Over the past 15 years in our clinic, there have been significant improvements in the use of antiretroviral therapy and in the laboratory and clinical responses to therapy. These benefits have similarly affected our patients regardless of sex, race, and human immunodeficiency virus transmission risk group.
Abstract
Background. Despite advances in human immunodeficiency virus (HIV) treatment, major challenges remain in achieving access, retention, and adherence. Our inner-city HIV clinical practice in Baltimore has a diverse patient population with high rates of poverty, black race, and injection drug use (IDU), providing us the opportunity to compare health process and outcomes.
Methods. Using data collected in a clinical HIV cohort in Baltimore, we compared receipt of combination antiretroviral therapy (ART), HIV type 1 (HIV-1) RNA, CD4, incidence of opportunistic illness, and mortality from 1995 to 2010. Comparisons were made of these outcomes by HIV risk group, sex, and race (black, white).
Results. From 1995 to 2010, we followed 6366 patients comprising 27 941 person-years (PY) of follow-up. By 2010, 87% of patients were receiving ART; median HIV-1 RNA was <200 copies/mL, median CD4 was 475 cells/mm3, opportunistic illness rates were 2.4 per 100 PY, and mortality rates were 2.1 per 100 PY, with no differences by demographic or HIV risk group. The only differences were that the IDU risk group had a median CD4 that was 79 cells/mm3 lower and HIV-1 RNA 0.16 log10 copies/mL higher compared with other risk groups (P < .01). In 2009 a 28-year-old HIV-infected person was estimated to have 45.4 years of life remaining, which did not differ by demographic or behavioral risk group.
Discussion. Our results emphasize that advances in HIV treatment have had a positive impact on all affected demographic and behavioral risk groups in an HIV clinical setting, with an expected longevity for HIV-infected patients that is now 73 years.
(See the Editorial Commentary by Saag on pages 1252–4.)
Since 1995, the care of human immunodeficiency virus (HIV)–infected persons in the United States has advanced dramatically, led by the availability of a growing number of classes of effective antiretroviral agents and management guidelines that have been based on advancing scientific evidence [1]. Several studies have demonstrated improvements in the clinical outcomes of HIV infection as management has improved, documenting a decline in opportunistic infection and in mortality, and improvements in the HIV type 1 (HIV-1) RNA level and the CD4 T-cell count [2–5]. However, these studies have generally reported this experience in relatively homogeneous populations that are often predominantly male, white race, and in the men who have sex with men (MSM) HIV transmission risk group.
Despite these advances in HIV treatment, major challenges remain in achieving access, retention in care, and adherence to antiretroviral treatment regimens. Improvements in HIV care and outcomes may not have equally affected women, minority races and non-MSM HIV transmission risk groups and persons across all income strata [6]. Disparities in healthcare are particularly concerning with HIV because infection rates are particularly high in African Americans [7], persons below the poverty line [8], and injection drug users (IDUs) [9]. This is especially challenging because HIV treatment is complex, lifelong, expensive, and demanding, as nonadherence leads to virologic failure and resistance [10]. Studies to date have not been able to directly compare these demographic and behavioral groups although population assessments suggest major obstacles to successful treatment attributed to inadequate access to care, retention in care, and adherence to contemporary guidelines in HIV treatment. These issues are often attributed to healthcare disparities. Our inner-city HIV clinical practice in Baltimore, Maryland, provides us with the opportunity to compare health processes and outcomes among these demographic and behavior groups within our clinic.
METHODS
The Johns Hopkins HIV/AIDS Service has provided HIV care to a majority of HIV-infected individuals who have sought care in Baltimore since the service opened in 1984. Its patients are principally from the Baltimore metropolitan region, an East Coast urban region with the fifth-highest incidence of HIV of US urban centers and in a state with the third-highest incidence of HIV infection in the United States [11]. The population has a high proportion of individuals who were infected through injection drug use, and the majority have incomes below the poverty line. This analysis used longitudinal data collected in the Johns Hopkins HIV Clinical Cohort. This is a nested clinical cohort of persons who have received care from the Johns Hopkins HIV/AIDS Service. All patients who present to the clinic for longitudinal HIV care are approached to enroll in the cohort, usually at their second visit, and the rate of refusal is <1%. Patients who present only for consultation are not included, and those whose HIV primary care is elsewhere are not included. Data on all measures used in this analysis have been collected over time since 1990. The methods used have not changed with data collected prospectively by trained abstractors from the patient, the medical record, and electronic institutional sources. Quality assurance mechanisms have been in place to ensure complete and accurate collection of these data [12]. Mortality is tracked through clinical records supplemented by both state and national vital statistics. We search the online Social Security Death Index twice yearly as well as Web-based local death announcements.
The demographic and behavioral stratifying variables of primary interest in this study were patient-reported HIV transmission risk group (MSM, IDU, heterosexual), patient-reported race (white, black) and sex (male, female). The clinic has had relatively low numbers of patients from other racial groups (<2%) and transgender patients (<1%), and these categories were not included. We also assessed retention in care, which was defined as having a primary care visit in the first, second, and third years after the calendar year of analysis (eg, a primary care visit in 2000 and subsequent visits in 2001, 2002, and 2003). We assessed the patient's medical insurance at first visit in a calendar year (available beginning in calendar year 2005, and classified as Medicaid, Medicare, private/commercial, and self-pay).
Outcome measures included utilization of combination antiretroviral therapy (ART) as measured by provider prescribing at any time during the year, HIV-1 RNA level, CD4 T-cell count (the earliest date the laboratory value was measured during that calendar year), incidence of opportunistic illness (OI) [13] and mortality (that occurred during that calendar year). We assessed each of these measures from 1995 through 2010 in those patients who were enrolled into the clinic prior to the midpoint of that calendar year and had at least 1 clinic visit in that calendar year. The percentage of patients prescribed ART, defined as a regimen that included a protease inhibitor, nonnucleoside reverse transcriptase inhibitor (RTI), integrase strand transfer inhibitor or CD4 T-cell entry inhibitor, or the use of triple nucleoside RTI therapy (which was an acceptable therapy during part of this time), was calculated for each calendar year for all individuals in care during that year. Early use of dual nucleoside RTI therapy was not assessed. The median HIV-1 RNA level and CD4 T-cell count was computed for each calendar year for all individuals in care during that year. The values closest to the midpoint of the year were used as most representative of patients in care during that entire year. OI incidence rates and mortality rates were computed for each calendar year as the number of events divided by the number of person-years (PY) patients in that year contributed. Life expectancy was calculated using a standard abridged (5-year age grouping) life table–based actuarial formula in 2009 (to allow for 1 year of follow-up person-time to compute mortality rates).
Descriptive comparison of demographic and behavioral risk group trends over time was done by Mantel-Haenszel test for trend for categorical variables (sex, race, transmission risk group, insurance, and retention in care) and the Kruskal-Wallis test for age. We conducted multivariate analyses of the associations of demographic (age, race, sex) and transmission risk group variables with the outcome variables (use of ART, HIV-1 RNA level, CD4 T-cell count, and OI and mortality rates) for calendar year 2010. Negative binomial regression was used to compare mortality and OI rates by demographic and behavioral risk categories. Linear regression was used to compare CD4 T-cell levels and log10 HIV-1 RNA level by these categories, and logistic regression was used to compare use of ART by these categories. In addition to the demographic and risk variables, these multivariate analyses also included the CD4 T-cell level at enrollment into the clinic and insurance. These analyses were done using Proc Genmod (SAS Institute).
RESULTS
From 1995 to 2010, we followed 6366 patients with 27 941 PY of follow-up. HIV transmission risk was 45% for IDUs, 26% for MSM, and 30% for heterosexual individuals. Seventy-six percent of patients were black, 22% were white, and 2% other races, and 32% were women. Table 1 shows the demographic and clinical characteristics of the population from 1995 to 2010 by 5-year intervals. Over time, the population was older, with an increased proportion of women and heterosexual HIV transmission. These characteristics reflect the changing demography of HIV infection in Maryland during this time. From 2005 to 2010, there were fewer uninsured patients and a concomitant increase in Medicare-insured patients. There is an increasing trend in the CD4 T-cell count at clinic enrollment, although the median entry CD4 T-cell count in 2010 remained <350 cells/mm3. Retention in care improved over time but remained lowest after 3 years. The only significant differences within sex, race, or HIV transmission risk groups was for a higher rate of retention in women compared with men in 1995 and in IDU compared to non-IDU patients in 1995 and 2000. This difference was no longer present after 2005.
Table 1.
Characteristics of the Sample, 1995–2010
| Characteristic | Calendar Year |
P Value | |||
|---|---|---|---|---|---|
| 1995 | 2000 | 2005 | 2010 | ||
| Patients in care, No. | 1041 | 1936 | 2140 | 2087 | |
| Age, median, y | 38 | 40 | 44 | 48 | <.01 |
| Sex, Male, % | 67 | 67 | 66 | 66 | .40 |
| Race, % | |||||
| Black | 79 | 77 | 75 | 75 | .04 |
| White | 21 | 23 | 25 | 25 | .04 |
| Transmission riska, % | |||||
| IDU | 58 | 49 | 41 | 36 | <.01 |
| MSM | 30 | 30 | 29 | 30 | .93 |
| Heterosexual | 46 | 50 | 54 | 56 | <.01 |
| Retained in care, % | |||||
| 1 y later | 85 | 89 | 89 | 90 | <.01 |
| 2 y later | 73 | 81 | 81 | … | <.01 |
| 3 y later | 64 | 74 | 73 | … | <.01 |
| Insurance (%) | |||||
| Medicaid | NA | NA | 42 | 44 | .21 |
| Self-pay (uninsured) | 23 | 18 | <.01 | ||
| Private/commercial | 15 | 15 | .99 | ||
| Medicare | 19 | 23 | <.01 | ||
Abbreviations: IDU, injection drug user; MSM, men who have sex with men; NA, not available.
a Categories are not mutually exclusive.
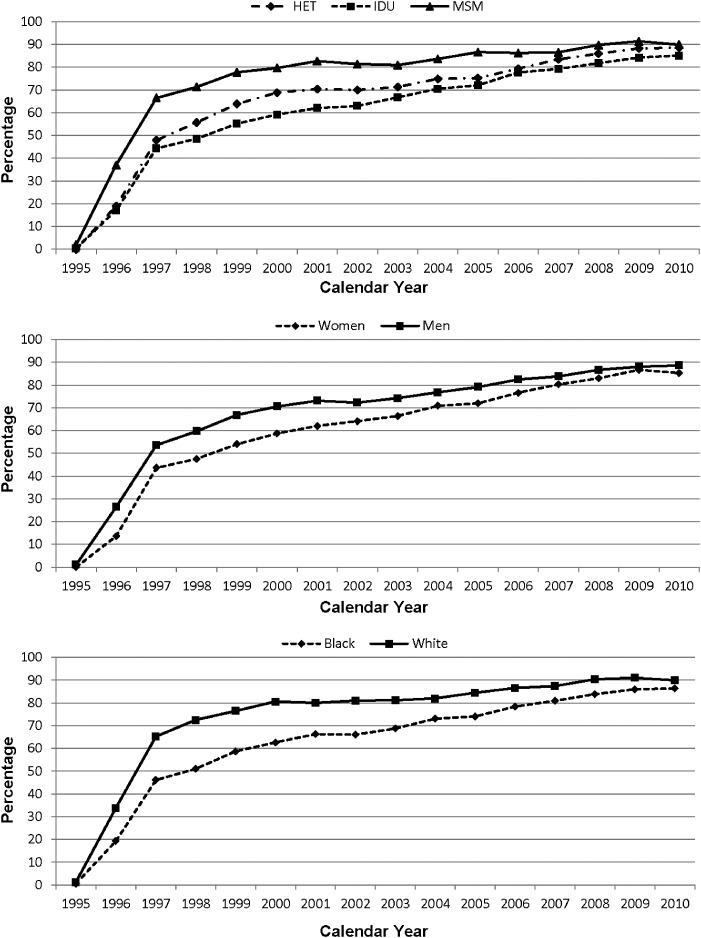
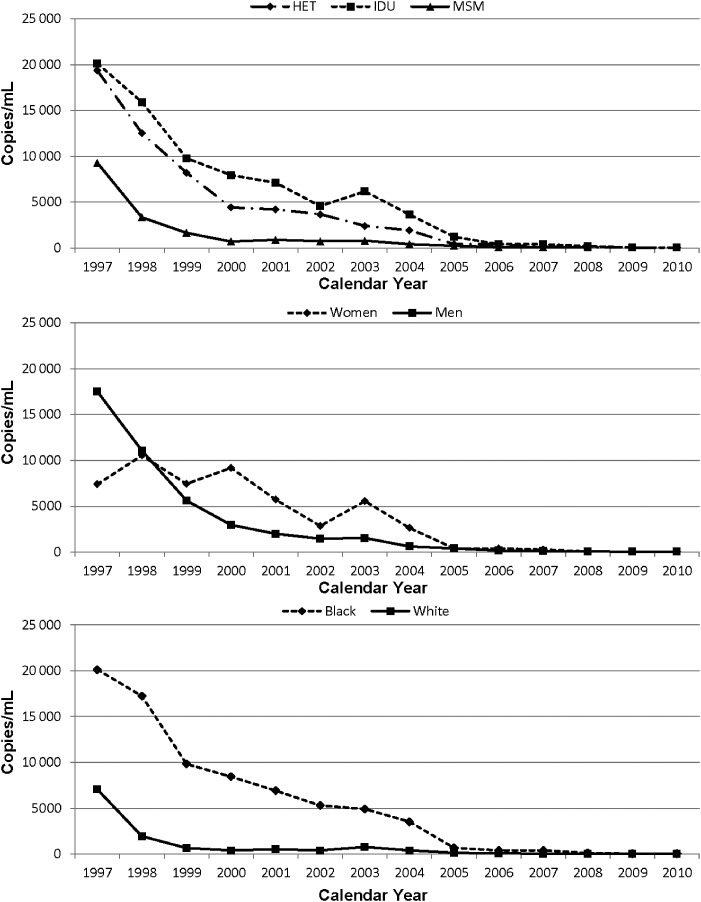
The annual percentage of patients prescribed combination ART is shown in Figure 1. There is an increasing trend in receipt of ART in all demographic and behavioral risk groups, with the earliest increase in use in MSM, men, and white patients. By 2010, the percentage of patients receiving ART was 87%. In multivariate analysis adjusting for age and enrollment CD4 T-cell level, there were no significant differences in ART receipt by HIV transmission risk group, sex, or race categories. The trend in the median annual HIV-1 RNA level over time is shown in Figure 2 for HIV transmission risk group, sex, and race. Notably, the trend in the HIV-1 RNA level was dramatically downward over time, with a median HIV-1 RNA level of <200 copies/mL in all demographic and behavioral groups in 2010. In multivariate analysis, the log10 HIV-1 RNA level was 0.28 logs higher in the IDU compared to the MSM HIV transmission group in 2010 (P < .001), although there was no significant difference between MSM and heterosexual risk groups. The log10 HIV-1 RNA level was 0.24 logs higher in blacks compared to whites in 2010 (P < .001). There was no difference by sex.
Figure 1.
Percentage of patients on combination antiretroviral therapy over time. Abbreviations: HET, heterosexual; IDU, injection drug user; MSM, men who have sex with men.
Figure 2.
Human immunodeficiency virus type 1 RNA level in copies/mL over time. Abbreviations: HET, heterosexual; IDU, injection drug user; MSM, men who have sex with men.
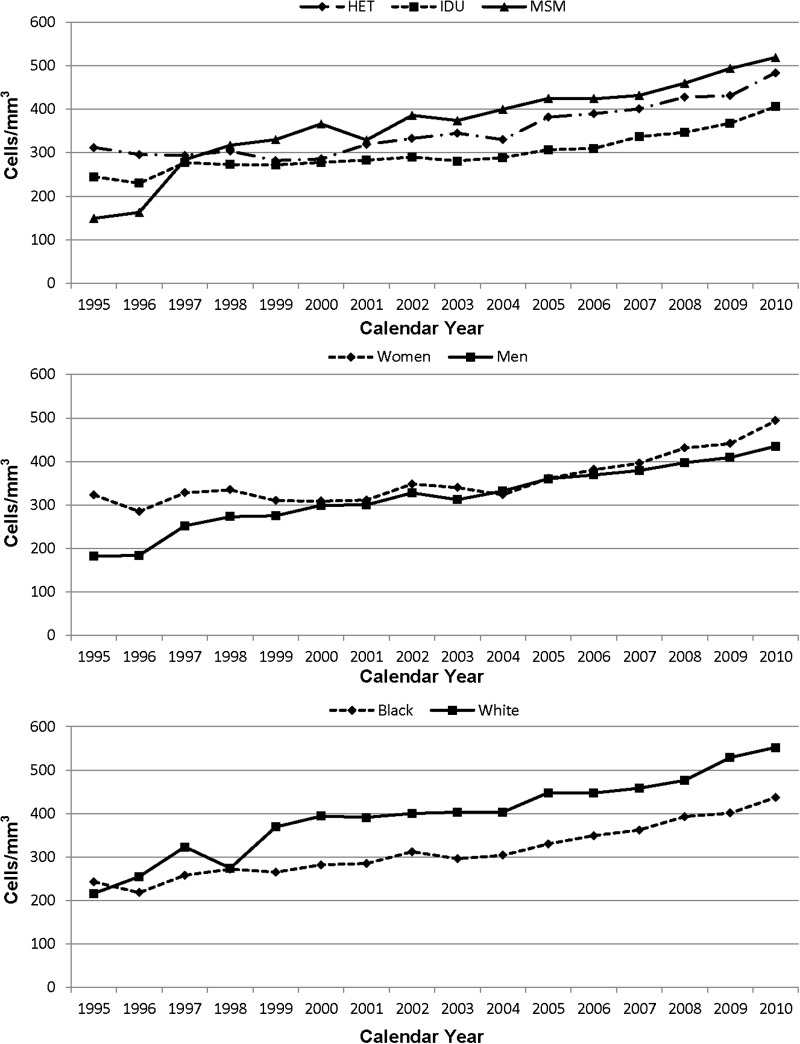
The trend in the median annual CD4 T-cell count is shown by HIV transmission risk group, sex, and race in Figure 3. There were upward trends in all demographic and behavioral groups, with an overall median CD4 T-cell level of 475 cells/mm3 in 2010. In multivariate analysis, the IDU risk group had a CD4 T-cell level that was 76 cells/mm3 less than that in MSM (P < .001), with no significant difference between MSM and heterosexual risk groups. The CD4 T-cell count was 55 cells/mm3 lower in men compared to women (P = .001). There was no racial difference.
Figure 3.
CD4 T-cell count in cells/mm3 over time. Abbreviations: HET, heterosexual; IDU, injection drug user; MSM, men who have sex with men.
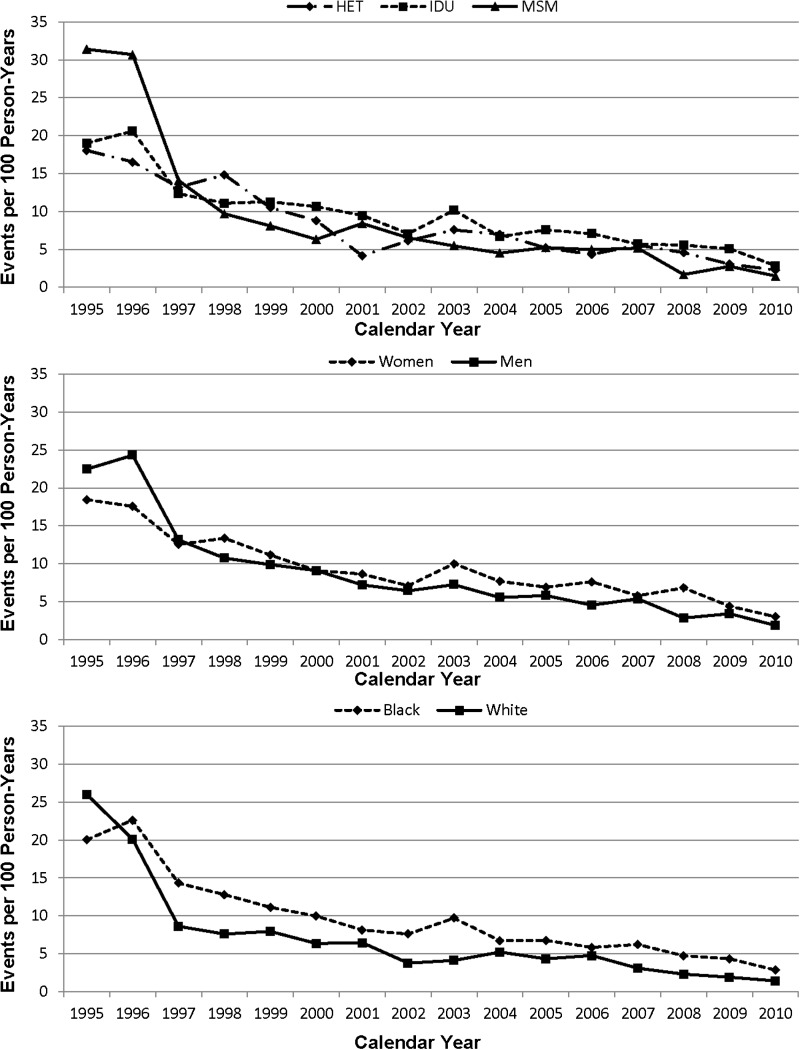
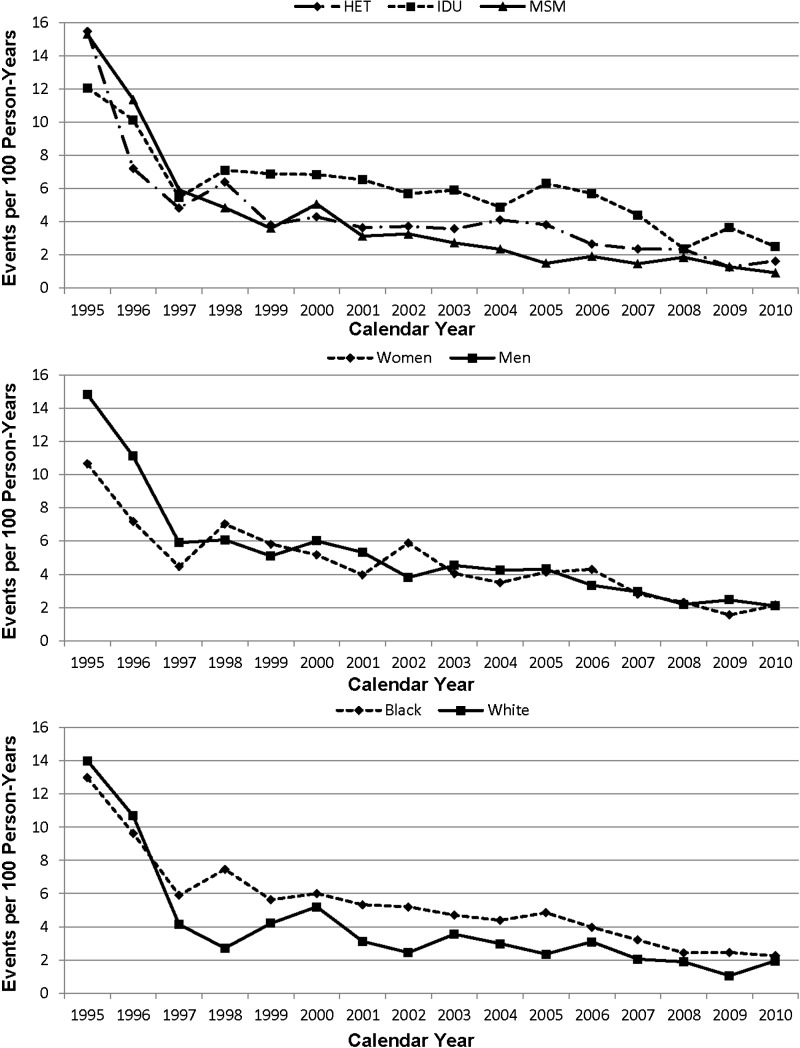
The annual incidence rate of opportunistic illness is shown in Figure 4 for HIV transmission risk group, sex, and race. There again was a dramatic decline in OI incidence rates over time to 2.4 per 100 PY by 2010. The annual mortality rate is shown for HIV transmission risk group, sex, and race in Figure 5. Mortality rate decline was greatest from 1995 to 1998, with a smaller decrease thereafter. In 2010, the mortality rate was 2.1 per 100 PY. In multivariate analysis, there was no significant difference in OI mortality rate by risk group, race, or sex. Adjusting further for medical insurer did not significantly alter these associations.
Figure 4.
Incidence of opportunistic illness in events per 100 person-years over time. Abbreviations: HET, heterosexual; IDU, injection drug user; MSM, men who have sex with men.
Figure 5.
Mortality rate per 100 person-years over time. Abbreviations: HET, heterosexual; IDU, injection drug user; MSM, men who have sex with men.
Because there was no significant difference in mortality, life expectancy was computed for the entire sample for patients in care in 2009. For a 28-year-old HIV-infected person in care in 2009, remaining life expectancy was calculated to be 45.4 years (95% confidence interval, 39.6–51.3 years).
DISCUSSION
Our clinic-wide analysis shows that substantial improvements have occurred in the health of those infected with HIV irrespective of HIV transmission risk group, race, and sex. As of calendar year 2010, there is no difference by demographic or behavioral risk groups in ART prescription, OI rates, or mortality rates. This likely reflects the remarkable advances in the development of ART, coupled with continual improvements in the management of HIV-infected individuals based on evidence-based guidelines [1]. In a previous study, we found that the HIV-1 RNA level had declined markedly over time but we did not analyze individual demographic and HIV transmission risk groups [14]. Our group previously published results showing disparities in comorbidity and mortality rates by race, sex, and risk group using data through 2005 [15, 16]. Others have also shown higher mortality rates in blacks vs whites, women vs men, and IDUs vs other HIV transmission risk groups in HIV care through 2005 [17]. A recent study with more recent data from HOPS, a consortium of HIV care sites in the United States, did not show differences in mortality rates by race for patients with a CD4 T-cell count >200 cells/mm3 [18].
Though markedly improved, there remained some differences in 2010 in both the HIV-1 RNA and the CD4 T-cell levels between the IDU risk group and the other HIV transmission risk groups. Approximately half of our patients who have IDU as a risk factor for HIV transmission continue to episodically use illicit drugs, primarily opiates [19]. This behavior may have an impact on adherence to prescribed ART and on subsequent HIV-1 RNA and CD4 response. It is also possible that use of opiates may have a direct effect on the immune system [20]. However, these differences did not appear to translate into differences in OI or mortality rates. This would not appear to be confounding related to retention in care since there were no significant differences between IDUs and other risk groups in retention after 2005. It is possible that receipt of ART and associated general medical management led to improved clinical outcomes, but this is speculative. Studies have shown that management of HIV-infected opioid-dependent patients will have better HIV outcomes associated with opioid substitution therapy [21–24]. Our clinic has had programs for treatment of opioid and other addictions for much of its history, which may have influenced the clinical outcomes in our patients.
Health disparities/inequalities has been defined as “potentially avoidable differences in health (or in health risks that policy can influence) between groups of people who are more and less advantaged socially; these differences systematically place socially disadvantaged groups at further disadvantage on health” [25]. An analysis of HIV in the United States by age, sex, and race for the post–highly active antiretroviral therapy era to 2005 showed the death rate was 7.92-fold higher for blacks compared with whites and 2.72-fold higher for low compared with high socioeconomic status [26]. Numerous reports have documented inequalities in healthcare receipt based on income, race, and, to a lesser extent, sex [27–31]. These differences are dramatized and more challenging by the demographics and cost of HIV infection. In the United States the prevalence of HIV is reported at 6-fold greater levels in blacks compared with whites [7], and the infection rate in persons below the poverty line is substantially higher [32]. There are probably few major medical conditions that have so selected minorities and “have nots.” The advances in HIV care since 1996 are also remarkably unique, but the cost for contemporary medications averaged about $12 000/year [33].
These disparities in the population at risk and challenges in healthcare delivery are further exaggerated in Baltimore, where 86% of reported HIV cases are in blacks and 32% are IDUs. The Centers for Disease Control and Prevention released its 2011 report outlining the continuing disparities in both the access to care and health outcomes based on a number of comorbidities including race/ethnicity, sex, and behaviors [36]. An important public health goal for Healthy People 2020 is to achieve health equity, eliminate disparities, and improve the health of all groups in the United States [34]. Optimizing health outcomes among people living with HIV and reducing HIV-related disparities is a major goal of the National HIV/AIDS Strategy for the United States [35]. Specifically in regard to HIV/AIDS, disparities are based not only upon race and sex, but also non-MSM transmission risk groups, particularly IDUs, with concern about adherence to treatment and retention in care.
One reason for our ability to deliver HIV care to patients who might otherwise have financial and other barriers to care is the Ryan White HIV/AIDS program. Since 1990, this program, administered by the Health Resources and Services Administration of the US Department of Health and Human Services, has provided federal financial assistance to the clinic to deliver HIV care using a care model that combines primary, specialty (substance abuse and mental health), and supportive care (case management, nutrition, treatment adherence, emergency assistance, transportation) into an integrated multidisciplinary program of care [36]. The Ryan White HIV/AIDS Program is designed to provide financial support for HIV care for the economically disadvantaged, and among the patients who received Ryan White support in fiscal year 2010 in our clinic, 73% had verified incomes less than the federal poverty guidelines and another 19% had incomes between 101%–200% of the federal poverty guidelines (personal communication, J. C. Keruly, August 2011). A recent policy paper describes the components of effective HIV care, with an emphasis on the contributions that the Ryan White HIV/AIDS Program has made in making medical and supportive services accessible [37].
An important caveat in our analysis is that these are data from patients who have engaged in care sufficiently to have laboratory testing and clinical follow-up. We did not assess patients who come to the clinic and then subsequently disengage from care. A recent estimate suggests that 36% of HIV-infected adults aged 18–64 who are linked to HIV care in the US are retained in care over 18–48 months [38], approximately the same as seen in our clinic. We do not know how many of the patients who were not retained in care transferred their care to another facility or did not receive any further HIV care. We also do not know whether our results would generalize to other HIV care settings in the United States, and they certainly do not generalize to HIV-infected people in the United States who have not engaged in HIV care. Nevertheless, we believe that our results are an important demonstration of what can be achieved by contemporary HIV care in patients who are retained in care.
In summary, we have shown a dramatic improvement in receipt of ART and in HIV outcomes in every demographic and HIV transmission risk groups in our Baltimore clinic, with relatively small remaining disparities in HIV-1 RNA and CD4 T-cell levels as of calendar year 2010. We believe that our results reflect what is possible when HIV care is delivered based on state-of-the-art care guidelines with support from the Ryan White HIV/AIDS Program to address the challenge to deliver treatment that is highly effective, but also expensive, complex, and requires continuous patient engagement by populations that are often underserved by healthcare disparities. These results may not reflect the care received by HIV-infected individuals across the entire United States, and particularly in patients with an IDU history, further improvement is needed. Nevertheless, we believe that our results reflect an effective model of care, and should continue in the United States if individuals with HIV infection are to have the maximal benefit possible from modern HIV care.
Notes
Financial support. This work was supported by the National Institutes of Health (grant numbers R01 DA11602, K24DA00432, P30 AI094189, and R01 AA16893).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; 2011. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf . Accessed 14 November 2011. [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Buchacz K, Baker RK, Palella FJ, et al. HOPS Investigators. AIDS-defining opportunistic illnesses in US patients, 1994–2007: a cohort study. AIDS. 2010;24:1549–59. doi: 10.1097/QAD.0b013e32833a3967. [DOI] [PubMed] [Google Scholar]
- 4.Moore RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. AIDS. 1999;13:1933–42. doi: 10.1097/00002030-199910010-00017. [DOI] [PubMed] [Google Scholar]
- 5.Braithwaite RS, Kozal MJ, Chang CC, et al. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21:1579–89. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Vol. 60. MMWR Morb Mortal Wkly Rep; 2011. CDC health disparities and inequalities report–United States, 2011; pp. 1–124. suppl. [Google Scholar]
- 7.Hall HI, Hughes D, Dean HD, Mermin HG, Fenton K Centers for Disease Control and Prevention (CDC) HIV infection–United States, 2005 and 2008. MMWR Surveill Summ. 2011;60(suppl):87–9. [PubMed] [Google Scholar]
- 8.Song R, Hall HI, Harrison KM, Sharpte TT, Lin LS, Dean HD. Identifying the impact of social determinants of health on disease rates using correlation analysis of area-based summary information. Public Health Rep. 2011;126(suppl 3):70–80. doi: 10.1177/00333549111260S312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) HIV infection among injection-drug users–34 states, 2004–2007. MMWR Morb Mortal Wkly Rep. 2009;58:1291–5. [PubMed] [Google Scholar]
- 10.Gebo KA, Fleishman JA, Conviser R, et al. HIV Research Network. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010;24:2705–15. doi: 10.1097/QAD.0b013e32833f3c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maryland Department of Health and Mental Hygiene. Fourth quarter; 2009. Maryland HIV/AIDS epidemiological profile. Available at:http://dhmh.state.md.us?AIDS/Data&Statistics?marylandHIVEpiPRofile12-2009.pdf . Accessed 24 November 2011. [Google Scholar]
- 12.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr. 1998;17(suppl 1):S38–41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC) 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR. 1992;41:RR17. [PubMed] [Google Scholar]
- 14.Moore RD, Bartlett JB. Dramatic decline in the HIV-1 RNA level over calendar time in a large urban HIV practice. Clin Infect Dis. 2011;53:600–4. doi: 10.1093/cid/cir467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poundstone KE, Chaisson RE, Moore RD. Differences in HIV disease progression by injecting drug use and by sex in the era of highly active antiretroviral therapy. AIDS. 2001;15:1115–23. doi: 10.1097/00002030-200106150-00006. [DOI] [PubMed] [Google Scholar]
- 16.Moore RD, Keruly JC, Chaisson RE. Differences in HIV disease progression by injecting drug use in HIV-infected persons in care. J Acquir Immune Defic Syndr. 2004;35:46–51. doi: 10.1097/00126334-200401010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Lemly DC, Shepherd BE, Hulfan T, et al. Race and sex differences in antiretroviral therapy used and mortality among HIV-infected persons in care. J Infect Dis. 2009;199:991–8. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palella FJ, Baker RK, Buchacz K, et al. HOPS investigators. Increased mortality among publicly insured participants in the HIV Outpatient Study despite HAART treatment. AIDS. 2011;25:1865–76. doi: 10.1097/QAD.0b013e32834b3537. [DOI] [PubMed] [Google Scholar]
- 19.Lucas G, Griswold M, Gebo KA, Keruly J, Chaisson RE, Moore RD. Illicit drug use and HIV-1 disease progression: a longitudinal study in the era of highly active antiretroviral therapy. Am J Epidem. 2006;163:412–20. doi: 10.1093/aje/kwj059. [DOI] [PubMed] [Google Scholar]
- 20.Cabral GA. Drugs of abuse, immune modulation, and AIDS. J Neuroimmune Pharmacol. 2006;1:280–95. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- 21.Altice FL, Bruce RD, Lucas GM, et al. BHIVES Collaborative. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr. 2011;56(suppl 1):S22–32. doi: 10.1097/QAI.0b013e318209751e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palepu A, Tyndall MW, Joy R, Kerr T, Wood E, Press N. Antiretroviral adherence and HIV treatment outcomes among HIV/HCV co-infected injection drug users: the role of methadone maintenance therapy. Drug Alcohol Depend. 2006;84:188–94. doi: 10.1016/j.drugalcdep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Kapadia F, Vlahov D, Wu Y, Cohen MH, Greenblatt RM, Howard AA. Impact of drug abuse treatment modalities on adherence to ART/HAART among a cohort of HIV seropositive women. Am J Drug Alcohol Abuse. 2008;34:161–70. doi: 10.1080/00952990701877052. [DOI] [PubMed] [Google Scholar]
- 24.Wood E, Hogg RS, Kerr T, Palepu A, Zhang R, Montaner JS. Impact of accessing methadone on the time to initiating HIV treatment among antiretroviral-naive HIV-infected injection drug users. AIDS. 2005;19:837–9. doi: 10.1097/01.aids.0000168982.20456.eb. [DOI] [PubMed] [Google Scholar]
- 25.Braveman P. Health disparities and health equity: concepts and measurement. Ann Rev Public Health. 2006;27:167–94. doi: 10.1146/annurev.publhealth.27.021405.102103. [DOI] [PubMed] [Google Scholar]
- 26.Rubin M, Cohen CG, Link BG. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. Am J Public Health. 2010;100:1053–9. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine RS, Rust GS, Pisu M, et al. Increased black-white disparities in mortality after the introduction of lifesaving innovations: a possible consequence of US federal laws. Am J Public Health. 2010;100:2176–84. doi: 10.2105/AJPH.2009.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGinnis KA, Fine MJ, Sharma RK, et al. Veterans Aging Cohort 3-Site Study (VACS 3) Understanding racial disparities in HIV using data from the Veterans Aging Cohort 3-site study and VA administrative data. Am J Public Health. 2003;93:1228–33. doi: 10.2105/ajph.93.10.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin DK, Stokes CS. Income inequality and mortality in US counties: does minority racial concentration matter? Am J Public Health. 2002;92:99–104. doi: 10.2105/ajph.92.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh GK. Area deprivation and widening inequalities in US mortality,1996–1998. Am J Public Health. 2003;93:1137–43. doi: 10.2105/ajph.93.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palacio H, Kahn JG, Richards TA, Morin SF. Effect of race and/or ethnicity in use of antiretrovirals and prophylaxis for opportunistic infection: a review of the literature. Public Health Rep. 2002;117:233–51. doi: 10.1093/phr/117.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lansky A, Brooks JT, DiNenno E, Heffelfinger J, Hall HI, Mermin J. Epidemiology of HIV in the United States. J Acquir Immune Defic Syndr. 2010;55(suppl 2):S64–8. doi: 10.1097/QAI.0b013e3181fbbe15. [DOI] [PubMed] [Google Scholar]
- 33.Gebo KA, Fleishman JA, Conviser R, et al. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010;24:2705–15. doi: 10.1097/QAD.0b013e32833f3c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.US Department of Health and Human Services. The Secretary's Advisory Committee on National Health Promotion and Disease Prevention Objectives for 2020. Available at: http://www.healthypeople.gov/2020/about/DisparitiesAbout.aspx . Accessed 28 November 2011. [Google Scholar]
- 35.Millett GA, Crowley JS, Koh H, et al. A way forward: the national HIV/AIDS strategy and reducing HIV incidence in the United States. J Acquir Immune Defic Syndr. 2010;55(suppl 2):S144–7. doi: 10.1097/QAI.0b013e3181fbcb04. [DOI] [PubMed] [Google Scholar]
- 36.HIV/AIDS Bureau, Health Resources and Services Administration, US Department of Health and Human Services. Going the distance. The Ryan White HIV/AIDS Program: 20 years of leadership. 2010. A legacy of care, August. Accessed 22 November 2011.
- 37.Gallant JE, Adimora AA, Carmichael JK. Essential components of effective HIV care: a policy paper of the HIV Medicine Association of the Infectious Diseases Society of American and the Ryan White Medical Providers Coalition. Clin Infect Dis. 2011;53:1043–50. Available at:. doi: 10.1093/cid/cir689. Accessed 14 November 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment—United States. Morb Mortal Weekly Rep. 2011;60:1–6. [PubMed] [Google Scholar]