Abstract
When multivalent ligands attach to IgEs bound to the receptors with high affinity for IgE on mast cells, the receptors aggregate, tyrosines on the receptors become phosphorylated, and a variety of cellular responses are stimulated. Prior studies, confirmed here, demonstrated that the efficiency with which later events are generated from earlier ones is inversely related to the dissociation rate of the aggregating ligand. This finding suggests that the cellular responses are constrained by a “kinetic proofreading” regimen. We have now observed an apparent exception to this rule. Doses of the rapidly or slowly dissociating ligands that generated equivalent levels of tyrosine-phosphorylated receptors comparably stimulated a putatively distal event: transcription of the gene for monocyte chemoattractant protein 1. Possible explanations of this apparent anomaly were explored.
When aggregated, the receptors with high affinity for IgE (FcɛRI), like other members of the family of multichain immune recognition receptors (1), initiate cellular responses by promoting the phosphorylation of tyrosines in their own immunoreceptor tyrosine-based activation motifs (2). These esterifications are mediated by Lyn kinase (3). We and our collaborators, and others, are attempting to define the factors that are quantitatively most important in regulating these initiating events (4–8).
In the 2H3 line of the rat basophilic leukemia tumor (9)—the cells in which the FcɛRI have been studied most intensively—the amount of kinase available to the receptors appears to be limiting (5, 6). While exploring the consequences of this constraint, we also examined whether some of the cellular signaling pathways stimulated by FcɛRI are subject to a kinetic proofreading regimen. In such a regime, the lifetime of the ligand-receptor complexes as well as their concentration determine the intensity of downstream signals (10, 11). This proved to be the case with three homologous ligands we examined (12). Cells incubated with IgE directed toward the 2,4-dinitrophenyl (DNP) ligand were stimulated with a protein conjugated alternatively with multiple DNP, 2,4-dinitro-6-carboxyphenyl, or 2-nitrophenyl (NP) groups. These haptenic constituents have relative intrinsic affinities of 1:0.037:0.0006 for the IgE (12), and their complexes are expected to have progressively shorter lifetimes (13).§ At doses stimulating comparable phosphorylation of the FcɛRI, the more weakly binding derivatives were deficient in stimulating distal responses. Moreover, the synergy between the competition for limited kinase and the kinetic proofreading control of the signaling caused the low-affinity ligands to act as noncompetitive antagonists (12). Those studies were performed largely on cell suspensions. Because adherent cells are more responsive and maintain the level of certain activated components longer (14), we tested whether this difference in the experimental protocol would influence the kinetic proofreading. As reported here, quantitative differences were observed, but the principal finding related to a response we had not previously examined: transcription of the gene for the monocyte chemoattractant protein 1 (MCP-1) (15–17). At appropriate doses, the low-affinity ligand unexpectedly stimulated this late response about as well as the high-affinity ligand.
Materials and Methods
Antibodies and Reagents.
The anti-DNP monoclonal mouse IgE (18) and anti-β subunit of FcɛRI have been described (19, 20). Goat anti-mouse IgE was from ICN, horseradish peroxidase-conjugated 4G10, a mouse monoclonal anti-phosphotyrosine (PY) was from Upstate Biotechnology (Lake Placid, NY), and rabbit polyclonal anti-Syk was a gift from Uli Blank, Institut Pasteur, Paris. The antigens were from a single batch of Fab fragments of bovine IgG conjugated alternatively with DNP- or NP-ɛNH2-caproate to ≈3 mol hapten/mol protein (21). For some experiments either the IgE or the antigens were iodinated with carrier-free 125I by using iodogen tubes (Pierce).
Cells.
Rat basophilic leukemia-2H3 cells were maintained as described (9). They were detached with trypsin before seeding in the desired plates and then grown to confluence.
Stimulation.
Adherent cells were incubated with IgE (at 0.3 to 0.5 μg/ml) overnight, and then washed with prewarmed buffer before addition of antigen at 37°C. Except where stated otherwise, there were 1.5 to 2 × 106 adherent cells in a well containing 1 ml medium. Binding studies with 125I-labeled IgE revealed ≈3 × 105 FcɛRI/cell (data not shown) so that there was the equivalent of 0.75 to 1 × 10−9 M receptors and bound IgE. Therefore, at 50 ng/ml antigen (molecular weight 50,000) we added roughly one molecule of antigen/receptor-bound IgE. In a few experiments, we measured the binding of (iodinated) antigen directly by adding it for a specified time, washing the cells twice, and counting the radioactivity in a detergent lysate of the cells. There was negligible loss of the bound DNP-modified antigen in the washing, but the NP-modified antigen dissociated completely and its binding could not be measured.
Lysates, Immunoprecipitation, and Western Blotting.
Except for those experiments in which RNA was extracted, cells were lysed with a Triton X-100-containing 3× solubilization buffer (6). Aliquots of lysate were immunoprecipitated with anti-mouse IgE or anti-Syk antibody. Fifty microliters of a 50% suspension of Protein-A-Sepharose (Amersham Pharmacia) was added at the same time, and the mixture was incubated at 4°C for 2 h. After centrifugation the bound proteins were released from the washed immunoprecipitates with hot Na dodecyl sulfate sample buffer, the eluted proteins were separated by electrophoresis, and the proteins were transferred and then detected by Western blotting using enhanced chemiluminescence (6). Autophotographs of Western blots were quantitated by computerized densitometry (Molecular Dynamics).
Degranulation.
Rat basophilic leukemia-2H3 cells were plated into a 24-well cluster dish at 2 × 105 cells/0.4 ml/well and incubated with IgE overnight. After washing twice some of the cells were stimulated with antigen. At successive times, 20 μl of supernatant was analyzed for released hexosaminidase and compared with total cellular enzyme by an enzyme-linked immunoabsorbent assay (22).
Isolation of RNA.
The supernatants from stimulated and control cells were aspirated, and the cells were directly lysed by addition of 0.5 ml/well of Trizol reagent (GIBCO/BRL). Total RNA was extracted according to the manufacturer's instruction.
c-fos mRNA.
mRNA was purified with a MessageMaker kit (GIBCO/BRL) and quantitated by UV spectroscopy. Approximately 0.5 μg from each sample was electrophoresed on a 1% agarose formaldehyde gel and transferred onto Nytran membranes (Schleicher & Schuell). The probe DNA was prepared by amplification of specific rat c-fos DNA fragment with PCR (sense primer: 5′-TTCTCGGGTTTCAACGCGGACTA-3′; antisense primer: 5′-CCAGTCTGCTGCATAGAAGGAAC-3′) and labeled by LofStrand Lab (Rockville, MD) with [α-32P] dCTP using random primer extension. Hybridization (16 h at 65°C) used probe DNA with a total radioactivity of ≈0.5 μCi. Autoradiographs of the washed membranes were prepared and the appropriate bands were scanned. The membranes were dehybridized with 0.1% SDS by boiling for 20 min twice, before reprobing with a rat β-actin probe.
MCP-1 mRNA.
Five micrograms total RNA from each sample was dot-blotted onto Nytran membranes by using a 77000X Easy-Titer ELIFA System (Pierce). The probe DNA-rat mcp-1 DNA fragment (5′-AAATGCATCTACATCTTGCATTTAAGGATTTC-TAGTATTCATG-3′) was end-labeled by LofStrand Lab. Hybridization (16 h at 65°C) used probe DNA containing about 0.5 μCi [32P]. Autoradiographs of the membranes were prepared, and the spots were quantitated by scanning. The membranes were reprobed with a rat β-actin probe as described above.
Secretion of MCP-1.
The supernatants from resting or stimulated cells were tested as described (23) with an enzyme-immunoassay kit from BioSource International (Camarillo, CA) according to the accompanying instructions.
Results
Kinetics of Binding of Antigen.
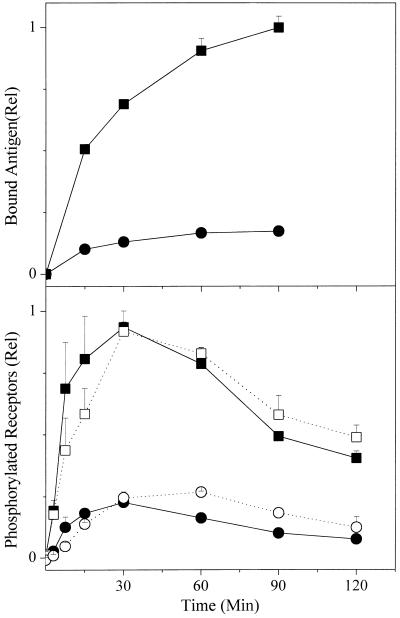
We examined the binding of iodinated antigens to the IgE-sensitized cells under the conditions used to assess the cellular responses. Fig. 1 Upper shows a typical result for the DNP-modified antigen. At the higher dose of antigen, equilibrium is achieved relatively slowly with a half-time of roughly 20 min. The k+1 calculated from the individual time points was lower at successive times but initially was ≈5 × 105 M−1⋅sec−1. As noted above, we couldn't perform a similar analysis with the NP-modified antigen.
Figure 1.
Time course of antigen binding and phosphorylation of receptors. (Upper) Binding of DNP-conjugated antigen to adherent cells. Cells were incubated with anti-DNP IgE and washed. Radiolabeled DNP-Fab was added and at the appropriate times the supernatant was aspirated. The cells were rapidly washed twice with medium, and then lysed with Triton X-100. The cell-bound radioactivity was determined and the data were normalized to the maximum value observed with the higher dose of antigen. On the basis of the number of cells and the specific activity of the antigen, the molecules of antigen bound per cell for this point was calculated to be ≈1.2 × 105 molecules/cell. Shown is one of three experiments, each of which gave very similar results. The points are the averages of duplicate determinations with the range shown. Here and in subsequent figures, where the range is not seen, it was smaller than the size of the symbol. DNP-Fab (ng/ml): ■, 42; ●, 7.6. (Lower) Time course of phosphorylation of tyrosines on FcɛRI. Cells were incubated with anti-DNP IgE and then stimulated with either the DNP- or NP-conjugated antigen for varying times. The FcɛRIs were immunoprecipitated and the PY on the β chain and the dimer of γ chains were quantitated by Western blotting. In this figure and Figs. 2 and 4, the data are from three or four separate experiments and have been normalized relative to the maximum signal observed on one of the samples reacted with 50 ng/ml of the DNP conjugate. The error bars show the standard errors. Antigen (ng/ml): DNP, ■ 50, ● 10; NP, □ 500, ○ 100.
Phosphorylation of Tyrosines on Receptors.
In preliminary experiments we determined the concentrations of each of the antigens that would engender equally modest or substantial tyrosine-phosphorylated FcɛRI. Fig. 1 Lower shows the time course of phosphorylation for the two doses of each antigen we regularly used on the basis of those preliminary results. The mean values plus the standard errors from three experiments are shown for the relative PY on the receptors resulting from incubation of the cells with 10 or 50 ng/ml of the DNP-conjugate (filled symbols) and 100 or 500 ng/ml of the NP-conjugate (open symbols). All of the data were normalized to the maximum PY observed with the higher dose of the DNP antigen in the individual experiments. Either dose of the low-affinity ligand appears to modify the FcɛRI somewhat more slowly. Compared with cells in suspension stimulated at a 6-fold higher concentration (cf. figure 1A in ref. 12), the maximum concentration of phosphorylated FcɛRI is observed at ≥30 min rather than ≈5 min, the kinetics of increasing phosphorylation approximating the rate of binding of iodinated DNP antigen to the cells measured in separate experiments (Fig. 1 Upper). The “spontaneous” rate of decline is also more gradual. At these doses of the paucivalent antigen, the maximum phosphorylated FcɛRI observed is approximately linearly proportional to the dose of antigen to which the cells were exposed and it takes a roughly 10 times higher concentration of the low-affinity ligand to induce comparable phosphorylation of FcɛRI. In our prior study with different preparations of similar conjugates, it took about seven times the concentration (12).
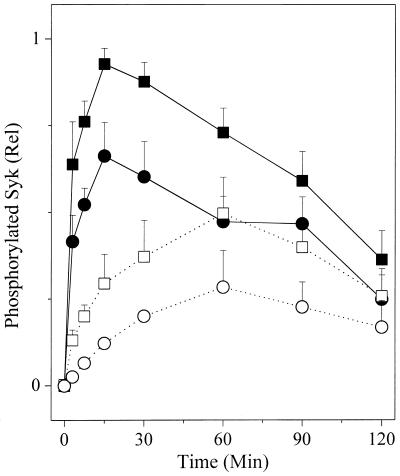
Phosphorylation of Tyrosines on Syk.
Fig. 2 shows the phosphorylation of Syk kinase observed in four experiments in which cells were incubated with the same doses of the same preparations of the antigens described in Fig. 1 Lower. With the high-affinity ligand, the amount of phosphorylated Syk observed is not linearly proportional to the dose of antigen. Based on the integrated values, the 5-fold increase in the concentration of antigen induced less than a 50% increase in the phosphorylation of Syk (cf. Fig. 1 Lower). In the cells exposed to the low-affinity ligand, a 5-fold increase in the concentration of antigen induces only a 2-fold increase in the phosphorylation of Syk. Consistent with our prior observations, the low-affinity ligand stimulates phosphorylation of Syk less efficiently than the high-affinity ligand per phosphorylated FcɛRI. Finally, we note that the rate at which the maximum phosphorylation of Syk is stimulated by the low-affinity ligand is clearly slower.
Figure 2.
Time course of phosphorylation of tyrosines on Syk. Cells were incubated with anti-DNP IgE and then stimulated with either a DNP- or NP-modified antigen for varying times. The Syk kinase was immunoprecipitated and the PY on the kinase was quantitated by Western blotting. Antigen (ng/ml): DNP, ■ 50, ● 10; NP, □ 500, ○ 100.
Degranulation.
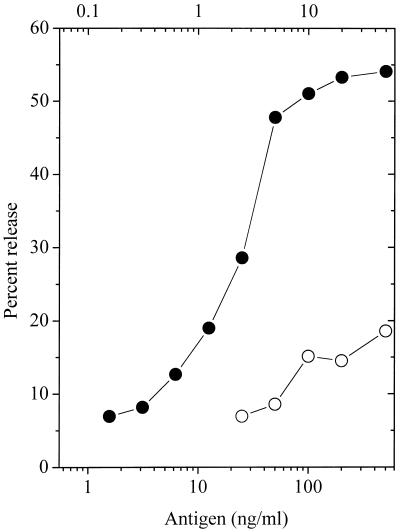
Fig. 3 summarizes several experiments that examined the release of hexosaminidase. With the DNP ligand (●), close to maximal release is observed by addition of 10 ng/ml. With the low-affinity NP antigen (○) ≈10 times as much was required to achieve maximum release but this release was only 1/3 of that observed with the high-affinity ligand. Because these doses lead to comparable phosphorylation of FcɛRI, it is apparent that receptors aggregated by the low-affinity ligand are less efficient in triggering this downstream signal also.
Figure 3.
Release of hexosaminidase from cells stimulated with varying doses of the alternative antigens. The amount of release plotted is the average observed at 60, 90, and 120 min after addition of either antigen. Upper abcissa: DNP antigen, ●; Lower abcissa: NP antigen, ○.
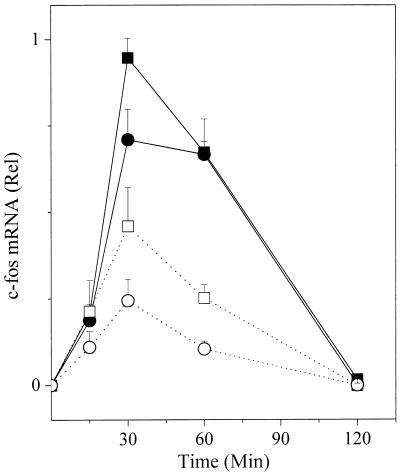
c-fos mRNA.
It has been reported that transcription of the gene for c-fos stimulated by FcɛRI does not depend on persistent aggregation of the FcɛRI (24). If so, transcription of c-fos might not be constrained by kinetic proofreading. Fig. 4 summarizes several experiments in which FcɛRI-stimulated increases in the c-fos mRNA were assayed. The results resemble those described so far for the other manifestations that follow phosphorylation of FcɛRI. At doses that yield comparable levels of tyrosine-phosphorylated FcɛRI, the low-affinity ligand is less efficient in stimulating the distal event. As with the other signals, the maximum level of mRNA is initiated by levels of phosphorylated FcɛRI far below those that can be achieved. Finally, as is also observed with the other responses, the maximum level achieved by the low-affinity ligand is substantially below that achieved by the high-affinity ligand. However, in this instance, the downstream signal followed similar kinetics for the two ligands.
Figure 4.
FcɛRI-stimulated increases in c-fos mRNA. After the desired stimulation, RNA was extracted, separated on gels, and transferred, and after blotting with the appropriate probe, the latter was quantitated. Antigen (ng/ml): DNP, ■ 50, ● 10; NP, □ 500, ○ 100.
MCP-1 mRNA.
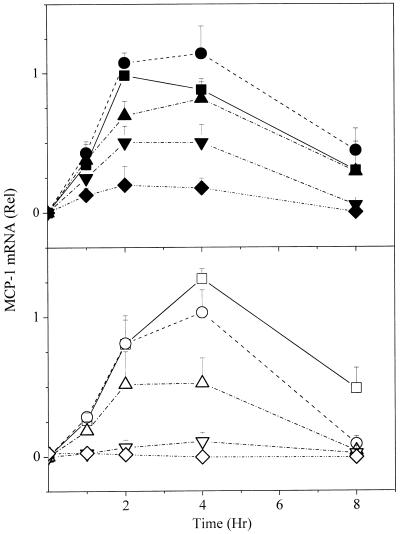
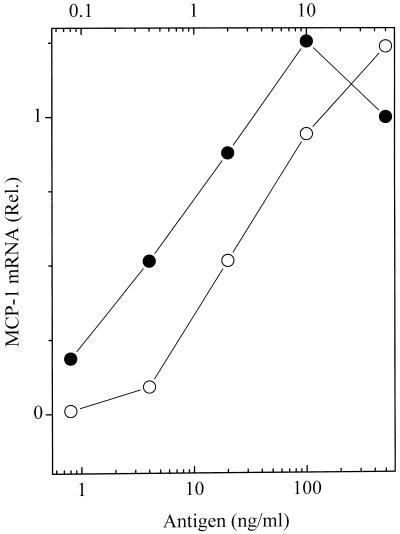
mRNA for MCP-1 is strongly induced upon aggregation of the FcɛRI on rat basophilic leukemia cells (17). Like responses seen for other downstream signals, maximal mRNA was seen at doses of antigen below those that stimulate increasing phosphorylation of FcɛRI (compare data for the two highest doses, squares and circles in Figs. 1 and 5). The rise and fall of mRNA for MCP-1 followed similar kinetics for the two antigens, the maximum level achieved being between 2 and 4 h (Fig. 5). But surprisingly, when normalized to the higher doses of antigen yielding comparable levels of phosphorylated FcɛRI, the levels of the mRNA stimulated by the low- and high-affinity ligands were similar (Fig. 6). It was as if this response was not subject to kinetic proofreading. At doses of antigen sufficiently low so that even the high-affinity ligand stimulated only a partial response, a proportionately reduced dose of the low-affinity ligand stimulated disproportionately diminished synthesis of mRNA for MCP-1. However, at these low doses we cannot be certain that the 10-fold differences in concentrations of the high- and low-affinity antigens stimulated equal amounts of phosphorylated FcɛRI.
Figure 5.
Kinetics of accumulation of MCP-1 mRNA. Cells were stimulated with the alternative high- and low-affinity ligands and the MCP-1 mRNA quantitated by ELIFA analysis (Materials and Methods) with a [32P]-probe. After correction for loading based on the amount of mRNA for β-actin, all of the data were normalized relative to the maximum mRNA observed in one of the samples from the cells reacted with the 50 ng/ml dose of the DNP conjugate. (Upper) DNP conjugate (ng/ml): ■, 50; ●, 10; ▴, 2; ▾, 0.4; ⧫, 0.08. (Lower) NP conjugate (ng/ml): □, 500; ○, 100; ▵, 20; ▿, 4; ◊, 0.8.
Figure 6.
MCP-1 mRNA stimulated by variable doses of the low- and high-affinity ligands. The values shown are the time integrated amounts observed in the experiments shown in Fig. 5, normalized to the response by the cells reacted with 50 ng/ml of the DNP antigen. Upper abcissa: DNP antigen, ●; Lower abcissa: NP antigen, ○.
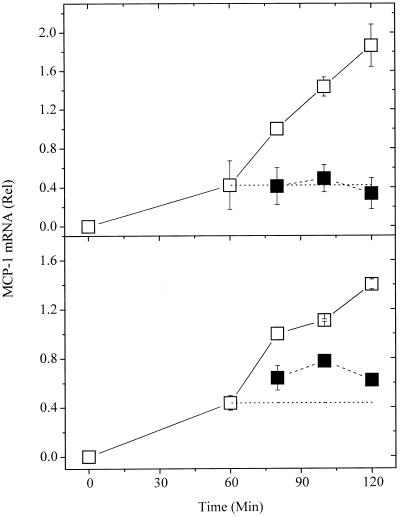
As documented in Fig. 5, the level of mRNA falls fairly sharply after 2–3 h. Possibly, by measuring mRNA levels, we were grossly underestimating the rate of transcription. To test this, actinomycin D was added to some of the wells 1 h after addition of antigen. As seen in Fig. 7 Upper, actinomycin D rapidly blocked further accumulation of mRNA, but the existing mRNA was stable over the time interval assessed. Therefore, in the 1–2 h after addition of antigen, the increase in MCP-1 mRNA accurately reflects the enhanced transcription.
Figure 7.
Effect of actinomycin and hapten on accumulation of MCP-1 mRNA. The values shown are the net differences between the mRNA for the unstimulated vs. stimulated cells. The data are the pooled results from two experiments in which samples for each time point were collected and from a third experiment in which no samples were collected at 100 and 120 min. The data for the cells to which no antigen was added are shown at time “zero” even though they were collected at 60 min. The values for the MCP-1 mRNA were corrected for the content of β-actin mRNA and then normalized to the amount found at 80 min. (□) Positive controls; no inhibitor added. (Upper) Actinomycin added at 60 min (■). (Lower) Hapten added at 60 min (■).
To determine whether the synthesis of MCP-1 mRNA depended on the persistence of aggregated receptors we added a monovalent hapten instead of actinomycin D (Fig. 7 Lower). It is apparent that further accumulation of mRNA was abruptly reduced, although not instantaneously halted.
Finally, we checked whether the doses of the high- and low-affinity antigen that stimulated equivalent phosphorylation of FcɛRI and transcription of the MCP-1 gene also stimulated equivalent secretion of the MCP-1 protein. This proved to be the case: In three experiments, the amount of MCP-1 secreted in response to 50 and 100 ng/ml of NP relative to the amount released after stimulation with a 10-fold lower dose of DNP averaged 0.77 and 0.95, in striking contrast to the receptor-induced release of hexosaminidase (Fig. 3). However, similar to what was observed with respect to transcription of mRNA, at doses where the high-affinity ligand triggered only a partial response, a proportionately lower dose of the low-affinity ligand stimulated disproportionately lower secretion of MCP-1.
Discussion
Purpose of Study.
Our interest is in some of the generic aspects of how surface receptors trigger cellular responses. The mast cells we use to explore such general questions respond to the aggregation of particular cell surface receptors, FcɛRI, when the receptor-associated IgE binds a multivalent ligand. Recently, we observed that the efficiency with which later events were generated from earlier ones correlated with the length of time an individual receptor would be expected to remain in an aggregate due to its association with the ligand through the bound IgE (12). Such differential efficiency is expected for a system subject to a kinetic proofreading regimen (10, 11). In the context of the system we study, such a regimen would apply if individual receptor(s) had to persist in a ligand-induced aggregate throughout the time necessary to generate the distal response being monitored. That the cellular responses are immediately aborted upon hapten-induced disaggregation of the receptors is consistent with such a model.
In the current study we first wanted to see the effects of a change in protocol that, by mechanisms still unclear, enhances the responses of the cells (14). Would this difference affect the extent to which the signaling pathway(s) were constrained by kinetic proofreading? Would the enhanced responsiveness alter the impact of the dissociation rate constant on later events? Second, we wanted to examine some putatively later cellular events that are more easily monitored on the adherent cells used here.
Comparison to Prior Results.
Like the suspended cells studied previously, the adherent cells showed disproportionately diminished responses to the low-affinity (more rapidly dissociating) ligand. Nevertheless, in both protocols, certain quantitative aspects are not easily explained by the simplest type of model. For example, all of the responses show a waxing and waning of the response. As yet, there are few experimental data that account for the decline (6), and it means that at least some of the responses measured likely represent the sum of opposing reactions.
The decreased efficiency by which the rapidly dissociating ligand converts proximal signals into distal effects is characteristic for a system subject to kinetic proofreading. However, in the adherent cells the phenomenon was less pronounced than that observed with cells in suspension. In the latter studies where we used concentrations of antigen comparable to the higher dose used in the current work, the normalized ratio of phosphorylated Syk per phosphorylated receptor was little more than 0.1 for the low affinity compared with the high affinity ligand (see figure 1A in ref. 12); with the adherent cells, the ratios were ≈0.5 and 0.4 for the maximal phosphorylations achieved at the high and low doses (Table 1).
Table 1.
Comparative responses of cells stimulated with slowly or rapidly dissociating ligands
| DNP
|
NP
|
Relative response*
|
||||
|---|---|---|---|---|---|---|
| High dose | Low dose | High dose | Low dose | High dose | Low dose | |
| PY-FcɛR | 1 | 0.21 | 1.01 | 0.28 | 1.01 | 1.3 |
| PY-Syk | 1 | 0.70 | 0.54 | 0.29 | 0.54 | 0.42 |
| Degranulation | 1 | 0.94 | 0.34 | 0.28 | 0.34 | 0.30 |
| c-fos mRNA | 1 | 0.88 | 0.45 | 0.21 | 0.45 | 0.24 |
| MCP-1 mRNA | 1 | 1.26 | 1.24 | 0.94 | 1.24 | 0.75 |
| MCP-1 secretion | N.D. | 1 | N.D. | 0.95 | — | 0.95 |
Cells were stimulated with either 50 (high) or 10 (low) ng/ml of the DNP antigen or 500 (high) or 100 (low) ng/ml of the NP antigen. The responses were integrated over time and then normalized to the response of the cells exposed to the high dose of the DNP antigen. N.D., no data.
Response to NP/response to DNP at high or low dose of antigen.
Several of the responses also showed an unexplained saturation effect. Thus, whereas the higher dose of antigen stimulated a 4-fold increase in the phosphorylation of the receptors, the phosphorylation of Syk it induced was at most 50% greater (Fig. 2 and Table 1). This finding suggests that there was insufficient Syk available to become phosphorylated yet the responses to the two doses of the low-affinity ligand show a similar saturation effect but at much lower absolute levels of phosphorylated Syk. Moreover, direct analysis showed that ≤10% of Syk was precipitable by anti-PY even when the cells were stimulated with the higher dose of the high-affinity ligand (I. Reischl, personal communication). Possibly, the anomalous results reflect an inactivation of phosphorylated Syk (25). However, the model described in the accompanying paper does not predict the results if such a step is introduced (26). Likewise, the data are not explained by postulating that some of the phosphorylated Syk dissociates from the aggregated receptors but remains phosphorylated for a time consistent with results from experiments in which receptors were disaggregated with hapten (26). Similarly, neither the basic nor modified models predict the delay in the maximum phosphorylation of Syk achieved by the low-affinity ligand (Fig. 2). Clearly, additional experiments and modeling will be necessary if these results are to be rationalized.
Transcription of MCP-1.
The most unexpected finding was that at least at the higher doses of antigen, the phosphorylated FcɛRIs engendered by the low-affinity ligand were about as efficient as those produced by the high-affinity ligand in initiating transcription of the gene for MCP-1. This result was particularly notable because of the relatively late appearance of this cellular response. Nevertheless, the response wholly depended on aggregation of the receptors because addition of hapten rapidly aborted the response even when added as late as an hour after the initiation of aggregation and when there is only a small amount of additional binding of antigen (cf. Figs. 1 and 7). These results indicate that a major portion of the transcription of the gene for MCP-1 that occurs between 1 and 2 h after stimulation requires the presence of the ligand:receptor complexes.
Our provisional interpretation of this phenomenon is as follows: Certain biochemical pathways stimulated by FcɛRI, such as those leading to secretion of hexosaminidase and the phosphorylation of cellular constituents such as Pyk2 and Erk2 (12), appear to involve multiple steps that require the continued presence of the particular liganded receptors that initiated the sequence of events.
We suppose that other events, such as the stimulated transcription of the gene for MCP-1, may not be so constrained. That is, that a form of liganded receptor promotes the formation of a component (X′) that in turn stimulates distal steps in a separate pathway without having to be physically associated with the liganded receptor. Because the concentration of this component is only maintained by the continued presence of the receptor-associated intermediate, the system nevertheless remains sensitive to the receptors' state of ligation. The accompanying paper (26) presents a formal analysis of such a branch in a pathway that is otherwise under kinetic proofreading control (figure 5a in ref. 26).
Ca2+ is a plausible candidate for a component no longer constrained by kinetic proofreading in such a scheme. Recent evidence shows that Ca2+-ATPase inhibitors, which raise intracellular Ca2+, promote both transcription of the gene for MCP-1 and secretion of the chemokine (23, 27). Clearly it will now be relevant to see whether ligand affinity influences the changes in intracellular Ca2+ levels induced by aggregation of FcɛRI as well as the downstream events that are in turn affected. Obviously, at some stage the transcription of a gene must be physically independent of the receptor:ligand complex because of their discrete cellular localization in the nucleus and plasma membrane, respectively.
Acknowledgments
We gratefully acknowledge the gift of polyclonal anti-Syk antibody from Dr. Uli Blank. We also thank Drs. Byron Goldstein and Carla Wofsy for comments and suggestions on the manuscript.
Abbreviations
- FcɛRI
high-affinity receptor for IgE
- MCP-1
monocyte chemoattractant protein 1
- DNP
2,4-dinitrophenyl
- NP
2-nitrophenyl
- PY
phosphotyrosine
Footnotes
See commentary on page 6989.
In most anti-hapten systems the forward rate constants, k+1, are more or less similar and the intrinsic affinity is largely governed by the reverse rate constant, k−1 (13). We confirmed this for the DNP and NP antigens used here by measuring the binding of the iodinated derivatives. We obtained a k+1 of ≈5 × 105 M−1⋅sec−1 for the binding of the DNP antigen to adherent cells such as used here (Results). That calculation was based on solving the equation for the rate at which equilibrium is achieved for a reversible reaction between reactants having an affinity (Ka) of ≥1010 M−1. The latter value was estimated from separate experiments in which the binding of the DNP-modified antigen to cells in suspension was examined (data not shown). We were unable to determine k+1 for the NP antigen because of the high concentration of this antigen required to observe appreciable binding and the rapid rate of dissociation during washing of adherent cells. However, it is notable that the stimulation of the initial phosphorylation of FcɛRI induced by the NP-conjugated antigen occurs with similar kinetics as with the high affinity derivative (Fig. 1 Lower). Using cell suspensions, we estimated an avidity of ≈5 × 106 M−1 for the NP antigen.
References
- 1.Keegan A D, Paul W E. Immunol Today. 1992;13:63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- 2.Cambier J C. Immunol Today. 1995;16:110–110. doi: 10.1016/0167-5699(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 3.Eiseman E, Bolen J B. Nature (London) 1992;355:78–80. doi: 10.1038/355078a0. [DOI] [PubMed] [Google Scholar]
- 4.Wofsy C, Torigoe C, Kent U M, Metzger H, Goldstein B. J Immunol. 1997;159:5985–5992. [PubMed] [Google Scholar]
- 5.Wofsy C, Vonakis B M, Metzger H, Goldstein B. Proc Natl Acad Sci USA. 1999;96:8615–8620. doi: 10.1073/pnas.96.15.8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torigoe C, Goldstein B, Wofsy C, Metzger H. Proc Natl Acad Sci USA. 1997;94:1372–1377. doi: 10.1073/pnas.94.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheets E D, Holowka D, Baird B. Curr Opin Chem Biol. 1999;3:95–99. doi: 10.1016/s1367-5931(99)80017-9. [DOI] [PubMed] [Google Scholar]
- 8.Peirce M J, Metzger H. J Biol Chem. 2000;275:34976–34982. doi: 10.1074/jbc.M005819200. [DOI] [PubMed] [Google Scholar]
- 9.Barsumian E L, Isersky C, Petrino M G, Siraganian R P. Eur J Immunol. 1981;11:317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- 10.Hopfield J J. Proc Natl Acad Sci USA. 1974;71:4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKeithan T W. Proc Natl Acad Sci USA. 1995;92:5042–5046. doi: 10.1073/pnas.92.11.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Torigoe C, Inman J K, Metzger H. Science. 1998;281:568–572. doi: 10.1126/science.281.5376.568. [DOI] [PubMed] [Google Scholar]
- 13.Day L A, Sturtevant J M, Singer S J. Ann NY Acad Sci. 1963;103:611–616. doi: 10.1111/j.1749-6632.1963.tb53721.x. [DOI] [PubMed] [Google Scholar]
- 14.Hamawy M M, Oliver C, Mergenhagen S E, Siraganian R P. J Immunol. 1992;149:615–621. [PubMed] [Google Scholar]
- 15.Gu L, Tseng S C, Rollins B J. Chem Immunol. 1999;72:7–29. doi: 10.1159/000058723. [DOI] [PubMed] [Google Scholar]
- 16.Gordon J R. In: IgE Receptor (FcɛRΙ) Function in Mast Cells and Basophils. Hamawy M M, editor. Austin, TX: Landes; 1997. pp. 209–242. [Google Scholar]
- 17.Chen H, Centola M, Altschul S F, Metzger H. J Exp Med. 1998;188:1657–1668. doi: 10.1084/jem.188.9.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F T, Bohn J W, Ferry E L, Yamamoto H, Molinaro C A, Sherman L A, Klinman N R, Katz D H. J Immunol. 1980;124:2728–2737. [PubMed] [Google Scholar]
- 19.Holowka D, Metzger H. Mol Immunol. 1982;19:219–227. doi: 10.1016/0161-5890(82)90334-0. [DOI] [PubMed] [Google Scholar]
- 20.Rivera J, Kinet J-P, Kim J, Pucillo C, Metzger H. Mol Immunol. 1988;25:647–661. doi: 10.1016/0161-5890(88)90100-9. [DOI] [PubMed] [Google Scholar]
- 21.Stein K E, Kanellopoulos-Langevin C, Cohen D I, Inman J K. J Immunol Methods. 1980;37:83–88. doi: 10.1016/0022-1759(80)90183-0. [DOI] [PubMed] [Google Scholar]
- 22.Alber G, Kent U M, Metzger H. J Immunol. 1992;149:2428–2436. [PubMed] [Google Scholar]
- 23.Onose J, Teshima R, Sawada J. Immunol Lett. 1998;64:17–22. doi: 10.1016/s0165-2478(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 24.Grupp S A, Campbell K, Mitchell R N, Cambier J C, Abbas A K. J Biol Chem. 1993;268:25776–25779. [PubMed] [Google Scholar]
- 25.Lupher M L J, Rao N, Lill N L, Andoniou C E, Miyake S, Clark E A, Druker B, Band H. J Biol Chem. 1998;273:35273–35281. doi: 10.1074/jbc.273.52.35273. [DOI] [PubMed] [Google Scholar]
- 26.Hlavacek W S, Redondo A, Wofsy C, Metzger H, Goldstein B. Proc Natl Acad Sci USA. 2001;98:7295–7300. doi: 10.1073/pnas.121172298. . (First Published June 5, 2001; 10.1073/pnas.121172298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teshima R, Onose J, Okunuki H, Sawada J. Int Arch Allergy Immunol. 2000;121:34–43. doi: 10.1159/000024295. [DOI] [PubMed] [Google Scholar]