Abstract
Background
Clinical factors predicting pulmonary complications after lung resection have been well described, whereas the role of genetics is unknown. The vascular endothelial growth factor (VEGF) signaling pathway has been linked to acute lung injury. We hypothesized that genetic variations in this pathway may be associated with postoperative pulmonary complications after lung resection.
Methods
One hundred ninety-six single nucleotide polymorphisms (SNPs) in 17 genes in the VEGF pathway were genotyped in a discovery set of 264 patients and a replication set of 264 patients who underwent lobectomy for lung cancer. Multivariable analysis adjusting for baseline clinical factors was used to identify SNPs associated with pulmonary complications. Cumulative and classification and regression tree (CART) analyses were used to further stratify risk groups.
Results
The overall number of pulmonary complications was 164/528 (31%). The effects of 6 SNPs were consistent in the discovery and replication sets (pooled p value < 0.05). The rs9319425 SNP in the VEGF receptor gene FLT1 resulted in a 1.50-fold increased risk (1.15–1.96; p = 0.003). A cumulative effect for the number of risk genotypes and complications was also evident (p < 0.01). Patients carrying 5 risk genotypes had a 5.76-fold increase in risk (2.73–12.16; p = 4.44 × 10−6). Regression tree analysis identified potential gene-gene interactions between FLT1:rs9319425 and RAF1:rs713178. The addition of the 6 SNPs to the clinical model increased the area under the receiver operating characteristic curve by 6.8%.
Conclusions
Genetic variations in the VEGF pathway are associated with risk of pulmonary complications after lobectomy. This may offer insight into the underlying biological mechanisms of pulmonary complications.
Since Graham’s [1] report of the first successful pneumonectomy in 1933, thoracic surgeons have attempted to identify patients at higher risk for complications after lung resection. A variety of clinical models have been created to help predict postoperative complications and aid in patient selection [2–5]. These models have included factors such as patient smoking history, spirometry, diffusion capacity, and exercise testing. These clinical models, however, often fail to identify patients who have postoperative complications. Modern series still report morbidity rates of 15% to 50% and mortality rates of 3% to 6% [5, 6]. Many patients with good pulmonary function on testing experience severe complications. Conversely, many patients with marginal pulmonary function perform better than expected.
Previous studies have suggested a link between genetic variations and pulmonary complications after lung resection, esophagectomy, and coronary artery bypass procedures [7–9]. Likewise, inflammatory pathways are important mediators of pulmonary complications [10 – 12]. Specifically, the vascular endothelial growth factor (VEGF) pathway has been associated with acute respiratory distress, emphysema, and pulmonary hypertension [13–16]. Thus we hypothesized that genetic variations in the VEGF pathway might be associated with increased risk of postoperative pulmonary complications after lung resection.
Patients and Methods
We analyzed a prospectively maintained database of patients with lung cancer at a single institution from 1994 to 2009. We identified patients who underwent anatomic lobectomy and had germline DNA available. Sublobar resections, pneumonectomies, emergency procedures, and redo operations were excluded. To minimize the effect of population substructure on the genetic analyses, we restricted our analysis to non-Hispanic white individuals. A total of 528 patients were identified and randomly assigned to the discovery and replication populations with matching by age at diagnosis, sex, smoking status, and year of operation. Patients provided written informed consent and this study was approved by MD Anderson’s Institutional Review Board.
Definition of Pulmonary Complication
Pulmonary complication was defined as previously described [5]. It included any or all of the following: acute lung injury (defined as arterial oxygen partial pressure–inspired oxygen fraction ratio less than 300), acute respiratory distress syndrome (defined as arterial oxygen partial pressure–inspired oxygen fraction ratio less than 200), both without evidence of central volume overload; pneumonia (defined as at least 3 of the following: leukocytosis > 10,000/mm3 or < 3,000/mm3, temperature > 38.5°C, purulent sputum, persistent infiltrate on chest roentgenogram, or pathogenic microorganisms from endotracheal aspirate); prolonged air leak (air leak > 5 days); bronchopleural fistula; pleural effusion requiring treatment; pneumothorax requiring treatment; pulmonary embolus; reintubation; respiratory arrest; initial ventilator support longer than 48 hours; discharge with home oxygen; discharge with chest tube; or atelectasis requiring bronchoscopy. Complications were entered into the database at the time of discharge.
Genotyping
Genomic DNA for each patient was isolated from lymphocytes of whole blood using the QIAamp DNA extraction kit (Qiagen, Valencia, CA). A total of 196 SNPs from 17 genes of the VEGF pathway were selected for genotyping and genotyped on a custom iSelect BeadChip (Illumina, San Diego, CA). For each candidate gene, tagging SNPs were selected from 10 kb upstream and 10 kb downstream using Tagger (http://www.broadinstitute.org/mpg/tagger/) (r2 > 0.8 and MAF > 0.05) with data from the HapMap CEU population (http://www.hapmap.org). Genotyping was performed following the standard Infinium II assay protocol (Illumina, San Diego, CA). All laboratory personnel were blinded to the status of the study subjects.
Statistical Analysis
Associations between pulmonary complications and demographics, clinical variables, and treatments were assessed by the χ2 and Fisher’s exact tests. To decide whether potential clinical confounders should be included as covariates in the multivariate logistic regression model, we first identified epidemiologic and clinical variables significantly associated with complication status using univariate logistic regression; next, for all the variables found significant, we performed a stepwise regression to select the final variables to be included for adjustment. Logistic regression was then used to identify SNPs independently associated with pulmonary complications after adjusting for age at operation, sex, intraoperative transfusion, percent diffusing capacity of lung for carbon monoxide (DLCO) predicted, and chest wall resection under the 3 models of inheritance (dominant, recessive, and additive). The multivariable analysis was then repeated for the replication set. To summarize results for the discovery set and the replication set, we performed pooled analysis to obtain the summary odds ratio (OR) and 95% confidence interval (CI). Receiver operating characteristic (ROC) curves were generated to determine ability to predict pulmonary complications based on a model composed of clinical variables and then a model including the SNPs with significant main effects. Areas under the curves were calculated for both ROC curves. Bootstrap resampling was then performed 1,000 times to determine the difference between the 2 ROC curves. All analysis was performed using STATA software (College Station, TX).
For higher order gene-gene interactions, classification and regression tree (CART) analysis was done using HelixTree software (Golden Helix, Bozeman, MT). CART is a binary recursive partitioning method that produces a decision tree to identify subgroups of subjects at different risk. ORs and corresponding 95% CIs were calculated for each of the terminal nodes to determine risk of pulmonary complications in that subgroup of patients. Cumulative effects of SNPs were assessed by summing the putative risk genotypes showing significant association with pulmonary complications in the pooled main effect analysis (p < 0.05). The results were then divided by quartiles, with ORs and corresponding 95% CI calculated for each group.
Results
Clinical Characteristics
Five hundred twenty-eight non-Hispanic white patients underwent lobectomy as part of their treatment for lung cancer at MD Anderson and had DNA available. Clinical details and the rates of pulmonary complications for the discovery and replication sets are shown in Table 1. The populations were well matched for demographic and clinical variables. No statistically significant differences were found between the 2 groups for any of the variables. There were 6 postoperative deaths, 3 in the discovery set and 3 in the validation set.
Table 1.
Clinical Characteristics of Patients in Discovery and Replication Sets
| Variable | Discovery N = 264 |
Replication N = 264 |
Total N = 528 |
|---|---|---|---|
| Mean age (y) | 65 | 66 | 66 |
| Sex | |||
| Male | 141 (53%) | 139 (53%) | 280 (53%) |
| Female | 123 (47%) | 125 (47%) | 248 (47%) |
| Smoking Status | |||
| Never smoker | 54 (21%) | 47 (18%) | 101 (19%) |
| Former smoker | 142 (54%) | 151 (57%) | 293 (55%) |
| Current/recent smoker | 65 (25%) | 64 (24%) | 129 (24%) |
| Pack years (mean) | 53 | 49 | 51 |
| FEV1 ≤ 50% predicted | 137 (53%) | 127 (49%) | 264 (51%) |
| DLCO ≤ 50% predicted | 118 (49%) | 127 (51%) | 245 (50%) |
| Preoperative chemotherapy | 37 (14%) | 39 (15%) | 76 (14%) |
| Thoracoscopic approach | 45 (17%) | 45 (17%) | 90 (17%) |
| Chest wall resection | 12 (5%) | 17 (6%) | 29 (6%) |
| Intraoperative transfusion | 12 (5%) | 11 (4%) | 23 (4%) |
| Pathologic tumor stage | |||
| T1 | 114 (43%) | 115 (44%) | 229 (43%) |
| T2 | 124 (47%) | 121 (46%) | 245 (46%) |
| T3 | 14 (5%) | 11 (4%) | 25 (43%) |
| T4 | 8 (3%) | 13 (5%) | 21 (4%) |
| Any pulmonary complication | 79 (31%) | 85 (33%) | 164 (31%) |
| Prolonged air leak | 39 (15%) | 43 (16%) | 82 (16%) |
| Discharge on home oxygen | 32 (12%) | 29 (11%) | 61 (12%) |
| Pneumonia | 24 (9%) | 26 (10%) | 50 (10%) |
| Atelectasis requiring bronchoscopy | 10 (4%) | 7 (3%) | 17 (4%) |
There were no significant differences between the groups with respect to epidural use, preoperative radiation, tumor location, body mass index, diabetes, or other comorbidities (p > 0.05). The following pulmonary complications each occurred in less than 1.5% of patients: aspiration, acute respiratory distress syndrome, bronchopleural fistula, pulmonary embolus, and initial ventilatory support longer than 48 hours.
DLCO = diffusing capacity of lung for carbon monoxide; FEV1 = forced expiratory volume in the first second of expiration.
The overall number of pulmonary complications was 164/516 (31%). Prolonged air leak was the most common complication, occurring in 82/516 (16%) patients. In the variable selection process, DLCO < 50% predicted, intraoperative transfusion, and en bloc chest wall resection were independently associated with significantly increased risk of pulmonary complications.
Main Effect Analysis
After adjustment for clinical variables, 27 SNPs from 13 genes were associated with the risk of pulmonary complications at a significance level of p < 0.1. Of these, 6 had consistent associations in the replication set at a pooled significance p < 0.05 (Table 2). The pooled adjusted OR was 1.50 (95% CI, 1.15–1.96) for FLT1:rs9319425, 0.62 (95% CI, 0.46 – 0.86) for NRAS:rs10489525, and 0.72 (95% CI, 0.54 – 0.97) for VEGFB:rs2429455 in additive models. The pooled ORs were 0.19 (95% CI, 0.05–0.70) for RAF1: rs713178, 2.18 (95% CI, 1.08 – 4.39) for VEGFC:rs3775195, and 1.84 (95% CI, 1.05–3.21) for FLT4:rs307811 in recessive models. FLT1 and FLT4 code for VEGF receptors. NRAS and RAF1 are genes involved in VEGF signal transduction.
Table 2.
Association of Genotypes in VEGF Pathway With Pulmonary Complications
| Gene | SNP | Chromosome | Model of Inheritance | Discovery Set
|
Replication Set
|
Pooled Analysis
|
|||
|---|---|---|---|---|---|---|---|---|---|
| ORa (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | ||||
| FLT1 | rs9319425 | 13 | Additive | 1.48 (1.01–2.17) | 0.04 | 1.53 (1.05–2.24) | 0.03 | 1.50 (1.15–1.96) | 0.003 |
| NRAS | rs10489525 | 1 | Additive | 0.66 (0.41–1.05) | 0.08 | 0.56 (0.36–0.88) | 0.01 | 0.62 (0.46–0.86) | 0.004 |
| RAF1 | rs713178 | 3 | Recessive | 0.13 (0.02–1.14) | 0.07 | 0.30 (0.06–1.45) | 0.13 | 0.19 (0.05–0.70) | 0.01 |
| VEGFB | rs2429455 | 11 | Additive | 0.68 (0.43–1.05) | 0.08 | 0.78 (0.52–1.16) | 0.21 | 0.72 (0.54–0.97) | 0.03 |
| VEGFC | rs3775195 | 4 | Recessive | 2.57 (1.03–6.40) | 0.04 | 2.00 (0.66–6.05) | 0.22 | 2.18 (1.08–4.39) | 0.03 |
| FLT4 | rs307811 | 5 | Recessive | 2.33 (1.05–5.17) | 0.04 | 1.42 (0.64–3.13) | 0.39 | 1.84 (1.05–3.21) | 0.03 |
OR = odds ratio adjusted for age at operation, sex, intraoperative transfusion, % DLCO predicted, and chest wall resection.
DLCO = diffusing capacity of lung for carbon monoxide; CI = confidence interval; SNP = single nucleotide polymorphism; VEGF = vascular endothelial growth factor.
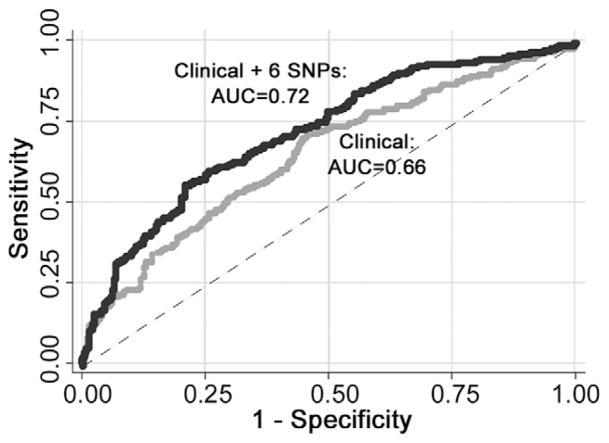
The ability of the model to predict patients with pulmonary complications was assessed by plotting ROC curves using the clinical factors with and without the genotyping factors. The area under the curve (AUC) for the clinical factors only was 0.66, compared with 0.72 with the addition of the genotyping data (Fig 1). This difference in AUCs between the 2 ROC curves remained significant after bootstrap resampling with the calculated 95% CI not including 1 (95% CI, 0.027–0.108). When we excluded patients who underwent thoracoscopic resection, the difference in the AUC was even greater. In this group, the AUC for the clinical factors remained 0.66, compared with 0.75 for the clinical factors with the genotyping data.
Fig 1.
Receiver operating characteristic curves for the ability to predict pulmonary complications with and without the genotype information. (AUC = area under the curve; SNP = single nucleotide polymorphism.)
CART Analysis
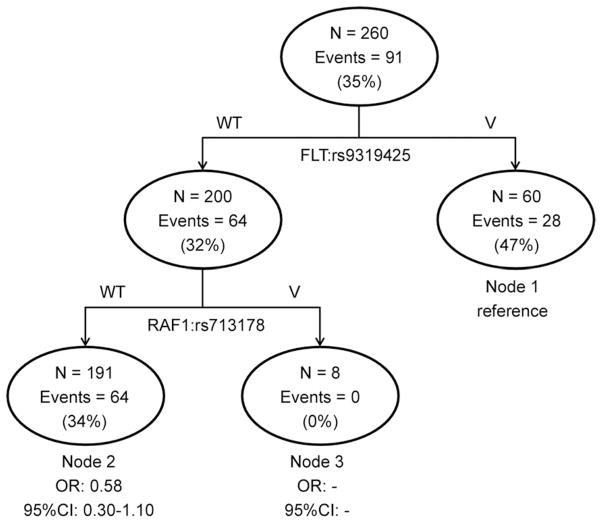
To further explore potential gene-gene interactions among the validated 6 VEGF pathway variants, we performed CART analysis in the discovery population. The analysis identified significant higher order gene-gene interactions for the FLT1:rs9319425 and RAF1:rs713178 polymorphisms as shown in Fig 1. In the highest risk group defined by the FLT1:rs9319425 variant genotype, 28/60 (47%) patients experienced pulmonary complications, compared with 0/8 in the lowest risk group that included patients carrying the common genotype for FLT1:rs9319425 and the variant RAF1:rs713178 genotype (Fig 2). A significant trend was observed for the 3 terminal nodes (p = 0.01). These predicted gene-gene interactions continued to be significant in the replication set (p = 0.02).
Fig 2.
Cumulative and classification and regression tree (CART) analysis of VEGF pathway genetic polymorphisms; p = 0.01. (CI = 95% confidence interval; OR = odds ratio; V = variant genotype; VEGF = vascular endothelial growth factor; WT = wild type.)
Cumulative Effect
The validated 6 SNPs also displayed a significant cumulative effect on the risk of pulmonary complications. As the number of risk genotypes increased in the discovery population, so did the risk of complications (p = 5.77 × 10–6). Those with 4 or 5 risk genotypes had a complication rate of nearly 60% and were at a 9.43 times higher risk (95% CI, 3.05–29.20) compared with patients with a 0 or 1 risk genotype. This significant dose-response relationship was also evident in the replication population (p-trend < 0.01), and in the pooled population (Table 3).
Table 3.
Cumulative Effect of Unfavorable Genotype
| No. of Risk Genotypes | Complication (%) | ORa (95% CI) | p Value |
|---|---|---|---|
| Discovery | |||
| 0–1 | 9 (18.37) | 1 (reference) | |
| 2 | 32 (30.19) | 1.69 (0.69–4.15) | 0.25 |
| 3 | 32 (44.44) | 4.19 (1.64–10.66) | 0.0027 |
| 4–5 | 19 (59.38) | 9.43 (3.05–29.20) | 9.9 × 10−5 |
| p trend | 5.77 × 10−6 | ||
| Replication | |||
| 0–1 | 14 (23.73) | 1 (reference) | |
| 2 | 39 (42.39) | 2.48 (1.06–5.78) | 0.035 |
| 3 | 35 (49.30) | 3.93 (1.67–9.28) | 0.0018 |
| 4–5 | 14 (42.42) | 3.99 (1.43–11.13) | 0.0081 |
| p trend | 0.0017 | ||
| Pooled | |||
| 0–1 | 23 (21.30) | 1 (reference) | |
| 2 | 71 (35.86) | 1.95 (1.07–3.56) | 0.030 |
| 3 | 67 (46.85) | 3.89 (2.09–7.26) | 1.92 × 10−4 |
| 4–5 | 33 (50.77) | 5.76 (2.73–12.16) | 4.44 × 10−6 |
| p trend | 7.64 × 10−8 | ||
OR = odds ratio adjusted for age at operation, sex, intraoperative transfusion, % DLCO predicted, and chest wall resection.
CI = confidence interval; OR = odds ratio.
Comment
A number of clinical factors may be used to assess risk and select patients for pulmonary resection [2–5, 17]. Despite the use of these factors, it is often difficult to predict which patients will have postoperative pulmonary complications. There is growing evidence that genetic factors play a role in pulmonary complications and in lung injury [8, 9, 11, 12]. Our study found that genetic variations in the VEGF pathway were associated with increased risk of pulmonary complications, independent of identified demographic or clinical factors. These results are based on associations found in a discovery set of patients and replicated in another set of patients.
Six variants displayed consistent results in the discovery and replication populations. This includes variants in the genes encoding the 2 VEGF receptors FLT1 and FLT4, as well as 2 VEGF molecules, VEGFB and VEGFC. The FLT1, FLT4, and VEGFC SNPs are located in introns, whereas the VEGFB SNP is located in the 5′ flanking region. The FLT1 gene encodes the FLT1 tyrosine kinase receptor, which binds the VEGFB ligand [18]. FLT1 has been implicated in lung development as well as acute and chronic lung disease [19]. The FLT4 receptor binds the VEGFC ligand. Both have been shown to be expressed in developing lungs [20].
Other significant SNPs include those in NRAS and RAF1, 2 well known cancer-related signaling molecules. The NRAS SNP is located in the 5′ flanking region of the gene, and the RAF1 SNP is located in the 3′ flanking region of its gene. The locations of these SNPs suggest that they mediate NRAS and RAF1 activity through regulatory elements that remain unclear. It is also possible that these variants are in linkage disequilibrium with other unknown SNPs that directly alter the function of these genes. Both molecules are downstream mediators of the VEGF signaling pathway and have been shown to be involved in the development of lung cancer [21, 22]. Their role in lung injury is less well established.
This study did not examine the functional significance of the SNPs or the yet-unidentified potential causal SNPs in linkage with these candidates. Our laboratory is currently exploring the underlying mechanisms by which the genotypes may affect VEGF pathway expression and provide a biological basis for these findings.
Together, these variants significantly improved predictive ability compared with the clinical variable model alone. The ROC of the clinical variables alone results in an AUC of only 0.66, which is relatively poor. However the addition of the genotyping data increases the AUC to 0.72, reaching a level of discrimination that has the potential for clinical translation [23]. An AUC of 0.50 indicates a model no better than random prediction. An AUC of 1 is a perfect model with 100% sensitivity and specificity. Most clinical stratification models have AUCs of 0.70 to 0.85 [24].
Particularly for patients who may have borderline risk, the addition of genotyping data could more accurately stratify risk. Given the relative ease of obtaining genomic DNA and the small number of genotypes involved in this model, this type of genetic testing for risk prediction would be relatively accessible. Currently, the phase III trial ACOSOG (American College of Surgeons Oncology Group) Z4099 is comparing stereotactic body radiotherapy versus sublobar resection for patients with early-stage lung cancer at high risk for undergoing surgical intervention. In the future, the addition of this type of genotyping data for borderline cases may help identify patients better suited for radiotherapy or for surgical treatment.
Furthermore, our results imply a cumulative effect of the risk genotypes, with the risk of pulmonary complications growing substantially with the number of risk genotypes. This highlights the need to understand the entire spectrum of genetic variation in each patient to more accurately predict the risk for pulmonary complications.
CART analysis also found significant gene-gene interactions between 2 of the risk genotypes—FLT1 and RAF1. These 2 molecules function through the pathway to increase pathway activity and propagate VEGF signals. Although they do not directly interact physically, it is biologically plausible that these genetic interactions act synergistically to modulate pulmonary complications. In addition, given the importance of the VEGF pathway in the development of chronic lung disease as well as lung cancer, future studies could explore higher order interactions between genes and clinical variables.
A previous study from our group indicated that genetic variations in inflammatory pathways are associated with postoperative complications after lung resection [9]. Our previous work had a more heterogeneous population, including sublobar resections as well as pneumonectomies. The end point was also more heterogeneous, as it included nonpulmonary complications such as atrial fibrillation. The strengths of this study include a more focused hypothesis specifically examining the VEGF pathway and pulmonary complications, as well as a more homogeneous population. The study design also included a discovery and replication set, helping decrease the possibility of a false-positive finding. However it should be noted that the study population is from a single group from a single institution. Validation of these results from a different institution with a different population is warranted.
Another potential criticism of the study is that the range of complications studied is broad in both cause and severity. However all the complications required interventions and in most cases prolonged hospitalization. Many of the complications overlapped, and some patients had multiple pulmonary complications. We did not have enough patients to perform a subset analysis examining just 1 of the end points, such as prolonged air leak. However we did perform an analysis excluding patients whose only complication was prolonged air leak. This analysis was consistent with the data from the entire dataset, albeit with higher p values because of the fewer number of events (data not shown).
In addition, inflammation or tissue repair, or both, were involved in all the complications. The diverse range of complications is reflective of the broad role of the VEGF pathway as an important regulator of inflammation and response to multiple mechanisms of lung injury, including pulmonary edema, pulmonary hypertension, and acute respiratory distress syndrome [11, 15, 16, 25–27]. In addition, VEGF has been shown to promote physical wound repair in lung epithelial cells, which may be important in healing air leaks [25].
Previous studies suggest that VEGF mediates increased capillary permeability in acute respiratory distress syndrome while also serving a protective role in preserving pulmonary endothelium [28]. Genetic polymorphisms in the VEGF pathway have been found to be associated with increased risk of the development of acute respiratory distress syndrome (ARDS) in ventilated patients. Medford and colleagues [29] found that the VEGF + 936 T allele is associated with a lower level of VEGF in epithelial lining fluid but not in plasma. They speculate that this may lead to increased susceptibility to ARDS. Although we did not find a corresponding effect for this SNP in our data, similar mechanisms may be involved in the pathogenesis of postoperative pulmonary complications.
In summary, our results indicate that genetic variations in the VEGF pathway may impact the risk of pulmonary complications after lung resection. There is a cumulative effect of these variations, with a significantly increased risk for patients with multiple risk genotypes. These findings may help risk stratify patients and provide more accurate assessments of perioperative risk.
Acknowledgments
This work was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Sponsored Program of Research Excellence grant CA70907 and through grant R01CA111646.
Footnotes
Presented at the Forty-eighth Annual Meeting of The Society of Thoracic Surgeons, Fort Lauderdale, FL, Jan 28–Feb 1, 2012.
References
- 1.Graham EA. Successful removal of an entire lung for carcinoma of the bronchus. JAMA. 1933;101:1371–4. doi: 10.1001/jama.251.2.257. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson MK, Lehman AG, Bolliger CT, Brunelli A. The role of diffusing capacity and exercise tests. Thorac Surg Clin. 2008;18:9–17. v. doi: 10.1016/j.thorsurg.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson MK, Reeder LB, Mick R. Optimizing selection of patients for major lung resection. J Thorac Cardiovasc Surg. 1995;109:275–81. doi: 10.1016/S0022-5223(95)70389-6. discussion 281–3. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson MK, Vigneswaran WT. Diffusing capacity predicts morbidity after lung resection in patients without obstructive lung disease. Ann Thorac Surg. 2008;85:1158–64. doi: 10.1016/j.athoracsur.2007.12.071. discussion 1164–5. [DOI] [PubMed] [Google Scholar]
- 5.Vaporciyan AA, Merriman KW, Ece F, et al. Incidence of major pulmonary morbidity after pneumonectomy: association with timing of smoking cessation. Ann Thorac Surg. 2002;73:420–5. doi: 10.1016/s0003-4975(01)03443-9. discussion 425–6. [DOI] [PubMed] [Google Scholar]
- 6.Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg. 2008;86:2008–16. doi: 10.1016/j.athoracsur.2008.07.009. discussion 2016–8. [DOI] [PubMed] [Google Scholar]
- 7.Gaudino M, Di Castelnuovo A, Zamparelli R, et al. Genetic control of postoperative systemic inflammatory reaction and pulmonary and renal complications after coronary artery surgery. J Thorac Cardiovasc Surg. 2003;126:1107–12. doi: 10.1016/s0022-5223(03)00396-9. [DOI] [PubMed] [Google Scholar]
- 8.Lee JM, Lo AC, Yang SY, Tsau HS, Chen RJ, Lee YC. Association of angiotensin-converting enzyme insertion/deletion polymorphism with serum level and development of pulmonary complications following esophagectomy. Ann Surg. 2005;241:659–65. doi: 10.1097/01.sla.0000157132.08833.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw AD, Vaporciyan AA, Wu X, King TM, et al. Inflammatory gene polymorphisms influence risk of postoperative morbidity after lung resection. Ann Thorac Surg. 2005;79:1704–10. doi: 10.1016/j.athoracsur.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Cox RA, Burke AS, Traber DL, Herndon DN, Hawkins HK. Production of pro-inflammatory polypeptides by airway mucous glands and its potential significance. Pulm Pharmacol Ther. 2007;20:172–7. doi: 10.1016/j.pupt.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Medford AR, Ibrahim NB, Millar AB. Vascular endothelial growth factor receptor and coreceptor expression in human acute respiratory distress syndrome. J Crit Care. 2009;24:236–42. doi: 10.1016/j.jcrc.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai R, Gong MN, Zhou W, et al. Genotypes and haplotypes of the VEGF gene are associated with higher mortality and lower VEGF plasma levels in patients with ARDS. Thorax. 2007;62:718–22. doi: 10.1136/thx.2006.069393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahm T, Crisostomo PR, Markel TA, Wang M, Lillemoe KD, Meldrum DR. The critical role of vascular endothelial growth factor in pulmonary vascular remodeling after lung injury. Shock. 2007;28:4–14. doi: 10.1097/shk.0b013e31804d1998. [DOI] [PubMed] [Google Scholar]
- 14.Lee CG, Ma B, Takyar S, et al. Studies of vascular endothelial growth factor in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2011;8:512–5. doi: 10.1513/pats.201102-018MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61:621–6. doi: 10.1136/thx.2005.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuder RM, Chacon M, Alger L, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol. 2001;195:367–74. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 17.Walsh GL, Morice RC, Putnam JB, Jr, et al. Resection of lung cancer is justified in high-risk patients selected by exercise oxygen consumption. Ann Thorac Surg. 1994;58:704–10. doi: 10.1016/0003-4975(94)90731-5. discussion 711. [DOI] [PubMed] [Google Scholar]
- 18.Shibuya M. Structure and dual function of vascular endothelial growth factor receptor-1 (Flt-1) Int J Biochem Cell Biol. 2001;33:409–20. doi: 10.1016/s1357-2725(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 19.Lassus P, Turanlahti M, Heikkila P, et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001;164 (10 Pt 1):1981–7. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- 20.Janer J, Lassus P, Haglund C, Paavonen K, Alitalo K, Andersson S. Pulmonary vascular endothelial growth factor-C in development and lung injury in preterm infants. Am J Respir Crit Care Med. 2006;174:326–30. doi: 10.1164/rccm.200508-1291OC. [DOI] [PubMed] [Google Scholar]
- 21.Reynolds SH, Anna CK, Brown KC, et al. Activated proto-oncogenes in human lung tumors from smokers. Proc Natl Acad Sci U S A. 1991;88:1085–9. doi: 10.1073/pnas.88.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerkhoff E, Fedorov LM, Siefken R, Walter AO, Papadopoulos T, Rapp UR. Lung-targeted expression of the c-Raf-1 kinase in transgenic mice exposes a novel oncogenic character of the wild-type protein. Cell Growth Differ. 2000;11:185–90. [PubMed] [Google Scholar]
- 23.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–6. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 24.Barua A, Handagala SD, Socci L, et al. Accuracy of two scoring systems for risk stratification in thoracic surgery. Interact Cardiovasc Thorac Surg. 2012;14:556–9. doi: 10.1093/icvts/ivs021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts JR, Perkins GD, Fujisawa T, et al. Vascular endothelial growth factor promotes physical wound repair and is anti-apoptotic in primary distal lung epithelial and A549 cells. Crit Care Med. 2007;35:2164–70. doi: 10.1097/01.ccm.0000281451.73202.f6. [DOI] [PubMed] [Google Scholar]
- 26.Voelkel NF, Tuder RM. Hypoxia-induced pulmonary vascular remodeling: a model for what human disease? J Clin Invest. 2000;106:733–8. doi: 10.1172/JCI11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CG, Link H, Baluk P, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abadie Y, Bregeon F, Papazian L, et al. Decreased VEGF concentration in lung tissue and vascular injury during ARDS. Eur Respir J. 2005;25:139–46. doi: 10.1183/09031936.04.00065504. [DOI] [PubMed] [Google Scholar]
- 29.Medford AR, Godinho SI, Keen LJ, Bidwell JL, Millar AB. Relationship between vascular endothelial growth factor + 936 genotype and plasma/epithelial lining fluid vascular endothelial growth factor protein levels in patients with and at risk for ARDS. Chest. 2009;136:457–64. doi: 10.1378/chest.09-0383. [DOI] [PubMed] [Google Scholar]