (Saravia et al. 1990; de Rossell et al. 1992; Mendonça et al. 2004).
Dermal species of Leishmania exhibit parasitism of some organs, such as the spleen and liver, in animal models of infection (Nicolas et al. 2000; Aguilar Torrentera et al. 2002). Here parasites were confirmed in the spleen and liver as well as in almost all tested organs, including the pancreas, adrenal glands, thyroid, salivary glands, thymus, kidneys, blood, stomach, intestines, and lymphoid nodules, such as the splenic nodule. In fact, the only exception was the brain. The distribution of Leishmania in several organs has been documented for visceral Leishmania acquired naturally by dogs, giving rise to the following pathogenic conditions: colitis (Adamama-Moraitou et al. 2007), nephropathy (Costa et al. 2003), liver fibrosis (Melo et al. 2008), genital lesions (Diniz et al. 2005), and transplacental transmission of parasites (Pangrazio et al. 2009). This indicates a broad spectrum for this disease, which may be true for dermal species as well.
We conclude that a temperature of 22°±1°C is an important regulator of pathogenic expression of leishmaniasis in a rodent model, and may favor an atypical presentation of leishmaniasis, characterized by dry exfoliative dermatitis with alopecia.
Acknowledgment
This work was supported by the Instituto Nacional de Perinatología.
Author Disclosure Statement
No competing financial interests exist.
References
- Adamama-Moraitou KK. Rallis TS. Koytinas AF, et al. Asymptomatic colitis in naturally infected dogs with Leishmania infantum: a prospective study. Am J Trop Med Hyg. 2007;76:53–57. [PubMed] [Google Scholar]
- Aguilar Torrentera F. Lambot MA. Laman DJ, et al. Parasitic load and histopathology of cutaneous lesions, lymph mode, spleen, and liver from Balb/c and C57BL/6 mice infected with Leishmania mexicana. Am J Trop Med Hyg. 2002;66:273–279. doi: 10.4269/ajtmh.2002.66.273. [DOI] [PubMed] [Google Scholar]
- Akiyama HJ. Taylor JC. Effect of macrophage engulfment and temperature on the transformation process of Leishmania donovani. Am J Trop Med Hyg. 1970;19:747–754. doi: 10.4269/ajtmh.1970.19.747. [DOI] [PubMed] [Google Scholar]
- Barral A. Pedral-Sampaio D. Grimaldi G, Jr, et al. Leishmaniasis in Bahia, Brazil: evidence that Leishmania amazonensis produces a wide spectrum of clinical disease. Am J Trop Med Hyg. 1991;44:536–546. doi: 10.4269/ajtmh.1991.44.536. [DOI] [PubMed] [Google Scholar]
- Barral A. Petersen EA. Sacks L, et al. Late metastatic leishmaniasis in the mouse. A model for mucocutaneous disease. Am J Trop Med Hyg. 1983;32:277–285. doi: 10.4269/ajtmh.1983.32.277. [DOI] [PubMed] [Google Scholar]
- Berman JD. Neva AF. Effect of temperature on multiplication of Leishmania amastigotes within human monocyte-derived macrophages in vitro. Am J Trop Med Hyg. 1981;30:318–321. doi: 10.4269/ajtmh.1981.30.318. [DOI] [PubMed] [Google Scholar]
- Besteiro S. Williams RA. Morrison LS, et al. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J Biol Chem. 2006;281:11384–11396. doi: 10.1074/jbc.M512307200. [DOI] [PubMed] [Google Scholar]
- Binhazim AA. Chapman WL. Latimer KS, et al. Canine leishmaniasis caused by Leishmania leishmania infantum in two Labrador retrievers. J Vet Diagn Invest. 1992;4:299–305. doi: 10.1177/104063879200400312. [DOI] [PubMed] [Google Scholar]
- Blum J. Desjeux P. Schwartz E, et al. Treatment of cutaneous leishmaniasis among travellers. J Antimicrob Chemother. 2004;53:158–166. doi: 10.1093/jac/dkh058. [DOI] [PubMed] [Google Scholar]
- Brachelente C. Muller N. Doherr MG, et al. Cutaneous leishmaniasis in naturally infected dogs is associated with a T helper-2-biased immune response. Vet Pathol. 2005;42:166–175. doi: 10.1354/vp.42-2-166. [DOI] [PubMed] [Google Scholar]
- Costa FAL. Goto H. Saldanha LC, et al. Histopathologic patterns of nephropathy in naturally acquired canine visceral leishmaniasis. Vet Pathol. 2003;40:677–684. doi: 10.1354/vp.40-6-677. [DOI] [PubMed] [Google Scholar]
- Daboul MW. Is the amastigote form of Leishmania the only form found in humans infected with cutaneous leishmaniasis? LabMedicine. 2008;39:38–41. [Google Scholar]
- Das VN. Pandey K. Verma N, et al. Short report: development of post-Kala-azar dermal leishmaniasis (PKDL) in miltefosine-treated visceral leishmaniasis. Am J Trop Med Hyg. 2009;80:336–338. [PubMed] [Google Scholar]
- de Rossell RA. de Duran RJ. Rossell O, et al. Is leishmaniasis ever cured? Trans R Soc Trop Med Hyg. 1992;86:251–253. doi: 10.1016/0035-9203(92)90297-p. [DOI] [PubMed] [Google Scholar]
- Diniz SA. Melo MS. Borger AM, et al. Genital lesions associated with visceral leishmaniasis and shedding of Leishmania sp. in semen of naturally infected dogs. Vet Pathol. 2005;42:650–658. doi: 10.1354/vp.42-5-650. [DOI] [PubMed] [Google Scholar]
- Doherty TM. Coffman RL. Leishmania major: effect of infectious dose on T cell subset development in BALB/c mice. Exp Parasitol. 1996;84:124–135. doi: 10.1006/expr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Fong D. Chang K. Tubulin biosynthesis in the development cycle of a parasitic protozoan, Leishmania mexicana: changes during differentiation of motile and nonmotile stages. Proc Natl Acad Sci USA. 1981;78:7624–7628. doi: 10.1073/pnas.78.12.7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Sevilla N. Soto N. Mancilla J, et al. Low serum levels of dehydroepiandrosterone and cortisol in human diffuse cutaneous leishmaniasis by Leishmania mexicana. Am J Trop Med Hyg. 2007;76:566–572. [PubMed] [Google Scholar]
- Goldman GH. dos Reis Marques E. Duarte Ribeiro DC, et al. Expressed sequence tag analysis of the human pathogen Paracoccidioides brasiliensis yeast phase: identification of putative homologues of Candida albicans virulence and pathogenicity genes. Eukaryot Cell. 2003;2:34–48. doi: 10.1128/EC.2.1.34-48.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo JA. Qiu Y. Lora JM, et al. Coordinated involvement of mast cells and T cells in allergic mucosal inflammation: critical role of the CC chemokine ligand 1:CCR8 axis. J Immunol. 2007;179:1740–1750. doi: 10.4049/jimmunol.179.3.1740. [DOI] [PubMed] [Google Scholar]
- Greer A. Ng V. Fisman D. Climate change and infectious diseases in North America: the road ahead. Can Med Assoc J. 2008;178:715–722. doi: 10.1503/cmaj.081325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangai M. Tanihara H. Honda Y, et al. In vivo delivery of phosphorothioate oligonucleotides into murine retina. Arch Ophthalmol. 1998;116:342–348. doi: 10.1001/archopht.116.3.342. [DOI] [PubMed] [Google Scholar]
- Hill JO. North RJ. Collins FM. Advantages of measuring changes in the number of viable parasites in murine models of experimental cutaneous leishmaniasis. Infect Immun. 1983;39:1087–1094. doi: 10.1128/iai.39.3.1087-1094.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane GZ. Samaras N. Spithill TW. Cloning of developmentally regulated genes from Leishmania major and expression following heat induction. J Biol Chem. 1989;264:4244–4250. [PubMed] [Google Scholar]
- Kimblin N. Peters N. Debrabant A, et al. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proc Natl Acad Sci USA. 2008;105:10125–10130. doi: 10.1073/pnas.0802331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T. Courret N. Cole J, et al. The levels and patterns of cytokines produced by CD4 T lymphocytes of BALB/c mice infected with Leishmania major by inoculation into the ear dermis depend on the infectiousness and size of the inoculum. Infect Immun. 2003;71:2674–2683. doi: 10.1128/IAI.71.5.2674-2683.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira R. Doherty M. Modi G, et al. Evolution of lesion formation, parasitic load, immune response, and reservoir potential in C57BL/6 mice following high- and low-dose challenge with Leishmania major. Infect Immun. 2000;68:5176–5182. doi: 10.1128/iai.68.9.5176-5182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M. Kostka SL. Siebenhaar F, et al. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006;20:2460–2467. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- Maresca B. Lambowitz AM. Kumar VB, et al. Role of cysteine in regulating morphogenesis and mitochondrial activity in the dimorphic fungus Histoplasma capsulatum. Proc Natl Acad Sci USA. 1981;78:4596–4600. doi: 10.1073/pnas.78.7.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rojano H. Mancilla-Ramirez J. Quiñonez L, et al. Anti-Leishmanial activity of hydroxyurea on Leishmania mexicana. Antimicrob Agents Chemother. 2008;52:3642–3647. doi: 10.1128/AAC.00124-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo F. Amaral M. Oliveira P, et al. Diffuse intralobular liver fibrosis in dogs naturally infected with Leishmania (Leishmania) chagasi. Am J Trop Med Hyg. 2008;79:198–204. [PubMed] [Google Scholar]
- Mendonca MG. de Brito MEF. Rodrigues EHG, et al. Persistence of Leishmania parasites in scars after clinical cure of American cutaneous Leishmaniasis: Is there a sterile cure? J Infect Dis. 2004;189:1018–1023. doi: 10.1086/382135. [DOI] [PubMed] [Google Scholar]
- Menon JN. Bretscher PA. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur J Immunol. 1998;28:4020–4028. doi: 10.1002/(SICI)1521-4141(199812)28:12<4020::AID-IMMU4020>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Nickerson KW. Atkin AL. Hornby JM. Quorum sensing in dimorphic fungi: farnesol and beyond. Appl Environ Microbiol. 2006;72:3805–3813. doi: 10.1128/AEM.02765-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas L. Sidjanski S. Colle J, et al. Leishmania major reaches distant cutaneous sites where it persists transiently while persisting durably in the primary dermal site and its draining lymph node: a study with laboratory mice. Infect Immun. 2000;68:6561–6566. doi: 10.1128/iai.68.12.6561-6566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangrazio KK. Costa EA. Amarilla SP, et al. Tissue distribution of Leishmania chagasi and lesions in transplacentally infected fetuses from symptomatic and asymptomatic naturally infected bitches. Vet Parasitol. 2009;165:327–331. doi: 10.1016/j.vetpar.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Roberts M. Alexander J. Blackwell JM. Influence of Lsh, H-2, and an H-11-linked gene on visceralization and metastasis associated with Leishmania mexicana infection in mice. Infect Immun. 1989;57:875–881. doi: 10.1128/iai.57.3.875-881.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney PJ. Klein BS. Linking fungal morphogenesis with virulence. Cell Microbiol. 2002;4:127–137. doi: 10.1046/j.1462-5822.2002.00179.x. [DOI] [PubMed] [Google Scholar]
- Saravia NG. Segura I. Labrada LA, et al. Recurrent lesions in human Leishmania braziliensis infection-reactivation or reinfection? Lancet. 1990;336:398–402. doi: 10.1016/0140-6736(90)91945-7. [DOI] [PubMed] [Google Scholar]
- Shapira M. McEwen JG. Jaffe CL. Temperature effects on molecular processes which lead to stage differentiation in Leishmania. EMBO J. 1988;7:2895–2901. doi: 10.1002/j.1460-2075.1988.tb03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Gallego L. Rodriguez-Cortes A. Iniesta L, et al. Cross-sectional serosurvey of feline leishmaniasis in ecoregions around the northwestern Mediterranean. Am J Trop Med Hyg. 2007;76:676–680. [PubMed] [Google Scholar]
- Sutherst RW. Global change and human vulnerability to vector-borne diseases. Clin Microbiol Rev. 2004;17:136–173. doi: 10.1128/CMR.17.1.136-173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travi BL. Osorio EY. Saldarriaga OA. Cadena H, et al. Clinical, parasitologic, and immunologic evolution in dogs experimentally infected with sand fly-derived Leishmania chagasi promastigotes. Am J Trop Med Hyg. 2009;81:994–1003. doi: 10.4269/ajtmh.2009.09-0229. [DOI] [PubMed] [Google Scholar]
- Vallim MA. Nichols CB. Fernandez L, et al. A Rac homolog functions downstream of Ras1 to control hyphal differentiation and high-temperature growth in the pathogenic fungus Cryptococcus neoformans. Eukaryot Cell. 2005;4:1066–1078. doi: 10.1128/EC.4.6.1066-1078.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE. Tull DI. Spurck T, et al. Leishmania adaptor protein-1 subunits are required for normal lysosome traffic, flagellum biogenesis, lipid homeostasis, and adaptation to temperatures encountered in the mammalian host. Eukaryot Cell. 2008;7:1256–1267. doi: 10.1128/EC.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RA. Tetley L. Mottram JC, et al. Cysteine peptidases CPA and CPB are vital for autophagy and differentiation in Leishmania mexicana. Mol Microbiol. 2006;61:655–674. doi: 10.1111/j.1365-2958.2006.05274.x. [DOI] [PubMed] [Google Scholar]
- Wonde T. Honigberg BM. Morphology and infectivity of Leishmania donovani cultivated in nonliving media at elevated temperatures. Am J Trop Med Hyg. 1971;20:828–838. doi: 10.4269/ajtmh.1971.20.828. [DOI] [PubMed] [Google Scholar]
- Young KD. The selective value of bacterial shape. Microbiol Mol Biol Rev. 2006;70:660–703. doi: 10.1128/MMBR.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein D. Shapira M. The role of pH and temperature in the development of Leishmania parasites. Annu Rev Microbiol. 1994;48:449–470. doi: 10.1146/annurev.mi.48.100194.002313. [DOI] [PubMed] [Google Scholar]
- Zijlstra EE. El-Hassan AM. Ismael A. Endemic Kala-azar in eastern Sudan: post-kala-azar dermal leishmaniasis. Am J Trop Med Hyg. 1995;52:299–305. doi: 10.4269/ajtmh.1995.52.299. [DOI] [PubMed] [Google Scholar]