Abstract
Chemotherapy with BCNU and temozolomide (TMZ) is commonly used for the treatment of glioblastoma multiforme (GBM) and other cancers. In preparation for a clinical gene therapy study in patients with glioblastoma, we wished to study whether these reagents could be used as a reduced-intensity conditioning regimen for autologous transplantation of gene-modified cells. We used an MGMT(P140K)-expressing lentivirus vector to modify dog CD34+ cells and tested in 4 dogs whether these autologous cells engraft and provide chemoprotection after transplantation. Treatment with O6-benzylguanine (O6BG)/TMZ after transplantation resulted in gene marking levels up to 75%, without significant hematopoietic cytopenia, which is consistent with hematopoietic chemoprotection. Retrovirus integration analysis showed that multiple clones contribute to hematopoiesis. These studies demonstrate the ability to achieve stable engraftment of MGMT(P140K)-modified autologous HSCs after a novel reduced-intensity conditioning protocol using a combination of BCNU and TMZ. Furthermore, we show that MGMT(P140K)-HSC engraftment provides chemoprotection during TMZ dose escalation. Clinically, chemoconditioning with BCNU and TMZ should facilitate engraftment of MGMT(P140K)-modified cells while providing anti-tumor activity for patients with poor prognosis glioblastoma or alkylating agent sensitive tumors, thereby supporting dose-intensified chemotherapy regimens.
Keywords: Hematopoietic stem cells, gene therapy, conditioning regimens, cancer, glioblastoma
Introduction
Successful transfer of chemotherapy resistance genes into hematopoietic stem cells (HSCs) holds tremendous therapeutic promise for patients with malignancies by conferring myeloprotection during chemotherapy and allowing for dose-intensified chemotherapy regimens and/or combination chemotherapy to maximize the anti-tumor effect. The level of myeloprotection directly correlates with the level of gene-modified cell engraftment (for review see ref. 1). In order to improve HSC engraftment and chemoprotection, ex vivo gene transfer protocols and conditioning regimens must be optimized. Genetic modification of HSCs with retroviral vectors has progressed to the point at which stable, long-term engraftment of - modified cells and therapeutic levels of transgene expression are routinely achieved in large-animal models2,3 and clinical applications.4–7 Therapeutic gene expression levels are augmented when the cells have an inherent selective survival or growth advantage5–7 or by conditioning with myeloablative doses of radiation or chemical agents such as cyclophosphamide and busulfan.8–11 Under circumstances in which the incoming graft does not have a growth advantage, conditioning with myeloablative radiation or high-dose chemotherapy may be required to achieve a clinically beneficial level of gene marking.
In the setting of genetic disease in which the gene-modified cells provide an alternative or experimental treatment option, an aggressive conditioning regimen is not easily justified despite the potential therapeutic benefit, as it deviates from the traditional standard of care and increases the risk of toxicity to the patient. Candidate patients for HSC gene therapy, who present with serious medical co-morbidities, may not be able to tolerate myeloablative conditioning with DNA damaging agents. Such patients may therefore require a reduced-intensity conditioning regimen to achieve gene-modified cell engraftment. Studies in large animals and in patients demonstrate that reduced-intensity conditioning decreases the severity of myelosuppression and time to hematopoietic recovery.12,13 Although reduced-intensity conditioning with busulfan14 and cyclophosphamide15 extends HSC transplantation to patients who would otherwise be ineligible, novel disease-specific chemical regimens that simultaneously condition for transplantation and have an anti-tumor effect are needed.16 To address this in the context of malignant disease, a more aggressive conditioning regimen with a disease-specific chemotherapeutic is appropriate, provided that the conditioning regimen is tailored to provide both a potent anti-tumor effect and sufficient myelosuppression to support engraftment of gene-modified cells. In preparation for a clinical trial for patients with GBM, the goal of the studies described herein is to test a clinically relevant conditioning regimen in a large-animal model that meets the following criteria: (1) documented HSC toxicity17,18 to facilitate engraftment of MGMT(P140K) gene-modified cells, (2) reduced extramedullary toxicity, and (3) a documented anti-glioma effect.19–22 Temozolomide (TMZ) and BCNU are commonly used to treat GBM, the most common subtype of primary brain tumors in adults and children, but even with aggressive treatment median survival after diagnosis is approximately 12 months.23,24 Chemotherapy with a nitrosourea (BCNU), methylating agents (procarbazine or TMZ), or other agents is effective and can prolong survival. Phase I and II clinical trials have shown that the combination of BCNU and TMZ results in a partial response in tumor regression in patients suffering from glioblastoma, while establishing the maximum tolerated doses as combination neoadjuvant therapy.18,25 However, the benefit of prolonging survival is attenuated by the hematopoietic toxicity of chemotherapeutic agents like BCNU and TMZ, which prevents chemotherapy dose-escalation. In addition, a subset of patients with glioblastoma who exhibit high levels of MGMT expression would greatly benefit from the addition of the wild type MGMT inhibitor O6-benzylguanine (O6BG). Although the addition of O6BG to the alkylating agent regimen has the potential to improve tumor cell killing by BCNU or TMZ, this drug combination exacerbates hematopoietic toxicity.26–28
To assess the extent of chemoprotection provided by drug resistance gene therapy for glioblastoma patients and to alleviate pancytopenia due to combinations of O6BG and TMZ or BCNU, we previously evaluated engraftment and in vivo selection/chemoprotection of MGMT(P140K)-modified HSCs in clinically relevant dog and monkey models. These studies demonstrated that MGMT(P140K)-HSC engraftment leads to effective multilineage in vivo selection and chemoprotection.2,3,29,30 As an extension of these findings, the goal of the current autologous transplantation study in dogs is to evaluate a novel reduced intensity conditioning regimen with BCNU and TMZ tailored for patients with malignant diseases that are known to be responsive to this drug combination, thus facilitating engraftment while also treating the underlying malignancy
MATERIALS AND METHODS
Animals
Dogs were raised and housed at the Fred Hutchinson Cancer Research Center under conditions approved by the American Association for Accreditation of Laboratory Animal Care. Animal experiments were reviewed and approved by the Fred Hutchinson Cancer Research Institutional Animal Care and Use Committee. For CD34 mobilization and priming, dogs were treated with canine stem cell factor (cSCF) (25 µg/kg body weight subcutaneously, once daily) and canine granulocyte-colony stimulating factor (cG-CSF) (5 µg/kg body weight subcutaneously, twice daily) for 4 consecutive days. CD34+ cells were then isolated from leukapheresis or bone marrow products using established methods.
Lentivirus vectors
The self-inactivating (SIN) lentivirus vector WPT-P140K contains the short form of the elongation factor alpha 1 (EF1α) promoter expressing MGMT(P140K) transgene with the wpre (described above), and was kindly provided by Dr. Stanton Gerson. The lentivirus vector RSCEMw2 was generated by excising the EF1α-MGMT(P140K) (EM) cassette from the WPT-P140K backbone with XhoI and ligated upstream of the phosphoglycerate kinase promoter (PGK) yellow fluorescent protein (YFP) cassette, and wpre in the RRL lentivirus backbone (described in ref. 31) that had been linearized with XhoI. After in vitro testing for MGMT(P140K)-mediated selection, the PGK-YFP cassette was excised with XcmI and BsrGI, end-polished with T4 polymerase and ligated. Elimination of the PGK-YFP cassette was confirmed by sequencing. The WPTαΔ-P140K vector was generated by excising the EF1α promoter with ClaI and BamHI, end polishing with T4 polymerase, and ligation of the backbone. Elimination of the EF1α promoter from WPTαΔ-P140K was confirmed by sequencing. Vector stocks were generated by transient transfection, concentrated, and titered, as previously described.31
Dog CD34+ cell isolation and transduction
The method has been described previously.32,33 Briefly, peripheral blood or bone marrow cells were labeled with biotinylated monoclonal antibody 1H6 at 4°C for 30 minutes. The cells were washed twice, incubated with streptavidin-conjugated microbeads for 30 minutes at 4°C, washed again, and then separated by means of an immunomagnetic column technique (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. In the instances where CD34-selected cells were prestimulated (see Table 1), cells were cultured in Isocove’s Modified Dulbecco’s Medium (IMDM) supplemented with 10% fetal bovine serum (FBS) (Gibco BRL, Gaithersburg, MD), 1% penicillin/streptomycin (GIBCO, BRL) in the presence of cG-CSF, cSCF, and human Flt3-ligand, each at 50 ng/mL; CD34+ cells were then exposed to lentivirus vector virus-conditioned media (VCM) at a multiplicity of infection (MOI) indicated in Table 1 in 75 cm2 non-TC treated flasks (Corning, Corning NY) coated with CH-296 (Retronectin; Takara Shuzo, Otsu, Japan) at a concentration of 2 µg/cm2. Culture media is the same noted above with the additional supplement of protamine sulfate [8 µg/ml]. Flow-through cells were stored at 4°C for the duration of the procedure. Following the last exposure to VCM, nonadherent and adherent cells were pooled, counted, and prepared for infusion. Both transduced and non-transduced cells were reinfused intravenously into the original donor (autologous) dog at least 24 hours after BCNU and TMZ administration.
Table 1.
Conditioning chemotherapy, retroviral vectors, transduction conditions and engraftment
| Dog | Conditioning Regimen | Vector | Transduction (total MOI) | Initial Gene Transfer (% by Q- PCR) |
Cells infused × 106 / kg |
Days ANC <100 / µL |
Day of Initial Gene Marking Assessment |
Initial Gene Marking (% by Q- PCR) |
|
|---|---|---|---|---|---|---|---|---|---|
| BCNU (mg/m2) |
TMZ (mg/m2) |
||||||||
| G547 | 50 | 550 | WPT-P140K | 24 hr prestimulation, 4 hr + ON (37×2) | 66.3 | 19.2 | 1 | 41 | 2.8 |
| G834 | 50 | 550 | WPT-P140K | No prestimulation, ON (10) | 12.8 | 1.5 | 4 | 35 | 0.7 |
| G867 | 50 | 550 | WPT-P140K | No prestimulation, ON (10) | 33.5 | 3.8 | 6 | 32 | 1.2 |
| G593 | 30 | 850 | WPT-P140K | 24 hr prestimulation, 4 hr × 2 (25×2) | 62 | 6.2 | 4 | 27 | 2.1 |
| G580 | 30 | 700 | WPT-P140K/ WPTαΔ-P140K | 24a, 48b hr prestimulation, 4 hr × 2 (52×2 / 10×2) | 65 / 45 | 16.5 / 20 | 2 | 46 | 5.5 |
CD34+ cells collected from apheresis product
CD34+ cells collected from bone marrow
“Initial Gene Marking (% by Q-PCR)” is reported from the day of the first post-transplant chemotherapy or the closest time point just prior to treatment, which is the date indicated in column entitled “Day of Initial Gene Marking Assessment”. Dog that never received post-transplant chemotherapy (G580) values are reported from ~3 weeks following transplantation
Abbreviations: ANC = absolute neutrophil count; BCNU = 1,3-bis (2-chloroethyl)-1-nitrosourea; MOI = multiplicity of infection; ON = overnight; Q-PCR = quantitative PCR; TMZ = temozolomide.
Conditioning with BCNU and temozolomide
The maximum tolerated dose (MTD) of BCNU and temozolomide in combination (no pre-treatment with O6BG) is 150 mg/m2 and 550 mg/m2 respectively.18,25 Based on data from our lab and personal communications with Eileen Dolan (University of Chicago) that dogs are about 2–3 times more sensitive to BCNU than humans, we adjusted the conditioning dose of BCNU accordingly. To account for this difference in drug sensitivity, we treated with a chemical conditioning protocol of 30–50 mg/m2 BCNU and 550–850 mg/m2 temozolomide as outlined in Table 1. As preparation for transplantation, the animals received a single dose of BCNU (Bristol-Myers Squibb Co., Princeton, NJ) and TMZ (Schering-Plough, Kenilworth, NJ) (Table 1). BCNU was diluted according to the manufacturer’s instructions and was further diluted in normal saline to a total volume of 18 mL and infused over approximately four minutes, followed two hours later by PO administration of TMZ. Post-transplantation immunosuppression which is administered in order to prevent a transgene-specific immune reaction, consisted of cyclosporine (CSP), 15 mg/kg orally, twice daily, and mycophenolate mofetil (MMF) (10 mg/kg, subcutaneously, twice daily) to block T lymphocyte activation and proliferation, respectively.
Quantitative PCR (TaqMan)
Relative gene marking levels with the vector in total bone marrow and white blood cells were analyzed with the TaqMan 5´ nuclease quantitative real-time (Q)-PCR assay. The gene marking percentages were calculated based on the assumption that the marrow and peripheral blood cells contain one copy of the corresponding vector per cell. Genomic DNA (300 ng) from each dog was amplified in duplicate with a lentivirus-specific primer/probe combination (5’-TGA AAG CGA AAG GGA AAC CA-3’; 5’-CCG TGC GCG CTT CAG-3’; probe: AGC TCT CTC GAC GCA GGA CTC GGC-3’). A dog interleukin 3 (IL-3)-specific primer/probe combination (5´-ATG AGC AGC TTC CCC ATC C-3´; 5´-GTC GAA AAA GGC CTC CCC-3´; probe: 5´-FAM-TCC TGC TTG GAT GCC AAG TCC CAC-TAMRA-3´) was used to adjust for equal loading of genomic DNA per reaction. Standards consisted of dilutions of DNA extracted from cell lines transduced with a single copy of a lentiviral vector and DNA from a normal dog. Samples were mixed with ABI master mix (Applied Biosystems, Branchburg. NJ) and reactions run on the ABI Prism 7500 sequence detection systems (Applied Biosystems) under the following thermal cycling conditions: 50°C for 2 minutes and 95°C for 10 minutes, then 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Following Q-PCR samples were plotted against the cell line standards and the percentage of gene marking was calculated from provirus copy number making the assumption that each clone has only a single provirus copy, so as an example, 0.1 provirus copies equals 10% gene marking.
Post-transplantation O6BG/temozolomide treatment
Fifty milligrams of O6BG (Sigma Aldrich, St Louis, MO) was dissolved in 30 ml of 40% polyethylene glycol in phosphate buffered saline (PBS), and the concentration was adjusted to 1 mg/ml with prewarmed (37°C) PBS. The drug was further diluted in normal saline to a final volume of 150 mL and was infused over 15 minutes. BCNU (Bristol-Myers Squibb Co., Princeton, NJ) was diluted according to the manufacturer’s instructions and was further diluted in normal saline to a total volume of 18 mL. Immediately after administration of O6BG temozolomide was given PO at the appropriate dose based on animal surface area calculations. The O6BG dose was fixed throughout the course of the study at 5 mg/kg, with a maximum of 50 mg per administration. TMZ doses ranged between 600 and 700 mg/m2.
Colony forming unit assay
Bone marrow for colony-forming units (CFUs) was isolated, grown in 2-layer soft agar, and DNA was isolated from individual CFUs as previously described.3
CFU retrovirus-specific PCR
To determine the level of gene marking in bone marrow-derived CFUs, DNA was amplified to detect provirus sequences and overall DNA as previously described.3
Linear amplification-mediated PCR
Retrovirus integration site (RIS) analysis by linear amplification-mediated (LAM)-PCR was performed on dog DNA isolated from peripheral blood leukocytes (PBLs) as previously described.3
Results
Reduced-intensity conditioning with BCNU and temozolomide is well-tolerated in dogs
Both BCNU and TMZ are extensively used in the treatment of patients with brain tumors and BCNU is also widely used for other malignancies like lymphoma. Thus, in preparation for a clinical study using chemoprotected hematopoietic cells in patients with glioblastoma, the goal of these studies is to examine whether the combination of BCNU and TMZ would allow for efficient engraftment of gene-modified cells. In this study, we show that a novel reduced-intensity conditioning regimen with BCNU and TMZ is well tolerated in the clinically relevant dog model of gene-modified drug resistant autologous HSC transplantation. Based on published clinical data in patients with glioblastoma,18,25 we evaluated the combination of BCNU, followed by TMZ for pre-transplant conditioning. The maximum tolerated dose (MTD) of BCNU and TMZ in combination (no pre-treatment with O6BG) is 150 mg/m2 and 550 mg/m2, respectively.18,25 Given that dogs exhibit 2 to 3-fold-higher chemosensitivity to alkylating agents compared to human patients (unpublished data and personal communications with Eileen Dolan), we adjusted the dose of BCNU to reflect these differences. Before transplantation, dogs received single treatments of intravenous (IV) BCNU (30–50 mg/m2) and oral (PO) TMZ (550–850 mg/m2). Dogs received G-CSF (5 µg/kg IV twice a day BID) for up to 18 days to facilitate neutrophil recovery after gene-modified HSC transplantation. Neutrophil and platelet nadirs occurred within 7 and 16 days post-transplantation, respectively (Table 1, Figure 1). In all dogs, following hematopoietic recovery directly after autologous transplantation, all peripheral blood counts remained within the normal range, apart from transient decreases in complete blood cell counts (CBCs) during post-transplantation chemotherapy. Aside from mild transaminitis following post-transplantation chemotherapy that resolved without intervention no significant extramedullary toxicity was observed. After chemoconditioning, all dogs received gene-modified cells that were cultured ex vivo for less than 48 hours, in order to maximize engraftment potential.
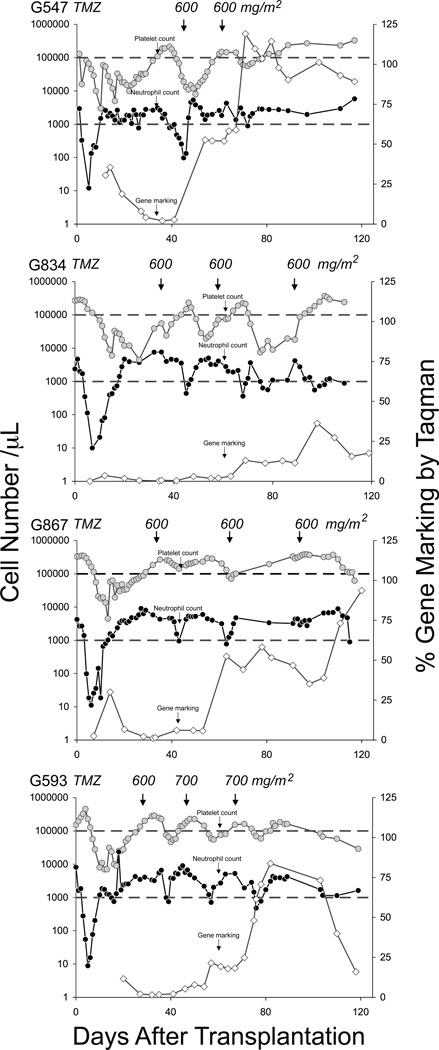
Figure 1. Hematopoietic reconstitution, gene marking and in vivo selection in dogs after reduced intensity alkylating-based conditioning and transplantation with MGMT(P140K) gene-modified cells.
Absolute platelet ( ) and neutrophil (
) and neutrophil ( ) counts in all dogs transplanted with autologous gene-modified cells after preconditioning chemotherapy. Small black arrows denote post-transplantation O6BG and temozolomide (TMZ) or BCNU administration. Upper and lower gray horizontal lines correspond to lower threshold of platelet counts and absolute neutrophil counts, respectively. In vivo selection of MGMT(P140K) gene marking (
) counts in all dogs transplanted with autologous gene-modified cells after preconditioning chemotherapy. Small black arrows denote post-transplantation O6BG and temozolomide (TMZ) or BCNU administration. Upper and lower gray horizontal lines correspond to lower threshold of platelet counts and absolute neutrophil counts, respectively. In vivo selection of MGMT(P140K) gene marking ( ) in white blood cells (WBCs) during the first 120 days after transplantation as determined by qPCR analysis. Small black vertical arrows denote chemotherapy administration.
) in white blood cells (WBCs) during the first 120 days after transplantation as determined by qPCR analysis. Small black vertical arrows denote chemotherapy administration.
Sustained in vivo selection and chemoprotection after stable engraftment and repopulation of MGMT(P140K)-modified hematopoietic cells
In this study, we wanted to determine whether MGMT(P140K) gene-modified hematopoietic cells infused after reduced-intensity conditioning support in vivo selection and provide protection from post-transplantation chemotherapy. Following transplantation and once peripheral blood counts recovered to levels within the normal range (ANC>1,000 cells/µL, PLT>100,000 cells/µL), dogs were treated with two to four cycles of O6BG (5 mg/kg) and TMZ (600–700 mg/m2). Despite transient decreases in peripheral blood counts following each chemotherapy cycle, the dogs showed marking-dependent chemoprotection from TMZ-induced myelotoxicity (Figure 1). All dogs exhibited long-term gene marking which supported in vivo selection after post-transplantation treatment with O6BG/TMZ (Table 1, Figure 1). Q-PCR analysis for detection of proviral integrants showed that multiple cycles of O6BG plus TMZ increased gene marking 15 to 250-fold, compared to gene marking observed in white blood cells before chemotherapy (Figure 1, Figure 2A). To verify high gene marking observed in white blood cells from dog G547, bone marrow was also collected, CFUs plated and then provirus-specific PCR performed on individual bone marrow-derived CFUs on day 90 after transplantation and two cycles of O6BG/TMZ. The high level gene marking in peripheral blood was confirmed in bone marrow samples, with 71% of the CFUs scored as provirus positive. This level of gene marking is similar to that calculated using Q-PCR at the same time point, 90.4%, and indicates ~1.3 provirus copies per clone.
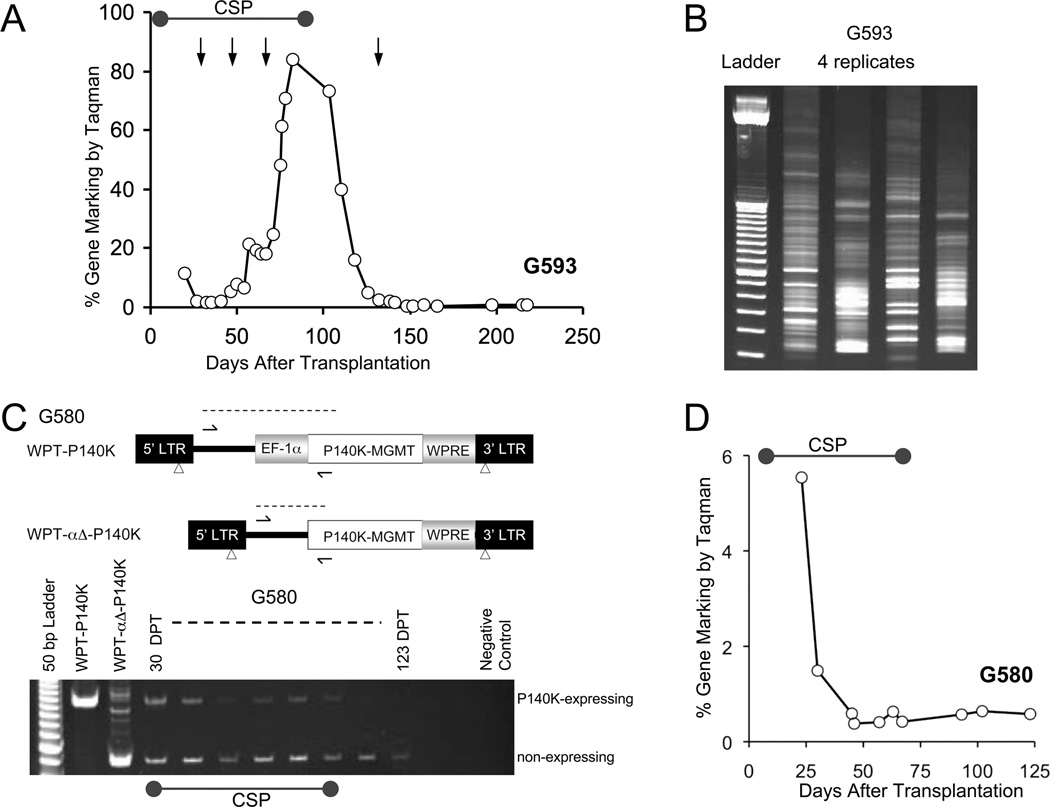
Figure 2. Loss of MGMT(P140K)-gene modified cells following withdrawal of immunosuppression.
(A) Progressive decline in gene marking in white blood cells of G593 after cessation of cyclosporine (100 days post-transplantation) and despite multiple treatments with O6BG and temozolomide (TMZ). (B) Representative gel of retroviral insertion sites (RIS) amplified by LAM-PCR from white blood cells from a single time point in dog G593 before cyclosporine was discontinued. (C) Upper panel: Schematic for SIN lentivirus vectors used for transduction and competitive repopulation of dog G580, which are identical except for deletion of the EF-1α promoter in WPTαΔ-P140K. Arrows denote PCR primers that distinguish between the different proviral integrants based on alternate product sizes (dashed line). Lower panel: Ethidium bromide stained gel of differential PCR products in dog G580. Left-Right: 50 bp ladder, PCR products from HT1080 cells transduced with WPT-P140K or WPTαΔ-P140K, and white blood cells taken from dog G580 at multiple days post-transplantation (DPT). (D) Total lentivirus-specific gene marking by in G580. LAM-PCR = linear amplification mediated PCR.
While gene marking in dogs G547 and G867 increased to levels approaching 100% after 2–3 treatments with O6BG and TMZ, G547 was also chemoprotected from myelotoxicity, as platelet and neutrophil counts did not fall significantly below 100,000 cells/µL and 1,000 cells/µL after chemotherapy treatment, respectively (Figure 1 black vertical arrow). In addition, while a single treatment of O6BG/TMZ led to a transient decrease in neutrophil and platelet counts, gene marking increased from 2.8% to 58.3%. When the dog was treated with a second cycle of an identical dose of O6BG/TMZ after an increase in gene-modified cells, no significant myelosuppression was observed (Figure 1, dog G547). These data provide evidence to suggest that a single treatment of O6BG/TMZ may support in vivo selection to increase the proportion of chemoresistant hematopoietic cells, which then provide bona fide chemoprotection to the transplant recipient. Furthermore, in vivo selection and chemoprotection were achieved within the context of CD34+ cell transplantation following nonmyeloablative reduced intensity conditioning with BCNU and TMZ. LAM-PCR analysis of retroviral insertion sites confirmed polyclonal repopulation was achieved in all dogs analyzed that received reduced-intensity conditioning and gene-modified hematopoietic cell transplantation (Figure 2B and data not shown).
Differential engraftment of promoterless and transcriptionally regulated human MGMT(P140K) gene-modified cells
Long-term engraftment of gene-modified cells was achieved in 3 of the 4 animals transplanted with MGMT(P140K)-transduced CD34+ cells (Figure 1 and data not shown). However, gene marking in dog G593 decreased after cessation of cyclosporine treatment on day 100 (Figure 2A). Although LAM-PCR results revealed multiple clone repopulation prior to day 100, a stable selective effect was not achieved in vivo, despite multiple cycles of O6BG and TMZ (Figure 2B). To determine whether the loss of gene-modified cells could be influenced by an immune response to the human variant of MGMT(P140K) transgene expression, a competitive repopulation assay was established in dog G580. G580 was infused with CD34+ transduced with a lentivirus vector containing human MGMT(P140K) with (WPT-P140K) or without (WPT-αΔ-P140K) the human EF-1α promoter (Figure 2C). Differential PCR analysis, which amplifies the region flanking the EF-1α promoter, showed that detection of the PCR product corresponding to MGMT(P140K) promoter-containing and promoterless proviruses was qualitatively similar in both arms 30 DPT. The PCR product specific for the promoterless MGMT(P140K) provirus was detectable out to 123 DPT, despite cyclosporine cessation. In contrast, the WPT-P140K PCR product band intensity decreased over time and was not detectable after cyclosporine removal. Consistent with the PCR data, MGMT(P140K) marking by Q-PCR, which detects both MGMT-expressing and non-expressing populations, decreased gradually during the first two months after transplantation, stabilizing around 1% (Figure 2D and Supplemental Figure 1). These data imply that following reduced-intensity transplantation, expression of the human MGMT(P140K) in dogs may lead to loss of the gene-modified cells over time, possibly due to other factors including failure to transduce long-term repopulating HSCs, toxicity associated with transgene overexpression, failure of highly marked HSCs to differentiate or an immune response to gene-modified cells. Based on our ongoing studies in patients with glioblastoma, we do not believe that there are immune responses to the human MGMTP140K in patients.
Discussion
Here we show that a reduced-intensity conditioning regimen based on BCNU and TMZ was well tolerated and allowed for efficient engraftment of autologous hematopoietic repopulating cells genetically modified with a lentivirus vector to express MGMT(P140K). We show in this clinically relevant dog model that multiple clones engrafted and contributed to hematopoiesis and that gene-modified cell engraftment resulted in multilineage chemoprotection proportional to the level of gene marking.
Historically, conditioning regimens for patients enrolled in clinical trials using gene-modified hematopoietic cells have been tailored to minimize myelosuppression to reduce the risk to the patient. Therapeutic benefit in these clinical trials has been aided by at least some modest lineage-specific selective growth advantage of the gene-modified cells (for review see ref. 34) following conditioning with reduced-intensity busulfan for ADA-SCID,35 CGD,6 and WAS36 or without any conditioning for SCID-X1.7 More recently, a successful clinical trial using gene-modified hematopoietic cells for adrenoleukodystrophy (ALD) used a more aggressive ablative conditioning regimen consisting of cyclophosphamide and busulfan because a relative high level of gene marking was required for therapeutic benefit, and the gene-modified cells do not have an intrinsic growth advantage.4 This latter example is a more appropriate comparison for therapeutic chemoprotection of gene-modified hematopoietic cells in the context of malignant disease. Collectively, these gene therapy clinical trials highlight the importance of tailoring a conditioning regimen and gene transfer strategy that fit best with the patient’s disease status and the desired therapeutic risk/benefit ratio. Although appropriate for gene correction studies, the lack of documented anti-tumor affects associated with busulfan and cyclophosphamide may not be clinically relevant for drug resistance gene therapy applications in the context of malignancies. Here, we took this into consideration and developed a reduced-intensity conditioning regimen that would also provide a disease-specific anti-tumor effect for patients with malignant disease where alkylating agents (i.e. BCNU and TMZ) would be standard of care chemotherapy.
The primary goal of chemotherapy resistance gene therapy within the context of patients suffering from malignant disease is to protect the hematopoietic system from chemotherapy-associated dose-limiting hematologic toxicity to allow for increased dose intensity and maximize anti-tumor effect. In order to achieve this goal, each chemosensitive solid tumor malignancy must be evaluated within the framework of the specific chemotherapy resistance genes and treatment regimens. We focused on evaluating a reduced-intensity conditioning regimen to support anti-tumor activity and engraftment of drug-resistance gene-modified hematopoietic cells directed for the treatment of glioblastoma multiforme. To this end, dogs were conditioned with a combination of TMZ and BCNU and subsequently transplanted with MGMT(P140K) gene-modified cells, delivered by a lentiviral vector. The reduced-intensity conditioning was well tolerated and supported long-term engraftment of gene-modified cells and supported in vivo selection that provided progressively more durable chemoprotection from subsequent doses of O6BG/TMZ combination chemotherapy. Effective engraftment of MGMT(P140K) gene-modified cells that led to successful in vivo selection and chemoprotection was achieved with a relatively short ex vivo transduction protocol using lentivirus vectors. This could be due to a variety of factors included but not limited to improved maintenance of HSC repopulation capacity, improved initial HSC gene transfer levels, or a combination of both.
Relative to historical control dogs, the experimental dogs in this study that received MGMT(P140K) lentivirus transduced cells were protected from pronounced myelosuppression attributed to post-transplant chemotherapy when gene marking levels were greater than 40%, as the extent of chemoprotection is directly proportional to the level of gene marking. Although the initial gene marking level is lower compared to animals the received myeloablative preconditioning, we are able to approach the higher gene marking levels through in vivo selection. Importantly, LAM-PCR analysis confirmed repopulation of the hematopoietic system by multiple clones. We also found loss of expression of the human variant of MGMT(P140K), in some dogs coincided with cessation of immunosuppressive therapy (cyclosporine), which indicates loss of the gene-modified cell population. Although we cannot conclusively attribute loss of the gene-modified graft to an immune response against the human transgene, it is important to consider use of species-specific, optimized transgenes and vectors to improve the likelihood of long-term stable in vivo gene expression.
In addition, these studies provide an important proof-of-concept platform for identifying appropriate reduced-intensity conditioning regimens coupled to specific drug resistance gene transfer for the treatment of other malignancies. This model may be translated to improve outcome in patients with hematopoietic malignancies following relapse, by coupling conditioning with high-dose cytosine arabinoside (Ara-C) to infusion of bone marrow cells modified to express cytidine deaminase,37 thereby combining chemoprotection of allogeneic cells from post-transplant Ara-C with a graft-versus-leukemia/lymphoma effect.38 With respect to multidrug resistance gene-1 (MDR1) gene transfer, chemotherapy regimens (vinblastine, colchicine, doxorubicin, paclitaxel) traditionally used to treat MDR1-expressing chemoresistant solid tumors (i.e. neuroblastoma, ovarian, lung and mammary cell carcinoma),39–43 may be evaluated in the dog model with reduced-intensity conditioning to facilitate engraftment of chemoresistant MDR1-modified HSCs. Finally, the antifolate resistance gene variant, dihydrofolate reductase (DHFR[L22Y]), may be more useful in the context of transient expansion of DHFR(L22Y)-modified allogeneic T-cells and in the elimination of unmodified T lymphocyte populations to facilitate engraftment of allogeneic HSCs early after transplantation.
In conclusion, these results demonstrate that reduced-intensity BCNU/TMZ conditioning coupled to lentivirus-mediated gene transfer of MGMT(P140K) into autologous HSCs supports in vivo selection, O6BG/TMZ post-transplant dose-escalation which may be translated to a clinical setting in support more aggressive treatment, and possibly extend survival in patients suffering from glioblastoma multiforme. Furthermore, the lessons learned from this model of reduced-intensity chemical conditioning and drug resistance gene transfer may be extrapolated for use in other drug resistance chemotherapy platforms, for the treatment of other malignancies and for potential use in alternative therapeutic applications.
Acknowledgements
We thank Helen Crawford, Laura Farren, and Bonnie Larson for help in preparing this manuscript. We also thank Michele Spector and the Fred Hutchinson Cancer Research Center Dog Lab staff for the care of the dogs. This work was supported in part by National Institutes of Health (Bethesda, MD) grants P01HL036444, R01HL074162, and P30DK056465. H.P.K. is a Markey Molecular Medicine Investigator and the recipient of the Jose Carreras/E.E Thomas Endowed Chair for Cancer Research.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
Conflict of interest
The authors declare no competing financial interests.
References
- 1.Neff T, Beard BC, Kiem H-P. Survival of the fittest: in vivo selection and stem cell gene therapy (Review) Blood. 2006;107:1751–1760. doi: 10.1182/blood-2005-06-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beard BC, Trobridge GD, Ironside C, McCune JS, Adair JE, Kiem H-P. Efficient and stable MGMT-mediated selection of long-term repopulating stem cells in nonhuman primates. J Clin Invest. 2010;120:2345–2354. doi: 10.1172/JCI40767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beard BC, Sud R, Keyser KA, Ironside C, Neff T, Gerull S, et al. Long-term polyclonal and multilineage engraftment of methylguanine methyltransferase P140K gene-modified dog hematopoietic cells in primary and secondary recipients. Blood. 2009;113:5094–5103. doi: 10.1182/blood-2008-09-176412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 5.Aiuti A, Cassani B, Andolfi G, Mirolo M, Biasco L, Recchia A, et al. Multilineage hematopoietic reconstitution without clonal selection in ADA-SCID patients treated with stem cell gene therapy. J Clin Invest. 2007;117:2233–2240. doi: 10.1172/JCI31666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- 7.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 8.Andion M, Molina B, Gonzalez-Vicent M, Alonso L, Hernandez C, Lassaletta A, et al. High-dose busulfan and cyclophosphamide as a conditioning regimen for autologous peripheral blood stem cell transplantation in childhood non-Hodgkin lymphoma patients: a long-term follow-up study. J Pediatr Hematol Oncol. 2011;33:e89–e91. doi: 10.1097/MPH.0b013e3181fd6c79. [DOI] [PubMed] [Google Scholar]
- 9.Gozdzik J, Pituch-Noworolska A, Skoczen S, Czogala W, Baran J, Krasowska-Kwiecien A, et al. Allogeneic haematopoietic stem cell transplantation as therapy for chronic granulomatous disease-single centre experience. J Clin Immunol. 2011;31:332–337. doi: 10.1007/s10875-011-9513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escalon MP, Stefanovic A, Venkatraman A, Pereira D, Santos ES, Goodman M, et al. Autologous transplantation for relapsed non-Hodgkin's lymphoma using intravenous busulfan and cyclophosphamide as conditioning regimen: a single center experience. Bone Marrow Transplant. 2009;44:89–96. doi: 10.1038/bmt.2008.429. [DOI] [PubMed] [Google Scholar]
- 11.McCune JS, Batchelder A, Deeg HJ, Gooley T, Cole S, Phillips B, et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant. 2007;13:853–862. doi: 10.1016/j.bbmt.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Gerull S, Beard BC, Peterson LJ, Neff T, Kiem H-P. In vivo selection and chemoprotection after drug resistance gene therapy in a nonmyeloablative allogeneic transplantation setting in dogs. Hum Gene Ther. 2007;18:451–456. doi: 10.1089/hum.2006.039. [DOI] [PubMed] [Google Scholar]
- 13.Cancrini C, Ferrua F, Scarselli A, Brigida I, Romiti ML, Barera G, et al. Role of reduced intensity conditioning in T-cell and B-cell immune reconstitution after HLA-identical bone marrow transplantation in ADA-SCID. Haematologica. 2010;95:1778–1782. doi: 10.3324/haematol.2010.025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bashey A, Owzar K, Johnson JL, Edwards PS, Kelly M, Baxter-Lowe LA, et al. Reduced-intensity conditioning allogeneic hematopoietic cell transplantation for patients with hematologic malignancies who relapse following autologous transplantation: a multi-institutional prospective study from the Cancer and Leukemia Group B (CALGB trial 100002) Biol Blood Marrow Transplant. 2011;17:558–565. doi: 10.1016/j.bbmt.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shea T, Johnson J, Westervelt P, Farag S, McCarty J, Bashey A, et al. Reduced-intensity allogeneic transplantation provides high event-free and overall survival in patients with advanced indolent B cell malignancies: CALGB 109901. Biol Blood Marrow Transplant. doi: 10.1016/j.bbmt.2011.01.016. 9999; prepublished online February 3, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho AYL, Pagliuca A, Kenyon M, Parker JE, Mijovic A, Devereux S, et al. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan and alemtuzumab (FBC) conditioning. Blood. 2004;104:1616–1623. doi: 10.1182/blood-2003-12-4207. [DOI] [PubMed] [Google Scholar]
- 17.Quinn JA, Pluda J, Dolan ME, Delaney S, Kaplan R, Rich JN, et al. Phase II trial of carmustine plus O(6)-benzylguanine for patients with nitrosourea-resistant recurrent or progressive malignant glioma. J Clin Oncol. 2002;20:2277–2283. doi: 10.1200/JCO.2002.09.084. [DOI] [PubMed] [Google Scholar]
- 18.Schold SCJ, Kuhn JG, Chang SM, Bosik ME, Robins HI, Mehta MP, et al. A phase I trial of 1,3-bis(2-chloroethyl)-1-nitrosourea plus temozolomide: a North American Brain Tumor Consortium study. Neuro-oncol. 2000;2:34–39. doi: 10.1093/neuonc/2.1.34. [erratum appears in Neuro-oncol 2001 Apr;3(2)123] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vinjamuri M, Adumala RR, Altaha R, Hobbs GR, Crowell EB., Jr Comparative analysis of temozolomide (TMZ) versus 1,3-bis (2-chloroethyl)-1 nitrosourea (BCNU) in newly diagnosed glioblastoma multiforme (GBM) patients. J Neurooncol. 2009;91:221–225. doi: 10.1007/s11060-008-9702-6. [DOI] [PubMed] [Google Scholar]
- 20.Nishikawa R. Standard therapy for glioblastoma--a review of where we are. Neurologia Medico-Chirurgica. 2010;50:713–719. doi: 10.2176/nmc.50.713. [DOI] [PubMed] [Google Scholar]
- 21.Nieder C, Grosu AL, Molls M. A comparison of treatment results for recurrent malignant gliomas (Review) Cancer Treat Rev. 2000;26:397–409. doi: 10.1053/ctrv.2000.0191. [DOI] [PubMed] [Google Scholar]
- 22.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. Journal of Neurosurgery. 2009;110:583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrie M, Couprie C, Dufour H, Figarella-Branger D, Muracciole X, Hoang-Xuan K, et al. Temozolomide in combination with BCNU before and after radiotherapy in patients with inoperable newly diagnosed glioblastoma multiforme. Ann Oncol. 2005;16:1177–1184. doi: 10.1093/annonc/mdi225. [DOI] [PubMed] [Google Scholar]
- 24.Chang SM, Butowski NA, Sneed PK, Garner IV. Standard treatment and experimental targeted drug therapy for recurrent glioblastoma multiforme (Review) Neurosurgical Focus. 2006;20:E4. [PubMed] [Google Scholar]
- 25.Chang SM, Prados MD, Yung WK, Fine H, Junck L, Greenberg H, et al. Phase II study of neoadjuvant 1, 3-bis (2-chloroethyl)-1-nitrosourea and temozolomide for newly diagnosed anaplastic glioma: a North American Brain Tumor Consortium Trial. Cancer. 2004;100:1712–1716. doi: 10.1002/cncr.20157. [DOI] [PubMed] [Google Scholar]
- 26.Chinnasamy N, Rafferty JA, Hickson I, Ashby J, Tinwell H, Margison GP, et al. O6-benzylguanine potentiates the in vivo toxicity and clastogenicity of temozolomide and BCNU in mouse bone marrow. Blood. 1997;89:1566–1573. [PubMed] [Google Scholar]
- 27.Westerhof GR, Down JD, Blokland I, Wood M, Boudewijn A, Watson AJ, et al. O6-Benzylguanine potentiates BCNU but not busulfan toxicity in hematopoietic stem cells. Exp Hematol. 2001;29:633–638. doi: 10.1016/s0301-472x(01)00631-2. [DOI] [PubMed] [Google Scholar]
- 28.Friedman HS, Keir S, Pegg AE, Houghton PJ, Colvin OM, Moschel RC, et al. O6-benzylguanine-mediated enhancement of chemotherapy. Molecular Cancer Therapeutics. 2002;1:943–948. [PubMed] [Google Scholar]
- 29.Neff T, Horn PA, Peterson LJ, Thomasson BM, Thompson J, Williams DA, et al. Methylguanine methyltransferase-mediated in vivo selection and chemoprotection of allogeneic stem cells in a large-animal model. J Clin Invest. 2003;112:1581–1588. doi: 10.1172/JCI18782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neff T, Beard BC, Peterson LJ, Anandakumar P, Thompson J, Kiem H-P. Polyclonal chemoprotection against temozolomide in a large-animal model of drug resistance gene therapy. Blood. 2005;105:997–1002. doi: 10.1182/blood-2004-08-3169. [DOI] [PubMed] [Google Scholar]
- 31.Horn PA, Keyser KA, Peterson LJ, Neff T, Thomasson BM, Thompson J, et al. Efficient lentiviral gene transfer to canine repopulating cells using an overnight transduction protocol. Blood. 2004;103:3710–3716. doi: 10.1182/blood-2003-07-2414. [DOI] [PubMed] [Google Scholar]
- 32.Goerner M, Horn PA, Peterson L, Kurre P, Storb R, Rasko JEJ, et al. Sustained multilineage gene persistence and expression in dogs transplanted with CD34+ marrow cells transduced by RD114-pseudotype oncoretrovirus vectors. Blood. 2001;98:2065–2070. doi: 10.1182/blood.v98.7.2065. [DOI] [PubMed] [Google Scholar]
- 33.Neff T, Horn PA, Valli VE, Gown AM, Wardwell S, Wood BL, et al. Pharmacologically regulated in vivo selection in a large animal. Blood. 2002;100:2026–2031. doi: 10.1182/blood-2002-03-0792. [DOI] [PubMed] [Google Scholar]
- 34.Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M. Gene therapy for primary adaptive immune deficiencies. J Allergy Clin Immunol. 2011;127:1356–1359. doi: 10.1016/j.jaci.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 36.Boztug K, Schmidt M, Schwarzer A, Banerjee PP, Avedillo I, Dewey RA, et al. Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N Engl J Med. 2010;363:1918–1927. doi: 10.1056/NEJMoa1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rattmann I, Kleff V, Sorg UR, Bardenheuer W, Brueckner A, Hilger RA, et al. Gene transfer of cytidine deaminase protects myelopoiesis from cytidine analogs in an in vivo murine transplant model. Blood. 2006;108:2965–2971. doi: 10.1182/blood-2006-03-011734. [DOI] [PubMed] [Google Scholar]
- 38.Budak-Alpdogan T, Alpdogan O, Banerjee D, Wang E, Moore MA, Bertino JR. Methotrexate and cytarabine inhibit progression of human lymphoma in NOD/SCID mice carrying a mutant dihydrofolate reductase and cytidine deaminase fusion gene. Molecular Therapy. 2004;10:574–584. doi: 10.1016/j.ymthe.2004.06.115. [DOI] [PubMed] [Google Scholar]
- 39.de Cremoux P, Jourdan-Da-Silva N, Couturier J, Tran-Perennou C, Schleiermacher G, Fehlbaum P, et al. Role of chemotherapy resistance genes in outcome of neuroblastoma. Pediatric Blood and Cancer. 2007;48:311–317. doi: 10.1002/pbc.20853. [DOI] [PubMed] [Google Scholar]
- 40.Martin LP, Schilder RJ. Management of recurrent ovarian carcinoma: current status and future directions (Review) Semin Oncol. 2009;36:112–125. doi: 10.1053/j.seminoncol.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Stewart DJ. Tumor and host factors that may limit efficacy of chemotherapy in non-small cell and small cell lung cancer (Review) Critical Reviews in Oncology-Hematology. 2010;75:173–234. doi: 10.1016/j.critrevonc.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noguchi S. Predictive factors for response to docetaxel in human breast cancers (Review) Cancer Science. 2006;97:813–820. doi: 10.1111/j.1349-7006.2006.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer (Review) Biochim Biophys Acta. 2008;1785:96–132. doi: 10.1016/j.bbcan.2007.10.004. [DOI] [PubMed] [Google Scholar]