Abstract
Bone marrow-derived endothelial progenitor cells (EPCs) constitute an important endogenous system in the maintenance of endothelial integrity and vascular homeostasis. Cardiovascular risk factors are associated with a reduced number and functional capacity of EPCs. Here we investigated the effect of transplantation of bone marrow-derived cells from Dahl salt-resistant rat into age-matched Dahl salt-sensitive (DS) rat on blood pressure, endothelial function, and circulating EPC number. The recipient DS rats were fed a normal (0.5% NaCl, NS) or high salt (4% NaCl, HS) diet for 6 weeks after BMT. DS rats on a NS or a HS diet without BMT were used as controls. Hypertensive DS (HS-DS) rat (systolic blood pressure: 213 ± 4 mmHg vs. 152 ± 4 mmHg in NS, p<0.05) manifested impaired endothelium-dependent relaxation to acetylcholine (EDR), increased gene expression of vascular oxidative stress and proinflamamtory cytokines, and decreased eNOS expression. BMT on HS-DS rat significantly improved EDR and eNOS expression, reduced oxidative stress without reduction in SBP (206 ± 6 mmHg). Flow cytometry analysis showed that there was no difference in the number of circulating EPCs, demonstrated by expression of EPC markers CD34, cKit, and vascular endothelial growth factor, between hypertensive and normotensive rats. Surprisingly, BMT resulted in a 5–10 fold increase in the above-mentioned EPC markers in hypertensive, but not normotensive rat. These results suggest that DS rat has an impaired ability to increase bone marrow-derived EPCs in response to HS diet challenge, which may contribute to endothelial dysfunction.
Keywords: Bone marrow transplantation, Endothelial progenitor cells, Endothelial function, Salt-sensitive hypertension
Introduction
It is increasingly recognized that vascular endothelium plays an important role in the maintenance of cardiovascular health1,2. Impaired endothelium function is predictable for the development of cardiovascular diseases. Endothelial injury has been implicated in atherosclerosis, thrombosis, and hypertension. The balance between endothelial injury and endothelial recovery is of paramount importance for reducing cardiovascular events1,3. Bone marrow-derived EPCs play an integral role in the regulation and protection of the endothelium. Peripheral circulating EPC number and function are robust biomarkers of vascular risk for cardiovascular diseases4,5. An increasing body of evidence suggests that cardiovascular risk factors affect the number and properties of EPCs, and that the number and functional activity of EPCs correlate inversely with cardiovascular risk factors including hypertension, smoking, hyperlipidemia, and diabetes mellitus2,4,5.
Endothelial dysfunction contributes to the pathogenesis and progression of hypertension and is an independent predictor of cardiovascular risk. Experimental and Clinical studies have indicated that hypertension is inversely correlated with EPC reduction and dysfunction6,7. The Dahl salt-sensitive (DS) rat is an animal model of salt-sensitive (SS) hypertension in humans. Others and we have shown that DS rat exposure to high-salt diet develops hypertension and exhibits a vascular diathesis characterized by the impairment of endothelial nitric oxide-dependent vasorelaxation, upregulation of vascular tissue angiotensin II action and reactive oxygen species production, increased vascular and systemic inflammation, accompanied by severe cardiovascular and renal injury3,8,9. Whereas, its counter-partner, Dahl salt-resistant (DR) rat, is resistant to blood pressure and preserves endothelial and vascular function when under high salt diet10,11. In the present study, we used this animal model to test hypothesis that dysfunctional or inadequately increased bone marrow-derived EPCs in response to high salt diet challenge contribute to the pathogenesis of hypertension and vascular endothelial dysfunction in hypertensive DS rat.
Methods
Animals and experimental protocols
The animals were housed in facilities accredited by the American Association for Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee at the Miami Veterans Affairs Medical Center approved the studies. Six-week-old inbred DS (DS/JR) or DR (DR/JR) male rats were purchased from Harlan Sprague Dawley Inc. (Indianapolis, IN) and maintained under controlled conditions of light, temperature, and humidity. Both DS and DR rats are developed from Sprague-Dawley stocks by sister and brother inbred breeding based on their salt-sensitivity. After 2-week acclimatization to the new environment, DR rat was used as donor rat for the extraction of bone marrow cells (BMCs), BMCs were harvested from the femurs and tibias of 8-week-old DR rats and the nucleated cells were enriched by lysing red blood cells with ACK buffer. The recipient DS rats were lethally irradiated with a single dose of 9 Gy using a cobalt-60 gamma source on the day of transplantation. BMCs (2 × 107) were injected via tail vein into recipient rats 4 hours after irradiation12. The irradiation at the lethal dose kills all recipient's BMCs, and the recipient rat survives only if they receive enough fresh replacement BMCs from the donor. As a routine procedure, we performed the same lethal irradiation on the rats who received an injection of PBS instead of donor cells after the radiotherapy. All rats (5) received PBS died within one week after irradiation therapy. Whereas only six from total 16 recipient DS rats received BMCs died with first week after BMT, the method for BMT has been accepted12–14. Ten survived recipient DS rats were fed a NS (0.5% NaCl) or HS (4% NaCl) diet for 6 weeks (5 rats/each group). DS rats on a NS or HS diet without BMT were used as controls (n=6). Systolic blood pressure (SBP) was measured in conscious rats by the tail-cuff method as described in our previous studies. At the end of the study, peripheral blood (0.2 ml) was withdrawn from tail vein for FACS analysis. The rats were sacrificed by decapitation and the heart, kidneys, and aorta were harvested. Heart weight (normalized by body weight) and aortic weight/cm (arch of thoracic aorta to the origin of mesenteric artery) were used as indices of cardiac hypertrophy and aortic hypertrophy.
Organ chamber bath experiments
Endothelial function was examined in aortic rings using an organ chamber bath, as previously described8. Endothelium-dependent relaxation (EDR) to acetylcholine (Ach, 10−9 to 10−5mol/L) was studied in aortic rings pre-contracted to 70% of maximal contraction to norepinephrine as previously described8.
Flow cytometry analysis of EPCs
The peripheral blood was withdrawn from rat tail vein into tubes containing 15% EDTA. Red blood cell lysis buffer (1.2 ml, Sigma) was added into the tubes containing 0.1 ml blood and incubated at 37°C for 5 minutes followed by addition of 10 ml PBS and centrifugation to remove the lysed red blood cells. The cells (106 cells/ml) were resuspended in 100μl protein blocking solution with 5 μl fluorescent conjugated antibodies, anti-CD34-FITC, anti-cKit-PE, and anti-VEGFR2 (vascular endothelial growth factor)—APRC-Cy7, and incubated at 4°C for 1 hour. The fluorescence on the cell surface was determined by flow cytometry (FACS)13 using cells labeled with isotype IgG as control. A morphologic gate was used to exclude granulocytes. Then, CD34+, cKit+, or VEGF2+ cells were gated. Double-positive cells of CD34+cKit+, CD34+VEGF2+, or cKit+VEGF2+ were identified at the intersection of two cell gates.
Real-Time PCR
Primers and probes for rat endothelial nitric oxide synthasis (eNOS), NADPH oxidase subunits gp91phox, p22phox, monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor (TNF) α were designed by using Primer Express Software (Applied Biosystems (ABI)). Real-time PCR was performed in a 50 μl reaction mixture containing an appropriately diluted cDNA solution, 0.1 μmol/L of each primer, 0.2 μmol/L of probe, and PCR Master Mix assay (ABI) under the following conditions: at 50 °C for 2 min, at 95 °C for 10 min, and 40 cycles at 95 °C for 15 seconds and at 60 °C for 1 min. A housekeeping gene (GADPH) was determined as an internal control. The data was expressed as fold increase vs. NS group.
Data Analysis
The results were expressed as mean ± SEM. The maximal response (Emax) to acetylcholine and the concentration of acetylcholine required for a half-maximal CR response (ED50) was determined from the concentration-response curves, using the best fit to a logistic sigmoid function. Statistical analyses were performed by ANOVA with Bonferonni's correction for multiple comparisons, followed by Scheffe's test. Significance was assumed at P< 0.05.
Results
SBP, body weight, aortic and heart weight
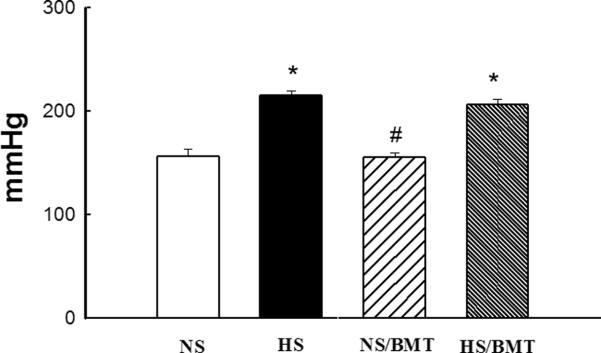
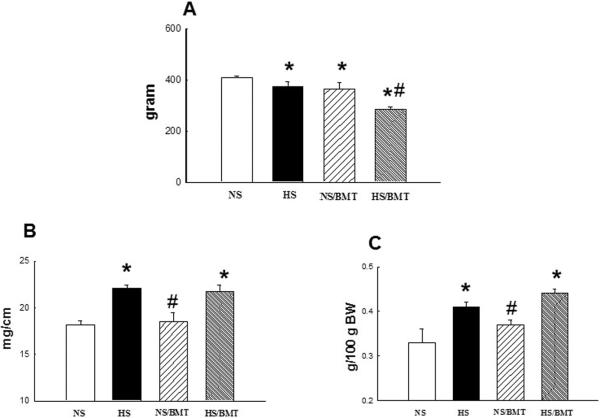
HS diet for 6 weeks significantly increased SBP in DS rats compared with NS-DS control (213 ± 5 mmHg in HS vs. 152 ± 5 mmHg in NS; P<0.05). BMT from DR rat into DS rat did not reduce SBP in both hypertensive (206 ± 6 in HS/BMT, p>0.05) and normotensive (155 ± 7 mmHg in NS/BMT, P>0.05) DS rats (Fig. 1). Hypertensive DS rat exhibited a slight inhibition of body weight gain (−8%), and a significant increase in aortic (21%) and heart (22%) weight compared with NS control. BMT resulted in a significant inhibition of body weight gain in both of hypertensive (24%) and normotensive (11%) DS rats, but did not significantly affect aortic and cardiac hypertrophy (Fig. 2).
Figure 1. Effects of bone marrow transplantation (BMT) on systolic blood pressure (SBP)in the recipient DS rats.
High salt diet intake for 6 weeks significantly increased SBP in DS rats, compared with normotensive DS rats. BMT from DR rat into DS rat did not affect SBP in both normotensive (NS/BMT) and hypertensive (HS/BMT) DS rats. N=5–6. *P<0.05 vs. NS group, #p<0.05 vs. HS group.
Figure 2. Effects of BMT on body weight (BW, A), aortic weight (AW, B), and heart weight (HW/100 g BW, C) in the recipient DS rats.
Hypertensive DS rats had a slight but significant inhibition in body weight gain compared with NS-DS rats. BMT from DR rat into DS rat further inhibited body weight gain in both normotensive (NS/BMT) and hypertensive (HS/BMT) DS rats. Hypertensive DS rats manifested an increase in AW (aortic hypertrophy) and HW (cardiac hypertrophy) compared with normotensive DS rats, BMT did not affect AW and HW in both normotensive and hypertensive DS rats. N=5–6, *P<0.05 vs. NS group, #p<0.05 vs. HS group.
EPC markers
There was no difference in circulating blood EPC number, identified by single-positive EPC marker expressing CD34+, cKit+ or VEGFR2+, or by double-positive EPC markers expressing CD34+/ckit+, CD34+/VEGFR2+, or cKit+/VEGFR2+, between DS and DR rats. There was also no difference in circulating blood EPC number between normotensive and hypertensive DS rats. Surprisingly, DS rat receiving BMCs from DR rat and a HS diet for 6 weeks (HS/BMT) exhibited 5–10 times increase in the circulating EPC number, compared with DS rat on a HS diet without BMT (Table 1 & Fig. 3). However, BMT did not affect the expression of the EPC markers in DS rat on a NS diet (NS/BMT).
Table 1.
Single positive cells for EPC Markers
| NS/DR | NS | HS | NS/BMT | HS/BMT | |
|---|---|---|---|---|---|
| CD34+ | 0.05 ± 0.05 | 0.08 ± 0.03 | 0.11 ± 0.03 | 0.05 ± 0.03 | 1.18 ± 0.45*# |
| cKit+ | 3.7 ± 0.7 | 3.4 ± 0.5 | 4.4 ± 0.3 | 4.8 ± 0.7 | 5.8 ± 0.7 |
| VEGFR2+ | 27.2 ± 4.0 | 26.9 ± 0.6 | 29.6 ± 4.7 | 40.3 ± 1.5*# | 37.3 ± 1.8*# |
p<0.05, vs. NS/DR or NS;
p<0.05, vs. correspondence NS or HS group without BMT therapy. N=4.
Figure 3. The circulating endothelial progenitor cell (EPC) number in DR and recipient DS rats.
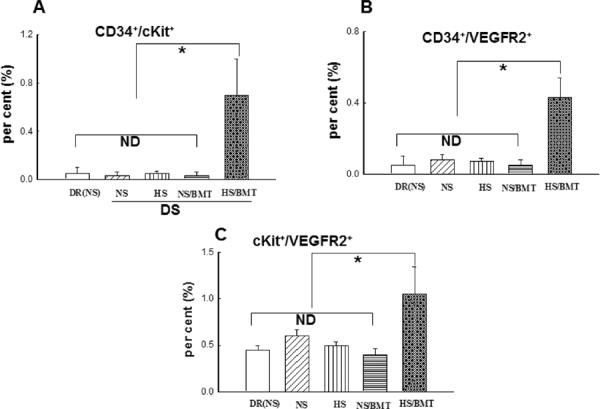
EPCs were characterized by flow cytometry using double markers: CD34+/cKit+ (A), CD34+/VEGFR2+(B), cKit+/VEGFR2+ (C). There is no significant difference in EPCs markers including CD34+/cKit+, CD34+/VEGFR2+, or cKit+/VEGFR2+ among DR, normotensive or hypertensive DS rats. BMT from DR rats into DS rats significantly increased all EPCs markers in hypertensive DS rats (HS/BMT) but not in normotensive DS rats (NS/BMT). N=4–5. *P<0.05. ND: no significant difference.
EDR to acetylcholine
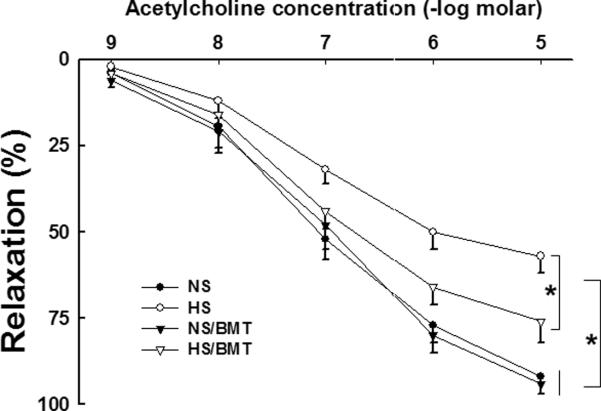
As shown in our previous studies3,8, EDR to acetylcholine was significantly attenuated in the aortic rings of hypertensive DS rats. BMT from DR rat into DS rat resulted in a significant improvement in EDR to acetylcholine in hypertensive DS rat (HS/BMT), as demonstrated by improvement in Emax (77 ± 7 % in HS/BMT vs. 60 ± 5% in NS, p<0.05 ) and ED50 (7.2 ± 0.1 in HS/BMT vs. 6.9 ± 0.2 -log molar in HS, p<0.05). BMT did not significantly affect EDR to acetylcholine in normotensive DS rats (Fig. 4).
Figure 4. Effects of BMT on endothelium-dependent relaxation (EDR) in the recipient DS rats.
EDR to acetylcholine was significantly reduced in hypertensive DS rats, compared with normotensive DS rats. BMT from DR rats into DS rats significantly improved EDR in hypertensive DS rats but did not affect EDR in normotensive DS rats. N=5–6, *P<0.05.
Vascular gene expression
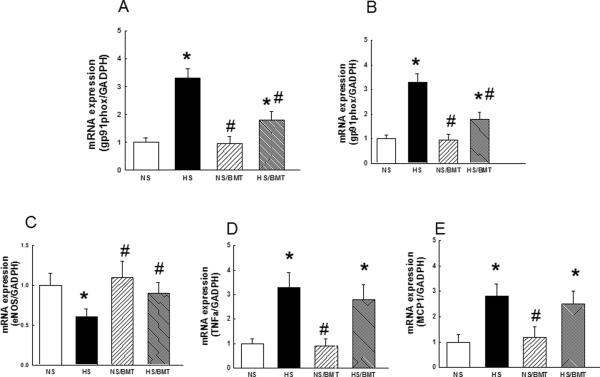
Hypertensive DS rat manifested increased mRNA expression of aortic NADPH oxidase subunits gp91phox and p22phox and reduced expression of eNOS, compared with that of normotensive DS rat. BMT on HS-DS rat significantly reduced the expression of aortic gp91phox and p22phox, improved eNOS expression, compared with that of normotensive rat. mRNA expression of the proinflammatory cytokines, including MCP1 and TNFα, in hypertensive DS rats, was higher than that of normotensive DS rats. BMT did not affect the expression of the proinflammatory genes in HS-DS rat (Fig. 5).
Figure 5. Effects of BMT on aortic gene expression in the recipient DS rats.
Hypertensive DS rats had a significant increase in mRNA expression of gp91phox (A), p22phox (B) compared with normotensive DS rats', BMT significantly reduced mRNA expression of gp91phox and p22 phox in hypertensive but not in normotensive DS rats. Hypertensive DS rats exhibited a reduction in eNOS (C) expression and an increase in the expression of proinflammatory cytokine TNFa (D) and MCP1 (E), BMT normalized eNOS expression but not inflammatory cytokines in hypertensive DS rats. N=4, *P<0.05 vs. NS group, #p<0.05 vs. HS group.
Discussion
A growing evidence supports the concept that bone marrow-derived EPCs enter the circulation and contribute to postnatal angiogenesis, neovascularization, and endothelial renewal, which may play an important role in the maintenance of vascular homeostasis15,16. Several clinical studies have shown that age and cardiovascular disease risk factors reduce the availability of circulating EPCs and impair their function to varying degrees17,18, whereas protective interventions, including statin therapy and exercise, appear to increase the supply of these cells4,7,17. These studies suggest that circulating EPCs may participate not only in forming new blood vessels but also in maintaining the integrity and function of vascular endothelium, thereby mitigating disease processes such as atherosclerosis and hypertension13,17,19.
It is well established that endothelial dysfunction underlies all of the major cardiovascular diseases20. Regeneration of endothelium is a fundamental process in vascular repair14. Mature endothelial cells have limited ability to regenerate damaged endothelium because these cells are terminally differentiated. Bone marrow-derived EPCs have the potential to differentiate into mature, functional endothelial cells, and may play a significant role in vascular repair and healing21,22.
Hypertension is a vascular disease characterized by impaired endothelium-dependent relaxation3,8. Hypertension is a strong predictor of EPC dysfunction and migratory impairment6,7. Clinical studies has shown that circulating EPCs are inversely correlated with blood pressure5,7,23. Giannotti et al6 have shown that endothelial repair capacity from EPCs is reduced in patients with prehypertension and hypertension, which is associated with impairment of endothelial function. DS rat is a paradigm of salt-sensitive hypertension in humans11, and we and others have shown that DS rat on HS diet developed hypertension and severe endothelial dysfunction, whereas DR rat had normal blood pressure and preserved endothelial function during HS intake8. It is still unclear whether endothelial dysfunction is related to impairment of bone marrow-derived EPCs in this genetic hypertensive animal model. Our data showed that there was no difference in peripheral circulating EPC number between normotensive DS and DR rats, and there was also no difference in EPC number between normotensive and hypertensive DS rats, although hypertensive DS rat had severe endothelial dysfunction. Surprisingly, DS rat that received BMCs from DR rat and was maintained on a HS diet resulted in a 5–10 fold increase in peripheral circulating EPCs. Transplantation of BMCs from DR rat into DS rat improved endothelial function in hypertensive DS rat without significant reduction in SBP. However, the EPCs in DS rat that received BMT and was maintained on a NS diet were not changed. These results suggest that hypertensive DS rat may need higher level of EPCs to maintain endothelial renewal and endothelial function in response to a HS diet challenge although it has normal circulating EPCs, and that DS rat has an impaired ability to increase EPCs in response to environmental challenges such as high salt intake or hypertension. In addition, Our data showed that transplantation of EPCs from DR rat into DS rat reduced vascular NADPH oxidase-derived oxidative stress and increased eNOS expression, which may at least in part contribute to improvement of endothelial function in hypertensive DS rats.
In summary, our data for the first time demonstrates that bone marrow therapy from DR rat into DS rat increase circulating EPCs and partially improve endothelial function in hypertensive DS rat, suggesting that dysfunction of bone marrow derived EPCs play an important role in endothelial dysfunction of this genetic salt-sensitive hypertension.
Limitation: The findings in EPCs may be limited by identification of EPCs markers. EPCs are defined by constitutive and inducible markers. Constitutive agents, such as CD31, CD34, ckit, VEGFR2, CD133 and CD144 are used for EPCs marker24,25. However, these markers are not solely restricted to endothelial progenitor cells and can, likewise, be detected on hematopoietic lineage and stromal cells. In the present study24,25, we used three different combinations: CD34+/cKit+, CD34+/VEGFR2+, cKit+/VEGFR2+ to represent the EPC level and minimize non-EPC phenotype identification.
Acknowledgments
This work was supported by grants from America Heart Association #0635303N (MSZ), #0855311 (HY), Florida Department of Health JEK Biomedical Research Program (MSZ, and HY), Minister of Science and Technology of China (Project 2012CBA1305, HY) and the National Institutes of Health #HLl95101 (NMT). The authors thank Dr Leopoldo Raij for his critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: None
References
- 1.Schulman IH, Zhou MS, Raij L. Interaction between nitric oxide and angiotensin II in the endothelium: role in atherosclerosis and hypertension. J Hypertens. 2006;24:S45–50. doi: 10.1097/01.hjh.0000220406.46246.f2. [DOI] [PubMed] [Google Scholar]
- 2.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 3.Zhou MS, Hernandez Schulman I, Pagano PJ, Jaimes EA, Raij L. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension. 2006;47:81–6. doi: 10.1161/01.HYP.0000197182.65554.c7. [DOI] [PubMed] [Google Scholar]
- 4.Werner N, Koriol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–7. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 6.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horváath T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Lèuscher TF, Drexler H, Landmesser U. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: relation to endothelial dysfunction. Hypertension. 2010;55:1389–97. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 7.Pirro M, Schillaci G, Menecali C, Bagaglia F, Paltriccia R, Vaudo G, Mannarino MR, Mannarino E. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34+ cells of hypertensive patients. J Hypertens. 2007;25:2093–9. doi: 10.1097/HJH.0b013e32828e506d. [DOI] [PubMed] [Google Scholar]
- 8.Zhou MS, Adam AG, Jaimes EA, Raij L. In salt-sensitive hypertension, increased superoxide production is linked to functional upregulation of angiotensin II. Hypertension. 2003;42:945–51. doi: 10.1161/01.HYP.0000094220.06020.C8. [DOI] [PubMed] [Google Scholar]
- 9.Zhou MS, Schulman IH, Raij L. Vascular inflammation, insulin resistance, and endothelial dysfunction in salt-sensitive hypertension: role of nuclear factor kappa B activation. J Hypertens. 2010;28:527–35. doi: 10.1097/HJH.0b013e3283340da8. [DOI] [PubMed] [Google Scholar]
- 10.Zhou MS, Nishida Y, Chen QH, Kosaka H. Endothelium-derived contracting factor in carotid artery of hypertensive Dahl rats. Hypertension. 1999;34:39–43. doi: 10.1161/01.hyp.34.1.39. [DOI] [PubMed] [Google Scholar]
- 11.Dahl LK, Knudsen KD, Iwai J. Humoral transmission of hypertension: evidence from parabiosis. Circ Res. 1969;24(Suppl):21–33. [PubMed] [Google Scholar]
- 12.Shao H, Xu Q, Wu Q, Ma Q, Salqueiro L, Wang J, Eton D, Webster KA, Yu H. Defective CXCR4 expression in aged bone marrow cells impairs vascular regeneration. J Cell Mol Med. 2011;15:2046–56. doi: 10.1111/j.1582-4934.2010.01231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao H, Tan Y, Eton D, Yang Z, Uberti MG, Li S, Schulick A, Yu H. Statin and stromal cell-derived factor-1 additively promote angiogenesis by enhancement of progenitor cells incorporation into new vessels. Stem Cells. 2008;26:1376–84. doi: 10.1634/stemcells.2007-0785. [DOI] [PubMed] [Google Scholar]
- 14.Wu Q, Shao H, Eton D, Li J, Li J, Yang B, Webster KA, Yu H. Extracellular calcium increases CXCR4 expression on bone marrow-derived cells and enhances pro-angiogenesis therapy. J Cell Mol Med. 2009;13:3364–73. doi: 10.1111/j.1582-4934.2009.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzau VJ, Gnecchi M, Pachori AS, Morello F, Melo LG. Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension. 2005;46:7–18. doi: 10.1161/01.HYP.0000168923.92885.f7. [DOI] [PubMed] [Google Scholar]
- 16.Itoh Y, Toriumi H, Yamada S, Hoshino H, Suzuki N. Resident endothelial cells surrounding damaged arterial endothelium reendothelialize the lesion. Arterioscler Thromb Vasc Biol. 2010;30:1725–32. doi: 10.1161/ATVBAHA.110.207365. [DOI] [PubMed] [Google Scholar]
- 17.Jialal I, Devaraj S, Singh U, Huet BA. Decreased number and impaired functionality of endothelial progenitor cells in subjects with metabolic syndrome:implications for increased cardiovascular risk. Atherosclerosis. 2010;211:297–302. doi: 10.1016/j.atherosclerosis.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pirro M, Schillaci G, Paltriccia R, Bagaglia F, Menecali C, Mannarino MR, Capanni M, Velardi A, Mannarino E. Increased ratio of CD31+/CD42 microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:2530–5. doi: 10.1161/01.ATV.0000243941.72375.15. [DOI] [PubMed] [Google Scholar]
- 19.Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Gorger M, Fialka-Moser V, Gschwandtner M, Koppensteiner R, Steiner S. Excerise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in periphjeral arterial disease: A randomized controlled trail. Atherosclerosis. 2011;217:240–8. doi: 10.1016/j.atherosclerosis.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 21.Sen S, McDonald SP, Coates PT, Bonder CS. Endothelial progenitor cells: novel biomarker and promising cell therapy for cardiovacular disease. Clin Sci (lond) 2011;120:263–83. doi: 10.1042/CS20100429. [DOI] [PubMed] [Google Scholar]
- 22.Kown SM, Lee YK, Yokoyama A, Jung SY, Masuda H, Kawamoto A, Lee YM, Asahara T. Differential activity of bone marrow hematopoietic stem cell subpopulations fro EPC development and ischemic neovascularization. J Mol Cell Cardiol. 2011;51:308–17. doi: 10.1016/j.yjmcc.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Chen DD, Dong YG, Yuan H, Chen AF. Endothelin 1 activation of endothelin A /NADPH oxidase pathway and dimished antioxidant contribute to endothelial progenitor critically cell reduction and dysfunction in salt-senstive hypertension. Hypertension. 2012;59:1037–43. doi: 10.1161/HYPERTENSIONAHA.111.183368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinmetz M, Nickenig G, Werner N. Endothelial-regenerating cells. Hypertension. 2010;55:593–9. doi: 10.1161/HYPERTENSIONAHA.109.134213. [DOI] [PubMed] [Google Scholar]
- 25.Garlanda C, Dejana E. Heterogeneity of endothelial cells: specific markers. Arterioscler Thromb Vasc Biol. 1997;17:1193–202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]