Abstract
Purpose.
The purpose of this study was to determine the Ca2+-dependent cellular signaling pathways used by histamine to stimulate conjunctival goblet cell secretion.
Methods.
Cultured rat goblet cells were grown in RPMI 1640. Goblet cell secretion of high molecular weight glycoconjugates was measured by an enzyme-linked lectin assay. Intracellular [Ca2+] ([Ca2+]i) was measured by loading cultured cells with the Ca2+ sensitive dye fura-2. The level of [Ca2+]i was measured using fluorescence microscopy. Extracellular regulated kinase (ERK) 2 was depleted using small interfering RNA (siRNA).
Results.
Histamine-stimulated conjunctival goblet cell secretion of high molecular weight glycoproteins was blocked by removal of extracellular Ca2+ and depletion of ERK2 by siRNA. Histamine increase in [Ca2+]i was desensitized by repeated addition of agonist and blocked by a phospholipase C antagonist. Histamine at higher doses increased [Ca2+]i by stimulating influx of extracellular Ca2+, but at a lower dose released Ca2+ from intracellular stores. Activation of each histamine receptor subtype (H1–H4) increased [Ca2+]i and histamine stimulation was blocked by antagonists of each receptor subtype. The H2 receptor subtype increase in [Ca2+]i was cAMP dependent.
Conclusions.
We conclude that histamine activates phospholipase C to release intracellular Ca2+ that induces the influx of extracellular Ca2+ and activates ERK1/2 to stimulate conjunctival goblet cell mucous secretion, and that activation of all four histamine receptor subtypes can increase [Ca2+]i.
In this study the signaling pathways utilized by histamine to stimulate mucin secretion in conjunctival goblet cells are identified.
Introduction
Allergic conjunctivitis is a prevalent ocular surface disease affecting between 15% to 20% of Americans.1 Symptoms of allergic conjunctivitis include chemosis, excess tearing, increase tear mucus, and itching. In some forms of allergic conjunctivitis excess tear mucus secretion predominates and is acerbated by mucous fishing syndrome, in which the patient's attempts to clear away mucus causes additional mucus secretion.2 There are no completely effective treatments for this type of allergic conjunctivitis and new approaches to treatment are warranted.
Histamine, a major mediator of allergy, is secreted by mast cells recruited into the conjunctival stroma.3 Although antihistamines, especially anti-H1 and -H2 antihistamines, are used to treat allergic conjunctivitis, the role of histamine in activating conjunctival goblet cells has only recently been explored. We recently published a study, in which we demonstrated that histamine directly stimulates secretion of high molecular weight glycoproteins from both human and rat conjunctival goblet cells in culture.4 Surprisingly, all four histamine receptor subtypes (H1–H4) are present on goblet cells, and their activation by agonists stimulates secretion and inhibition by antagonists blocks histamine-induced goblet cell secretion.4 Thus, conjunctival goblet cells are a direct target of histamine, suggesting that these cells play an integral role in the pathogenesis of allergic conjunctivitis.
The signaling pathways activated by histamine have not been investigated in goblet cells from any tissue including conjunctiva, lung, gastrointestinal tract, and nasal cavity. The typical signaling pathways activated by histamine receptor subtypes, however, are an increase in [Ca2+]i by H1 and H4 receptors, activation of adenylyl cyclase and production of cAMP by H2 receptors, and a decrease in intracellular [Ca2+] ([Ca2+]i) and cAMP by H3 receptors.5
Known stimuli of conjunctival goblet cell secretion, including cholinergic agonists, vasoactive intestinal peptide (VIP), and cysteinyl leukotrienes, stimulate goblet cell secretion by increasing the [Ca2+]i and activating extracellular regulated kinase (ERK)1/2.6,7 In the present study, we used rat conjunctival goblet cells in culture to determine if histamine increases [Ca2+]i and activates ERK1/2, if histamine-induced mucin secretion is dependent upon [Ca2+]i and activation of ERK1/2, and if so what cellular mechanisms histamine uses to increase [Ca2+]i, and which histamine receptor subtypes are used. We concluded that histamine activates phospholipase C to release intracellular Ca2+ that induces the influx of extracellular Ca2+ and activates ERK1/2 to stimulate conjunctival goblet cell mucous secretion. Interestingly, activation of all four histamine receptor subtypes increases [Ca2+]i, but the H2 receptor uses cAMP and protein kinase A (PKA) to elevate [Ca2+]i.
Materials and Methods
Materials
Histamine and the histamine receptor agonists 2-([3-trifluoromethyl]phenyl) histamine dimaleate (H1), amthamine dihydrobromide (H2), and 4-methylhistamine dihydrochloride (H4), the histamine receptor antagonists chlorpheniramine (H1), cimetidine (H2), and JNJ7777120 (H4) as well as histamine and thapsagargin were from Sigma (St. Louis, MO) while (R)α-methylhistamine dihydrochloride, the H3 receptor agonist, conessine, the H3 receptor antagonist, and U73122 were purchased from Tocris Bioscience (Ellisville, MO). VIP was from EMD Chemicals (Rockville, MA). H89 was purchased from R&D Systems (Minneapolis, MN). Amplex Red and fura2/AM were purchased from Invitrogen (Grand Island, NY). The MUC5AC ELISA kit was purchased from Biotang Inc. (Waltham, MA).
Animals
Male Sprague-Dawley rats weighing between 125 and 150 g were obtained from Taconic Farms (Germantown, NY). Rats were anesthetized with CO2 for 1 minute followed by decapitation and the bulbar and forniceal conjunctiva removed from both eyes. All experiments conformed to ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Schepens Eye Research Institute Animal Care and Use Committee.
Cell Culture
Goblet cells from excised rat conjunctiva were grown in organ culture as described previously.8,9 Pieces of minced tissue dissected from excised conjunctiva were placed in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, and 100 μg/mL penicillin-streptomycin. The tissue plug was removed after nodules of cells were observed. First passage goblet cells were used in all experiments. The identity of cultured cells was periodically confirmed by evaluating staining with antibody to cytokeratin 7 (that detects goblet cell bodies) and the lectin Ulex europaeus agglutinin (UEA)-1 or an antibody to MUC5AC (both detect goblet cell secretory products) to ensure that goblet cells predominated.
Secretion
Cultured goblet cells were seeded in 24-well plates and grown to confluence. Cells were serum starved for 2 hours before use, pre-incubated with antagonists for 30 minutes, and then stimulated with histamine for 2 hours in the presence of serum-free RPMI 1640, then supplemented with 0.5% bovine serum albumin (BSA). For small interfering (siRNA) experiments, goblet cells were incubated for 18 hours with 100 nM siRNA for ERK 2. The media was replaced and cells were allowed to grow for a total of 48 hours before use. Goblet cell secretion was measured using an enzyme-linked lectin assay (ELLA) with the lectin UEA-I. UEA-1 detects high molecular weight glycoconjugates including mucins produced by rat goblet cells. The media were collected and analyzed for the amount of lectin-detectable glycoconjugates, which quantifies the amount of goblet cell secretion. The standards and supernatant were spotted onto Nunc microplates (Thermo Scientific, Waltham, MA) and dried overnight at 60°C. The ELLA was performed according to a protocol from Pierce, Inc. (Rockford, IL) using UEA-I conjugated to horseradish peroxidase. The UEA-1 was then detected using Amplex Red (Invitrogen, Carlsbad, CA), which when oxidized by peroxidase in the presence of hydrogen peroxide, produces a highly fluorescent molecule. The fluorescence was quantified on a fluorescent ELISA reader (Synergy MX; Bio-Tek, Winooski, VT) with an excitation wavelength of 530 nm and an emission wavelength of 590 nm. The cells were removed and sonicated, and the cell homogenate analyzed for the total amount of protein by using the Bradford protein assay. High molecular weight glycoconjugate secretion was normalized to total protein in the homogenate. Bovine submaxillary mucin was used for the standard curve. High molecular weight glycoconjugate secretion was expressed as fold increase over basal that was set to 1. As a control we used a commercial rat MUC5AC ELISA kit to demonstrate that MUC5AC secretion is a component of high molecular weight glycoprotein secretion measured by ELLA (data not shown).
Measurement of [Ca2+]i
Goblet cells were incubated for 1 hour at 37°C with Krebs-Ringer bicarbonate buffer with HEPES (KRB-HEPES) (119 mM NaCl, 4.8 mM KCl, 1.0 mM CaCl2, 1.2 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 10 mM HEPES, and 5.5 mM glucose [pH 7.45]), plus 0.5% BSA containing 0.5 μM fura-2/AM, 8 μM pluronic acid F127, and 250 μM sulfinpyrazone, followed by washing in KRB-HEPES containing sulfinpyrazone. Calcium measurements were made with a ratio imaging system (In Cyt Im2; Intracellular Imaging, Cincinnati, OH) using wavelengths of 340 and 380 nm and an emission wavelength of 505 nm. At least 10 cells were used for each condition and experiments were repeated in at least three separate animals. Antagonists of histamine (H) receptor subtypes H1–H4 were dissolved in KRB-HEPES buffer and added 30 minutes before histamine. After addition of agonists data were collected in real time. Data are presented as the actual [Ca2+]i with time or as the change in peak [Ca2+]i. Change in peak [Ca2+]i was calculated by subtracting the average of the basal value (no added agonist) from the peak [Ca2+]i. Although data is not shown, the plateau [Ca2+]i was affected similarly to the peak [Ca2+]i.
Statistical Analysis
Results were expressed as the fold increase above basal or the percentage of inhibition of the net stimulation by agonist alone. Results are presented as mean ± SEM. Data were analyzed by Student's t-test. A P value less than 0.05 was considered statistically significant.
Results
Effect of Extracellular Ca2+ (Ca2+O) Removal and Inhibition of Erk 2 on Histamine-Stimulated High Molecular Weight Glycoconjugate Secretion
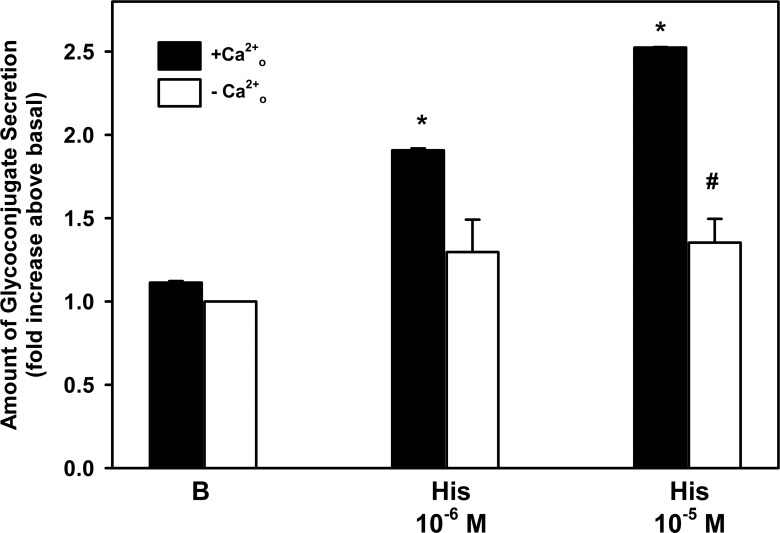
As an increase in [Ca2+]i and in ERK1/2 activity is a major mechanism by which agonists stimulate conjunctival goblet cell secretion,4,10–13 we investigated the extent to which histamine-stimulated secretion is dependent upon these signaling intermediates. We first determined the effect of extracellular (Ca2+o) removal on histamine-stimulated goblet cell secretion, as we previously showed that histamine increases [Ca2+]i (data not shown). When Ca2+o was removed, histamine-stimulated secretion was significantly blocked by 68% and 72% for histamine at 10−6 and 10−5 M, respectively (Fig. 1). Removal of Ca2+o did not alter basal secretion.
Figure 1. .
Effect of removal of extracellular (Ca2+o) on histamine-stimulated rat goblet cell high molecular weight glycoconjugate secretion. Cultured rat goblet cells were incubated in the presence (black bar) and absence (white bar) of extracellular Ca2+ and then stimulated for 2 hours with no additions (B) or histamine (His) at 10−6 and 10−5 M. High molecular weight glycoconjugate secretion was measured by enzyme-linked lectin assay. Data are mean ± SEM of three independent experiments. *Indicates statistical significance from no addition (B); #indicates statistical significance from histamine alone.
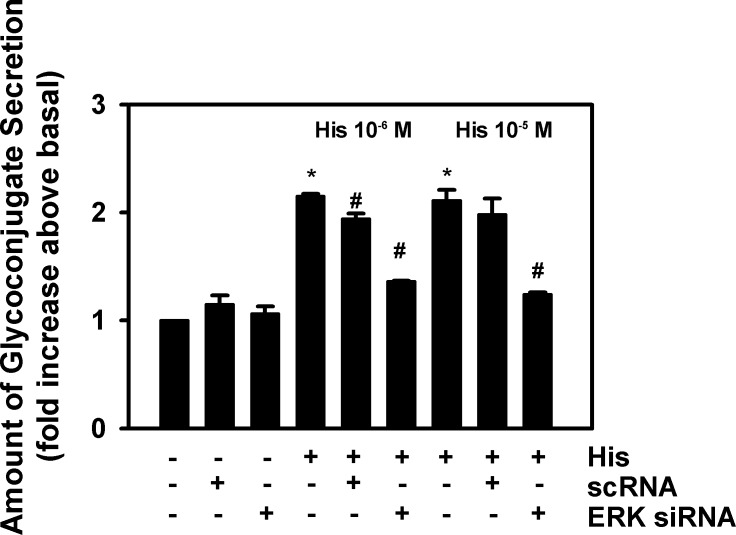
We then ascertained the extent that histamine-stimulated secretion is dependent upon activation of ERK1/2 by using siRNA for ERK2. We have shown that histamine activates ERK1/2 and, in addition, siRNA targeting ERK2, but not negative control, scrambled siRNA (small cytoplasmic RNA[scRNA]), decreased the amount of ERK 2.14 When ERK2 was depleted with siRNA, histamine-stimulated secretion was significantly decreased by 68% and 78% for histamine at 10−6 and 10−5M, respectively (Fig. 2). Incubation with scRNA did not alter the histamine-stimulated secretory response. In addition neither scRNA nor ERK2 siRNA altered basal secretion.
Figure 2. .
Effect of depletion of ERK2 on histamine-stimulated rat goblet cell high molecular weight glycoconjugate secretion. Cultured rat goblet cells were preincubated for 48 hours with no additions, siRNA for ERK2 (ERK siRNA), or siRNA for a scrambled sequence (scRNA), and then stimulated with histamine (His) at 10−6 and 10−5 M for 2 hours. High molecular weight glycoconjugate secretion was measured by enzyme-linked lectin assay. Data are mean ± SEM of three independent experiments. *Indicates statistical significance from no addition; #indicates statistical significance from histamine alone.
We conclude that in conjunctival goblet cells histamine-stimulated secretion is dependent upon both extracellular Ca2+ and activation of ERK 2.
Effect of Multiple Applications of Histamine on [Ca2+]i
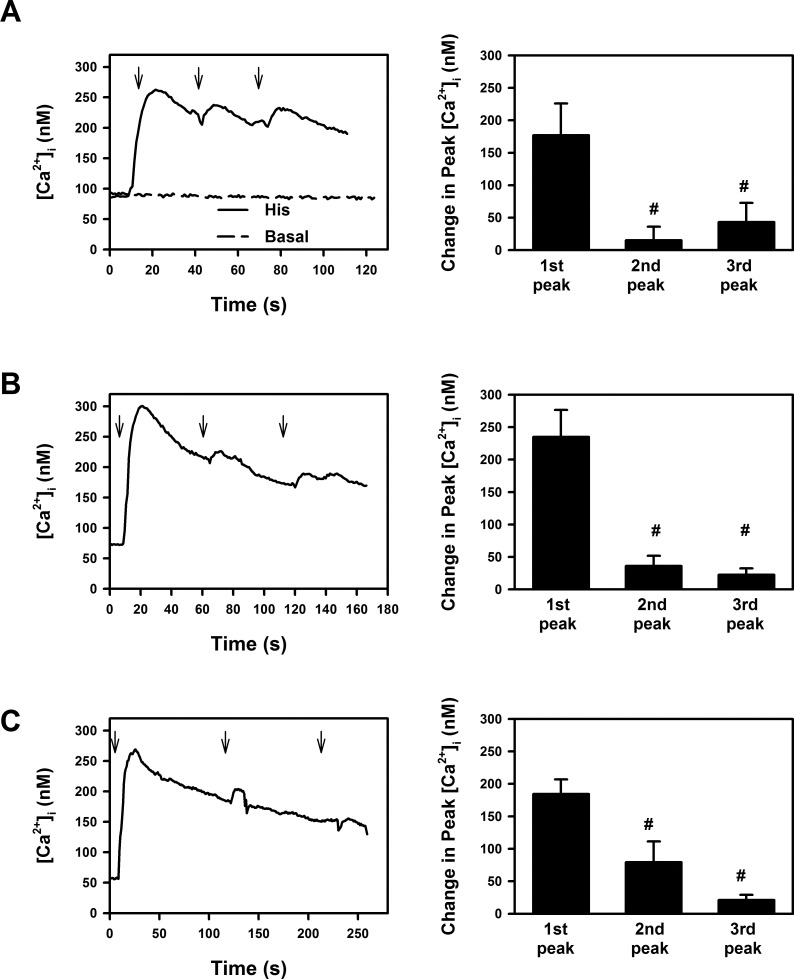
Histamine is a G protein-coupled receptor (GPCR) and one characteristic of GPCRs is desensitization. To explore whether activation of histamine receptors in conjunctival goblet cells desensitizes, histamine (10−5 M) was added sequentially three times at either 30 (Fig. 3A), 60 (Fig. 3B), or 120 (Fig. 3C) second intervals and [Ca2+]i measured. The second and third histamine responses were significantly decreased compared with the first, when histamine was added at 30, 60, or 120 second intervals, suggesting that histamine receptors in goblet cells desensitize and are GPCRs. Similar results were obtained if peak [Ca2+]i was expressed as a change compared with the basal [Ca2+]i before addition of histamine (data not shown) or the [Ca2+]i before each addition of histamine (Fig. 3A). Basal [Ca2+]i did not change over the course of the experiment (Fig. 3A).
Figure 3. .
Effect of repeated additions of histamine on intracellular [Ca2+] ([Ca2+]i) in rat conjunctival goblet cells. Cultured rat goblet cells were loaded with the Ca2+ indicator dye fura 2. Histamine (10−5 M) was then added at 30 (A), 60 (B), or 120 seconds (C) intervals (indicated by the arrows) and the [Ca2+]i measured. Traces shown in left panels are mean of five individual experiments. Peak [Ca2+]i was calculated from [Ca2+]i prior to histamine addition and shown as mean ± SEM in right panels. #Indicates statistical significance from first histamine addition.
Effect of Inhibition of Phospholipase C with U73122 on Histamine-Stimulated Increase in [Ca2+]i
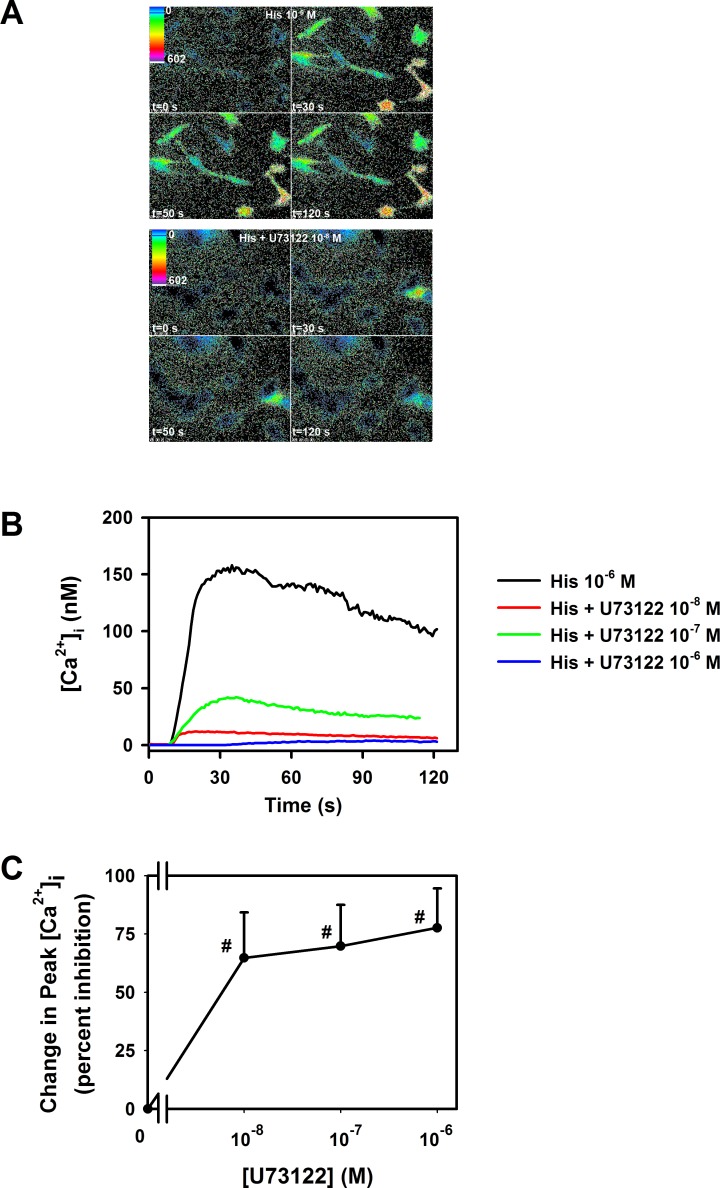
We next determined if, like other GPCRs, histamine in rat conjunctival goblet cells activates phospholipase C. Histamine at 10−6 M significantly increased [Ca2+]i by 170 ± 35.9 nM (Figs. 4A, 4B). When goblet cells were pre-incubated for 30 minutes with the phospholipase C inhibitor U73122 (10−6–10−8 M), the histamine-stimulated goblet cell increase in [Ca2+]i was significantly decreased. A maximum decrease of 78% was obtained (Fig. 4C). Thus, histamine interacts with GPCRs and activates phospholipase C to increase the [Ca2+]i.
Figure 4. .
Effect of a phospholipase C inhibitor on intracellular [Ca2+] ([Ca2+]i) stimulated by histamine in rat conjunctival goblet cells. Cultured rat goblet cells were loaded with the Ca2+ indicator dye fura 2 and pre-incubated for 30 minutes with the phospholipase C inhibitor U73122. The [Ca2+]i was visualized in single goblet cells by pseudocolor as indicated in (A). The top four panels indicate the [Ca2+]i response to histamine (His, 10−6 M) at 0, 30, 50, and 120 seconds. The bottom four panels indicate the [Ca2+]i response to histamine (10−6 M) at 0, 30, 50, and 120 seconds after pre-incubation with U73122 (10−8 M). Traces shown in (B) are mean of four individual experiments. The percent inhibition of peak [Ca2+]i was calculated and shown as mean ± SEM in (C). #Indicates statistical significance from histamine alone.
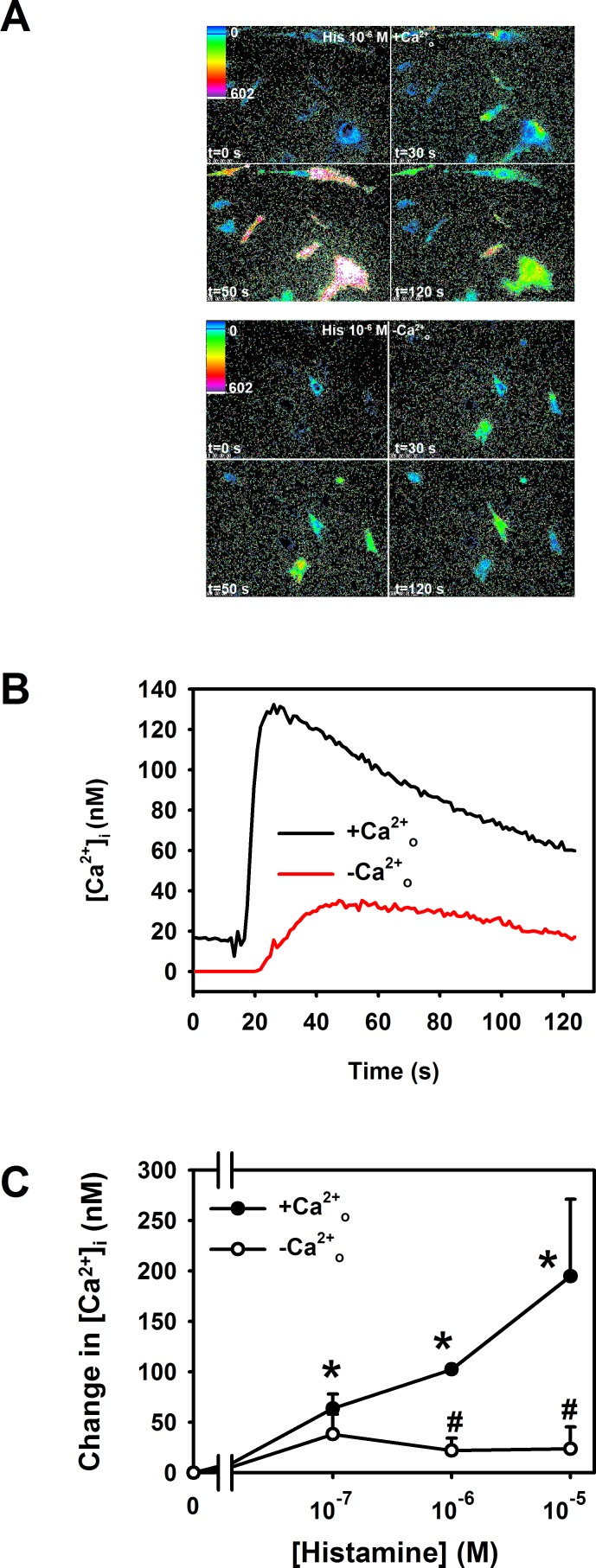
Effect of Removal of Ca2+O on Histamine-Stimulated Increase in [Ca2+]i
We next explored the cellular Ca2+ pools used by histamine to elevate [Ca2+]i. To determine the role of Ca2+o, we removed Ca2+o before stimulation with histamine. In the absence of Ca2+o the histamine (10−6 and 10−5 M) stimulated increase in [Ca2+]i was almost completely inhibited (Figs. 5A–C). At the lowest concentration of histamine (10−7 M) removal of Ca2+o did not change the level of [Ca2+]i caused by histamine (Fig. 5C), suggesting that at higher concentrations the histamine response was almost completely dependent on Ca2+o, but was not similarly dependent at the lower histamine concentration. Removal of Ca2+o blocks both the histamine-stimulated increase in [Ca2+]i (Fig. 5) and secretion (Fig. 1) at histamine concentrations of 10−5 and 10−6 M and links the two functions.
Figure 5. .
Effect of removal of extracellular Ca2+ on histamine-stimulated increase in intracellular [Ca2+] ([Ca2+]i) in rat conjunctival goblet cells. Cultured rat goblet cells were loaded with the Ca2+ indicator dye fura 2 and incubated in the presence and absence of extracellular Ca2+ (Ca2+o). Cells were then stimulated with histamine (10−7, 10−6, and 10−5 M). The [Ca2+]i was indicated in single goblet cells by pseudocolor as indicated in (A). The top four panels indicate the [Ca2+]i response to histamine (His, 10−6 M) at 0, 30, 50, and 120 seconds in the presence of extracellular Ca2+. The bottom four panels indicate the [Ca2+]i response to histamine (10−6 M) at 0, 30, 50, and 120 seconds in the absence of extracellular Ca2+. Traces shown in (B) represent the [Ca2+]i induced by histamine (10−6 M) in the presence and absence of extracellular Ca2+ and are the mean of three individual experiments. Peak [Ca2+]i was calculated and shown as mean ± SEM in (C). *Indicates statistical significance from no addition (0); #indicates statistical significance from histamine in the presence of extracellular Ca2+.
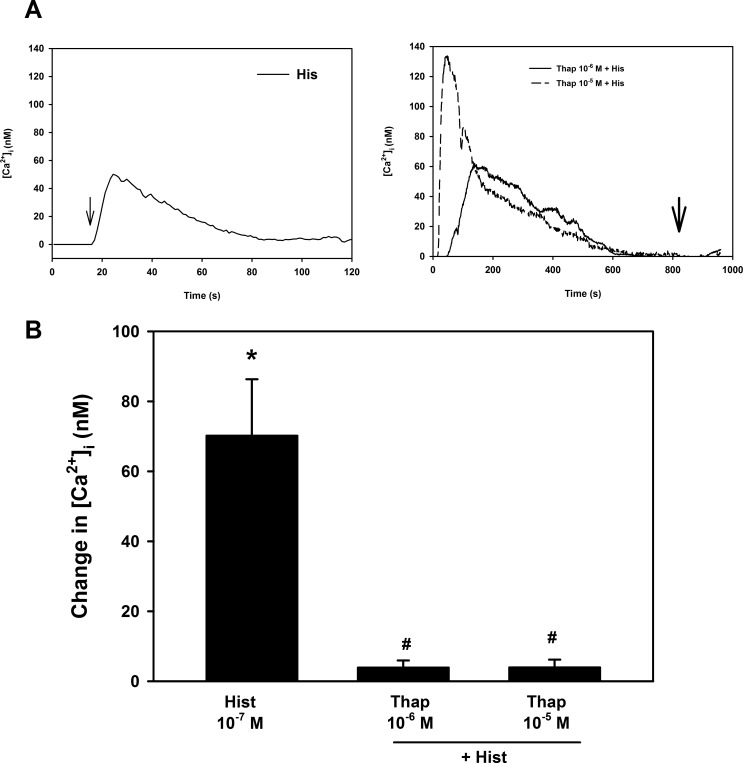
We then determined if the lower concentration of histamine released intracellular Ca2+ stores. As a control, goblet cells were stimulated with histamine at 10−7 M in the presence of Ca2+o which increased [Ca2+]i by 70.2 ± 16.1 nM (Figs. 6A, 6B). In the next set of experiments, goblet cells were first stimulated with thapsigargin (10−6 and 10−5 M) in the absence of Ca2+o, which blocks the Ca2+ATPase, a Ca2+ pump, of intracellular Ca2+ stores. This maneuver empties intracellular Ca2+ stores by preventing the re-uptake of Ca2+ after it passively leaks out. After the intracellular Ca2+ stores were depleted with thapsigargin, cells were stimulated by histamine. Compared with the histamine (10−7 M) stimulated increase in [Ca2+]i response in the absence of thapsigargin (Fig. 6A left panel, 6B) the histamine response in the presence of thapsigargin (10−6 and 10−5 M) was completely blocked (Fig. 6A right panel, 6B). We suggest that histamine at low concentrations releases intracellular Ca2+ stores that stimulate influx of extracellular Ca2+, but at higher concentrations the influx of extracellular Ca2+ overwhelms the release of intracellular Ca2+.
Figure 6. .
Effect of pre-incubation with thapsigargin on histamine-stimulated increase in intracellular [Ca2+] ([Ca2+]i) in rat conjunctival goblet cells. Cultured rat goblet cells were loaded with the Ca2+ indicator dye fura 2 and either stimulated with histamine (His) alone in the presence of Ca2+o or preincubated with thapsigargin and then stimulated with histamine indicated by the arrow in the absence of Ca2+o. The [Ca2+]i was measured. Traces shown in (A) are the mean of three individual experiments. Peak [Ca2+]i was calculated and shown as mean ± SEM in (B). *Indicates statistical significance from no addition (0); #indicates statistical significance from histamine alone.
Effect of Specific Histamine Receptor Subtype Agonists on [Ca2+]i
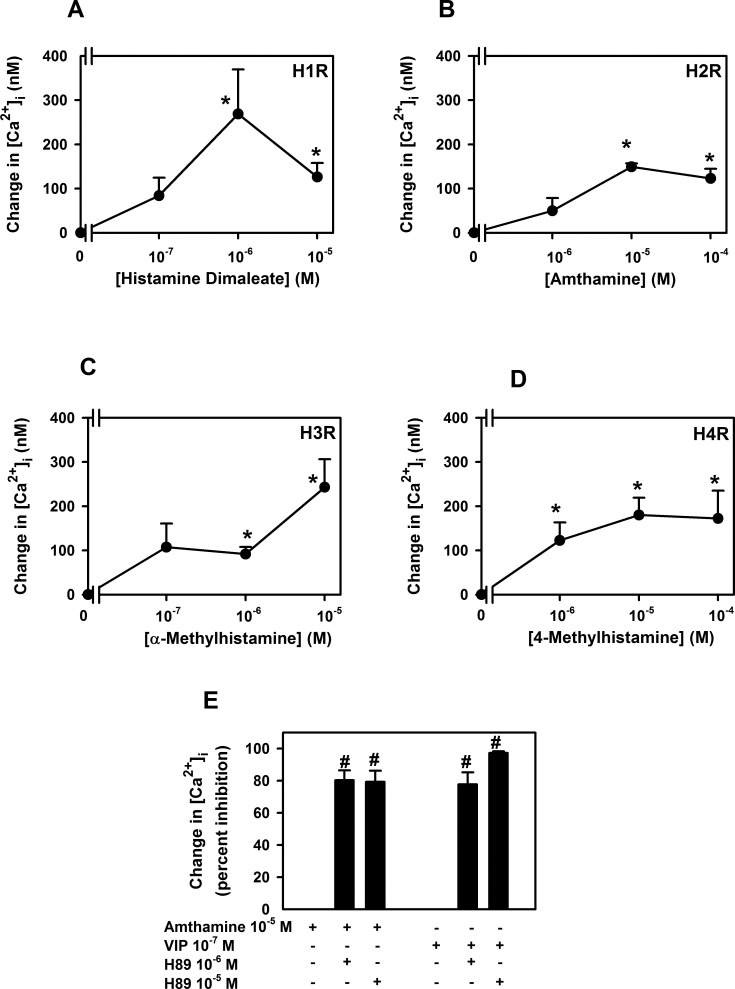
We previously found that all four histamine receptor subtypes, H1–H4, were present in human and rat conjunctival goblet cells and agonists for each receptor subtype stimulated rat goblet cell secretion.4 In the present experiments on rat conjunctival goblet cells in culture, agonists of each histamine receptor subtype significantly increased intracellular [Ca2+]i in a concentration-dependent manner (Figs. 7A–D).
Figure 7. .
Effect of histamine receptor subtype agonists on intracellular [Ca2+] ([Ca2+]i) in rat conjunctival goblet cells. Cultured rat goblet cells were loaded with the Ca2+ indicator dye fura 2 and either stimulated with an H1 agonist histamine dimaleate (A), an H2 agonist amthamine (B), an H3 agonist α-methylhistamine (C), or an H4 agonist 4-methylhistamine (D). Peak [Ca2+]i was calculated and shown as mean ± SEM from three independent experiments (A–D). Cultured rat goblet cells were also pre-incubated with H89 (10−6 and 10−5 M) for 30 minutes and stimulated with either amthamine (10−5 M) or VIP (10−7 M) (E). Mean ± SEM of the percent inhibition shown in (E) is from four independent experiments. *Indicates statistical significance from no addition (0). #Indicates statistical significance from amthamine alone.
As H2 receptors have been shown to increase cAMP, we determined if the rise in Ca2+ stimulated by the H2 receptor agonist amthamine was inhibited by H89, a specific inhibitor of cAMP-dependent protein kinase, known as protein kinase A (PKA). Rat goblet cells were pre-incubated for 30 minutes with H89 at 10−6 and 10−5 M. Amthamine (10−5 M) or the positive control VIP (10−7 M) was then added and [Ca2+]i measured. H89 at both concentrations significantly inhibited amthamine-stimulated increase in [Ca2+]i (Fig. 7E). VIP-stimulated increase in [Ca2+]i was also significantly inhibited by H89 at both concentrations (Fig. 7E).
Effect of Specific Histamine Receptor Subtype Antagonists on Histamine-Stimulated Increase in [Ca2+]i
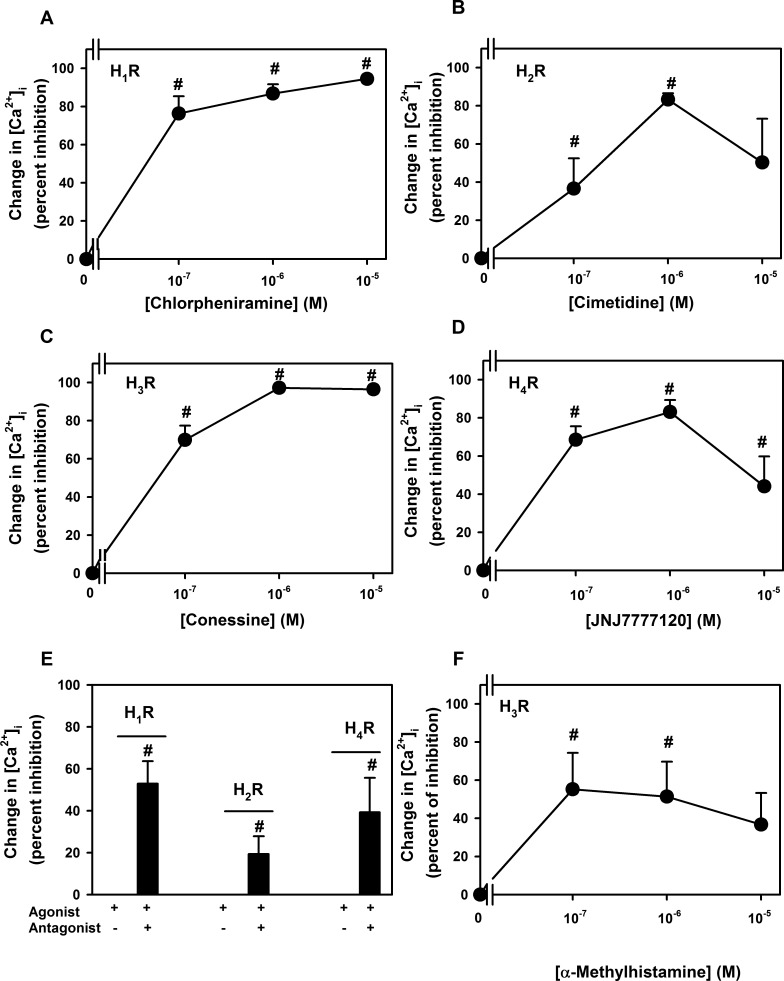
Similarly to the agonists, antagonists of all four histamine receptor subtypes significantly blocked the histamine-stimulated increase in [Ca2+]i in a concentration-dependent manner (Figs. 8A–D). A maximum inhibition of 86.8% ± 4.8% was obtained at 10−5 M chlorpheniramine the H1 antagonist, of 83.3% ± 3.3% by 10−6 M of cimetidine the H2 antagonist, of 97.3% ± 0.4% by 10−5 M of conessine the H3 antagonist, and of 83.1% ± 6.3% by 10−6 M of JNJ7777120 the H4 antagonist. As the antagonists could have non-specific effects we determined the effect of each histamine receptor subtype antagonist on its respective agonist. The H1 antagonist chlorpheniramine at 10−5 M inhibited the Ca2+i response to the H1 agonist histamine dimaleate (10−6 M) by 52.9% ± 10.7% (Fig. 8E). The H2 antagonist cimetidine at 10−6 M inhibited the Ca2+i response to the H2 agonist amthamine (10−5 M) by 19.3% ± 8.5 % (Fig. 8E). The H4 antagonist JNJ7777120 at 10−6 M inhibited the Ca2+i response to the H4 agonist 4-methylhistamine (10−4 M) by 39.2% ± 16.5% (Fig. 8E).
Figure 8. .
Effect of histamine receptor subtype antagonists on intracellular [Ca2+] ([Ca2+]i) stimulated by histamine in rat conjunctival goblet cells. Cultured rat goblet cells were loaded with the Ca2+ indicator dye fura 2 and pre-incubated with an H1 antagonist chlorpheniramine (A), an H2 antagonist cimetidine (B), an H3 antagonist conessine (C), and an H4 antagonist JNJ7777120 (D), and then stimulated with histamine (10−6 M). Peak [Ca2+]i was calculated and shown as mean ± SEM of the percent inhibition. Cultured goblet cells were loaded with the Ca2+ indicator dye fura 2 and preincubated with an H1 antagonist chlorpheniramine (10−5 M) prior to stimulation with H1 agonist histamine dimaleate (10−6 M) (E), an H2 antagonist cimetidine (10−6 M) prior to stimulation with an H2 agonist amthamine (10−5 M) (E), an H4 antagonist JNJ7777120 (10−6 M) prior to stimulation with an H4 agonist 4-methylhistamine (10−4 M) (E), and an H3 antagonist conessine (10−5 M) prior to stimulation with an H3 agonist α-methylhistamine (10−7, 10−6, and 10−5 M) (F). Peak [Ca2+]i was calculated and shown as mean ± SEM of the percent inhibition. #Indicates statistical significance from agonist alone.
Since the effect of the H3 agonist increased substantially between 10−6 and 10−5 M, we tested the effect of the H3 antagonist conessine on α-methylhistamine at 10−7−10−5 M. The H3 antagonist conessine at 10−6 M inhibited the Ca2+i response to the H3 agonist by a maximum 55.3% ± 19.1% at 10−7 M α–methylhistamine (Fig. 8F). The effect of the H3 antagonist did not differ with the concentration of H3 agonist used.
Although subtype receptor antagonists were not as effective on subtype receptor antagonists as on histamine, these data taken together suggest that activation of each of the four histamine receptor subtypes is a potent and effective stimulus of intracellular Ca2+. In conjunctival goblet cells histamine could activate all four histamine receptor subtypes to increase intracellular Ca2+.
Discussion
In the present study, we found that an increase in intracellular Ca2+ and activation of ERK1/2 is a major mechanism by which histamine stimulates conjunctival goblet cell secretion of MUC5AC and other high molecular weight glycoproteins. Furthermore, we demonstrated that histamine functions as a typical GPCR as it desensitizes, activates phospholipase C, releases Ca2+ from intracellular stores, and stimulates influx of extracellular Ca2+. Histamine also activates ERK1/2. These effects of histamine are in common with all stimuli of conjunctival goblet cell secretion studied to date.7,11 We also discovered that histamine activates all four histamine receptor subtypes to increase the [Ca2+]i.
Histamine also stimulates secretion from goblet cells located in other tissues including nasal epithelium, intestine, colon, and airways.15–20 However, there is contradictory evidence in colon, airway, and pancreatic duct goblet cells whether histamine increases [Ca2+]i.21–23 Unlike the conjunctival goblet cells, the cellular mechanisms used by histamine to increase [Ca2+]i in the goblet cells from these nonconjunctival tissues has yet to be published.
The role [Ca2+]i in the histamine-stimulated response has been investigated in nongoblet cells.24 In human conjunctival epithelial cells, which are likely to be stratified squamous cells, histamine activates phospholipase C and increases [Ca2+]i.25 In another cell type, astrocytoma cells, histamine increases [Ca2+]i.26 However, the increase in [Ca2+]i, in most tissues, depends upon the histamine receptor subtype. All four histamine receptor subtypes are present in conjunctival goblet cells4 and activation of each subtype increases [Ca2+]i (present study) and stimulates secretion (although H2 and H3 receptors are more effective than H1 and H4).4 Furthermore, inhibition of histamine activation of each receptor subtype by antagonists decreases [Ca2+]i (present study) and blocks secretion (except for H4).4 These results were unexpected as not all histamine receptor subtypes are known to increase [Ca2+]i. When histamine receptor subtypes were studied in conjunctival epithelial cells, unlike the conjunctival goblet cells of the present study, histamine stimulation of [Ca2+]i did not desensitize, and was blocked by H1, but not by H2 or H3 antagonists.25 H4 antagonists were not available at the time of these experiments. We suggest that both conjunctival cell types (stratified squamous and goblet cell) respond to histamine, but the subtypes of histamine receptors that are active differ. In other cell types activation of histamine H1 increased [Ca2+]i in salivary gland cells, cytotrophoblasts, and purkinje cell neurons, microvascular endothelial cells, and endothelial cells.27–31 When H4 receptors were induced in mast cells, [Ca2+]i increased.32 In contrast in cardiac sympathetic nerve endings, activation of H3 receptors decreased [Ca2+]i and H1 and H4 receptors increased [Ca2+]i.33 Thus, in most tissues H1 and H4 receptors increase [Ca2+]i, H3 receptors decrease [Ca2+]i, and H2 receptors have not been tested as they are expected to activate adenylate cyclase. In the current study, we showed that in conjunctival goblet cells activation of all four histamine receptor subtypes increases [Ca2+]i. Activation of H1 and H4 receptors in conjunctival goblet cells is in agreement with the results in other cell types. In contrast, however, activation of H2 receptors has not been shown to alter Ca2+ and activation of H3 receptors usually decreases [Ca2+]i. In fact, we found herein that the H2 agonist uses cAMP and PKA to increase in [Ca2+]i as the amthamine-stimulated Ca2+ response was blocked by the PKA inhibitor H89. Activation of H3 receptors usually decreases [Ca2+]i, but perhaps when co-expressed with H1 and H4 subtypes, as in conjunctival goblet cells, the H3 receptor subtype is either not linked to an inhibitory pathway or the inhibitory pathway is altered. Conjunctival goblet cells are, thus, unusual in possessing all four histamine receptor subtypes4 and by all four subtypes having the same effect on [Ca2+]i as shown in the present study.
Shown in Figure 9 is a schematic of the possible mechanisms the histamine subtype receptors could use to increase [Ca2+]i and activate ERK1/2 to stimulate conjunctival goblet cell secretion. We propose that H1 receptors are linked to the G protein (G) Gαq that activates phospholipase C to release Ca2+ from intracellular stores and stimulate Ca2+ influx. The increase in [Ca2+]i either by itself or by activating ERK1/2 stimulates goblet cell secretion. H2 receptors, in contrast, are couple to Gαs that activates adenylate cyclase. Activation of this cyclase produces cAMP from adenosine triphosphate (ATP) that activates PKA. PKA perhaps by interacting with the IP3 receptor releases intracellular Ca2+ and then stimulates Ca2+ influx. The increase in [Ca2+]i either by itself or by activating ERK1/2 stimulates goblet cell secretion. We propose that H3 and H4 receptors are each coupled to Gαi/o. By some mechanism, perhaps phospholipase C, these receptors increase [Ca2+]i that either by itself or by activating ERK1/2 stimulates goblet cell secretion.
Figure 9. .
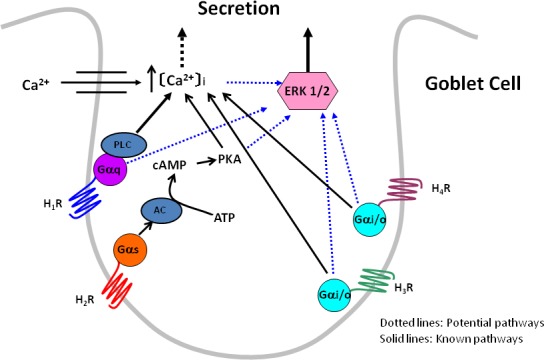
Proposed mechanism of the role of histamine receptor subtypes in goblet cell signaling and secretion. H1R to H4R, histamine receptor subtypes 1 to 4; Gαq, G protein of the αq subtype; Gαs, G protein of the αs subtype; Gαi/o, G protein of the αi/o subtype; PLC, phospholipase C; AC, adenylate cyclase; PKA, protein kinase A; ERK 1/2, extracellular regulated kinase 1/2.
When histamine was used as an agonist, each of the four histamine receptor subtype antagonists blocked the increase in [Ca2+]i by 83% to 97%. In contrast, when specific subtype agonists, and their respective antagonists, were used inhibition of [Ca2+]i was less. We suggest that the antagonists were more effective on histamine itself for two reasons. First, activation of all four histamine receptor subtypes in conjunctival goblet cells converge on increasing [Ca2+]i. Second, the selected histamine receptor subtype agonists and antagonists are not completely subtype specific. Thus, a non-specific effect of an antagonist on a second histamine receptor subtype would cause an additional inhibition of the [Ca2+]i response to histamine.
Histamine is also known to activate ERK1/2. In vascular smooth muscle cells histamine increases ERK1/2, an effect dependent upon [Ca2+]i.34 Histamine also activates ERK1/2 in nerves.35 Activation of several histamine receptor subtypes activates ERK1/2. In splenocytes, activation of all four histamine receptors increased ERK1/2 activity.36 H1 activation increased ERK1/2 phosphorylation in adrenal chromafin cells as did activation of H2 in HEK293 cells, and gastric epithelial cells stimulated ERK1/2 activity.37,38 Finally, stimulation of H3 receptors decreases ERK1/2 phosphorylation in cholangiocytes.39 Although the effect of the different histamine receptor subtypes on ERK1/2 was not tested in conjunctival goblet cells, we would predict that activation of each of the four histamine receptor subtypes would increase ERK1/2 activity as an increase in [Ca2+]i stimulates ERK1/2 activity.
The evidence that all four histamine receptors function in conjunctival goblet cells is robust as all four receptors are present by PCR, Western blotting analysis, and immunofluorescence microscopy4; agonist activation of all four receptors stimulates secretion (although H2 and H3 agonists are more effective than H1 and H4 agonists) and antagonists of all four receptors except H4 block histamine-stimulated secretion4; and agonist activation of all four receptor subtypes increases [Ca2+]i, and antagonists of all four receptor subtypes blocks histamine-induced elevation of [Ca2+]i, as shown in the present study. Involvement of multiple types of histamine receptors in one type of allergic conjunctivitis was shown in vernal keratoconjunctivitis. Leonardi et al.40 found that H1, H2, and H4, but not H3, receptors were overexpressed in the conjunctiva from patients with vernal keratoconjunctivitis than controls. In addition Leonardi et al.40 found that cytokines present in allergic conjunctivitis can upregulate H2, but not H4 receptors in conjunctival cells. Thus, the role of the histamine receptor subtypes may change in the setting of allergic conjunctivitis. Multiple histamine receptor subtypes appear to play a role in at least one type of allergic conjunctivitis.
Since goblet cell mucous secretion functions to protect the surface of the eye and, indeed, the entire eye from a variety of challenges from the external environment, it is not surprising that there are multiple redundant pathways for goblet cell secretion. Our findings with histamine suggest that goblet cell mucous secretion is an important component of the innate immune system that has layers of protective mechanisms designed to keep the eye safe from the environment. In diseases such as allergic conjunctivitis, stimulation of mucous secretion by histamine, and other mediators of the allergic response becomes uncontrolled or excessive. Thus, excess mucous secretion can be a component of the pathology of allergic conjunctivitis. In this case development of antagonists of multiple histamine receptor subtypes as treatments of ocular allergy would be beneficial to normalize conjunctival goblet cell secretion.
We conclude that histamine activates phospholipase C to release intracellular Ca2+ that induces the influx of extracellular Ca2+ and activates ERK1/2 to stimulate conjunctival goblet cell mucous secretion. Interestingly, activation of all four histamine receptor subtypes increases [Ca2+]i, but the H2 receptor uses cAMP and PKA for this effect.
Acknowledgments
The authors thank Charles N. Serhan, PhD, and Nan Chiang, PhD, for their helpful discussions and suggestions, and Gary Bird, PhD, and James Putney Jr, PhD, for their help in designing and interpreting experiments on measuring [Ca2+]i.
Footnotes
Supported by grants from the National Institutes of Health (EY019470).
Disclosure: D. Li, None; R.B. Carozza, None; M.A. Shatos, None; R.R. Hodges, None; D.A. Dartt, None
References
- 1.Nathan RA, Meltzer EO, Seiner JC, et al. Prevalence of allergic rhinitis in the United States. J Allergy Clin Immunol. 1997;99:S808–S814 [PubMed] [Google Scholar]
- 2.McCulley JP, Moore MB, Matoba AY. Mucus fishing syndrome. Ophthalmology. 1985;92:1262–1265 [DOI] [PubMed] [Google Scholar]
- 3.Anderson DF, Macleod JDA, Baddeley SM, et al. Seasonal allergic conjunctivitis is accompanied by increased mast cell numbers in the absence of leucocyte infiltration. Clin Exp Allergy. 1997;27:1060–1066 [DOI] [PubMed] [Google Scholar]
- 4.Hayashi D, Li D, Hayashi C, Shatos M, Hodges RR, Dartt DA. Role of Histamine and Its Receptor Subtypes in Stimulation of Conjunctival Goblet Cell Secretion. Invest Ophthalmol Vis Sci. 2012;53:2993–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bongers G, Bakker RA, Leurs R. Molecular aspects of the histamine H3 receptor. Biochem Pharmacol. 2007;73:1195–1204 [DOI] [PubMed] [Google Scholar]
- 6.Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011;186:4455–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rios JD, Zoukhri D, Rawe IM, Hodges RR, Zieske JD, Dartt DA. Immunolocalization of muscarinic and VIP receptor subtypes and their role in stimulating goblet cell secretion. Invest Ophthalmol Vis Sci. 1999;40:1102–1111 [PubMed] [Google Scholar]
- 8.Shatos MA, Rios JD, Horikawa Y, et al. Isolation and characterization of cultured human conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2477–2486 [DOI] [PubMed] [Google Scholar]
- 9.Shatos MA, Rios JD, Tepavcevic V, Kano H, Hodges R, Dartt DA. Isolation, characterization, and propagation of rat conjunctival goblet cells in vitro. Invest Ophthalmol Vis Sci. 2001;42:1455–1464 [PubMed] [Google Scholar]
- 10.Hodges RR, Horikawa Y, Rios JD, Shatos MA, Dartt DA. Effect of protein kinase C and Ca(2+) on p42/p44 MAPK, Pyk2, and Src activation in rat conjunctival goblet cells. Exp Eye Res. 2007;85:836–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horikawa Y, Shatos MA, Hodges RR, et al. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2003;44:2535–2544 [DOI] [PubMed] [Google Scholar]
- 12.Kanno H, Horikawa Y, Hodges RR, et al. Cholinergic agonists transactivate EGFR and stimulate MAPK to induce goblet cell secretion. Am J Physiol Cell Physiol. 2003;284:C988–998 [DOI] [PubMed] [Google Scholar]
- 13.Dartt DA, Rios JD, Kanno H, et al. Regulation of conjunctival goblet cell secretion by Ca(2+) and protein kinase C. Exp Eye Res. 2000;71:619–628 [DOI] [PubMed] [Google Scholar]
- 14.Hodges RR, Bair JA, Carozza RB, Li D, Shatos MA, Dartt DA. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp Eye Res. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu T, Shimizu S, Hattori R, Majima Y. A mechanism of antigen-induced goblet cell degranulation in the nasal epithelium of sensitized rats. J Allergy Clin Immunol. 2003;112:119–125 [DOI] [PubMed] [Google Scholar]
- 16.Halm DR, Halm ST. Secretagogue response of goblet cells and columnar cells in human colonic crypts. Am J Physiol Cell Physiol. 2000;278:C212–233 [DOI] [PubMed] [Google Scholar]
- 17.Lane AP, Prazma J, Gibbons PJ, Rose AS, Pillsbury HC. The role of nitric oxide in the neural control of nasal fluid production. Am J Rhinol. 1997;11:303–311 [DOI] [PubMed] [Google Scholar]
- 18.Tamaoki J, Nakata J, Takeyama K, Chiyotani A, Konno K. Histamine H2 receptor-mediated airway goblet cell secretion and its modulation by histamine-degrading enzymes. J Allergy Clin Immunol. 1997;99:233–238 [DOI] [PubMed] [Google Scholar]
- 19.Takeyama K, Tamaoki J, Nakata J, Konno K. Effect of oxitropium bromide on histamine-induced airway goblet cell secretion. Am J Respir Crit Care Med. 1996;154:231–236 [DOI] [PubMed] [Google Scholar]
- 20.Neutra MR, O'Malley LJ, Specian RD. Regulation of intestinal goblet cell secretion. II. A survey of potential secretagogues. Am J Physiol. 1982;242:G380–387 [DOI] [PubMed] [Google Scholar]
- 21.Hoffstein ST, Malo PE, Bugelski P, Wheeldon EB. Leukotriene D4 (LTD4) induces mucus secretion from goblet cells in the guinea pig respiratory epithelium. Exp Lung Res. 1990;16:711–725 [DOI] [PubMed] [Google Scholar]
- 22.Kamijo A, Terakawa S, Hisamatsu K. Neurotransmitter-induced exocytosis in goblet and acinar cells of rat nasal mucosa studied by video microscopy. Am J Physiol. 1993;265:L200–209 [DOI] [PubMed] [Google Scholar]
- 23.Hootman SR, de Ondarza J. Regulation of goblet cell degranulation in isolated pancreatic ducts. Am J Physiol. 1995;268:G24–32 [DOI] [PubMed] [Google Scholar]
- 24.MacGlashan D Jr. Histamine: A mediator of inflammation. J Allergy Clin Immunol. 2003;112:S53–59 [DOI] [PubMed] [Google Scholar]
- 25.Sharif NA, Xu SX, Magnino PE, Pang IH. Human conjunctival epithelial cells express histamine-1 receptors coupled to phosphoinositide turnover and intracellular calcium mobilization: role in ocular allergic and inflammatory diseases. Exp Eye Res. 1996;63:169–178 [DOI] [PubMed] [Google Scholar]
- 26.Arias-Montano JA, Berger V, Young JM. Calcium-dependence of histamine- and carbachol-induced inositol phosphate formation in human U373 MG astrocytoma cells: comparison with HeLa cells and brain slices. Br J Pharmacol. 1994;111:598–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JH, Park SH, Moon YW, et al. Histamine H1 receptor induces cytosolic calcium increase and aquaporin translocation in human salivary gland cells. J Pharmacol Exp Ther. 2009;330:403–412 [DOI] [PubMed] [Google Scholar]
- 28.Liu Z, Kilburn BA, Leach RE, Romero R, Paria BC, Armant DR. Histamine enhances cytotrophoblast invasion by inducing intracellular calcium transients through the histamine type-1 receptor. Mol Reprod Dev. 2004;68:345–353 [DOI] [PubMed] [Google Scholar]
- 29.Kirischuk S, Matiash V, Kulik A, Voitenko N, Kostyuk P, Verkhratsky A. Activation of P2-purino-, alpha 1-adreno and H1-histamine receptors triggers cytoplasmic calcium signalling in cerebellar Purkinje neurons. Neuroscience. 1996;73:643–647 [DOI] [PubMed] [Google Scholar]
- 30.Ikeda H, Kubo N, Nakamura A, Harada N, Minamino M, Yamashita T. Histamine-induced calcium released from cultured human mucosal microvascular endothelial cells from nasal inferior turbinate. Acta Otolaryngol. 1997;117:864–870 [DOI] [PubMed] [Google Scholar]
- 31.Jacob R, Merritt JE, Hallam TJ, Rink TJ. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988;335:40–45 [DOI] [PubMed] [Google Scholar]
- 32.Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther. 2003;305:1212–1221 [DOI] [PubMed] [Google Scholar]
- 33.Silver RB, Poonwasi KS, Seyedi N, Wilson SJ, Lovenberg TW, Levi R. Decreased intracellular calcium mediates the histamine H3-receptor-induced attenuation of norepinephrine exocytosis from cardiac sympathetic nerve endings. Proc Natl Acad Sci U S A. 2002;99:501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edwards C, Armstrong P, Goode G, et al. Cross-talking between calcium and histamine in the expression of MAPKs in hypertensive vascular smooth muscle cells. Cell Mol Biol (Noisy-le-grand). 2007;53:61–66 [PubMed] [Google Scholar]
- 35.Lipnik-Stangelj M. Multiple role of histamine H1-receptor-PKC-MAPK signalling pathway in histamine-stimulated nerve growth factor synthesis and secretion. Biochem Pharmacol. 2006;72:1375–1381 [DOI] [PubMed] [Google Scholar]
- 36.Dandekar RD, Khan MM. Regulation of ERK2 phosphorylation by histamine in splenocytes. Immunopharmacol Immunotoxicol. 2011;33:250–258 [DOI] [PubMed] [Google Scholar]
- 37.Xu AJ, Kuramasu A, Maeda K, et al. Agonist-induced internalization of histamine H2 receptor and activation of extracellular signal-regulated kinases are dynamin-dependent. J Neurochem. 2008;107:208–217 [DOI] [PubMed] [Google Scholar]
- 38.Ancha HR, Kurella RR, Stewart CA, Damera G, Ceresa BP, Harty RF. Histamine stimulation of MMP-1(collagenase-1) secretion and gene expression in gastric epithelial cells: role of EGFR transactivation and the MAP kinase pathway. Int J Biochem Cell Biol. 2007;39:2143–2152 [DOI] [PubMed] [Google Scholar]
- 39.Francis H, Franchitto A, Ueno Y, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest. 2007;87:473–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leonardi A, Di Stefano A, Vicari C, Motterle L, Brun P. Histamine H4 receptors in normal conjunctiva and in vernal keratoconjunctivitis. Allergy. 2011;66:1360–1366 [DOI] [PubMed] [Google Scholar]