Abstract
Annonaceous acetogenins, a large family of naturally occurring polyketides isolated from various species of the plant genus Annonaceae, have been found to exhibit significant cytotoxicity against a variety of cancer cells. Previous studies showed that these compounds could act on the mitochondria complex-I and block the corresponding electron transport chain and terminate ATP production. However, more details of the mechanisms of action remain ambiguous. In this study we tested the effects of a set of mimetics of annonaceous acetogenin on some cancer cell lines, and report that among them AA005 exhibits the most potent antitumor activity. AA005 depletes ATP, activates AMP-activated protein kinase (AMPK) and inhibits mTOR complex 1 (mTORC1) signal pathway, leading to growth inhibition and autophagy of colon cancer cells. AMPK inhibitors compound C and inosine repress, while AMPK activator AICAR enhances, AA005-caused proliferation suppression and subsequent autophagy of colon cancer cells. AA005 enhances the ATP depletion and AMPK activation caused by 2-deoxyglucose, an inhibitor of mitochondrial respiration and glycolysis. AA005 also inhibits chemotherapeutic agent cisplatin-triggered up-regulation of mTOR and synergizes with this drug in suppression of proliferation and induction of apoptosis of colon cancer cells. These data indicate that AA005 is a new metabolic inhibitor which exhibits therapeutic potentials in colon cancer.
Introduction
In tumor cells there is an increased glycolytic pathway enzymes and glucose transporters even in the presence of a high O2 concentration, ultimately leads to an elevated ATP production rate [1]. Clinical evidence has linked cell metabolism with cancer outcomes, and reprogramming energy metabolism has been approved to be an emerging hallmark of cancer [2]. These observations have raised interest in targeting energy metabolism for cancer therapy for both hypoxic (glycolytic) and oxidative tumors [1], albeit also concerns that these therapies would have unacceptable effects on normal cells. Intriguingly, some of the first cancer therapies targeting specific metabolic needs of cancer cells remain effective in the clinic today, re-inspiring efforts to target metabolic dependencies of cancer cells as a selective anticancer strategy [3].
AMP-activated protein kinase (AMPK) which exists in cells as a heterotrimeric complex composed of a catalytic kinase subunit (α) and two regulatory subunits (β and γ), is a sensor of energy status that maintains cellular energy homeostasis. It is activated by a fall in ATP (concomitant with a rise in ADP and AMP) or stimuli that increase the cellular AMP/ATP ratio, resulting in the activation of catabolic pathways and the inhibition of anabolic pathways [4], [5]. Essential to activation of AMPK is its phosphorylation at Thr-172 by an upstream AMPK kinases (AMPKKs) [6] and tumor suppressor LKB1 [7] which is a serine/threonine kinase associated with gastrointestinal polyposis and cancer [8] and lung cancer [9]. AMPK phosphorylates two rate-limiting enzymes in fatty acid and cholesterol synthesis: acetyl-CoA carboxylase (ACC) and HMG-CoA reductase, as well as other downstream targets, culminating in the inhibition of anabolic pathways and the activation of catabolic pathways [5]. AMPK activation directly limits translational initiation and protein synthesis [10], through inhibition of translation elongation factor 2 (EF2) [11], and indirectly through TSC2, leading to suppression of mammalian target of rapamycin (mTOR) [12] which can phosphorylate and activate p70 S6 kinase and 4E-binding protein (4EBP) [13].
AMPK activation by AMP analog 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) accumulates cyclin dependent kinase inhibitors p21 and p27 and down-regulates cyclin D1 in human hepatocellular carcinoma cells, leading to cell cycle arrest at G1 phase [14]. Population studies provide clues that the use of metformin which is an AMPK activator, may be associated with reduced incidence and improved prognosis of certain cancers [15], [16]. In breast cancer, metformin exerts inhibitory effects via inhibition of mTOR-dependent translation initiation [17], [18]. Metformin inhibits proliferation, decreases cell viability and blocks cell cycle in G1 phase in prostate cancer cells, and in vivo treatment with metformin leads to a significant reduction of tumor growth in mice bearing xenografts of prostate cancer cells [19]. Metformin and AICAR induce apoptosis and suppresses the tumor growth of colon cancer line HCT116 p53(−/−) xenografts, and trigger autophagy of HCT116 p53(+/+) cells [20]. AICAR and protein folding inhibitor 17-AAG, especially when combined, show efficacy against aneuploid human cancer cell lines [21]. These results indicate that AMPK could be a rational drug target and lead compounds should be identified or designed for the development of therapeutic avenues for cancers.
Annonaceous acetogenins represent a class of naturally occurring polyketides isolated from various species of the plant genus Annonaceae [22]. These compounds exhibit diverse bioactivities, including promising cytotoxicites and antiparasitic activities [23]. Though studies show that annonaceous acetogenins can disrupt mitochondrial function through blocking mitochondria complex I and ubiquinone-linked NADH oxidase [23], and bind the third matrix-side loop of ND1 subunit in mitochondrial NADH-ubiquinone oxidoreductase [23], [24], the mechanisms of action of these compounds in fighting cancer remain largely unknown. The chemistry group of our team had designed and synthesized a series of annonaceous acetogenin mimetics [25]–[28]. In this work we tested the biological activity of some new analogs and investigated the mechanisms underlying annonaceous acetogenins’ cytotoxicities, and reported that a mimetic AA005 which showed potent and selective inhibitory activities against a variety of cancer cells, was able to activate AMPK and induce cell cycle arrest followed by autophagy, demonstrating its therapeutic potentials.
Materials and Methods
Chemicals and Reagents
Annonaceous acetogenin mimetics (Table 1) were dissolved in DMSO and stored at −20°C. The 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Amresco Inc. (Solon, OH). Compound C, rapamycin, rotenone, inosine, Rhodamine 123, 2-DG and AICAR were purchased from Sigma. MitoTracker Deep Red FM was purchased from Invitrogen. ATP Assay Kit was purchased from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China).
Table 1. IC50 values of the derivatives of AA005 in various human cancer and noncancerous cell lines.
| No. | Compound | Structure | MW | IC50 (µM) | |||||
| MCF7 | SGC7901 | HCT116 | HT29 | BEAS-2B | HLF | ||||
| 1 | AA005 | <$>\scale 75%\raster="rg1"<$> | 554.8 | 0.26±0.05 | 0.06±0.02 | 0.11±0.008 | 0.35±0.09 | >50 | 20.9±7.4 |
| 2 | AA090 | <$>\scale 75%\raster="rg2"<$> | 320.3 | >50 | >50 | >50 | >50 | >50 | >50 |
| 3 | AA091 | <$>\scale 75%\raster="rg3"<$> | 498.4 | 4.4±0.042 | 1.5±0.3 | 4.3±0.47 | 19.5±3.15 | >50 | >50 |
| 4 | AA093 | <$>\scale 75%\raster="rg4"<$> | 570.45 | 0.18±0.05 | 0.03±0.01 | 0.13±0.01 | 0.38±0.1 | 31.2±5.18 | 18.3±2.78 |
| 5 | AA101 | <$>\scale 75%\raster="rg5"<$> | 678.5 | 5.95±0.59 | 2±0.39 | 5.18±0.18 | >50 | >50 | >50 |
| 6 | AA102 | <$>\scale 75%\raster="rg6"<$> | 766.6 | 1.8±0.18 | 0.44±0.01 | 2.3±0.16 | 9.9±1.47 | >50 | 5.8±0.62 |
| 7 | AA103 | <$>\scale 75%\raster="rg7"<$> | 723.0 | 1.9±0.66 | 0.27±0.03 | 1.1±0.08 | 3.3±0.97 | 9.3±0.98 | 4.9±0.17 |
| 8 | AA104 | <$>\scale 75%\raster="rg8"<$> | 811.1 | 2.5±0.59 | 0.18±0.02 | 0.6±0.02 | 6.5±1.33 | >50 | 3.8±0.61 |
| 9 | AA105 | <$>\scale 75%\raster="rg9"<$> | 855.2 | 3±0.2 | 0.9±0.14 | 2.7±0.13 | 16.1±0.78 | >50 | >50 |
Antibodies
The antibodies used in this study were as follows: anti-β-Actin (Sigma); anti-p-AMPKα1, anti-p-ACC, anti-p-mTOR, anti-p-S6K, anti-AMPKα1, anti-ACC, anti-mTOR, anti-S6K, anti-PARP, goat anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP antibody (Cell Signaling Technology); anti-CyclinD1 (Abcam), anti-CDK4 (Santa Cruz Biotechnology), and anti-LC3 (Sigma).
Cell Culture and Transfection
The lung cancer cell line A549, breast cancer cell line MCF-7, cervical cancer cell line HeLa, colon cancer cell lines LOVO, SW480, HCT116 and HT29, and human embryonic kidney HEK-293T cells were obtained from the American Tissue Culture Collection (ATCC). Human embryonic lung fibroblast MRC-5, gastric cancer cell line SGC7901, and hepatic cancer cell line BEL7402 were purchased from the Cell Resource Center, Chinese Academy of Medical Sciences (Beijing). The normal human bronchial epithelial cells (HBEpiC) were purchased from ScienCell (ScienCell Research Laboratories, San Diego, California). The 293T, A549, BEL7402, HeLa, MCF-7, SGC7901 and the human normal bronchial epithelial cells BEAS-2B [29] cells were cultured in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS; Gibco/BRL, Grand Island, NY), 100 U/ml penicillin and 100 µg/ml streptomycin. LOVO, SW480, HCT116 and HT29 cells were cultured in DMEM/F12 supplemented with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. MRC-5 cells were cultured in MEM/EBSS medium supplemented with non-essential amino acids, 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. HBEpiC cells were cultured in a serum-free bronchial epithelial cell medium (ScienCell Research Laboratories) containing bronchial epithelial cell growth supplement (ScienCell Research Laboratories). The pQCXIP-GFP-LC3 and pQCXIP-GFP plasmids [30] were transfected into LOVO cells using the Lipofectamine 2000 (Qiagen) according to the recommended protocol by the manufacturer.
Cell Viability, Cell Proliferation and Clonogenic Assays
Cancer cells (5×103) were seeded in each well of 96-well tissue culture plates (Coaster, Charlotte, NC) and treated with annonaceous acetogenin mimetics for 48 h at 37°C in a 5% CO2 atmosphere. MTT assay was performed as described [31], and the IC50 values were calculated using the CalcuSyn software (version 2.0, Biosoft, Cambridge, UK). Cell viability was estimated by trypan blue dye exclusion. The potential synergistic, additive or antagonistic effect between AA005 and 2-DG or cisplatin was carefully assessed using the Calcusyn Software (Biosoft, Cambridge, UK) as described [32]. The dose-effect curves of single or combined drug treatment were analyzed by the median-effect method [33], where the combination indexes (CI) less than, equal to, and greater than 1 indicate synergistic, additive, and antagonistic effects, respectively.
For foci formation, HT29, LOVO or HCT116 treated with AA005 were seeded in triplicate into 35 mm plates (200 cells per plate). After 8 days of culturing, cells were stained with Giemsa and clones containing more than 50 cells were counted [29].
Analysis of Cell Cycle and Apoptosis
To detect the cell cycle distribution, colon cancer cells were synchronized to G1/S boundary by a double thymidine block [31] and then exposed to AA005 at indicated concentrations for 24 h. Cells were harvested, fixed with 70% cold ethanol in 4°C overnight. The cells were centrifuged and washed with PBS, followed by incubation with RNase and propidium iodide (PI) (Sigma-Aldrich). Cell cycle distribution was analyzed by flow cytometry (BD FACS Vantage Diva, USA). Cell apoptosis was measured using PE Annexin V/PI Apoptosis Detection kit (BD Biosciences, San Jose, CA) according to manufacturer’s instruction.
Analysis of Cells with GFP-LC3 Vesicles
Cells were transfected with pQCX-IP-GFP-LC3 and pQCX-IP-GFP-expressing plasmids for 24 h, and then treated with various concentrations of AA005 for another 24 h. Cells were fixed with 4% paraformaldehyde/PBS for 15 min at room temperature and analyzed using confocal microscopy at 63× magnification. The percentage of GFP-positive vesicles cells was assessed [30].
Measurement of Mitochondrial Transmembrane Potential, ATP Content and NAD+/NADH Ratio
Cells were treated with different concentrations of annonaceous acetogenin mimetics for 24 h. The mitochondrial transmembrane potential was examined as described [34], ATP content was measured using an ATP Bioluminescence Assay Kit (Beyotime Institute of Biotechnology), and NAD+/NADH ratio was measured using Amplite™ Colorimetric NAD/NADH Assay Kit (AAT Bioquest, Inc., Sunnyvale, CA) according to the manufacturer’s instructions.
Confocal Microscopy Analyses
Cells were grown on coverslips and fixed in 4% paraformaldehyde. After a brief washing in PBS supplemented with 100 mM glycine, slides were blocked with 5% bovine serum albumin (BSA; Sigma) and 0.3% Triton X-100 in PBS for 30 min at room temperature, and stained for fluorescence microscopy as described [31]. Cells upon AA005-flu were examined by use of a Zeiss LSM 510 META microscope equipped with a 63× oil-immersion objective. Image processing and analysis were done with Zeiss LSM 510 software version 3.2, ImageJ Version 1.42 (National Institutes of Health), and Adobe Photoshop Version 7.0 (Adobe Systems).
Western Blotting
Cell pellets were lysed in RIPA buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% SDS, 1% deoxycholate, 1% TritonX-100, 1 mM EDTA, 5 mM NaF, 1 mM sodium vanadate, and protease inhibitors cocktail (Sigma). Cells were lysed on ice for 30 min in RIPA buffer, lysates were centrifuged, protein extracts were quantitated and loaded on 10% to 15% sodium dodecyl sulfate polyacrylamide gel, electrophoresed, and transferred to a nitrocellulose membrane (Whatman). The membrane was incubated with primary antibody, washed, and incubated with horseradish peroxidase (HRP)–conjugated secondary antibody. Detection was performed by using a chemiluminescent western detection kit (Cell Signaling Technology) [35].
Statistical Analysis
All experiments were repeated at least three times and the data are presented as the mean±SD unless noted otherwise. Differences between data groups were evaluated for significance using Student t-test of unpaired data or one-way analysis of variance and Bonferroni post-test. P values<0.05 were considered statistically significant.
Results
Structure activity Relationship (SAR) Analysis of Annonaceous Acetogenin Mimetics
A serial annonaceous acetogenin mimetics had been synthesized by replacement of both tetrahydrofuran (THF) rings of natural bullatacin with a simple diethylene glycol ether unit by the chemistry group of our team [25]–[28]. Nine novel analogs were synthesized in this work, and we found that the mimetics AA005 [25] and AA093, a compound with the introduction of a 10-hydroxy group onto AA005, represented the two compounds which had the lowest IC50 values in this setting (Table 1). The observation that compound AA090 and AA091 lacking the right lactone unit and left 11-carbon tail exhibited a 17 to >170 fold lower cytotoxicity in cancer cells suggested that these groups are essential for the cytotoxicity of AA005. AA101 with an additional lactone unit embedded in the left hydrocarbon chain part exhibited a 23–142 fold lower cytotoxicity in cancer cells, further confirming the importance of the long hydrophobic tail and the right terminal lactone in the mimicry. Adding a middle ether unit to the mimetics (compounds AA102-105) slightly enhanced their anti-proliferative activity as compared to AA101, suggesting that a diethylene glycol ether unit is essential for the anti-proliferative activity. The diverse biological activity of these mimetics indicates that the structural analogs may not be functional analogs.
Inhibitory Effects of AA005 on Cancer Cells
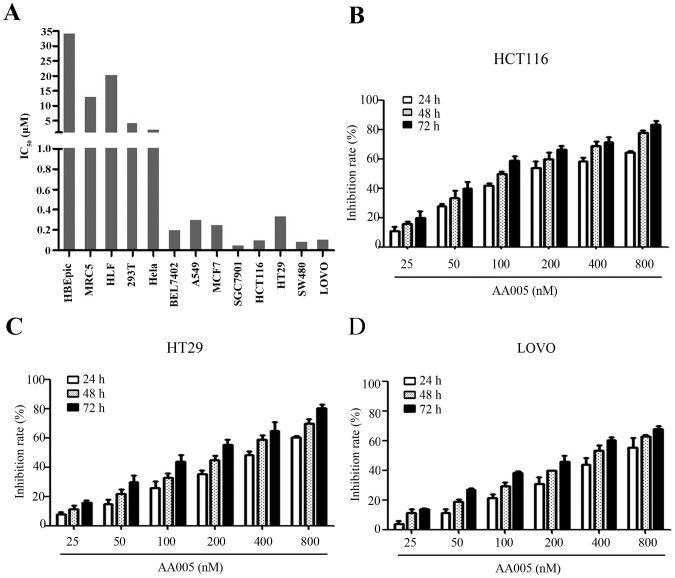
Because AA005 was the most potent cytotoxic agent among these mimetics, we further tested its effects on 11 human cancer cell lines and 4 noncancerous cell lines (HBEpiC, MRC5, HLF and 293T), and found that AA005 showed diverse effects on cancer cells in that it had potent inhibitory effect on colon (HCT116, HT29, LOVO and SW480), gastric (SGC7901), hepatic (BEL7402), lung (A549) and breast (MCF7) cancer lines, and weak effect on cervical (HeLa) cancer cells (Figure 1A). AA005 exhibited inhibitory effects on HCT116 (Figure 1B), HT29 (Figure 1C) and LOVO (Figure 1D) cells in a dose- and time-dependent fashion. Interestingly, AA005 showed an even weaker activity against noncancerous (HBEpiC, MRC5, HLF, BEAS-2B and 293T) cells (Figure 1A and Table 1). These results indicate that the relative selective inhibitory effects of AA005 on cancer cells warrant further investigation.
Figure 1. AA005 shows a relatively selective cytotoxicity against cancer cells.
(A) IC50 values of AA005 (in 48 h) for various human cancer and noncancerous cell lines. IC50 values (mean ± SD, µM) were calculated from 3 independent experiments. (B through D) MTT assays of HCT116 (B), HT29 (C) and LOVO (D) cells upon AA005 at indicated concentration and time points.
AA005 Suppresses Cell Proliferation and Colony Forming Activity of Colon Cancer Cells
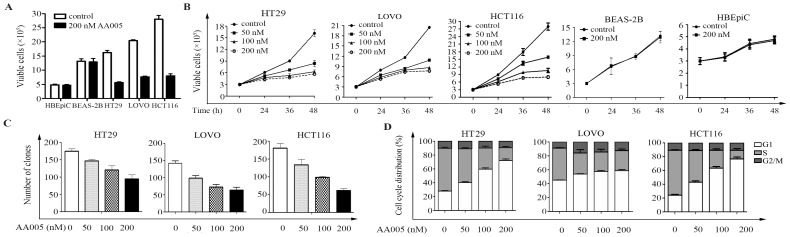
We further analyzed the effects of AA005 on colon cancer cells. By using the trypan blue exclusion analyses, we showed that treatment with AA005 at 50 to 200 nM for 24 to 48 h markedly inhibited proliferation of HT29, LOVO and HCT116, but not HBEpiC or BEAS-2B cells (Figure 2, A and B). Foci formation assay showed AA005’s potent inhibitory effects on colony forming activity of colon cancer cells (Figure 2C). We analyzed the effects of AA005 on cell cycle and found that AA005 caused a substantial increase in the percentage of colon cancer cells in G1 phase in a dose-dependent fashion (Figure 2D).
Figure 2. AA005 inhibits cell growth/proliferation, suppresses colony forming activity and arrests cell cycle in colon cancer cells.
(A, B) Indicated cells were treated with or without AA005 for 48 h or indicated time points, and analyzed by trypan blue exclusion assay. (C) Colony formation assay for the clonogenic activity of colon cancer cells treated with or without AA005. (D) Colon cancer cells were treated with AA005 at indicated concentrations for 24 h. Cell cycle distribution was determined by flow cytometry.
AA005 Targets Mitochondria, Depletes ATP and Activates AMPK in Colon Cancer Cells
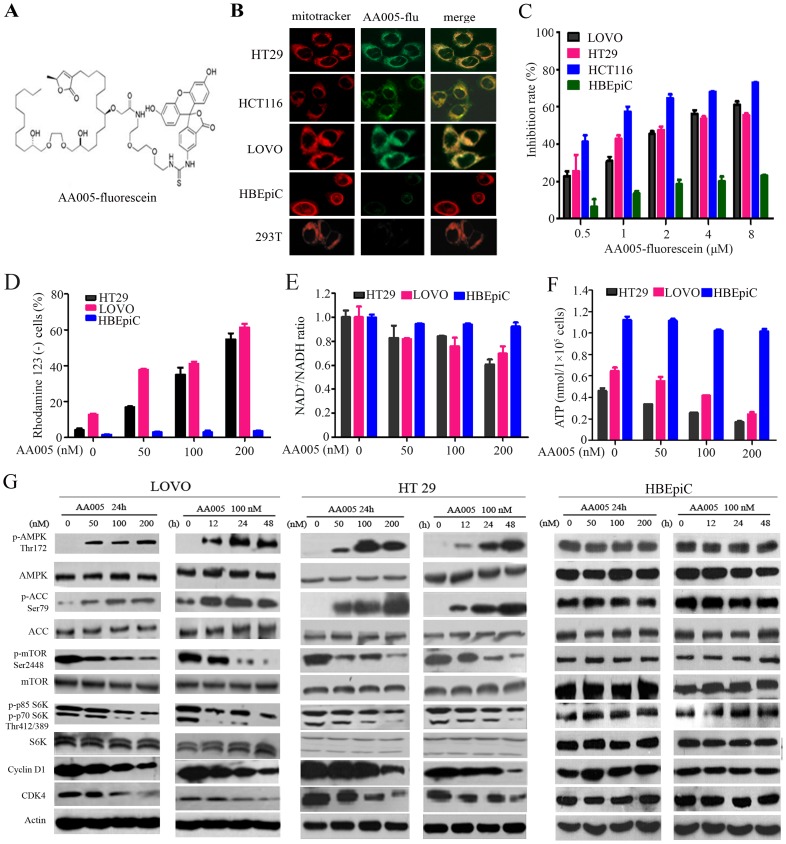
Fluorescein-labeled AA005 (AA005-flu, Figure 3A) was successfully accomplished by a biological activity assessment-aided protocol after examining a number of potential derivative positions in parallel [36]. AA005-flu was found to exhibit similar cell selectivity to its parental molecule, and accumulate in the mitochondria of hepatic cancer but not normal cells [36]. By using immunofluoresence confocal microscopy analysis, we demonstrated that AA005-flu could co-localize with mitochondria in HT29, HCT116 and LOVO cells (Figure 3B). However, AA005-flu signal was very weak in mitochondria of HBEpiC or 293T cells (Figure 3B). While AA005-flu inhibited proliferation of LOVO, HT29 and HCT116 cells in a dose-dependent fashion, its cytotoxic effect on HBEpiC was weak (Figure 3C). Furthermore, we reported that AA005 could increase Rhodamine 123-negative fractions in HT29 and LOVO but not HBEpiC cells (Figure 3D). These results suggest that AA005 might target mitochondrial molecules and thus perturb energetic pathway of cancer cells.
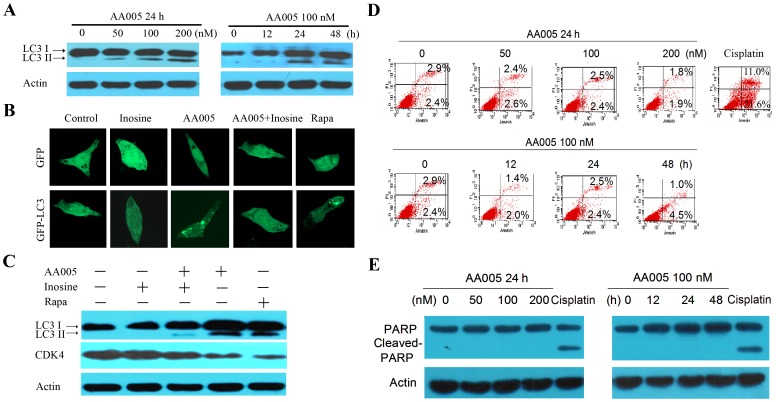
Figure 3. Effects of AA005 on ATP production and AMPK/mTOR signaling pathway.
(A) Chemical structure of AA005-fluorescein. (B) The intracellular localization of AA005. The cells were co-incubated with AA005-flu at 100 nM for 12 h, and analyzed by confocal microscopy using a mitotracker (red) to counter-stain mitochondria. (C) MTT assay of LOVO, HT29, HCT116 and HBEpiC cells upon AA005-flu at indicated concentrations for 48 h. (D) AA005 decreases the mitochondrial transmembrane potential of colon cancer cells revealed by increase in Rhodamine 123-negative cells. The cells were treated with AA005 at indicated concentration for 24 h and analyzed by Rhodamine 123 staining and flow cytometry. (E) The cells were treated with or without AA005 at indicated concentration for 24 h, NAD+/NADH ratio was measured using an AmpliteTM Colorimetric NAD/NADH Assay Kit. (F) The cells were treated with or without AA005 at indicated concentration for 24 h, and ATP content was measured using an ATP Bioluminescence Assay Kit. (G) The cells were treated with AA005 at indicated concentration and time points, lysed, and Western blot analysis was performed using indicated antibodies.
Mitochondria targeting agents (mitocans) are prone to disrupting oxidation phosphorylation pathway and reducing NAD+/NADH ratio, resulting in inhibition of ATP production and activation of AMPK that is reflected by phosphorylation and inactivation of Acetyl-CoA Carboxylase (ACC) (Ser79) which is an indicator for AMPK activity [37]. We tested the effect of AA005 on cellular NAD+/NADH ratio and ATP content, and found that in HT29 and LOVO cells, treatment with AA005 at 50 to 200 nM for 24 h reduced NAD+/NADH ratio (Figure 3E) and depleted ATP in a dose-dependent manner (Figure 3F), while interestingly, AA005 could not significantly decrease NAD+/NADH ratio and ATP content in HBEpiC cells (Figure 3E and F). Furthermore, AA005 markedly up-regulated p-AMPK and p-ACC in a dose- and time-dependent fashion (Figure 3G). However, these phenomena were not observed in HBEpiC cells (Figure 3G).
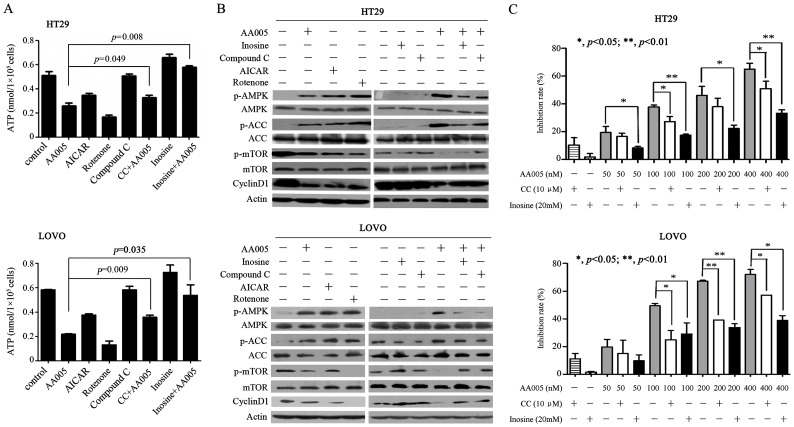
We showed that AA005 as well as the mitocans AICAR and rotenone caused ATP depletion in HT29 and LOVO cells, while AMPK inhibitors compound C and inosine attenuated this effect (Figure 4A). AA005, AICAR and rotenone up-regulated p-AMPK and p-ACC, while compound C and inosine reduced AA005-triggered up-regulation of the two molecules (Figure 4B). In addition, while AA005 inhibited proliferation of HT29 and LOVO cells, compound C and inosine reduced this effect (Figure 4C). These results suggest that AMPK activation is required for AA005-induced cytotoxicity to colon cancer cells.
Figure 4. AMPK activation is required for the effects of AA005 on colon cancer cells.
(A) The indicated cells were treated alone or combinatory with AA005 (100 nM), AICAR (AA, 1 mM), retenone (1 µM), compound C (CC, 10 µM), or inosine (20 mM) for 24 h, and ATP content was measured. (B) HT29 and LOVO cells were treated with indicated protocol, lysed, and Western blotting was conducted using indicated antibodies. (C) HT29 and LOVO cells were treated with different treatment regimens for 48 h, and cell viability was detected by MTT assay.
AMPK Activation Mediates AA005-induced mTOR Complex 1 (mTORC1) Inhibition in vitro
Activated AMPK phosphorylates TSC2 at Thr-1227 and Ser-1345 and increases the activity of TSC1–TSC2 complex to inhibit mTOR [12]. We tested the effects of AA005 on mTORC1, and reported that AA005 decreased p-mTOR and p-S6K (p85 and p70 S6K) in LOVO and HT29 but not HBEpiC cells (Figure 3G). AICAR and rotenone also down-regulated p-mTOR in HT29 and LOVO cells (Figure 4B). However, AA005-induced p-mTOR down-regulation could be partially repressed by inosine and compound C (Figure 4B). Compound C and inosine also partially attenuated cyclin D1 down-regulation caused by AA005 (Figure 4B).
AMPK/mTOR Signaling is Involved in AA005 Induced Autophagy in vitro
AMPK activation can induce autophagy via inhibition of mTOR (49). We tested whether AA005 could induce autophagy in colon cancer cells or not by detecting the changes of cytosolic form (LC3-I) and lipidated form (LC3-II) of the autophagy marker LC3. Interestingly, we found that AA005 induced accumulation of LC3-II in LOVO cells in a time- and dose-dependent fashion (Figure 5A). The pQCXIP-GFP-LC3 plasmid was transfected into LOVO cells which were then treated with AA005 at 100 nM for 24 h, followed by confocal microscopy assessment. We showed that while control cells displayed a diffuse staining, LOVO cells upon AA005 or mTOR inhibitor rapamycin (100 nM) exhibited a speckled fluorescent staining pattern, indicating the redistribution of LC3 to autophagosomes (Figure 5B). Interestingly, inosine attenuated AA005-caused formation of LC3 autophagosomes (Figure 5B) and accumulation of LC3-II (Figure 5C). These results indicate that AA005 induces autophagy of colon cancer cells via AMPK/mTOR signaling pathway. However, treatment with AA005 at 50–200 nM for 24 h or 100 nM for 12–48 h did not result in marked apoptosis of LOVO cells, reflected by Annexin V/PI staining and flow cytometry assessment (Figure 5D) or Western blot analysis of cleavage of PARP, a substrate of activated casp-3 (Figure 5E).
Figure 5. AA005 induces autophagy of colon cancer cells.
(A) Immunoblot analysis of LC3-I and LC3-II levels in LOVO cells treated with AA005. (B) LOVO cells transfected with pQCXIP-GFP or pQCXIP-GFP-LC3 plasmid were treated for 24 h with AA005 (100 nM) and/or inosine (20 mM), and rapamycin (100 nM), and assessed by immunofluorescence analyses. (C) LOVO cells were treated with indicated protocols, lysed, and Western blot assay was performed using indicated antibodies. (D) LOVO cells were treated with AA005 or cisplatin (20 µM) for 24 h, or AA005 at 100 nM for indicated time points. The cells were then analyzed by Annexin V/PI staining and flow cytometry. (E) Western blot analyses of lysates of LOVO cells treated with AA005 or cisplatin (20 µM for 24 h).
AA005 Synergizes with 2-Deoxyglucose and Cisplatin in Inhibiting Colon Cancer Cell Proliferation by Modification of AMPK and mTOR
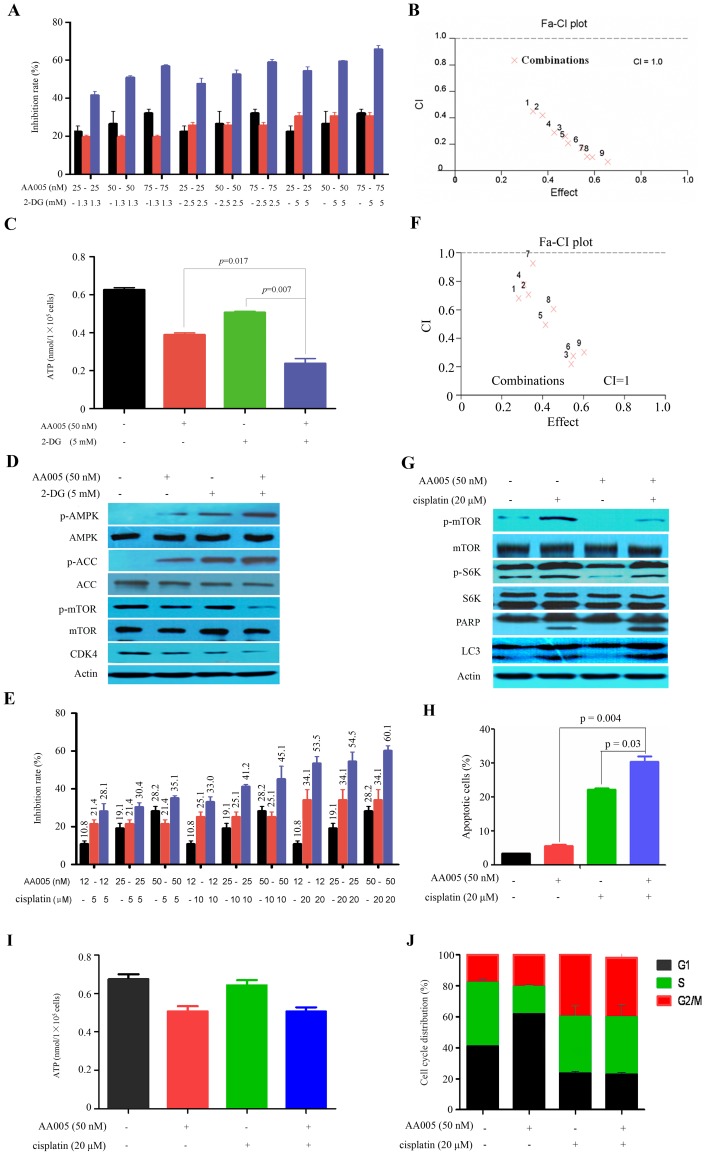
2-Deoxyglucose (2-DG) is a synthetic glucose analogue capable of inhibiting glycolysis and ATP production [38]. We tested the combined effects of AA005 and 2-DG in LOVO cells, and found that combined use of the two agents exerted synergism in inhibition of cell proliferation (Figure 6A and B) and suppression of ATP generation (Figure 6C). AA005 in combination with 2-DG led to enhanced up-regulation of p-AMPK and p-ACC, and down-regulation of p-mTOR and CDK4 (Figure 6D).
Figure 6. AA005 synergizes with 2-DG and cisplatin in colon cancer cells.
(A, B) LOVO cells were treated indicated protocols for 48 h, analyzed by MTT assay (A), and the combined effects were evaluated by the Chou-Talay method and Calcusyn software (B). The combination indexes (CI) less than, equal to, and greater than 1 indicate synergistic, additive, and antagonistic effects, respectively. (C) LOVO cells were treated with 50 nM AA005 or/and 5 mM 2-DG for 24 h, and ATP content was assessed as described above. (D) Western blot analyses of lysates of LOVO cells treated with AA005 or/and 2-DG using indicated antibodies. (E, F) LOVO cells were treated indicated protocols for 48 h, analyzed by MTT assay (E), and the combined effects were evaluated by the Chou-Talay method and Calcusyn software (F). (G) Western blot analyses of lysates of LOVO cells treated with AA005 or/and cisplatin using indicated antibodies. (H) LOVO cells were treated with AA005 and/or cisplatin, and analyzed by Annexin V/PI staining and flow cytometry. (I) The cells were treated with AA005 and/or cisplatin for 24 h, and ATP content was measured using an ATP Bioluminescence Assay Kit. (J) LOVO cells were treated with AA005 and/or cisplatin for 24 h, and cell cycle distribution was determined by flow cytometry.
Chemotherapeutic agent cisplatin can up-regulate mTOR survival pathway which confers drug resistance to cancer cells [39]. We showed that while AA005 and cisplatin caused synergistic inhibitory effect on LOVO cell proliferation (Figure 6E and F), AA005 decreased cisplatin-triggered up-regulation of mTOR and S6K (Figure 6G). Combined use of AA005 and cisplatin led to enhanced apoptotic effect on LOVO cells, reflected by Annexin V/PI staining and flow cytometry assessment (Figure 6H) or Western blot analysis of cleavage of PARP (Figure 6G). However, combination of these two agents did not cause synergy in depletion of ATP content (Figure 6I), cell cycle arrest (Figure 6J), or autophagy (reflected by the expression LC3-II, Figure 6G) in LOVO cells.
Discussion
Annonaceous acetogenins represent a series of C-35/C-37 natural products with hydroxylated THF and the γ-lactone rings structures [25], [26], [28]. Many members of this family display cytotoxic activity by perturbation of the terminal electron transfer step in complex I of mitochondria [40]. AA005 is a mimetic of annonaceous acetogenin in which both the THF rings are replaced by an ethylene glycol ether unit. AA005 retains the essential functionalities of the natural acetogenins and shows more powerful biological activity [41]. In this study, we report that AA005 is able to activate AMPK and inhibit mTOR, therefore arrests cell cycle at G1 phase and induces autophagy of colon cancer cells. AA005 synergies with glycolysis inhibitor 2-DG for marked ATP generation blockade, and antagonizes cisplatin-induced up-regulation of p-mTOR, leading to enhanced proliferation inhibition and apoptosis induction in colon cancer cells. These results indicate that AA005 bears therapeutic potentials at least in this kind of malignant neoplasm.
Targeting cancer cell metabolism, especially glycolysis inhibition, has emerged as a new promising strategy to fight cancer [42]–[44]. Mitochondria not only manage energy generation via citric acid cycle (tricarboxylic acid cycle, TCA cycle), but also play a key role in apoptosis regulation through release of cytochrome C. Although there has much debate on whether mitochondria have defects in oxidative phosphorylation pathway, it could be a potential target for cancer therapy [42], [45]. Some mitocans such as metaformin and vitamin E analogues which inhibit complex I and II show effective and selective anti-cancer activity. These agents induce cancer cell death through generation of superoxide and mitochondrial destabilization [46]. ATP could also be depleted to some extent upon treatment of mitocans [42], [45]. Mitochondria complex I has been shown to be targeted by annonaceous acetogenin [40]. We report that AA005 can co-localize with mitochondria in colon cancer but not HBEpiC or 293T cells (Figure 3A), suggesting that AA005 may reduce NAD+/NADH ratio and ATP production in cancer cells. This possibility is confirmed in LOVO, HT29 and HCT116 cells (Figure 3C). However, why mitochondria in cancer cells are more prone to be targeted by AA005 remains an open question. Inhibition of mitochondria by metaformin and rotenone may lead to activation of AMPK [42]. We show that AA005 can also suppress mitochondria and activate AMPK (Figure 3). At this point, inosine and compound C antagonize AA005-induced AMPK activation and cytotoxicity (Figure 4), indicating a direct link between ATP depletion through mitochondria inhibition and AMPK activation, which ultimately results in mTOR inhibition. AA005 also synergizes with 2-DG in ATP depletion and AMPK activation, indicating that AA005 bears therapeutic potentials for colon cancer.
The oncoprotein mTOR suppresses while activation of AMPK promotes autophagy [47]. We show that AA005 down-regulates p-mTOR as well as its downstream effector S6K (Figure 3), while compound C and inosine partially reverse this effect (Figure 4). Inosine also represses AA005-induced autophagy of LOVO cells reflected by decrease LC3-II accumulation (Figure 5C). While cisplatin up-regulates mTOR, AA005 attenuates this phenomenon (Figure 6G) and synergizes with this agent in inducing apoptosis of LOVO cells (Figure 6H). These results indicate that AA005 can inhibit mTOR via activation of AMPK, and further demonstrate the benefits of this annonaceous acetogenin mimetic for colon cancer cells.
Funding Statement
This work was supported in part by the National Key Program for Basic Research (2012CB910800, 2010CB833200 and 2009CB940900), the National Natural Science Foundation (No. 81071930, 81171925, 20972160 and 21172220), the Special Foundation of President and the Key Project of Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX1-YW-R-26 and KSCX2-YW-R-235), and the National Major Scientific and Technological Program for Drug Discovery (2009ZX09103-101). No additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E (2007) Energy metabolism in tumor cells. FEBS J 274: 1393–1418. [DOI] [PubMed] [Google Scholar]
- 2. Hnanhan D, Weinberg RA (2011) Hallmarks of Cancer: The Next Generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 3. Vander Heiden MG (2011) Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 10: 671–684. [DOI] [PubMed] [Google Scholar]
- 4. Hardie DG (2011) AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 25: 1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carling D, Mayer FV, Sanders MJ, Gamblin SJ (2011) AMP-activated protein kinase: nature’s energy sensor. Nat Chem Biol 7: 512–518. [DOI] [PubMed] [Google Scholar]
- 6. Hawley SA, Davison M, Woods A, Davies SP, Beri RK, et al. (1996) Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271: 27879–27887. [DOI] [PubMed] [Google Scholar]
- 7. Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, et al. (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A 101: 3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bardeesy N, Sinha M, Hezel AF, Signoretti S, Hathaway NA, et al. (2002) Loss of the Lkb1 tumour suppressor provokes intestinal polyposis but resistance to transformation. Nature 419: 162–167. [DOI] [PubMed] [Google Scholar]
- 9. Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, et al. (2007) LKB1 modulates lung cancer differentiation and metastasis. Nature 448: 807–810. [DOI] [PubMed] [Google Scholar]
- 10. Bolster DR, Crozier SJ, Kimball SR, Jefferson LS (2002) AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277: 23977–23980. [DOI] [PubMed] [Google Scholar]
- 11. Horman S, Browne G, Krause U, Patel J, Vertommen D, et al. (2002) Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Curr Biol 12: 1419–1423. [DOI] [PubMed] [Google Scholar]
- 12. Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590. [DOI] [PubMed] [Google Scholar]
- 13. Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, et al. (2001) Mammalian TOR: a homeostatic ATP sensor. Science 294: 1102–1105. [DOI] [PubMed] [Google Scholar]
- 14. Imamura K, Ogura T, Kishimoto A, Kaminishi M, Esumi H (2001) Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole- 4-carboxamide-1-beta-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem Biophys Res Commun 287: 562–567. [DOI] [PubMed] [Google Scholar]
- 15. Bowker SL, Majumdar SR, Veugelers P, Johnson JA (2006) Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care 29: 254–258. [DOI] [PubMed] [Google Scholar]
- 16. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD (2005) Metformin and reduced risk of cancer in diabetic patients. BMJ 330: 1304–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N (2007) Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res 67: 10804–10812. [DOI] [PubMed] [Google Scholar]
- 18. Zakikhani M, Dowling R, Fantus IG, Sonenberg N, Pollak M (2006) Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 66: 10269–10273. [DOI] [PubMed] [Google Scholar]
- 19. Ben S, I, Laurent K, Loubat A, Giorgetti-Peraldi S, Colosetti P, et al. (2008) The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene 27: 3576–3586. [DOI] [PubMed] [Google Scholar]
- 20. Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, et al. (2007) Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res 67: 6745–6752. [DOI] [PubMed] [Google Scholar]
- 21. Tang YC, Williams BR, Siegel JJ, Amon A (2011) Identification of Aneuploidy-Selective Antiproliferation Compounds. Cell 144: 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Landolt JL, Ahammadsahib KI, Hollingworth RM, Barr R, Crane FL, et al. (1995) Determination of structure-activity relationships of Annonaceous acetogenins by inhibition of oxygen uptake in rat liver mitochondria. Chem Biol Interact 98: 1–13. [DOI] [PubMed] [Google Scholar]
- 23. Carmen Zafra-Polo M, Figadere B, Gallardo T, Tormo J, Cortes D (1998) Natural acetogenins from annonaceae, synthesis and mechanisms of action. Phytochemistry 48: 1087–1117. [Google Scholar]
- 24. Nakanishi S, Abe M, Yamamoto S, Murai M, Miyoshi H (2011) Bis-THF motif of acetogenin binds to the third matrix-side loop of ND1 subunit in mitochondrial NADH-ubiquinone oxidoreductase. Biochim Biophys Acta 1807: 1170–1176. [DOI] [PubMed] [Google Scholar]
- 25. Jiang S, Li Y, Chen XG, Hu TS, Wu YL, et al. (2004) Parallel Fragment Assembly Strategy Towards Multiple-Ether Mimicry of Anticancer Annonaceous Acetogenins. Angew Chem Intl Ed 43: 329–334. [DOI] [PubMed] [Google Scholar]
- 26. Xiao Q, Liu Y, Qiu Y, Zhou G, Mao C, et al. (2010) Potent Antitumor Mimetics of Annonaceous Acetogenins Embedded with an Aromatic Moiety in the Left Hydrocarbon Chain Part. J Med Chem 54: 525–533. [DOI] [PubMed] [Google Scholar]
- 27. Xiao Q, Liu Y, Qiu Y, Yao Z, Zhou G, et al. (2011) Design, synthesis of symmetrical bivalent mimetics of annonaceous acetogenins and their cytotoxicities. Bioorgan Med Chemi Lett 21: 3613–3615. [DOI] [PubMed] [Google Scholar]
- 28. Yao ZJ, Wu YL (1995) Synthetic Studies toward Mono-THF Annonaceous Acetogenins: A Diastereoselective and Convergent Approach to Corossolone and (10RS)-Corossoline. J Org Chem 60: 1170–1176. [Google Scholar]
- 29. Ma L, Wen ZS, Liu Z, Hu Z, Ma J, et al. (2011) Overexpression and Small Molecule-Triggered Downregulation of CIP2A in Lung Cancer. PLoS ONE 6: e20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu XJ, Han QB, Wen ZS, Ma L, Gao J, et al. (2012) Gambogenic acid induces G1 arrest via GSK3b-dependent cyclin D1 degradation and triggers autophagy in lung cancer cells. Cancer Letters 322: 185–194. [DOI] [PubMed] [Google Scholar]
- 31. Liu Y, Chen XQ, Liang HX, Zhang FX, Zhang B, et al. (2011) Small Compound 6-O-Angeloylplenolin Induces Mitotic Arrest and Exhibits Therapeutic Potentials in Multiple Myeloma. PLoS ONE 6: e21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu Z, Pan XF, Wu FQ, Ma LY, Liu DP, et al. (2009) Synergy between Proteasome Inhibitors and Imatinib Mesylate in Chronic Myeloid Leukemia. PLoS ONE 4: e6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chou TC, Talalay P (1981) Generalized equations for the analysis of inhibitions of Michaelis-Menten and higher-order kinetic systems with two or more mutually exclusive and nonexclusive inhibitors. Eur J Biochem 115: 207–216. [DOI] [PubMed] [Google Scholar]
- 34. Zhou GB, Kang H, Wang L, Gao L, Liu P, et al. (2007) Oridonin, a diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion protein and shows potent antitumor activity with low adverse effects on t(8;21) leukemia in vitro and in vivo. Blood 109: 3441–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fang HT, Zhang B, Pan XF, Gao L, Zhen T, et al. (2012) Bortezomib interferes with C-KIT processing and transforms the t(8;21)-generated fusion proteins into tumor-suppressing fragments in leukemia cells. Proc Natl Acad Sci U S A 109: 2521–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Liu HX, Huang GR, Zhang HM, Jiang S, Wu JR, et al. (2007) Cover Picture: A structure-activity guided strategy for fluorescent labeling of annonaceous acetogenin mimetics and their application in cell biology. ChemBioChem 8: 153. [DOI] [PubMed] [Google Scholar]
- 37. Hardie DG (2003) Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology 144: 5179–5183. [DOI] [PubMed] [Google Scholar]
- 38. Kang HT, Hwang ES (2006) 2-Deoxyglucose: An anticancer and antiviral therapeutic, but not any more a low glucose mimetic. Life Sciences 78: 1392–1399. [DOI] [PubMed] [Google Scholar]
- 39. Peng DJ, Wang J, Zhou JY, Wu GS (2010) Role of the Akt/mTOR survival pathway in cisplastin resistance in ovarian cancer cells. Biochem Biophys Res Commun 394: 600–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zafra-Polo MC, Gonzalez MC, Estornell E, Sahpaz S, Cortes D (1996) Acetogenins from Annonaceae, inhibitors of mitochondrial complex I. Phytochemistry. 42: 253–271. [DOI] [PubMed] [Google Scholar]
- 41. Jiang S, Liu ZH, Sheng G, Zeng BB, Cheng XG, et al. (2002) Mimicry of annonaceous acetogenins: enantioselective synthesis of a (4R)-hydroxy analogue having potent antitumor activity. J Org Chem 67: 3404–3408. [DOI] [PubMed] [Google Scholar]
- 42. Ben Sahra I, Laurent K, Giuliano S, Larbret F, Ponzio G, et al. (2010) Targeting cancer cell metabolism: the combination of metformin and 2-deoxyglucose induces p53-dependent apoptosis in prostate cancer cells. Cancer Res 70: 2465–2475. [DOI] [PubMed] [Google Scholar]
- 43. Pelicano H, Martin DS, Xu RH, Huang P (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25: 4633–4646. [DOI] [PubMed] [Google Scholar]
- 44. Gogvadze V, Orrenius S, Zhivotovsky B (2008) Mitochondria in cancer cells: what is so special about them? Trends in Cell Biology 18: 165–173. [DOI] [PubMed] [Google Scholar]
- 45. Fath MA, Diers AR, ykin-Burns N, Simons AL, Hua L, et al. (2009) Mitochondrial electron transport chain blockers enhance 2-deoxy-D-glucose induced oxidative stress and cell killing in human colon carcinoma cells. Cancer Biol Ther 8: 1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neuzil J, Dyason J, Freeman R, Dong LF, Prochazka L, et al. (2007) Mitocans as anti-cancer agents targeting mitochondria: lessons from studies with vitamin E analogues, inhibitors of complex II. J Bioenerg Biomembr 39: 65–72. [DOI] [PubMed] [Google Scholar]
- 47. Inoki K, Kim J, Guan KL (2011) AMPK and mTOR in Cellular Energy Homeostasis and Drug Targets. Ann Rev Pharm Toxicol 52: 381–400. [DOI] [PubMed] [Google Scholar]