Abstract
Sarin, a highly toxic nerve gas, is believed to cause bronchoconstriction and even death primarily through respiratory failure; however, the mechanism underlying the respiratory failure is not fully understood. The goals of this study were to ascertain whether sarin affects baseline ventilation (VE) and VE chemoreflexes as well as airway resistance and, if so, whether these changes are reversible. Four groups of F344 rats were exposed to vehicle (VEH) or sarin at 2.5, 3.5, and 4.0 mg h m−3 (SL, SM, and SH, respectively). VE and VE responses to hypercapnia (7% CO2) or hypoxia (10% O2) were measured by plethysmography at 2 h and 1, 2, and 5 days after VEH or sarin exposure. Total pulmonary resistance (RL) also was measured in anesthetized VEH- and SH-exposed animals 2 h after exposure. Our results showed that within 2 h after exposure 11% of the SM- and 52% of the SH-exposed groups died. Although the SM and SH significantly decreased hypercapnic and hypoxic VE to similar levels (64 and 69%), SH induced greater respiratory impairment, characterized by lower baseline VE (30%; P < 0.05), and total loss of the respiratory frequency response to hypercapnia and hypoxia. VE impairment recovered within 1–2 days after sarin exposure; interestingly, SH did not significantly affect baseline RL. Moreover, sarin induced body tremors that were unrelated to the changes in the VE responses. Thus, LC50 sarin causes a reversible impairment of VE that is not dependent on the sarin-induced body tremors and not associated with changes in RL.
Keywords: acetylcholine, respiratory failure, chemoreflexes
Introduction
Sarin (O-isopropyl methylphosphonofluoridate, also known as GB) is an organophosphorus nerve agent that binds and irreversibly inactivates acetylcholinesterase (Grob and Harvey, 1958). Inhibition of acetylcholinesterase elevates the synaptic level of acetylcholine (ACh), causing cholinergic shock and clinical symptoms of sarin intoxication. Sarin is more volatile than other nerve gases such as soman, tabun, and VX (Lee, 2003), and combined with its lethality and low cost of production, it is the nerve agent of choice in the hands of terrorists and rouge nations. In the 1980s, Iraq used sarin against Iranian soldiers and Kurds, resulting in thousands of deaths; and a Japanese terrorist group used sarin in two attacks in the city of Matsumoto in 1994 and on a Tokyo subway in 1995, killing dozens and injuring over 6000 people (Okumura et al., 2003). At or near LC50, sarin causes miosis, bronchoconstriction, excessive secretions, vomiting, body tremors, seizures, and may lead to respiratory and cardiac arrest (Sidell and Borak, 1992; Sidell, 1994; Abu-Qare and Abou-Donia, 2002). The respiratory system is the major route of entry and absorption for nerve gases and plays a major role in the pathogenesis of nerve gas toxicity. Respiratory failure is the most common cause of death following nerve gas exposure (Rickett et al., 1986; Colwill et al., 2004), and it is generally thought that the severe bronchoconstriction from nerve agent exposure is involved in respiratory failure (Johnson et al., 1958; Holstege et al., 1997). However, there are no critical data to prove this premise.
Exposure to nerve agents has been reported to affect ventilatory (VE) responses (Adams et al., 1976; Rickett et al., 1986), and several lines of evidence suggest that VE changes in response to hypercapnia and hypoxia are critical in maintaining VE rhythm. First, CO2-chemoreception is critical for maintaining the VE rhythm in mammals, and reduction of the CO2-respiratory drive by lowing arterial blood CO2 or blunting CO2-chemosensitivity may lead to ventilatory arrest (Bruce and Cherniack, 1987; Nattie, 2000). Second, children with congenital central hypoventilation syndrome (CCHS), characterized by severely blunted or even absent VE responsiveness to hypercapnia, require artificial ventilation (Shea et al., 1993; Gozal et al., 1996). Similarly, severe chronic obstructive pulmonary disease (COPD) patients who exhibit blunted VE response to hypercapnia and hypoxia are likely to develop respiratory failure (Schaefer, 1949; Fahey and Hyde, 1983; Franciosi et al., 2006; Ucgun et al., 2006). Third, blunted VE response to hypoxia may lead to VE dysfunction, as was shown in an animal model of sudden infant death syndrome (SIDS) (Milerad et al., 1995; St-John and Leiter, 1999; Hafstrom et al., 2002; Murai et al., 2003).
Although respiratory failure is the major cause of sarin-induced mortality, the precise role of VE chemoreflexes in sarin-induced respiratory failure is essentially unknown. In this communication we present evidence that sarin decreases baseline VE and the VE responses to hypercapnia and hypoxia, and that the changes in the VE responses are not dependent on body tremors and not associated with significant changes in baseline airway resistance.
Materials and methods
Animals
Pathogen-free, 8-week-old male Fischer 344 rats were purchased from Harlan Sprague- Dawley Farms (Indianapolis, IN). Animals were quarantined for 2 weeks, and food and water were provided ad libitum. At the age of approximately 12–14 weeks, animals were exposed to sarin via inhalation. All studies were conducted at Lovelace Respiratory Research Institute (LRRI), a facility fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Sarin exposure
Sarin in isopropyl alcohol was obtained from the United States Army. Animals (n = 84) were exposed to either isopropyl alcohol (vehicle) or to various concentrations of sarin by the nose-only inhalation method developed at LRRI as described previously (Henderson et al., 2002). Briefly, prior to exposure animals were acclimatized to the nose-only inhalation chambers for 120 min day−1 for 3 consecutive days. Rats were divided into 4 groups and exposed to either vehicle at 35 ppm in fresh air (VEH, n = 23) or three doses of sarin: 2.5 (SL, n = 12), 3.5 (SM, n = 9), or 4.0 mg h m−3 (SH, n = 40) at the rate of 2.0 mg m−3 h−1. After degassing, animals were removed from exposure chambers and experiments began 2 h post exposure. Essentially, all sarin-related deaths occurred within 2 h of sarin exposure. Among 84 rats, one in SM and 21 in SH groups died after sarin exposure, the 62 surviving rats (23, 12, 8, and 19 for VEH, SL, SM, and SH groups, respectively) underwent the following tests.
Measurement of the VE responses in conscious rats
40 rats out of the surviving rats (n = 12, 12, 8, and 8, for VEH, SL, SM, and SH, respectively) were placed in an unrestrained whole-body plethysmograph system (Buxco Electronics, Sharon, CT) two hours after exposure. Respiratory activity, including airflow, tidal volume (VT), respiratory frequency (fR), and VE were continuously monitored and recorded. In addition to baseline VE and VE changes in response to 5 min of hypercapnia (7% CO2 and 30% O2 balanced with N2) or hypoxia (10% O2 balanced with N2) were measured in these animals at 2 h, and 1, 2, and 5 days after VEH or sarin exposure. The rectal temperature of the animals was monitored with a flexible temperature microprobe (MLT 1401 Thermocouple Probe; ADInstruments, Castle Hill, Australia) and maintained at 36–37°C by adjusting airflow temperature in the chamber and a placing a heating pad underneath the plethysmograph.
Measurement of the VE responses in two groups of anesthetized rats
Anesthetic (Nembutal®, 50 mg kg−1 i.p.) was administrated 2 h and 5 days post the exposures in the first and second group, respectively. Each group contains 6 VEH and 6 SH rats. It should be noted that the 12 rats in the second group came from those after completion of the plethysmographic VE response tests in conscious state. The left femoral artery was cannulated for monitoring arterial blood pressure (ABP) and heart rate (HR), and the femoral vein was used to administer supplemental anesthetic (combination of chloralose, 100 mg kg−1 and urethane, 500 mg kg−1) if the ABP, HR and fR responses to pinching the hind limb paw were 15% greater than the control values. The trachea was cannulated and connected to a pneumotachograph (Frank’s Mfg. Co., Albuquerque, NM) to record airflow. The pneumotachograph, as previously reported (Wang and Xu, 2006), was made of stainless steel with a linear flow-pressure relationship in the range of 0–10 ml s−1 and having a flow resistance equal to 0.046 cm H2O ml−1 s−1 with a dead space of 0.08 ml. The other end of the pneumotachograph was placed (~3 mm deep) in a plastic tube with a diameter five-fold greater than the pneumotachograph. A three-way stopcock was attached to the other side of the plastic tube and connected to a supplemental gases device that controlled the inhaled gas composition. Baseline VE and VE response to 2 min of hypercapnia (7% CO2) or 1 min hypoxia (10% O2) were measured. It should be noted that because the route of exposure to 7% CO2 or 10% O2 challenge was different between conscious and anesthetized rats (plethysmograph vs. tracheal cannulation), the duration of exposure was shorter in the latter. The interval between two stimulation-episodes was at least 5 min. The animal was supine and its body temperature was monitored with a rectal probe and maintained at approximately 36.5°C by a heating pad and a radiant heat lamp.
Measurement of arterial blood gases and pH
In the 24 anesthetized rats mentioned above, ~120 µl arterial blood was sampled from each animal before hypercapnia and hypoxia challenges. Blood samples were taken from 12 VEH- and 12 SH-exposed rats at 2 h or 5 days after exposure, and blood gases were measured immediately after collection by a blood gas analyzer (GEM Premier 3000, Instrumentation Lab., Lexington, MA).
Measurements of total pulmonary resistance (RL)
To measure airway resistance and responsiveness to methacholine, the RL was measured in 10 surviving rats (n = 5 each from VEH and SH groups) 2 h after exposure. The animals were anesthetized, tracheotomized, paralyzed and ventilated, and a small incision was made in the trachea through which a 20-gauge needle hub was inserted. The tracheal cannula was secured by suture thread and the skin was pulled back over the cannula and secured by cyanoacrylate adhesive. A small saline-filled catheter was placed in the thoracic esophagus via the mouth to obtain transthoracic pressure measurements. The rat was placed on the Flexivent apparatus (Scireq, Montreal, Canada) and ventilated through the tracheal cannula. Once on the ventilator, paralytics (doxicurium, 0.5 mg kg−1) were administered via the intraperitoneal route. Aerosolized methacholine was delivered in increasing doses (0, 1, 3, 6, 12, 25, and 50 mg ml−1) with a 6-min interval between two doses.
Data acquisition and analysis
Raw data from the conscious or the anesthetized (paralyzed) rats were recorded by a PowerLab/8sp data acquisition system connected to a computer employing the PowerLab Chart 5 software (ADInstruments, Australia). Respiratory variables including VT, fR, and VE were derived by the on-line calculation functions of the software. The baselines values were collected and averaged for 2 min immediately before 7% CO2 or 10% O2 challenge. The maximal responses during the challenge period and the percent change from the baseline values were calculated. Percent mortality following sarin exposure represents the number of dead animals divided by total number of animals at a given concentration of sarin multiplied by 100. Baseline RL and RL responses were determined by RL values 1 min before methacholine challenge and the peak response during each methacholine challenge, respectively.
Statistical analysis
Values for VE parameters, RL, and body weights were calculated as mean ± SE. Because the baseline VE and VE responses as well as the arterial blood gas data in anesthetized animals obtained from VEH rats at 2 h and on day 5 after exposure were not different statistically, they were combined into one group (VEH) in the tables and figures for simplicity. Comparison between mortality rates among various groups was evaluated by the Pearson’s Chi-Square test. Paired t-test was used to evaluate if a VE response was different from the baseline value. One-way analysis of variance (ANOVA) was used to examine the effects of various doses of sarin exposure on respiratory parameters, while two-way ANOVA with repeated tests was used to test the differences in the recovery times among the sarin groups and the RL responses to different levels of methacholine challenge between the VEH and SH groups. If the overall ANOVA had a P value less than 0.05, the Tukey’s method for multiple comparisons was followed. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK) was employed for statistical analysis. Difference is considered significant at a P value < 0.05.
Results
Mortality and morbidity is associated with acute sarin exposure
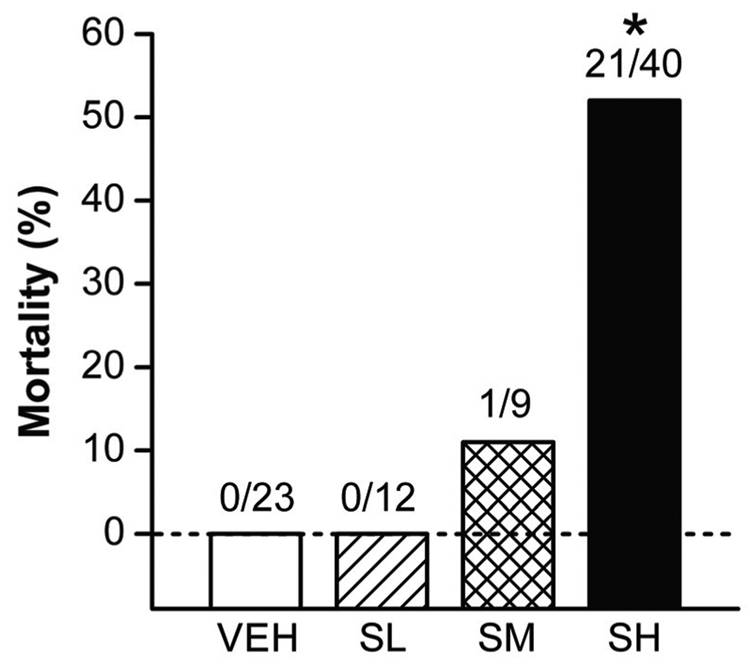
Among the rats exposed to VEH or SL, there was no apparent abnormal sign observed during the exposures. At higher levels of sarin exposures, however, the rats showed body tremors, hypersecretion of saliva, and seizures that were more severe in SH than SM group. Two h after the exposures, significant body tremor was still observed in SM and SH rats, while other signs were minimal. Of the 84 rats tested for sarin-related mortality, no deaths were observed among the rats exposed to VEH or SL. However, as shown in Fig. 1, 1 out of 9 rats exposed to SM and 21 out of 40 rats exposed to SH died within 2 h after sarin exposure, representing 11% and 52% mortality, respectively. Compared with the VEH rats, only the SH group showed significant mortality (P < 0.01). Mortality was defined as loss of heartbeat and respiratory rhythm, and usually occurred between 45–95 min after sarin exposure. Among the surviving animals there were no significant differences in the body weight between the SH and VEH groups before (212 ± 6 and 207 ± 3 g) and 5 days post sarin exposure (220 ± 9 and 215 ± 7 g). These results suggest that 4.0 mg h m−3 sarin approximates the LC50 for sarin in male F344 rats.
Figure 1.
Dose-dependency of acute sarin exposure-induced mortality. No death occurred in the rats exposed to vehicle only (VEH) or a low dose of sarin (SL). In contrast, 11% and 52% mortality was observed in the rats within 2 hours immediately after exposure to moderate (SM) and high doses (SH) of sarin, respectively. * P < 0.01, compared with VEH, SL and SM groups.
High-dose sarin affects baseline VE
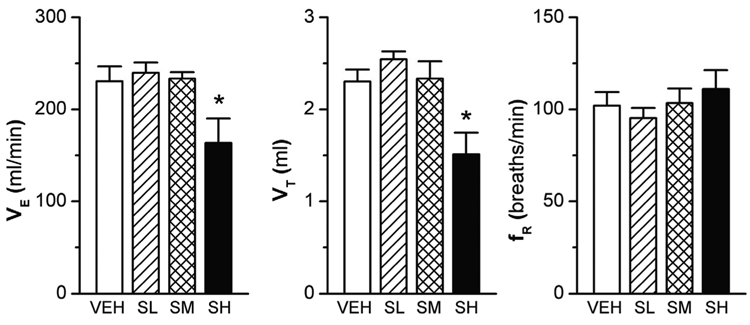
Two hours after sarin or VEH exposure, surviving rats were placed in the whole body plethysmograph. Among these rats, body tremors were noticed in the SM and SH groups and the severity of tremors was greater in latter than the former, but no tremors were observed in SL and VEH rats. The baseline fR in the rats exposed to the three sarin doses was similar to that of the VEH animals (Fig. 2, right panel). However, the baseline VE and VT of the SH animals were consistently lower (approximately 33%; p < 0.01) than those of the animals in the VEH, SL, or SM groups (Fig. 2, left and middle panel). Moreover the animals’ body temperature was not statistically different among the four groups. Thus, SH rather than SL and SM is able to suppress baseline VE and VT in conscious rats.
Figure 2.
Baseline ventilation (VE) in the surviving conscious rats 2 h after sarin exposure. The baseline VE, as compared with that in the vehicle (VEH)-exposed rats, was not changed by low and moderate levels of sarin (SL and SM), but was significantly reduced by high-level sarin (SH) due to a reduction of tidal volume (VT) with little effect on respiratory frequency (fR). Mean ± SE; n = 12, 12, 8, and 8 for VEH, SL, SM, and SH animals, respectively. * P < 0.01, SH vs. three other groups.
Sarin depresses VE responses to hypercapnia and hypoxia
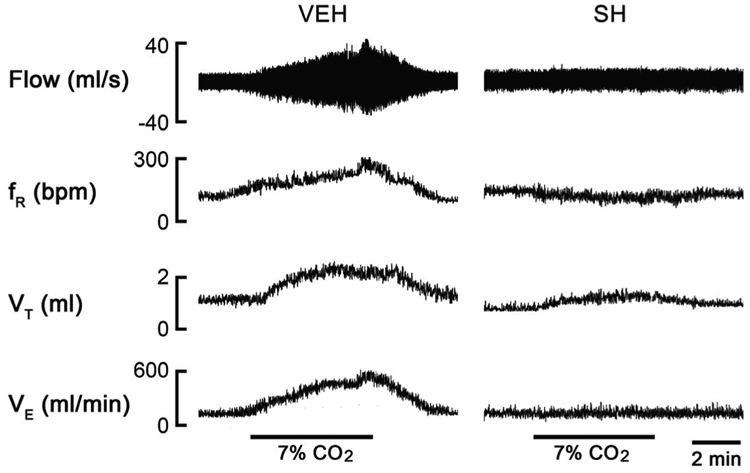
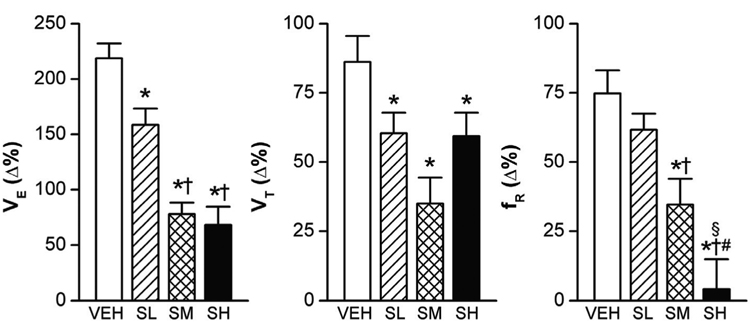
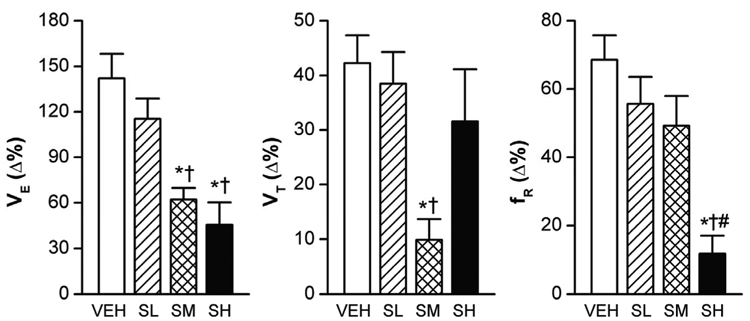
Normally, the increase in the partial pressure of CO2 (PCO2) leads to elevated VE, characterized by increasing VT and fR (Xu and Frazier, 2002). However, sarin exposure even at SL blunted the VE response to hypercapnia (7% CO2 for 5 min), as shown in Fig. 3 (an example of the recording data) and Fig. 4 (the group data). Compared with VEH, the SL, SM, and SH attenuated the VE responses by 28%, 64%, and 69%, respectively (P < 0.01). We observed an interesting correlation between the VE response to hypercapnia and the level of sarin exposure. While all the three doses of sarin attenuated VE responses through reducing the VT response, the fR response was unaffected by SL, reduced by SM, and totally abolished by SH. Exposure to hypoxia (10% O2 for 5 min) also significantly increased the ventilatory parameters in the VEH rats, with VE, VT, and fR increasing by 142 ± 16%, 42 ± 5%, and 69 ± 7%, respectively, (P < 0.01)(Fig. 5). The SM and SH attenuated the VE response by 56% and 68% (P < 0.01) as compared with VEH; however, differently from the VE response to hypercapnia, the VE response to hypoxia was not significantly affected by SL. The most surprising finding was that the SM diminished the hypoxia-induced VE response that resulted mainly from the decreased VT response. On the other hand, changes in VE in SH animals were primarily contributed by a marked decrease in the fR response, which is similar to the VE response to hypercapnia. Together, these results suggest that sarin blunts the VE responses to hypoxia and hypercapnia; however, while the SM-induced attenuation is achieved by impairing the VT response, in SH animals it is achieved by eliminating the fR response.
Figure 3.
Examples of experimental recordings showing the hypercapnic ventilatory responses in a vehicle (VEH)- and a high-dose of sarin (SH)-exposed rats 2 h after exposure by plethysmorgraphy. The traces from the top to bottom are airflow (Flow), respiratory frequency (fR), tidal volume (VT), and minute ventilation (VE). SH almost eliminated the VE response to hypercapnia.
Figure 4.
The hypercapnic ventilatory (VE) responses (7% CO2 for 5 min) in rats exposed to three different levels of sarin. As compared with vehicle only (VEH), high and moderate sarin exposure (SM and SH) decreased the VE responses more than the low level of sarin (SL). The respiratory frequency (fR) response was absent in SH-exposed rats. Mean ± SE; n = 12, 12, 8, and 8 for VEH, SL, SM, and SH animals, respectively. * P < 0.05, compared with VEH group; † P < 0.05, SM and SH vs. SL; # P < 0.05, SH vs. SM group; § P > 0.10, compared with baseline “0”.
Figure 5.
Group data of the hypoxic ventilatory (VE) responses (10% O2 for 5 min) in rats exposed to sarin. High- and moderate- (SM and SH), but not low-dose sarin exposure (SL), attenuated the VE responses to 10% O2 due to a reduction in either tidal volume (VT) or respiratory frequency (fR). Mean ± SE; n = 12, 12, 8 and 8 for vehicle (VEH), SL, SM and SH animals, respectively. * P < 0.05, compared with VEH; † P < 0.05, SM and SH vs. SL; # P < 0.05, SH vs. SM group.
Sarin also changes VE in anesthetized rats
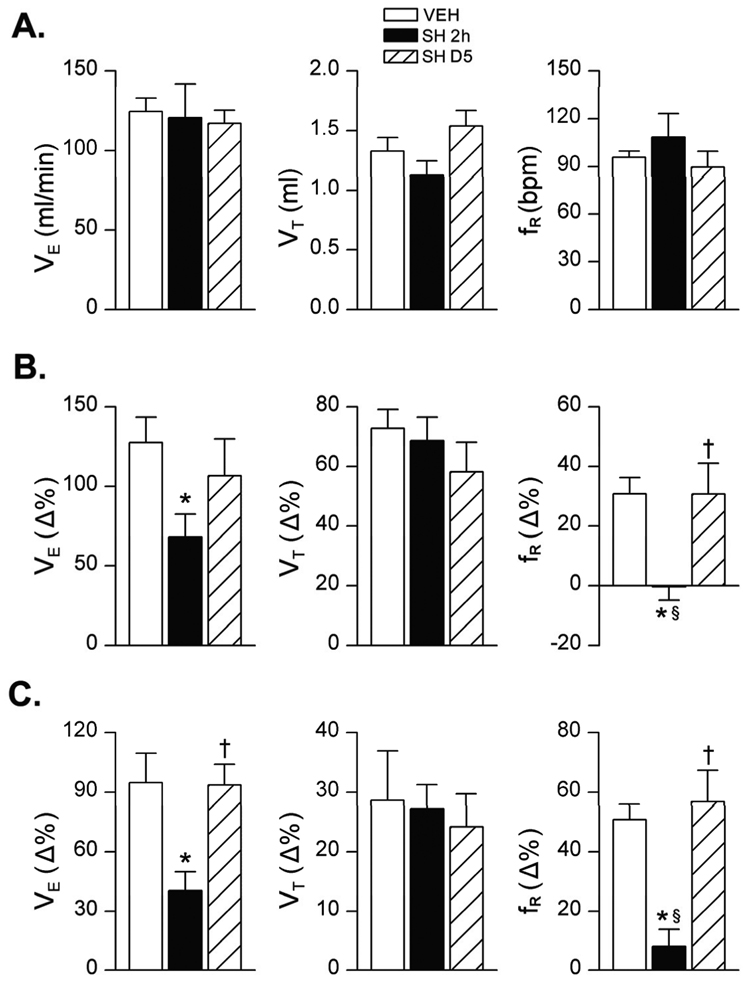
As mentioned above, the surviving SH rats exhibited body tremors 2 h post sarin exposure and severe respiratory impairment (essentially eliminating the fR response). Sarin-induced body tremors may impair respiratory activity (Shih et al., 2007), thereby blunting the VE responses. To evaluate the possible contribution of tremors to the sarin-induced changes in VE, we compared the baseline VE and VE responses to hypercapnia and hypoxia challenges in anesthetized VEH and SH rats. Interestingly, the anesthetized SH animals’ tremors ceased and their body temperatures were consistent in both VEH and SH rats. Moreover, sarin exposure did not significantly alter baseline VE but profoundly attenuated the VE responses to hypercapnia and hypoxia by 47% and 57%, respectively (Fig. 6). In addition, as in conscious rats, SH caused the loss of the fR response in the anesthetized rats. The absence of baseline VE depression and relatively smaller inhibitory VE responses in the anesthetized compared with conscious rats is very likely due to the anesthetic effect on VE (Wixson et al., 1987) and/or a relative shorter hypercapnic/hypoxic exposure. In the anesthetized state at 2 h and 5 days post sarin exposure, the baseline cardiovascular activities and their responses to hypoxia and hypercapnia were not significantly different between VEH and SH animals (Table 1).
Figure 6.
Group data of the baseline VE (panel A) and hypoxic VE responses to hypercapnia (7% CO2 for 2 min, panel B) and hypoxia (10% O2 for 1 min, panel C) in the anesthetized rats exposed to vehicle only (VEH) or high-dose sarin (SH), respectively. Mean ± SE; n = 12, 6, and 6 for VEH, 2 h after SH (SH 2h), and 5 days after SH (SH D5) groups, respectively. * P < 0.05, compared with VEH group; † P < 0.05, SH D5 vs. SH 2h groups; § P > 0.10, compared with baseline “0”.
Table 1.
Effects of SH exposure on cardiovascular responses to hypercapnia and hypoxia in anesthetized rats
| 2h |
D5 |
||||
|---|---|---|---|---|---|
| VEH | SH | VEH | SH | ||
| Baseline (mmHg) | 106 ± 7 | 98 ± 11 | 108 ± 8 | 112 ± 12 | |
| MABP | Hypercapnia (Δ%) | 3 ± 2 | 5 ± 2 | 4 ± 2 | 6 ± 4 |
| Hypoxia (Δ%) | −42 ± 4* | −44 ± 5* | −44 ± 4* | −41 ± 7* | |
| Baseline (bpm) | 394 ± 11 | 406 ± 7 | 398 ± 12 | 412 ± 25 | |
| HR | Hypercapnia (Δ%) | 0 ± 1 | −3 ± 3 | −1 ± 0 | −2 ± 1 |
| Hypoxia (Δ%) | −2 ± 1 | 2 ± 2 | −1 ± 1 | −1 ± 2 | |
2h and D5 represent the data obtained at 2 h and on day 5 after vehicle (VEH) or high-dose sarin (SH, 4.0 mg h m−3) exposure. Data are expressed in percentage change from baseline “0” (Δ%) in response to hypercapnia or hypoxia.
P < 0.05, compared with baseline “0”; MABP, mean arterial blood pressure; HR, heart rate.
Sarin-induced VE changes are relatively short-lived in surviving animals
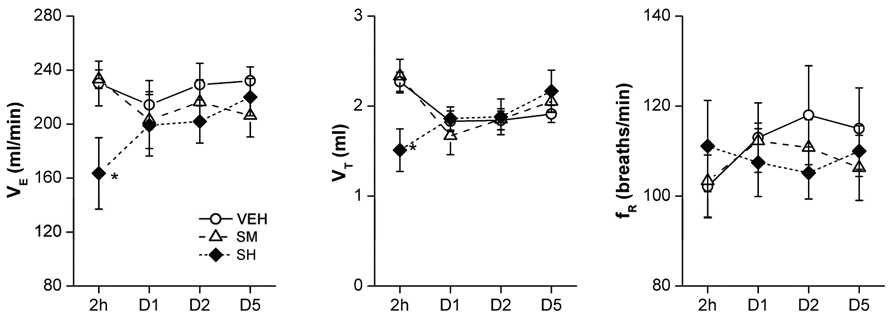
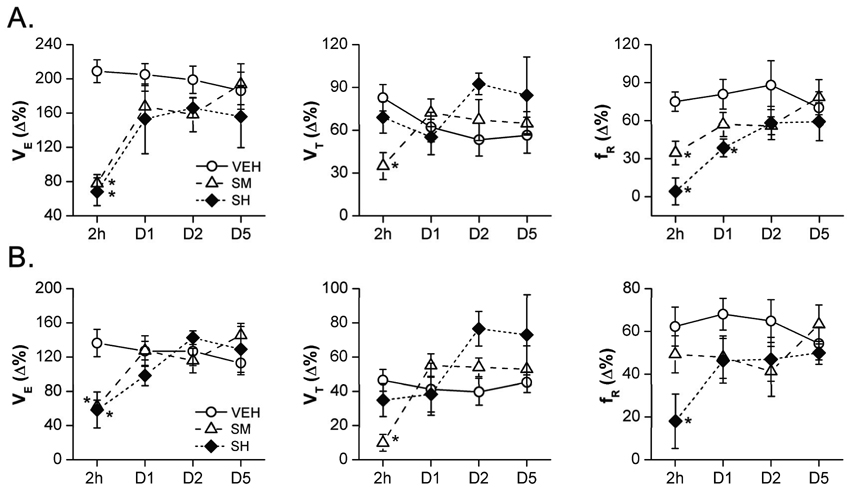
To ascertain the duration of sarin-induced VE changes, VEH and surviving SH animals were retested on days 1, 2, and 5 for baseline and hypercapnic/hypoxic VE. Results presented in Fig. 7 suggest that the baseline changes in VE and VT in SH animals observed 2 h post sarin exposure were resolved within 24 h after sarin exposure. Similarly, hypoxia-induced changes in VE, VT, and fR in SH animals also recovered within 24 h after sarin inhalation (Fig. 8B). However, the hypercapnia-stimulated changes in the fR response in SH animals remained significantly lower even at 24 h, but returned to normal within 2 days after sarin exposure (Fig. 8A). Thus, as in humans (Yanagisawa et al., 2006), the respiratory effects of sarin in rats are relatively short-lived.
Figure 7.
The recovery of baseline ventilation (VE) after sarin exposure in conscious rats. As compared with vehicle (VEH), the moderate- (SM) and high-dose sarin (SH)-induced reduction of baseline VE and tidal volume (VT) 2 h after exposure was recovered 1 day after sarin exposure. D1–D5 represents the tests applied 1, 2, and 5 days after exposure. Mean ± SE; n = 12, 8, and 8 for VEH, SM, and SH animals, respectively. * P < 0.01, SH vs. VEH and SM groups.
Figure 8.
The ventilatory (VE) responses to hypercapnia (7% CO2, panel A) and hypoxia (10% O2, panel B) in conscious rats 2 h, and 1, 2, and 5 days after exposure to vehicle (VEH), moderate-, or high-dose of sarin (SM and SH). The reductions of VE, tidal volume (VT) and/or respiratory frequency (fR) responses to hypercapnia or hypoxia immediately (2 h) after SM and SH exposure were recovered 1–2 days after sarin exposure. D1–D5 represents the tests applied 1, 2, and 5 days after exposure. Mean ± SE; n = 12, 8 and 8 for VEH, SM and SH animals, respectively. * P < 0.05, SH or SM vs. VEH group.
Sarin alters arterial blood gases
We measured the arterial blood CO2 partial pressure (PaCO2), oxygen saturation (SaO2), and pH in anesthetized rats. Data presented in Table 2 suggests that at 2 h after exposure, compared with VEH, SH animals exhibited higher PaCO2 and lower SaO2, although the differences did not reach statistical significance. This insignificant higher PaCO2 is likely due to that arterial blood samples were collected from anesthetized animals, in which the hypoventilation observed in conscious SH rats were presumably masked by the anesthesia. On the other hand, compared with the VEH group, the arterial blood pH, bicarbonate (HCO3−), and base excess (BE) were significantly lower in SH rats at 2 h after sarin exposure, suggesting a metabolic acidosis in rats post acute sarin exposure. At day 5 after sarin treatment, all the arterial blood gas parameters were similar in VEH and SH rats.
Table 2.
Baseline parameters of arterial blood gas in anesthetized rats exposed to VEH and SH
| VEH | SH 2h | SH D5 | |
|---|---|---|---|
| Paco2 (torr) | 36.8 ± 2.7 | 41.8 ± 0.5 | 36.8 ± 2.8 |
| Sao2 (%) | 93.5 ± 0.5 | 87.5 ± 3.3 | 92.5 ± 1.9 |
| pH (unit) | 7.41 ± 0.02 | 7.22 ± 0.04* | 7.43 ± 0.03† |
| HCO3− (mM) | 22.6 ± 1.5 | 15.9 ± 1.4* | 24.5 ± 0.5† |
| BE (mM) | −0.4 ± 1.4 | −10.8 ± 2.1* | 0.6 ± 0.5† |
SH 2h and SH D5 represent the data obtained at 2 h and on day 5 after high-dose (SH, 4.0 mg h m−3) exposures.
P < 0.05, compared to VEH
P < 0.05, compared to SH 2h. Paco2, arterial CO2 partial pressure; Sao2, arterial O2 saturation; HCO3−, bicarbonate; BE, base excess.
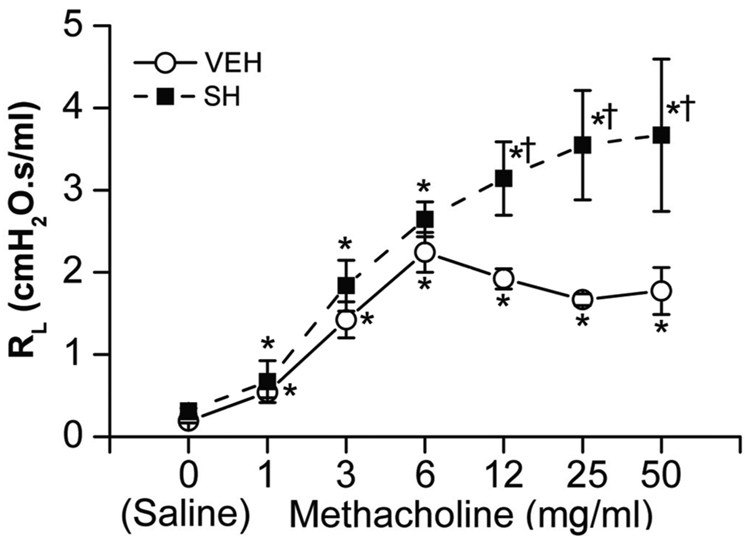
SH exposure fails to change baseline RL
Sarin is a potent cholinergic compound that causes bronchoconstriction (Grob and Harvey, 1958). To ascertain the effects of SH on airway resistance, we determined the baseline RL in VEH and SH animals and the effects of seven different concentrations of methacholine on RL. Results indicated that the baseline RL and the RL response to methacholine at concentrations less than 6 mg ml−1 were not significantly different between VEH and SH rats; however, the RL of SH rats at methacholine concentrations greater than 6 mg ml−1 were significantly higher than VEH rats (Fig. 9). These data indicated that sarin does not significantly alter the baseline RL, but makes the airways more sensitive to the bronchoconstricting effects of muscarinic receptor agonists.
Figure 9.
Total pulmonary resistance (RL) during different levels of methacholine challenge (0, 1, 3, 6, 12, 25 and 50 mg ml−1) in vehicle (VEH)- and high dose sarin (SH)-exposed rats (2 h after exposure). Baseline RL was not different between VEH and SH rats (see the variables after saline injection). RL responses to higher dose of methacholine (12, 25 and 50 mg ml−1) were significantly greater in the SH than VEH rats. Mean ± SE; n = 5 for each group. * P < 0.05, compared with saline; † P < 0.05, between SH and VEH group.
Discussion
The respiratory system is the major route of sarin entry and absorption and plays an important role in the pathogenesis of nerve agent toxicity. In animal experiments, even at low doses, sarin causes long-term neurological and immunological deficiencies (Abu-Qare and Abou-Donia, 2002; Kalra et al., 2002; Pena-Philippides et al., 2007); however, immediate death from high doses of nerve gas exposure is invariably due to respiratory failure. The latter is suggested to be resulted from respiratory muscle paralysis, increased lung secretion-induced airway obstruction, and dysfunction of central respiratory centers as a result of ACh over stimulation and disruption of the nervous system (Rickett et al., 1986; Colwill et al., 2004). To understand the mechanism of acute respiratory failure in sarin-exposed animals, we first established the LC50 dose for sarin in F344 rats. We compared mortality in the rats exposed to three different doses of sarin, SL, SM, and SH, and found that a significant mortality (52%) occurred within 2 h after exposure in SH animals. Our results also indicated that most animals that were alive at 2 h post sarin exposure lived until sacrificed at the next 2 weeks post sarin exposure. Similarly, in Japan, most human fatalities emanating from sarin terrorism occurred within a few hours of the attacks, and were attributed to respiratory failure. However, following respiratory stabilization, subsequent deaths among the sarin victims resulted primarily from neurotoxicity or causes indirectly related to sarin exposure (Yanagisawa et al., 2006).
While chemoreflexes to hypercapnia and hypoxia are critical for normal respiratory rhythm and life, the role of VE chemoreflexes in sarin-induced respiratory failure is essentially unknown. An important finding of this study is that sarin significantly blunts the VE responses to hypercapnia and hypoxia in conscious as well as anesthetized rats. The results unequivocally indicate that even an acute exposure to sublethal doses of sarin (SL and SM) blunts the VE responses, and the changes in VE from SH reflected mainly the effects of sarin on the fR response. Although, we did not present direct evidence demonstrating that sarin-induced VE dysfunction leads to respiratory failure and mortality, we observed that SH exposure not only caused significant mortality but it also produced a marked impairment of VE characterized by baseline VE inhibition and the absence of the fR responses to hypercapnia and/or hypoxia. To our knowledge, this is the first research showing that sarin affects the VE responses to hypercapnia and hypoxia. The loss of the fR responses to hypoxia and/or hypercapnia strongly suggests that sarin blunts the respiratory central drive, which could lead to respiratory failure and death. Indeed, it has been documented that high doses of physostigmine, another acetylcholinesterase inhibitor causes death through centrally mediated respiratory arrest (Anzueto et al., 1990; Futagawa et al., 2000; Duncan et al., 2001). Blunted chemoreflexes have been correlated to respiratory failure and death in other diseases, including COPD and CCHS, and in animal model of SIDS (Schaefer, 1949; Fahey and Hyde, 1983; Shea et al., 1993; Milerad et al., 1995; Gozal et al., 1996; St-John and Leiter, 1999; Hafstrom et al., 2002; Murai et al., 2003; Franciosi et al., 2006; Ucgun et al., 2006).
An interesting finding from this study is that although the magnitude of inhibition of VE by SH and SM is essentially similar, mortality is primarily associated with SH exposures. It is likely that this difference in lethality arises from the SH’s more potent effects on the central respiratory chemical drive, and this assumption is supported by the following observations: (1) Only SH significantly inhibited the baseline VE in conscious rats. (2) The fR responses to hypoxia and hypercapnia were almost completely abolished by SH exposures, but much less affected by SM. Several studies indicate that decreased fR responses (appearance of apnea) correlate with respiratory failure. For example, apnea (reduction in fR) caused by the stimulation of pulmonary C-fibers is strongly aggravated by respiratory syncytial virus, causing death in infected animals (Peng et al., 2007). Similarly, increased mortality associated with the blunted VE responses to hypercapnia and hypoxia in an animal model of COPD and SIDS is also characterized by a depressed fR response and respiratory arrest (St-John and Leiter, 1999; Xu et al., 2007). (3) Compared with SM, the slower recovery of VE impairment in SH rats suggests a more severe damage to the central respiratory system in the latter. That the VT responses to hypercapnic and hypoxic challenges were seemingly higher in SH than SM rats might stem from a compensatory response to SH-depressed fR responses, and/or a more severe acidic response to hypoxia and hypercapnia in SH rats (Kussmaul's respiration).
Sarin-induced body tremors could impair the respiration and in the present study the sarin-induced changes in VE in response to hypercapnia and hypoxia were invariably associated with body tremors. Therefore, it was important to determine whether the VE abnormality was secondary to the inception of body tremors. However, a significant reduction in the VE response to hypercapnia was also observed in the SL-exposed rats that had no tremors. Moreover, because the blunted VE responses also exist in anesthetized SH rats that do not exhibit tremors, it is unlikely that the two events (body tremors and VE changes) are causally related.
Our results indicated that SH exposures decrease arterial pH and HCO3−concentration. These changes may result from metabolic acidosis. Metabolic acidosis due to accumulation of lactic acid has been reported in sarin-exposed animals (Gold et al., 1957). Cerebral acidosis stimulates VE (Van de Ven et al., 2001); however, in spite of increased chemical drive in SH animals, VE was still significantly lower than that in VEH-exposed rats. This finding argues again that the SH-induced substantial respiratory impairment. VE function could also be affected by cardiovascular function (Turner, 1991; Dick et al., 2005); therefore, we compared the cardiovascular response at baseline and in response to hypercapnia and hypoxia in anesthetized VEH and SH rats. Our results did not indicate a significant difference in these responses between the two groups, suggesting that the VE abnormalities did not arise from sarin-induced cardiovascular dysfunction. Similarly, we did not find that the VE changes in SH animals were related to changes in sarin-induced airway resistance, because SH exposures failed to increase the baseline RL and, more importantly, the blunted VE responses to hypercapnia and hypoxia was also seen in anesthetized rats with tracheal cannulation that greatly limits RL changes.
It is plausible that sarin-induced VE changes arise from the effects of sarin on the function of the chemoreceptors. The central and peripheral chemoreceptors play a key role in control of breathing (Gonzalez et al., 1994; Nattie and Li, 1996; Burton et al., 1997; Xu and Frazier, 2002), and there is abundant evidence showing the importance of cholinergic mechanisms in the central CO2 chemoreception of mammals (Fukuda and Loeschcke, 1979; Loeschcke, 1982; Burton et al., 1989; Burton et al., 1990; Monteau et al., 1990; Lydic et al., 1991). For example, multiple brainstem areas with CO2-chemoreception express a high density of cholinergic receptors (Hyde et al., 1988; Mallios et al., 1995; Rosin et al., 2006), and the respiratory responses to focal stimulation of chemosensitive neurons are similar to those evoked by focal application of ACh agonists in the same regions (Fukuda and Loeschcke, 1979; Noakes et al., 2006). ACh is also involved in facilitating the carotid chemoreceptor-mediated VE response to hypoxia through nicotinic ACh receptors (van Lunteren et al., 1984). Taken together, these studies suggest that cholinergic stimulation increases the VE responses to hypercapnia and hypoxia and, therefore, the effects of sarin on the VE responses to hypercapnia and hypoxia seem to be paradoxical. While the precise mechanism is still not clear, it is conceivable that excessive synaptic levels of ACh from high-dose sarin treatment are toxic to target neurons, including those in the medulla oblongata — the seat of the major respiratory control network (Gonzalez et al., 1994; Nattie and Li, 1996; Burton et al., 1997; Xu and Frazier, 2002). In fact, neuronal damage in the central nervous system, including the cortex, hippocampal formation, and cerebellum has been observed in animals exposed to high doses of sarin (Shih, 1982; Li et al., 2000; Henderson et al., 2002; Abou-Donia, 2003). While some neuronal injury was observed in the cortex and hippocampus of SH rats, we were unable to find significant neuronal damage in the medulla oblongata of these animals (Razani-Boroujerdi et al., unpublished observation). Therefore, the sarin-induced blunted VE response to hypercapnia and hypoxia might reflect functional rather than overt loss of the target neurons in the central respiratory network. This possibility is further strengthened by the observations that the VE changes in SH animals as well as the humans surviving the Japanese sarin terrorism (Yanagisawa et al., 2006), were transitory and normalized within hours after the exposure.
While exposure of guinea pigs to the nerve agent VX was reported to cause pulmonary edema and increase emigration of inflammatory cells into the lung (Wright et al., 2006), we were unable to find any histopathological evidence of lung injury in SH-exposed rats at 24 or 7 days after sarin exposure (Razani-Boroujerdi et al., unpublished observation). Moreover, several arguments exclude a significant contribution of possible lung inflammation in the sarin-induced blunted VE responses. First, sarin exposure did not significantly change the baseline PaCO2, SaO2, and RL, suggesting that the sarin exposures did not affect pulmonary diffusion and pulmonary fluid exudation. Second, we found that the fR response, which is controlled by the neurons, was almost eliminated by SH. Third, while bleomycin-induced acute lung injury elevates hypoxic VE responses, it does not affect the hypercapnic VE response (Jacono et al., 2006).
In summary, these studies present three major observations: (1) The LC50 dose of sarin in adult Fischer 344 rat is approximately 4.0 mg h m−3. (2) While the concentrations of sarin that induce tremors also lower the baseline VE and the VE responses to hypercapnia and hypoxia, the changes in the VE are independent of tremor activity and predominantly contributed by the decreased fR response. (3) The blunted VE response is not associated with airway resistance and cardiovascular abnormality. (4) The altered baseline VE and the VE responses to hypercapnia and hypoxia by sarin recover within 2 days. Our results suggest that the sarin-induced respiratory impairment emanates, at least primarily, from a transitory dysfunction of the central respiratory network.
Acknowledgements
The study is supported by National Heart, Lung, and Blood Institute Grant 74183; and U.S. Army grant W81XWH-04-C-0071.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
Some of the findings reported in this paper were presented at Society for Neuroscience Meeting, San Diego, CA, 2007.
References
- Abou-Donia MB. Organophosphorus ester-induced chronic neurotoxicity. Arch Environ Health. 2003;58:484–497. doi: 10.3200/AEOH.58.8.484-497. [DOI] [PubMed] [Google Scholar]
- Abu-Qare AW, Abou-Donia MB. Sarin: health effects, metabolism, and methods of analysis. Food Chem Toxicol. 2002;40:1327–1333. doi: 10.1016/s0278-6915(02)00079-0. [DOI] [PubMed] [Google Scholar]
- Adams GK, 3rd, Yamamura HI, O'Leary JF. Recovery of central respiratory function following anticholinesterase intoxication. Eur J Pharmacol. 1976;38:101–112. doi: 10.1016/0014-2999(76)90206-5. [DOI] [PubMed] [Google Scholar]
- Anzueto A, deLemos RA, Seidenfeld J, Moore G, Hamil H, Johnson D, Jenkinson SG. Acute inhalation toxicity of soman and sarin in baboons. Fundam Appl Toxicol. 1990;14:676–687. doi: 10.1016/0272-0590(90)90293-s. [DOI] [PubMed] [Google Scholar]
- Bruce EN, Cherniack NS. Central chemoreceptors. J Appl Physiol. 1987;62:389–402. doi: 10.1152/jappl.1987.62.2.389. [DOI] [PubMed] [Google Scholar]
- Burton MD, Johnson DC, Kazemi H. CSF acidosis augments ventilation through cholinergic mechanisms. J Appl Physiol. 1989;66:2565–2572. doi: 10.1152/jappl.1989.66.6.2565. [DOI] [PubMed] [Google Scholar]
- Burton MD, Johnson DC, Kazemi H. Adrenergic and cholinergic interaction in central ventilatory control. J Appl Physiol. 1990;68:2092–2099. doi: 10.1152/jappl.1990.68.5.2092. [DOI] [PubMed] [Google Scholar]
- Burton MD, Johnson DC, Kazemi H. The central respiratory effects of acetylcholine vary with CSF pH. J Auton Nerv Syst. 1997;62:27–32. doi: 10.1016/s0165-1838(96)00104-x. [DOI] [PubMed] [Google Scholar]
- Colwill JM, Daniell WE, Goldman RH, Mayeux R, Potolicchio SJ, Rodricks JV. Toxicology. In: Sarin CoGWaHULRo., editor. Gulf War and Health: Updated Literature Review of Sarin. Washington, D.C.: National Academy of Sciences; 2004. pp. 26–46. [Google Scholar]
- Dick TE, Shannon R, Lindsey BG, Nuding SC, Segers LS, Baekey DM, Morris KF. Arterial pulse modulated activity is expressed in respiratory neural output. J Appl Physiol. 2005;99:691–698. doi: 10.1152/japplphysiol.01124.2004. [DOI] [PubMed] [Google Scholar]
- Duncan EJ, Conley JD, Grychowski KD, Conley SA, Lundy PM, Hamilton MG, Sawyer TW. A comparison of the effects of sarin and succinylcholine on respiratory parameters in anesthetized domestic swine. Mil Med. 2001;166:322–327. [PubMed] [Google Scholar]
- Fahey PJ, Hyde RW. "Won't breathe" vs "can't breathe". Detection of depressed ventilatory drive in patients with obstructive pulmonary disease. Chest. 1983;84:19–25. doi: 10.1378/chest.84.1.19. [DOI] [PubMed] [Google Scholar]
- Franciosi LG, Page CP, Celli BR, Cazzola M, Walker MJ, Danhof M, Rabe KF, Della Pasqua OE. Markers of exacerbation severity in chronic obstructive pulmonary disease. Respir Res. 2006;7:74. doi: 10.1186/1465-9921-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Loeschcke HH. A cholinergic mechanism involved in the neuronal excitation by H+ in the respiratory chemosensitive structures of the ventral medulla oblongata of rats in vitro. Pflugers Arch. 1979;379:125–135. doi: 10.1007/BF00586938. [DOI] [PubMed] [Google Scholar]
- Futagawa H, Kakinuma Y, Takahashi H. The role of cholinergic and noncholinergic mechanisms in the cardiorespiratory failure produced by N-methylcarbamate cholinesterase inhibitors in rabbits. Toxicol Appl Pharmacol. 2000;165:27–36. doi: 10.1006/taap.2000.8924. [DOI] [PubMed] [Google Scholar]
- Gold AJ, Weller JM, Freeman G. Metabolic and acid-base changes following acute cholinesterase inhibition. Am J Physiol. 1957;188:321–326. doi: 10.1152/ajplegacy.1957.188.2.321. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gozal D, Marcus CL, Ward SL, Keens TG. Ventilatory responses to passive leg motion in children with congenital central hypoventilation syndrome. Am J Respir Crit Care Med. 1996;153:761–768. doi: 10.1164/ajrccm.153.2.8564130. [DOI] [PubMed] [Google Scholar]
- Grob D, Harvey JC. Effects in man of the anticholinesterase compound sarin (isopropyl methyl phosphonofluoridate) J Clin Invest. 1958;37:350–368. doi: 10.1172/JCI103615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafstrom O, Milerad J, Sundell HW. Prenatal nicotine exposure blunts the cardiorespiratory response to hypoxia in lambs. Am J Respir Crit Care Med. 2002;166:1544–1549. doi: 10.1164/rccm.200204-289OC. [DOI] [PubMed] [Google Scholar]
- Henderson RF, Barr EB, Blackwell WB, Clark CR, Conn CA, Kalra R, March TH, Sopori ML, Tesfaigzi Y, Menache MG, Mash DC. Response of rats to low levels of sarin. Toxicol Appl Pharmacol. 2002;184:67–76. [PubMed] [Google Scholar]
- Holstege CP, Kirk M, Sidell FR. Chemical warfare. Nerve agent poisoning. Crit Care Clin. 1997;13:923–942. doi: 10.1016/s0749-0704(05)70374-2. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Gibbs M, Peroutka SJ. Distribution of muscarinic cholinergic receptors in the dorsal vagal complex and other selected nuclei in the human medulla. Brain Res. 1988;447:287–292. doi: 10.1016/0006-8993(88)91131-6. [DOI] [PubMed] [Google Scholar]
- Jacono FJ, Peng YJ, Nethery D, Faress JA, Lee Z, Kern JA, Prabhakar NR. Acute lung injury augments hypoxic ventilatory response in the absence of systemic hypoxemia. J Appl Physiol. 2006;101:1795–1802. doi: 10.1152/japplphysiol.00100.2006. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Gold AJ, Freeman G. Comparative lung-airway resistance and cardiovascular effects in dogs and monkeys following parathion and sarin intoxication. Am J Physiol. 1958;192:581–584. doi: 10.1152/ajplegacy.1958.192.3.581. [DOI] [PubMed] [Google Scholar]
- Kalra R, Singh SP, Razani-Boroujerdi S, Langley RJ, Blackwell WB, Henderson RF, Sopori ML. Subclinical doses of the nerve gas sarin impair T cell responses through the autonomic nervous system. Toxicol Appl Pharmacol. 2002;184:82–87. [PubMed] [Google Scholar]
- Lee EC. Clinical manifestations of sarin nerve gas exposure. JAMA. 2003;290:659–662. doi: 10.1001/jama.290.5.659. [DOI] [PubMed] [Google Scholar]
- Li L, Gunasekar PG, Borowitz JL, Isom GE. Muscarinic receptor-mediated pyridostigmine-induced neuronal apoptosis. Neurotoxicology. 2000;21:541–552. [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol. 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA, Wertz R, White DP. Cholinergic reticular mechanisms influence state-dependent ventilatory response to hypercapnia. Am J Physiol. 1991;261:R738–R746. doi: 10.1152/ajpregu.1991.261.3.R738. [DOI] [PubMed] [Google Scholar]
- Mallios VJ, Lydic R, Baghdoyan HA. Muscarinic receptor subtypes are differentially distributed across brain stem respiratory nuclei. Am J Physiol. 1995;268:L941–L949. doi: 10.1152/ajplung.1995.268.6.L941. [DOI] [PubMed] [Google Scholar]
- Milerad J, Larsson H, Lin J, Sundell HW. Nicotine attenuates the ventilatory response to hypoxia in the developing lamb. Pediatr Res. 1995;37:652–660. doi: 10.1203/00006450-199505000-00017. [DOI] [PubMed] [Google Scholar]
- Monteau R, Morin D, Hilaire G. Acetylcholine and central chemosensitivity: in vitro study in the newborn rat. Respir Physiol. 1990;81:241–253. doi: 10.1016/0034-5687(90)90049-5. [DOI] [PubMed] [Google Scholar]
- Murai M, Oyamada Y, Hakuno H, Yamaguchi K. Effects of prenantal nicotine exposure on ventilatory control in neonatal rats. Am J Respir Critic Care Med. 2003;167:A851. [Google Scholar]
- Nattie E. Multiple sites for central chemoreception: their roles in response sensitivity and in sleep and wakefulness. Respir Physiol. 2000;122:223–235. doi: 10.1016/s0034-5687(00)00161-4. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreception in the region of the ventral respiratory group in the rat. J Appl Physiol. 1996;81:1987–1995. doi: 10.1152/jappl.1996.81.5.1987. [DOI] [PubMed] [Google Scholar]
- Noakes PS, Hale J, Thomas R, Lane C, Devadason SG, Prescott SL. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur Respir J. 2006;28:721–729. doi: 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- Okumura T, Ninomiya N, Ohta M. The chemical disaster response system in Japan. Prehosp Disaster Med. 2003;18:189–192. doi: 10.1017/s1049023x00001047. [DOI] [PubMed] [Google Scholar]
- Pena-Philippides JC, Razani-Boroujerdi S, Singh SP, Langley RJ, Mishra NC, Henderson RF, Sopori ML. Long- and short-term changes in the neuroimmune-endocrine parameters following inhalation exposures of F344 rats to low-dose sarin. Toxicol Sci. 2007;97:181–188. doi: 10.1093/toxsci/kfm017. [DOI] [PubMed] [Google Scholar]
- Peng W, Zhuang J, Harrod KS, Xu F. Respiratory syncytial virus infection in anesthetized weanling rather than adult rats prolongs the apneic responses to right atrial injection of capsaicin. J Appl Physiol. 2007;102:2201–2206. doi: 10.1152/japplphysiol.01436.2006. [DOI] [PubMed] [Google Scholar]
- Rickett DL, Glenn JF, Beers ET. Central respiratory effects versus neuromuscular actions of nerve agents. Neurotoxicology. 1986;7:225–236. [PubMed] [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol. 2006;499:64–89. doi: 10.1002/cne.21105. [DOI] [PubMed] [Google Scholar]
- Schaefer KE. Respiration and acid-base balance during prolonged exposure to 3% CO2. Pflugers Archiv. 1949;251:689–715. [Google Scholar]
- Shea SA, Andres LP, Shannon DC, Banzett RB. Ventilatory responses to exercise in humans lacking ventilatory chemosensitivity. J Physiol. 1993;468:623–640. doi: 10.1113/jphysiol.1993.sp019792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih TM. Time course effects of soman on acetylcholine and choline levels in six discrete areas of the rat brain. Psychopharmacology (Berl) 1982;78:170–175. doi: 10.1007/BF00432257. [DOI] [PubMed] [Google Scholar]
- Shih TM, Rowland TC, McDonough JH. Anticonvulsants for nerve agent-induced seizures: The influence of the therapeutic dose of atropine. J Pharmacol Exp Ther. 2007;320:154–161. doi: 10.1124/jpet.106.111252. [DOI] [PubMed] [Google Scholar]
- Sidell FR. Clinical effects of organophosphorus cholinesterase inhibitors. J Appl Toxicol. 1994;14:111–113. doi: 10.1002/jat.2550140212. [DOI] [PubMed] [Google Scholar]
- Sidell FR, Borak J. Chemical warfare agents: II. Nerve agents. Ann Emerg Med. 1992;21:865–871. doi: 10.1016/s0196-0644(05)81036-4. [DOI] [PubMed] [Google Scholar]
- St-John WM, Leiter JC. Maternal nicotine depresses eupneic ventilation of neonatal rats. Neurosci Lett. 1999;267:206–208. doi: 10.1016/s0304-3940(99)00364-x. [DOI] [PubMed] [Google Scholar]
- Turner DL. Cardiovascular and respiratory control mechanisms during exercise: an integrated view. J Exp Biol. 1991;160:309–340. doi: 10.1242/jeb.160.1.309. [DOI] [PubMed] [Google Scholar]
- Ucgun I, Metintas M, Moral H, Alatas F, Yildirim H, Erginel S. Predictors of hospital outcome and intubation in COPD patients admitted to the respiratory ICU for acute hypercapnic respiratory failure. Respir Med. 2006;100:66–74. doi: 10.1016/j.rmed.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Van de Ven MJ, Colier WN, van der Sluijs MC, Oeseburg B, Folgering H. Ventilatory response in metabolic acidosis and cerebral blood volume in humans. Respir Physiol. 2001;124:105–115. doi: 10.1016/s0034-5687(00)00194-8. [DOI] [PubMed] [Google Scholar]
- van Lunteren E, Haxhiu MA, Deal EC, Jr, Perkins D, Cherniack NS. Effects of CO2 and bronchoconstriction on costal and crural diaphragm electromyograms. J Appl Physiol. 1984;57:1347–1353. doi: 10.1152/jappl.1984.57.5.1347. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu F. Postnatal development of right atrial injection of capsaicin-induced apneic response in rats. J Appl Physiol. 2006;101:60–67. doi: 10.1152/japplphysiol.00085.2006. [DOI] [PubMed] [Google Scholar]
- Wixson SK, White WJ, Hughes HC, Jr, Lang CM, Marshall WK. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on arterial blood pH, blood gases, mean arterial blood pressure and heart rate in adult male rats. Lab Anim Sci. 1987;37:736–742. [PubMed] [Google Scholar]
- Wright BS, Rezk PE, Graham JR, Steele KE, Gordon RK, Sciuto AM, Nambiar MP. Acute lung injury following inhalation exposure to nerve agent VX in guinea pigs. Inhal Toxicol. 2006;18:437–448. doi: 10.1080/08958370600563847. [DOI] [PubMed] [Google Scholar]
- Xu F, Frazier DT. Role of the cerebellar deep nuclei in respiratory modulation. Cerebellum. 2002;1:35–40. doi: 10.1080/147342202753203078. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhuang J, Wang R, Seagrave JC, March TH. Blunted ventilatory response to hypoxia/hypercapnia in mice with cigarette smoke-induced emphysema. Respir Physiol Neurobiol. 2007;158:5–13. doi: 10.1016/j.resp.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: acute toxicity and long-term effects. J Neurol Sci. 2006;249:76–85. doi: 10.1016/j.jns.2006.06.007. [DOI] [PubMed] [Google Scholar]