Abstract
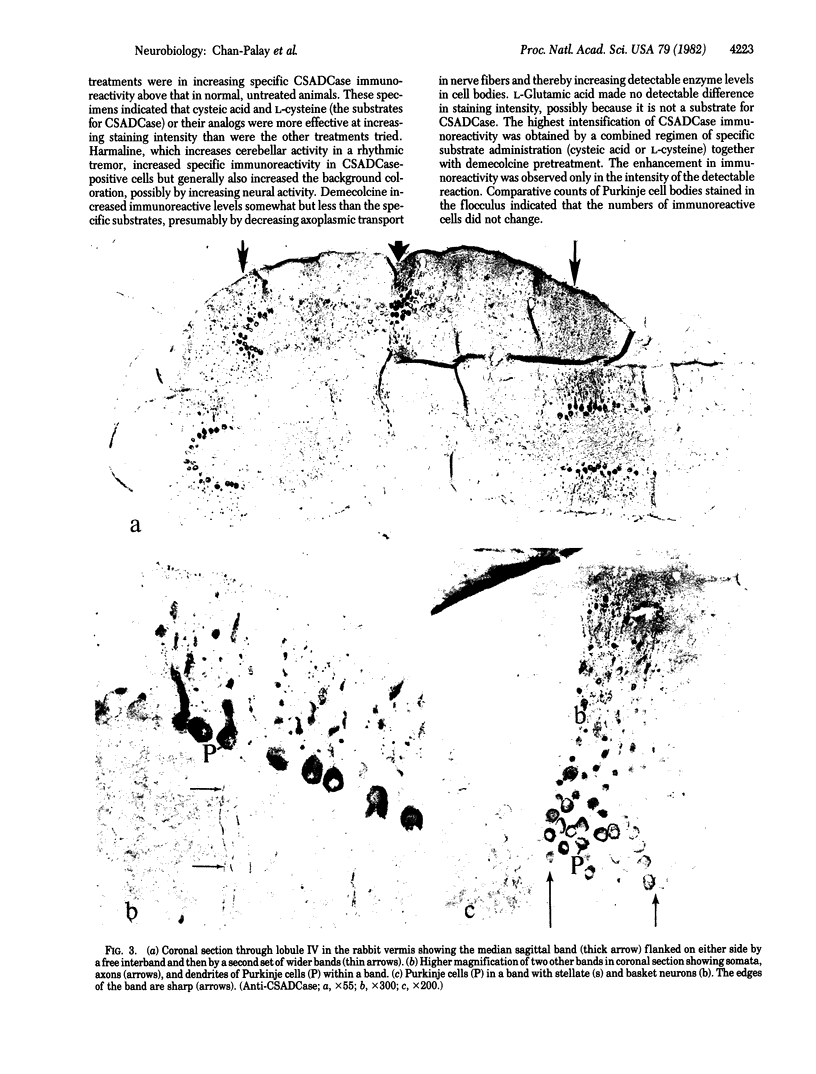
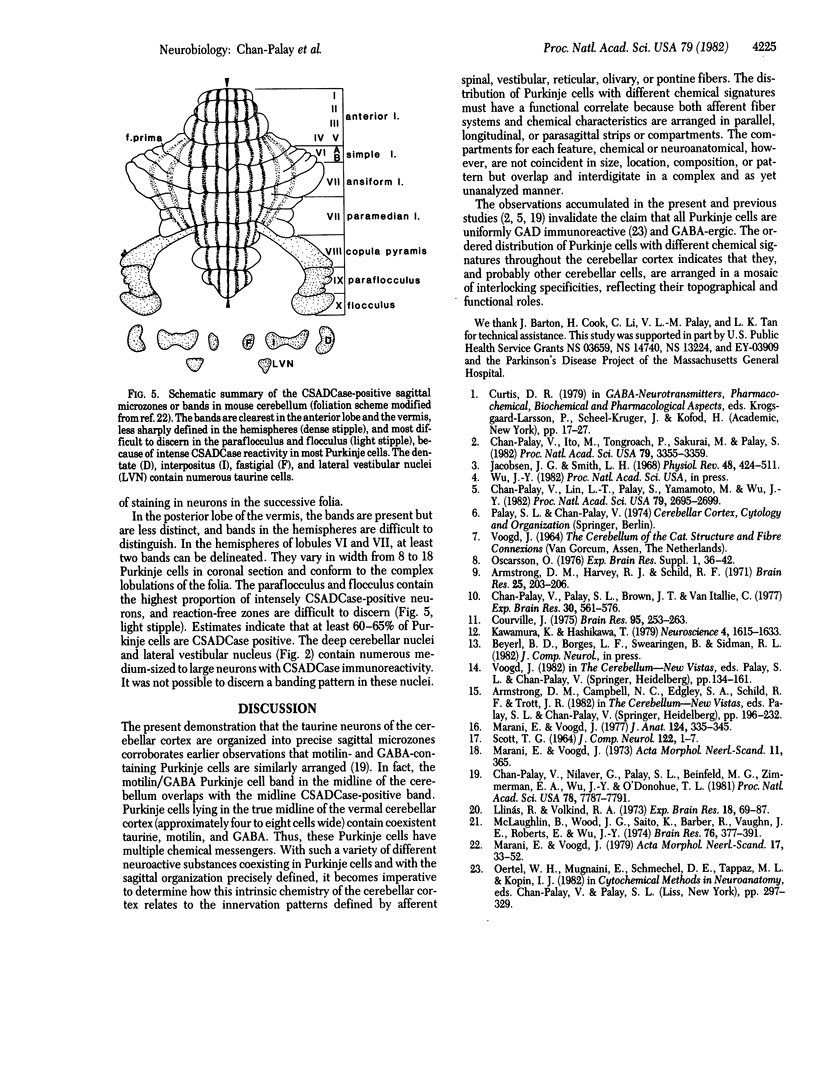
Taurine neurons in the cerebellum of rabbit, rat, and mouse were localized at the light microscope level by using polyclonal antibodies against cysteine sulfinic acid decarboxylase (CSADCase; EC 4.1.1.29), the enzyme responsible for the conversion of cysteine sulfinic acid to hypotaurine and of cysteic acid to taurine. The indirect peroxidase-antiperoxidase method was used on Vibratome sections and on serial sections of paraffin-embedded tissue. Intensification of CSADCase immunoreactivity was achieved by pretreatment of the animal with L-cysteine or L-cysteic acid intravenously 1-2 hr prior to perfusion. A combination of L-cysteic acid and demecolcine, which retards axoplasmic flow, was most effective in maximizing CSADCase immunoreactivity. Although these treatments intensified immunoreactivity in neurons, no more cells were reactive than in untreated controls. L-Glutamic acid did not increase CSADCase immunoreactivity but did increase immunoreactivity with antibodies against L-glutamic acid decarboxylase (GAD; EC 4.1.1.15), the synthetic enzyme for γ-aminobutyric acid. Specificity was established by negative results obtained with various control incubations including the use of CSADCase antiserum preabsorbed with the antigen. Taurine neurons of the cerebellar cortex are arranged in sagittal microbands, defined by intensely CSADCase-reactive Purkinje neurons and their axons and dendrites, together with stellate, basket, and Golgi cells and their processes. In the vermis there is a narrow midline band, flanked laterally by three wider bands on either side, each separated from the next by an unreactive zone. Although the zonal borders are sharp, the interzonal areas contain some CSADCase-immunoreactive axons but no cell bodies. The seven vermal bands are best observed in the anterior lobe. Others exist in the lateral hemispheres. The paraflocculus and flocculus contain numerous intensely immunoreactive neurons, and banding is difficult to discern. Lobule X of the vermis is also heavily endowed with taurine neurons. Numerous large and medium-sized deep cerebellar and vestibular nuclei are also immunoreactive. These observations indicate that cerebellar neurons are chemically heterogeneous but that neurons of similar chemical signature in the cerebellar cortex are organized into sagittal microbands. This corroborates our earlier evidence that Purkinje cells containing motilin and those containing both motilin and γ-aminobutyric acid are also arranged in vermal sagittal microbands. The midline vermal band contains Purkinje neurons with multiple neuroactive substances—taurine, γ-aminobutyric acid, and motilin. It remains to be determined how this chemical zonation in the cerebellar cortex relates to the banded afferent innervation from spinal, vestibular, reticular, and olivary sources.
Keywords: Purkinje cell, interneurons, vermis, flocculonodular
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Harvey R. J., Schild R. F. Distribution in the anterior lobe of the cerebellum of branches from climbing fibres to the paramedian lobule. Brain Res. 1971 Jan 8;25(1):203–206. doi: 10.1016/0006-8993(71)90583-x. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V., Ito M., Tongroach P., Sakurai M., Palay S. Inhibitory effects of motilin, somatostatin, [Leu]enkephalin, [Met]enkephalin, and taurine on neurons of the lateral vestibular nucleus: interactions with gamma-aminobutyric acid. Proc Natl Acad Sci U S A. 1982 May;79(10):3355–3359. doi: 10.1073/pnas.79.10.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V., Lin C. T., Palay S., Yamamoto M., Wu J. Y. Taurine in the mammalian cerebellum: demonstration by autoradiography with [3H]taurine and immunocytochemistry with antibodies against the taurine-synthesizing enzyme, cysteine-sulfinic acid decarboxylase. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2695–2699. doi: 10.1073/pnas.79.8.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V., Nilaver G., Palay S. L., Beinfeld M. C., Zimmerman E. A., Wu J. Y., O'Donohue T. L. Chemical heterogeneity in cerebellar Purkinje cells: existence and coexistence of glutamic acid decarboxylase-like and motilin-like immunoreactivities. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7787–7791. doi: 10.1073/pnas.78.12.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V., Palay S. L., Brown J. T., Van Itallie C. Sagittal organization of olivocerebellar and reticulocerebellar projections: autoradiographic studies with 35S-methionine. Exp Brain Res. 1977 Dec 19;30(4):561–576. doi: 10.1007/BF00237645. [DOI] [PubMed] [Google Scholar]
- Courville J. Distribution of olivocerebellar fibers demonstrated by a radioautographic tracing method. Brain Res. 1975 Sep 23;95(2-3):253–263. doi: 10.1016/0006-8993(75)90105-5. [DOI] [PubMed] [Google Scholar]
- Jacobsen J. G., Smith L. H. Biochemistry and physiology of taurine and taurine derivatives. Physiol Rev. 1968 Apr;48(2):424–511. doi: 10.1152/physrev.1968.48.2.424. [DOI] [PubMed] [Google Scholar]
- Kawamura K., Hashikawa T. Olivocerebellar projections in the cat studied by means of anterograde axonal transport of labelled amino acids as tracers. Neuroscience. 1979;4(11):1615–1633. doi: 10.1016/0306-4522(79)90024-1. [DOI] [PubMed] [Google Scholar]
- Llinás R., Volkind R. A. The olivo-cerebellar system: functional properties as revealed by harmaline-induced tremor. Exp Brain Res. 1973 Aug 31;18(1):69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- Marani E., Voogd J. An acetylcholinesterase band-pattern in the molecular layer of the cat cerebellum. J Anat. 1977 Nov;124(Pt 2):335–345. [PMC free article] [PubMed] [Google Scholar]
- Marani E., Voogd J. The morphology of the mouse cerebellum. Acta Morphol Neerl Scand. 1979 Feb;17(1):33–52. [PubMed] [Google Scholar]
- McLaughlin B. J., Wood J. G., Saito K., Barber R., Vaughn J. E., Roberts E., Wu J. Y. The fine structural localization of glutamate decarboxylase in synaptic terminals of rodent cerebellum. Brain Res. 1974 Aug 23;76(3):377–391. doi: 10.1016/0006-8993(74)90815-4. [DOI] [PubMed] [Google Scholar]