Abstract
In this study we examine the effects of aging on antigen presentation of B cells and monocytes. We compared the antigen presentation function of peripheral blood B cells from young and old subjects using a system that specifically measures the B cell receptor (BCR)-mediated MHC-II antigen presentation. Monocytes were studied as well. Overall the mean magnitude of antigen presentation of soluble antigen and peptide was not different in older and younger subjects for both B cells and monocytes. Older subjects, however, showed increased heterogeneity of BCR-mediated antigen presentation by their B cells. The magnitude and variability of peptide presentation, which does not require uptake and processing, was the same between groups. Presentation by monocytes had similar variability between the older and younger subjects. These data suggest that poor B cell antigen processing, which results in diminished presentation in some older individuals may contribute to poor vaccine responses.
Keywords: antigen presentation, antigen processing, geriatrics, B cells, monocytes, immunosenescence
1. Introduction
The aging population in the U.S. is on the rise. The population over 65 years of age is projected to increase from 40.2 million in 2010 to 88.5 million 2050 [1]. With the increasing geriatric population comes an increase in the prevalence of age-related morbidities. In 2007, influenza and pneumonia represented the 7th leading cause of death in Americans 65 years and older [2]. Thus, vaccination of geriatric populations, particularly for influenza and pneumococcus has become a major healthcare initiative. However, undermining even the most successful vaccination programs is the inadequate response of many geriatric patients to vaccines. Estimates of the clinical efficacy of the standard influenza vaccine in persons over 65 years old vary widely but in all cases they are substantially lower than in younger populations [3; 4; 5].
Underlying these impaired responses is the concept of immunosenescence, or age-related changes in the immune system and its effector functions. Immunosenescence is multi-factorial and may be related to quantitative changes in cell populations including those important for T cell dependent vaccines [6; 7; 8]. Immunosenescence of T cells has been studied most extensively, revealing reduced numbers of effector memory CD4 T cells, increased apoptosis and functional defects in T cells from older individuals [9; 10; 11; 12; 13]. Most of the studies on ex vivo antigen presenting cell (APC) function in older persons have focused on characterization of costimulatory receptors and HLA-DR expression or functionally measuring their ability to do mixed lymphocyte reaction (MLR). These studies have had conflicting results [14; 15]. There are conflicting results in APC function from dendritic cells derived in vitro from monocytes from older individuals as well [16; 17]. This current study focuses on ex vivo studies of two APC types (B cells and monoctyes). There are virtually no data on antigen presentation function by these cell types in older humans to date.
In humans the paucity of data that focuses specifically on antigen presentation function by APC is due to a lack of a standardized method to compare antigen presentation across multiple individuals. We have developed a novel T cell hybridoma system that can study antigen processing and presentation in appropriately HLA-DR matched individuals. This allows us to perform quantitative comparisons of antigen presentation function of B cells and monocytes across the age groups in this study.
2. Materials and Methods
2.1 Study Subjects
Recruitment and participation of older and younger subjects was approved by University Hospitals Case Medical Center and the Cleveland VA Institutional Review Boards. Informed consent was obtained from all subjects for peripheral blood sampling. Subjects were screened for HLA-DRB1 *01.01 with low and then confirmatory high resolution PCR analysis (Biosynthesis, Lewisville, TX). 14 older and 10 young healthy controls screened positive for HLA-DRB1 *01.01 and were available and enrolled in the study. Subjects with inflammatory conditions, using immunomodulatory drugs such as steroids, or with recent acute illness or hospitalization were excluded. 13 older subjects underwent frailty testing using the Fried frailty method [18].
2.2. Cell lines and antigens
HLA-DRB1 *01.01-restricted goat antibody specific (line 1C) and M. tuberculosis antigen 85b specific (line F9A6) T cell hybridiomas were generated and characterized using an IACUC approved protocol as previously described [19; 20]. Line 1C is specific for goat Fab and line F9A6 is specific for M. tuberculosis (MTB) antigen 85b aa 97-112.
Goat anti-human IgM was purchased from Lampire (Pipersville, PA). MTB antigen 85b was grown in E. coli and purified on nickel-columns using a plasmid given to us by Douglas Kernodle [21]. 85b peptide (aa 97–112) was purchased from Biosynthesis.
2.3 Isolation of B cells and monocytes
All blood was collected in heparin containing green top tubes (BEBiosciences). Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll Paque Plus (Amersham Biosciences) density gradient centrifugation according to the manufacture's instructions.
Monocytes were purified by positive selection with CD14 MicroBeads (Miltenyi) and B-cells were purified from the remaining cells using the EasySep Human B-cell Enrichment kit (StemCell). Purity was determined by flow cytometry as described below. Purified CD19+ B cells had <2% CD14+ contamination, and purified CD14+ monocytes had <2% CD19+ contamination.
2.4 Antigen Processing and Presentation Assay
Purified B cells (25,000/well) or purified monocytes (50,000/well) were plated in 96-well round-bottom plates in DMEM based medium with 10% FCS. T cell hybridoma (100,000/well) were added to each well with the appropriate soluble antigen or peptide and incubated at 37°C. After 22 h, supernatants were harvested and IL-2 levels measured by ELISA (eBioscience). Aliquots of a single batch of each T cell hybridoma line were frozen so that APC could be tested with an identical T cell line readout over the course of the experiments. Single batches of antigen and IL-2 ELISA standard were so that the B cells and monocytes were the only variables in the assay.
2.5 Flow Cytometry
B-cells and monocytes were stained with live-dead violet stain (Invitrogen), anti-CD14 (BD Biosciences), and anti-CD19, HLA-DR, IgM, LFA3 and ICAM1 (Biolegend) for 10 min at RT. Cells were then washed and fixed in 2% paraformaldehyde till analysis on a LSRI-II (BDBiosciences) flow cytometer. Cells were gated on CD14+ monocytes or CD19+ B cells and Mean Fluorescent Intensity (MFI) of the surface markers was analyzed using FlowJo software (Tree Star, Ashland, OR).
2.6 Statistical Analysis
Statistics were analyzed using SPSS software (IBM). Comparisons between mean differences in groups for antigen presentation assays and surface expression of HLA-DR, IgM, CD54, and CD58 were performed using independent samples t-tests after determination that the data were normally distributed. Differences in variability were determined by Levene's test of equality of error variances using two-tailed assumptions.
3. Results
3.1. Subjects
We obtained samples from 14 older individuals, aged 62-92. Table 1 summarizes the pertinent clinical data. The population is overall an older and more frail population with average age of 80 years. Frailty is a syndrome that has been associated with high risk of falls, disability, hospitalization and mortality [18]. Of the 13 older subjects tested, all of them were either pre-frail or frail as determine by the Fried frailty testing method. None of the subjects had diagnosis of any systemic inflammatory condition or were on immunomodulatory drugs such as steroids. Subject VH98 has early stage prostate adenocarcinoma diagnosed by needle biopsy that was confined to the prostate. He did not receive chemotherapy and was therefore left in the dataset. The 10 control subjects were healthy individuals from the VA and university employee community, aged 22-45.
Table 1. Older subject description.
| Subject | Age | Gender | Race | Frailty Status | Medical history |
|---|---|---|---|---|---|
| VH139 | 75 | Male | Black | frail | COPD, CHF |
| VF8 | 78 | Male | White | frail | COPD |
| VF13 | 90 | Female | White | frail | ESRD on HD |
| VF16 | 63 | Male | White | pre-frail | |
| VM77 | 81 | Male | White | frail | CHF |
| VM121 | 78 | Male | White | pre-frail | DM |
| VM98 | 92 | Female | White | pre-frail | |
| VM117 | 92 | Male | Black | pre-frail | ESRD on HD, COPD, CHF |
| VH80 | 88 | Male | White | unknown | COPD |
| VM97 | 88 | Male | Black | frail | COPD |
| VM29 | 88 | Male | White | pre-frail | COPD |
| VF78 | 65 | Male | White | pre-frail | DM |
| VH98 | 81 | Male | White | pre-frail | stage T1c prostate CA |
| VF20 | 62 | Male | White | pre-frail |
COPD=Chronic obstructive pulmonary disease, CHF=congestive heart failure, ESRD on HD= Endstage renal disease on hemodialysis, DM= diabetes mellitus, CA=cancer
3.2. Antigen presentation by B cells
The purpose of the study was to determine if there is a difference in antigen processing and presentation by B cells and monocytes from older individuals versus younger individuals. HLA-DR1-restricted T cell hybridomas were used to quantify antigen processing and presentation by the B cells. As it typical of T cell hybridomas, these lines were selected based on their ability quantatively secrete IL-2 in proportion to the amount of peptide-MHC-II on the surface of the APC. These hybridoma lines respond to human APC independent of CD80 and CD86 co-stimulation as previously described [19; 22]. As a result, they are more specific tools to measure differences in antigen presentation alone and not co-stimulatory function.
The T cell hybridoma system used to quantify B cell antigen presentation measures BCR-mediated MHC-II antigen presentation. This is important because antigen presentation after BCR-mediated endocytosis is much more efficient than after non-specific uptake of antigen [23; 24]. Presentation of the antigen specific to a B cells' BCR is necessary for the B cell to receive help from CD4+ T cells. The mean magnitude of BCR-mediated antigen presentation was the same in older and younger subjects (Fig. 1A-C). There was a trend or statistically significant increase in heterogeneity of BCR-mediated antigen presentation in older persons as determined by Levene's Test for Equality of Variances at the three concentrations of antigen tested (0.2 ng/ml at p= 0.15, 2 ng/ml at p=0.04, and 7 ng/ml at p=0.08, Fig. 1C). 4 of 11 older subjects demonstrated inferior processing and presentation at all concentrations of anti-BCR antigen (Fig. 1B red dotted lines) compared to the results from all younger subjects studied (Fig. 1A). We compared whole antigen (anti-BCR antigen) that requires uptake and processing with peptide that can be directly presented on the cell surface. Presentation of peptide on B cells was measured with the MTB antigen 85b-specific hyrbidoma since the exact peptide epitope of the anti-BCR antigen-specific hybridoma is not known. The magnitude and variability of peptide presentation was the same between groups (Fig 1d-F). The 4 older subjects whose anti-BCR antigen was poorly presented are marked in red dotted lines to demonstrate that presentation of peptide in these individuals was not different than the younger population (Fig. 1 D and E).
Fig.1. Antigen presentation by B cells in younger and older individuals.
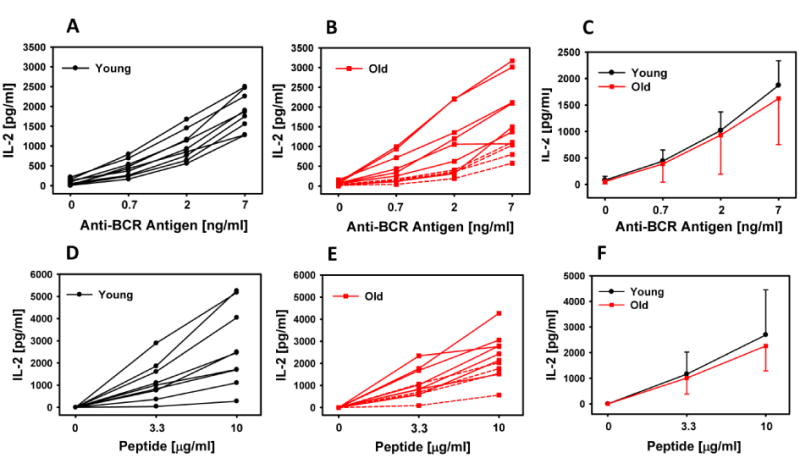
B cells (2.5×104/well) were incubated with the T cell hybridoma (1×105/well) and the appropriate antigen overnight. Antigen for A-C was anti-BCR antigen and D-F was peptide. Supernatants were harvested, and IL-2 was determined by ELISA. Each line represents an individual subject. The dotted lines in panels B and E indicate the 4 lowest responders to anti-BCR antigen. Panel C summarizes presentation of anti-BCR antigen from panels A and B and panel F summarizes presentation of peptide from panels D and E. Error bars depict SD of these groups.
3.3. Antigen Presentation by Monocytes
Monocytes were purified from donor PBMC by positive selection. Antigen processing and presentation was assessed using whole MTB antigen 85b, which requires uptake and processing to be presented. Presentation of peptide was determined as well. Figure 2A-F demonstrates there was no difference in the magnitude of soluble whole 85B antigen or peptide by monocytes from young and old subjects. Fig 2C demonstrates that there also was no difference in the variability of presentation of the soluble antigen as well. The 4 subjects that had the poorest B cell antigen processing and presentation were indicated with dotted lines in Fig. 2 B and E. These data show that their monocytes were not consistently inferior in their ability to process and present antigen.
Fig.2. Antigen presentation by monocytes in younger and older individuals.
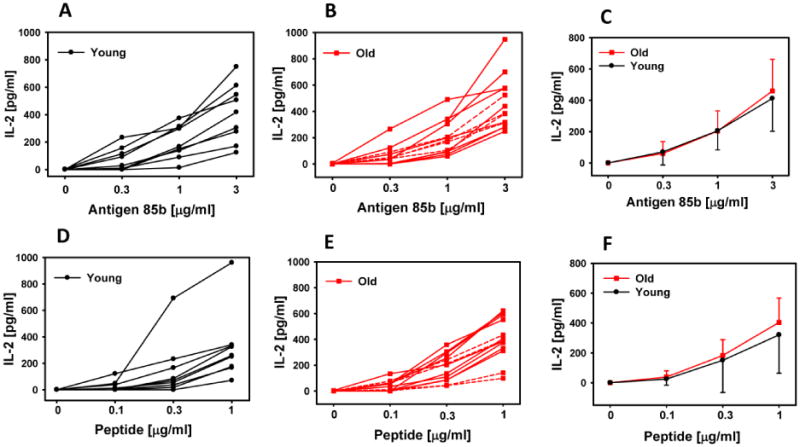
Monocytes (5×104/well) were incubated with the T cell hybridoma (1×105/well) and the appropriate antigen overnight. Antigen for A-C was soluble antigen 85b antigen and D-F was peptide. Supernatants were harvested, and IL-2 was determined by ELISA. Each line represents an individual subject. The dotted lines in panels B and E indicate the 4 lowest responders to anti-BCR antigen in Fig 1B. Panel C summarizes presentation of 85b antigen from panels A and B and panel F summarizes presentation of peptide from panels D and E. Error bars depict SD of these groups.
3.4. Surface Expression of MHC II and BCR
BCR is surface immunoglobulin. In our antigen presentation system surface IgM is the BCR molecule that must take up the antigen in B cells. HLA-DR is the molecule that presets peptide on both B cells and monocytes. Surface expression of HLA-DR (MHC-II) and IgM were assessed using flow cytometry. Live-Dead Violet was used to gate out non-viable cells. CD19 and CD14 were used to gate on of B-cells and Monocytes, respectively. The HLA-DR and IgM expression by flow cytometry was not statistically different between the older and younger groups for either B cells or monocytes. The MFI for HLA-DR on younger B cells was 30,800 (SD 9,730) and older B cells 25,400 (SD 6,920). The MFI for HLA-DR on younger MN was 26,900 (SD 11,100) and older MN 28,400 (SD 6,250). The MFI for IgM on younger B cells was 7,370 (SD 3,400) and on older B cells 25,000 (SD 33,100). The IgM level on older B cell were strongly affected by 2 older donors with MFI's of 88,300 and 94,200. The IgM levels were still statistically not different (p>0.13) with or without these two influential subjects. Interestingly both of these donors are in the group of 4 older subjects whose BCR mediated antigen presentation was below the antigen presentation of every control (Fig 1. dotted lines). Levels of adhesion molecules CD54 and CD58, which are involved in forming the immunological synapse between T cells and APC, were also assessed and had no significant difference between young and old subjects, for either B cells or monocytes (data not shown).
4. Discussion
There is very little data in the literature that focuses specifically on antigen processing and presentation function in human APC from older individuals. This is due primarily to difficulties in the experimental system to compare antigen processing and presentation function between groups of persons. Measurement of antigen presentation is routinely performed by a bioassay where the T cell responses reflect that magnitude of antigen presentation by the APC. The difficulty with this T cell bioassay system is having a T cell system that can work across multiple subjects with the caveat that T cells have an HLA-restriction for their recognition of APC. Our T cell hybridoma system allows the use of a highly characterized HLA-restricted T cell clones to study all of the HLA-DR1.01+ subjects and compare them. All of the T cells come from a common batch of T cells that was cryopreserved and is thawed and used immediately for each assay. This allows comparison between subjects with only the APC as the variable and not the antigen presentation “readout”. An additional advantageous feature of the system and data of the current study is that the T cell lines are co-stimulation (CD80/CD86) independent. This allows the T cell tool to reflect a more antigen presentation specific result due to lack of dependence on co-stimulatory molecules. A number of prior studies on human APC function had studied MLR activity [14; 15]. MLR is not an antigen processing and presentation assay but rather it is primarily an assay that depends on the co-stimulatory ability of the APC. The antigen processing and presentation specificity of these data in the current study is a novel contribution the field.
The B cell populations were evaluated using a system that measure BCR-mediated antigen presentation since this is extremely efficient and is the relevant mechanism in vivo. Presentation of peptide was also determined as it does not require processing and serves as a comparator of the capability of presentation vs. the entire process of antigen uptake and processing that results in presentation. In the BCR-mediated assays, the mean antigen presentation by B cells from the older group on average was maintained but did show increased heterogeneity in antigen presentation ability. Namely, a subset of 4 older subjects showed inferior antigen presentation compared to younger subjects at each concentration of antigen tested. This suggests that B cells from some older individuals may have a defect in antigen processing capability that could result in immune deficits.
B cells from older subjects show preserved ability to present peptide on MHC II molecules. Since presentation of peptide was preserved, even in the 4 subjects with poor BCR-mediated presentation, the reduced BCR-mediated antigen presentation in these 4 individuals may concern antigen uptake and/or intracellular processing. Possible mechanisms of poor antigen presentation were examined by measuring surface levels of HLA-DR, IgM (BCR), CD54 and CD58. There was no significant difference in expression levels among young and old subjects. These findings point to an internal process as the cause of the poor antigen presentation capability in some of the older subjects and not a lack of the uptake receptor (IgM) or the presentation molecule (HLA-DR). MHC II antigen processing is complex and defects could occur in degradation of antigen into peptides, loading of peptides onto MHC II molecules, or trafficking of molecules within the APC. Further mechanistic studies are beyond the scope of this study primarily due to limitations in sample for the older subjects.
We performed similar experiments with monocytes from old and young subjects and found no significant difference in the ability of monocytes to process or present antigen or peptide. Likewise, we observed no difference in surface expression of HLA-DR, CD54 or CD58. Also there was not increased heterogeneity of presentation in monocytes that was seen in the B cells. This suggests there may be B cell specific processes that are defective in some older donors that are not present in monocytes.
In the age range of most of the subjects in this study, T cell immunosenescence has been well described and is significant in magnitude [9; 10; 11; 12]. Our study sample overall was older, with a mean age of 80 and relatively frail, with no robust subjects as determined by the Fried method. 5 of 14 subjects were found to be frail. The 4 subjects with the worst B cell antigen presentation were all pre-frail and age 62, 65, 81, and 88 years old. So they were not among frail or oldest subjects. These data then show that generally B cell and monocyte presentation among even very old and frail person is preserved. This was an unexpected finding and underscores the described importance of T cell immunosenescence for the observed defects of older persons to respond to vaccination. However, other non-antigen processing and presentation functions of APC such as ability to become activated, costimulation, or cytokine/chemokine production may play a role as well in immunosenescence observed in older individuals.
Immunosenescence and poor vaccine response in older populations are likely multifactorial and our data suggest some explanation for the heterogeneity observed among older adults. B cell function underlies the ability to mount an effective and lasting immune response to infection or vaccination. The variability in antigen presentation capability by B cells of older subjects in this study may point to why some elderly patients respond poorly to common vaccines, such as the influenza and hepatitis B vaccines [4; 25]. Further investigation is required to elucidate the cause and potential significance of this poor processing ability in a subset of older individuals
Highlights.
Antigen presentation by B cells preserved with aging
Increased heterogeneity of antigen presentation in B cells from older persons
Antigen presentation by monocytes preserved with aging
Surface HLA-DR and IgM preserved with aging
Acknowledgments
Thanks to Htin Aung for assistance generating the figures. This work was supported by VA Merit, AI080313, AI077056, and AI36219.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Clark HL, Email: hlc36@case.edu.
Banks R, Email: Richard.Banks2@va.gov.
Jones L, Email: lxa4@case.edu.
Hornick TR, Email: Thomas.Hornick@va.gov.
Higgins PA, Email: pxg3@case.edu.
Burant CJ, Email: cxb43@case.edu.
References
- 1.Vincent GK, Velkoff VA. Current Population Reports. U.S. Department of Commerce Economics and Statistics Administreation: U.S. Census Bureau; 2010. The Next Four Decades. The Older Population in the United States: 2010 to 2050. [Google Scholar]
- 2.With Special Feature on Death and Dying, National Center for Health Statistics. Hyattsville, MD: 2011. [PubMed] [Google Scholar]
- 3.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 4.Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2010:CD004876. doi: 10.1002/14651858.CD004876.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24:1159–69. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 6.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117:57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 7.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 8.Grubeck-Loebenstein B, Della Bella S, Iorio AM, Michel JP, Pawelec G, Solana R. Immunosenescence and vaccine failure in the elderly. Aging Clin Exp Res. 2009;21:201–9. doi: 10.1007/BF03324904. [DOI] [PubMed] [Google Scholar]
- 9.Kang I, Hong MS, Nolasco H, Park SH, Dan JM, Choi JY, Craft J. Age-associated change in the frequency of memory CD4+ T cells impairs long term CD4+ T cell responses to influenza vaccine. J Immunol. 2004;173:673–81. doi: 10.4049/jimmunol.173.1.673. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal S, Gupta S. Increased apoptosis of T cell subsets in aging humans: altered expression of Fas (CD95), Fas ligand, Bcl-2, and Bax. J Immunol. 1998;160:1627–37. [PubMed] [Google Scholar]
- 11.Tamir A, Eisenbraun MD, Garcia GG, Miller RA. Age-dependent alterations in the assembly of signal transduction complexes at the site of T cell/APC interaction. J Immunol. 2000;165:1243–51. doi: 10.4049/jimmunol.165.3.1243. [DOI] [PubMed] [Google Scholar]
- 12.Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ T cells. Immunol Rev. 2005;205:220–8. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–22. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shurin MR, Shurin GV, Chatta GS. Aging and the dendritic cell system: implications for cancer. Crit Rev Oncol Hematol. 2007;64:90–105. doi: 10.1016/j.critrevonc.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varas A, Sacedon R, Hernandez-Lopez C, Jimenez E, Garcia-Ceca J, Arias-Diaz J, Zapata AG, Vicente A. Age-dependent changes in thymic macrophages and dendritic cells. Microsc Res Tech. 2003;62:501–7. doi: 10.1002/jemt.10411. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal A, Gupta S. Impact of aging on dendritic cell functions in humans. Ageing Res Rev. 2011;10:336–45. doi: 10.1016/j.arr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steger MM, Maczek C, Grubeck-Loebenstein B. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin Exp Immunol. 1996;105:544–50. doi: 10.1046/j.1365-2249.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Bartley MB, Canaday DH. T cell hybridomas to study MHC-II restricted B-cell receptor-mediated antigen presentation by human B cells. J Immunol Methods. 2011;370:35–42. doi: 10.1016/j.jim.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canaday DH, Gehring A, Leonard EG, Eilertson B, Schreiber JR, Harding CV, Boom WH. T-cell hybridomas from HLA-transgenic mice as tools for analysis of human antigen processing. J Immunol Methods. 2003;281:129–42. doi: 10.1016/j.jim.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Lakey DL, Voladri RK, Edwards KM, Hager C, Samten B, Wallis RS, Barnes PF, Kernodle DS. Enhanced production of recombinant Mycobacterium tuberculosis antigens in Escherichia coli by replacement of low-usage codons. Infect Immun. 2000;68:233–8. doi: 10.1128/iai.68.1.233-238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canaday DH, Chakravarti S, Srivastava T, Tisch DJ, Cheruvu VK, Smialek J, Harding CV, Ramachandra L. Class II MHC antigen presentation defect in neonatal monocytes is not correlated with decreased MHC-II expression. Cell Immunol. 2006;243:96–106. doi: 10.1016/j.cellimm.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rock KL, Benacerraf B, Abbas AK. Antigen presentation by hapten-specific B lymphocytes. I. Role of surface immunoglobulin receptors. J Exp Med. 1984;160:1102–13. doi: 10.1084/jem.160.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanzavecchia A, Bove S. Specific B lymphocytes efficiently pick up, process and present antigen to T cells. Behring Inst Mitt. 1985:82–7. [PubMed] [Google Scholar]
- 25.Wolters B, Junge U, Dziuba S, Roggendorf M. Immunogenicity of combined hepatitis A and B vaccine in elderly persons. Vaccine. 2003;21:3623–8. doi: 10.1016/s0264-410x(03)00399-2. [DOI] [PubMed] [Google Scholar]


