Abstract
Background
In rat middle cerebral and mesenteric arteries the KCa2.3 component of endothelium-dependent hyperpolarization (EDH) is lost following stimulation of thromboxane (TP) receptors, an effect that may contribute to the endothelial dysfunction associated with cardiovascular disease. In cerebral arteries, KCa2.3 loss is associated with NO synthase inhibition, but is restored if TP receptors are blocked. The Rho/Rho kinase pathway is central for TP signalling and statins indirectly inhibit this pathway. The possibility that Rho kinase inhibition and statins sustain KCa2.3 hyperpolarization was investigated in rat middle cerebral arteries (MCA).
Methods
MCAs were mounted in a wire myograph. The PAR2 agonist, SLIGRL was used to stimulate EDH responses, assessed by simultaneous measurement of smooth muscle membrane potential and tension. TP expression was assessed with rt-PCR and immunofluorescence.
Results
Immunofluorescence detected TP in the endothelial cell layer of MCA. Vasoconstriction to the TP agonist, U46619 was reduced by Rho kinase inhibition. TP receptor stimulation lead to loss of KCa2.3 mediated hyperpolarization, an effect that was reversed by Rho kinase inhibitors or simvastatin. KCa2.3 activity was lost in L-NAME-treated arteries, but was restored by Rho kinase inhibition or statin treatment. The restorative effect of simvastatin was blocked after incubation with geranylgeranyl-pyrophosphate to circumvent loss of isoprenylation.
Conclusions
Rho/Rho kinase signalling following TP stimulation and L-NAME regulates endothelial cell KCa2.3 function. The ability of statins to prevent isoprenylation and perhaps inhibit of Rho restores/protects the input of KCa2.3 to EDH in the MCA, and represents a beneficial pleiotropic effect of statin treatment.
Introduction
In rat middle cerebral arteries (MCA) endothelium-dependent hyperpolarization (EDH) responses (commonly called endothelium derived hyperpolarizing factor, EDHF, response) are observed in the presence of NO synthase (NOS) inhibitors, and can be abolished by inhibition of endothelial cell KCa3.1 (intermediate conductance, IKCa) channels, irrespective of the agonist used to stimulate EDH [1], [2]. In most other arterial beds, inhibition of both endothelial cell KCa3.1 and KCa2.3 (small conductance, SKCa) is necessary for block of EDH [3]. However, the MCA does expresses endothelial cell KCa2.3 [4], [5] which contribute to EDH in vessels still able to synthesise NO [5]. Following inhibition of NO synthase, input from KCa2.3 to EDH responses is restored in the middle cerebral artery by exposure to antagonists of thromboxane receptors (TP) [6]. As TP stimulation suppresses the KCa2.3 input to EDH in rat middle cerebral and mesenteric arteries [6], [7], endogenous stimulation may represent a significant influence on KCa2.3 function in the vasculature. The mechanism that ‘protects’ KCa2.3 function during NO signalling or TP inhibition remains unclear.
NO could potentially protect KCa2.3 channel function by direct interaction/stimulation of the channel [8]. Alternatively, NO might inhibit the synthesis of metabolites that affect KCa channels by binding to the heme groups of enzymes. For example, the cytochrome P450 metabolite 20-HETE inhibits EDH responses in coronary arteries [9]. Neither of these pathways is likely to explain the ‘protective’ effect of NO in cerebral arteries, as hyperpolarization evoked by exogenous NO is inhibited by the KCa1.1 blocker iberiotoxin and therefore does not involve KCa2.3 [10] and inhibition of 20-HETE synthesis did not influence KCa2.3 function [6]. However, as KCa2.3 function is restored by antagonizing TP [6], NO may protect KCa2.3 function by PKG dependent inhibition of these receptors [11] or by inhibiting the generation of metabolites that could stimulate this receptor by binding to heme groups [12].
A major signalling pathway associated with TP is activation of Rho kinase [13]. TP are expressed primarily on the smooth muscle cell layer but they can also be expressed in endothelial cells [14]. It is likely that TP signalling in endothelial cells also involves Rho kinase therefore they may regulate the KCa2.3 channels expressed selectively in these cells. The role of Rho kinase signalling on KCa2.3 channel function can be directly assessed using inhibitors of this kinase but the statin class of drugs are also reported to have effects on Rho mediated signalling. They improve endothelium-dependent relaxation via a mechanism that involves inhibition of Rho signalling [15], independently of their ability to lower cholesterol.
The aims of the current study were 1) to investigate if disrupting the Rho kinase pathway could protect KCa2.3 functionality following TP stimulation; 2) to establish if inhibition of Rho kinase signalling might restore the KCa2.3 component of the EDH response suppressed by the presence of NOS inhibitors, and 3) to assess if statins had a similar effects to inhibitors of Rho kinase.
Materials and Methods
Animals and Ethics Statement
Male Wistar rats (200–300 g) were humanely killed by cervical dislocation following institutional guidelines for animal welfare and schedule 1 of the Animals (scientific procedures) Act 1986. The brain was removed and immediately placed in ice-cold Krebs solution. Segments of the MCA (∼2 mm long) were dissected and stored in ice-cold Krebs for use within 30 min, with similar size vessels used in all experimental groups.
Simultaneous Measurement of Membrane Potential and Tension
Segments of MCA (internal diameter ∼150 µm) were mounted in a Mulvany-Halpern myograph (model 400A, Danish Myotechnology) in Krebs solution containing (mM): NaCl, 118.0; NaCO3, 24; KCl, 3.6; KH2PO4, 1.2; MgSO4⋅7H2O, 1.2; glucose, 11.0; CaCl2, 2.5; gassed with 95% O2, 5% CO2 and maintained at 37°C. After equilibration for 20 min, vessels were tensioned to 1–1.5 mN (approximates wall tension at 60 mmHg). Smooth muscle tension was recorded with an isometric force transducer and Powerlab software (ADI, Australia). Vessel viability was assessed by adding exogenous K+ (15–55 mM, total K+ concentration); only vessels developing tension of ≥3 mN were used. Endothelial cell viability was assessed by the ability of the agonist of protease-activated receptor 2 (PAR2) SLIGRL (serine, leucine, isoleucine, glycine, arginine, leucine-NH2; 20 µM) to relax spontaneous tone and to hyperpolarize the smooth muscle cell membrane by >15 mV. A concentration of 20 µM SLIGRL was used in all further experiments; our previous study characterized the potency of this agent to evoke endothelium dependent relaxation and this concentration evokes circa 75–80% of the maximal relaxation response [1]. Use of this single concentration also minimises potential desensitization of the PAR2 receptor.
In some experiments endothelium-dependent responses to SLIGRL were obtained in vessels that were able to synthesize NO but were pre-constricted to approximately 80% of maximal tone (obtained with 55 mM K+) with varying concentrations of the TP receptor agonist U46619 (circa 50–100 nM). Under these conditions EDH was assessed in the presence of the KCa channel blockers used at concentrations that should cause full channel inhibition with no off target effects at other KCa channels with respect to their published Ki values, apamin (KCa2.3, 100 nM) [16], TRAM-34 (KCa3.1, 1 µM) [17] and iberiotoxin (KCa1.1, 100 nM) [18] Previous studies have shown that the order in which these drugs are added has no effect on the experimental outcome [1], [5]. For clarity of presentation they were always added in the order: 1) TRAM-34; 2) The combination of TRAM-34 and apamin; 3) the combination of TRAM-34, apamin and iberiotoxin. The effect of KCa blockers on EDH responses was also assessed after addition of inhibitors of Rho kinase (Y27632; 1 and 10 µM) [19], and HMG-CoA reductase (simvastatin; 0.1 and 1 µM). We did not routinely measure relaxation in vessels able to synthesise NO, under these conditions maximal vasodilatation due to NO release persists after hyperpolarization is blocked [5]. In all other experiments, isolated EDH responses were obtained in the presence of the NO synthase inhibitor L-NAME (100 µM). We have previously reported that under these conditions only KCa3.1 contribute to EDH [1] thus the other KCa channel blockers were added in the sequence detailed above only if the previous KCa blocker(s) failed to prevent hyperpolarization. KCa channel blockers were assessed against EDH responses to SLIGRL (20 µM) in the presence of: 1. the Rho kinase inhibitors Y27632 (10 µM) [19] or SR5037 (1 µM) [20] as no kinase inhibitor is 100% selective we chose two structurally distinct inhibitors at concentrations approximately 100 times their respective Ki/IC50 for Rho kinase to ensure full inhibition with minimal effects on other, related kinases [19], [20]; 2. the HMG-CoA inhibitors simvastatin (0.1 and 1 µM) or lovastatin (100 nM).These concentrations of statins are similar to the concentrations found in human plasma (circa 25–90 nM) [21] and are in line with their Ki values of low to high nanomolar, reported in various rat cell types [22] In some experiments, in the presence of L-NAME and simvastatin (100 nM) the vessels were also incubated with the isoprenoid geranylgeranyl-pyrophosphate (GGPP, 1 µM). The concentration of GGPP used corresponds to those reported to reverse statin mediated inhibition of RhoA in vascular endothelial cells [15], [23] Papaverine (150 µM) was added at the end of each experiment to assess overall tone (previous experiments show this was sufficient to give a maximal relaxation indistinguishable from that obtained in the presence of zero calcium [24]. All drugs were allowed to equilibrate for 20 min before vasodilator responses were stimulated. The statins and GGPP were incubated for >60 min; longer than prenylation-dependent translocation of Rho measured in cerebral artery smooth muscle cells [25]. In most experiments smooth muscle membrane potential (Em) and tension were measured simultaneously (except where stated) at a sample rate of 100 Hz as previously described, using glass microelectrodes (filled with 2 M KCl; tip resistance, 80–120 MΩ) to measure Em [26].
Reverse Transcription-PCR
RNA from intact rat MCAs was isolated using Trizol (Life Technologies Ltd., Paisley, UK) and was reverse-transcribed using standard protocols with random hexamers and TaqMan reverse transcription reagents (Life Technologies Ltd.). Subsequent PCR reactions used primers specific for rat TP receptor (forward 5′- CTCCTGGTGCTTCTTGACTC-3′; reverse 5′-GAGCTGGGAAGTGAACCTTG-3′) and KCa2.3 (forward 5′-ACTTCAACACCCGATTCGTC-3′; reverse 5′- TTGACACCCCTCAGTTGGTG-3′). Control reactions omitted reverse transcriptase. PCR products were separated by electrophoresis, stained with ethidium bromide, and sequenced to confirm identity.
Immunofluorescence
Sources of antibodies were as follows: mouse anti-rat Platelet/endothelial cell adhesion molecule-1 (PECAM-1/CD31, TLD-3A12, Millipore, Watford, UK); rabbit anti-mouse thromboxane A2 receptor (101882; Cayman, Tallinn, Estonia) and donkey anti-mouse Cy5 and anti-rabbit Rhodamine Red-X (Stratech Scientific Limited, Newmarket, UK).
Arteries were cut longitudinally and pinned flat on sylgard plates and fixed with 100 mM PBS, pH 7.4 containing 4% paraformaldehyde (30 min, 4°C). Artery whole mounts were washed in PBS containing 10% normal horse serum and 0.3% Triton-X-100 (1 h, room temperature) and proteins localized using primary antibodies: Thromboxane A2 receptor (1∶100) and PECAM-1 (CD31, 1∶200) (24 h, 4°C). Whole mounts were washed (1 h, room temperature), incubated with secondary antibodies coupled to Rhodamine Red-X, or Cy5 (1∶500, 2 h, room temperature) and mounted in ProLong® Gold (Invitrogen, Paisley, UK) which contains DAPI.
Whole mounts were observed with a Zeiss laser-scanning confocal microscope (LSM Meta 510) using an EC Plan-Neofluar 40×/1.3 Oil DIC objective. Images were collected at a zoom of 1.5 and an iris of <3 µm, and at least five optical sections were taken at intervals of 0.5 µm. Single sections are shown. Images were processed using ImageJ and Adobe Photoshop software. The specificity of the immunostaining was evaluated by omission of the primary antibody and processed as above. Under these conditions, no staining was observed in any experimental situation.
Data Analysis and Statistical Procedure
Results are expressed as the mean±s.e.mean of n animals. Tension values are given in mN (always per 2 mm segment) and Em as mV. Vasodilatation is expressed as percentage reduction of the total vascular tone (spontaneous tone plus vasoconstrictor response), quantified by relaxation with papaverine (150 µM). Graphs were drawn and comparisons made using one-way ANOVA with Tukeys’ post-test (Prism, Graphpad). P≤0.05 was considered significant. pEC50 values were calculated using non-linear regression fitted to the Hill equation with variable slope (Prism, Graphpad).
Drugs, Chemicals, Reagents and Other Materials
Exogenous K+ was added as an isotonic physiological salt solution in which all the NaCl was replaced with an equivalent amount of KCl. Concentrations of K+ used are expressed as final bath concentration. GGPP, L-NAME (NG-nitro-L-arginine methyl ester), papaverine HCl, TRAM-34 (1-[(2-Chlorophenyl)diphenylmethyl]-1H-pyrazole), U46619 (9,11-Dideoxy-11α,9α-epoxymethanoprostaglandin F2α) and Y27632 ((R)-(+)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexane carboxamide dihydrochloride) were all obtained from Sigma (Poole, U.K.). Apamin and iberiotoxin, from Latoxan (Valence, France). SLIGRL-NH2 from Auspep (Parkville, Australia). Simvastatin and Lovastatin were from Cayman-Europe (Tallinn, Estonia). SR5037 ((R)-6-Methoxy-N-(2-(1-methylpiperidin-4-yloxy)-4-(1H-pyrazol-4-yl)phenyl)-1,2,3,4-tetrahydroisoquinoline-3-carboxamide) was a generous gift from Professor Phil LoGrasso (Scripps Research Institute, Florida, USA). All stock solutions (100 mM) were prepared in dimethylsulfoxide except L-NAME, apamin, iberiotoxin, papaverine and SLIGRL which were dissolved in 0.9% NaCl. Controls included appropriate vehicle. All blocking drugs were used at appropriate concentrations to ensure full inhibition of their target (normally 10–100 times the Ki/IC50 value) and minimal off target effects and are the recommended selective inhibitors as classified by IUPHAR [27].
Results
Thromboxane Receptors are Expressed in Endothelial Cells of Middle Cerebral Arteries
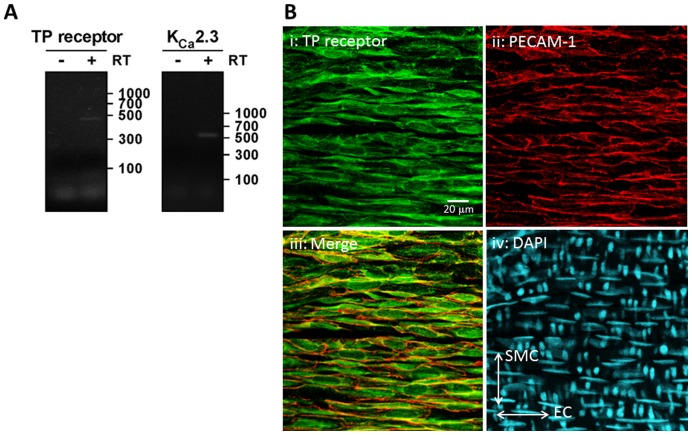
While TP receptors are known to be expressed in human endothelial cells derived from umbilical or saphenous veins [14], expression has never been demonstrated in the endothelium of the rat MCA. We examined expression of TP receptors and KCa2.3 in the MCA by RT-PCR (Figure 1A). We amplified mRNA transcripts of expected sizes and confirmed identity by sequencing. Next, we examined expression of TP receptors in whole mount preparations of rat MCA using immunofluorescence and confocal microscopy. We detected TP receptor-immunoreactivity in the endothelial cell layer (PECAM-1-positive) of rat MCA (Figure 1B–E). Nuclei orientation was confirmed using DAPI, with endothelial cell nuclei orientated in the horizontal plane, smooth muscle cell nuclei in the vertical. TP receptor-immunoreactivity was also detected in the smooth muscle cell layer (PECAM-1-negative) (data not shown). Together, these data suggest that TP receptors are appropriately localized on endothelial cells to regulate KCa2.3 channel function.
Figure 1. Expression of the TP receptor in the endothelium of rat middle cerebral arteries.
A) RT-PCR amplification of mRNA transcripts for TP receptor (439 bp) and KCa2.3 channels (514 bp) from rat MCA. bp = base pairs B) Localization of TP receptor and PECAM-1-immunoreactivity in whole mount preparations of rat MCA. TP receptor-immunoreactivity was present in the endothelial cell layer (PECAM-1-positive). Orientation of cell nuclei was determined using DAPI. The merged image demonstrates coexpression of TP receptors and PECAM-1, indicating TP receptor expression in the endothelial cells of rat MCAs. Scale bar, 20 µm.
Inhibiting Rho Kinase Prevents Loss of KCa2.3 Input to EDH during TP Receptor Stimulation
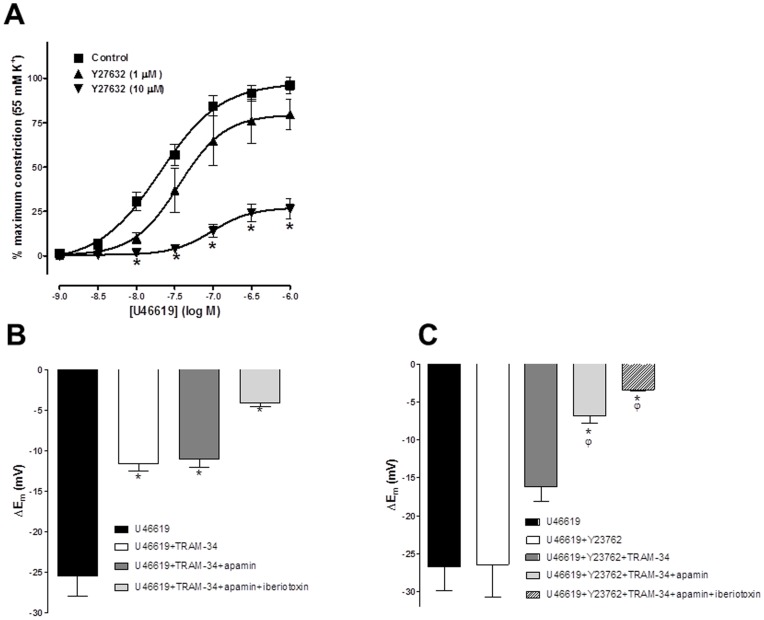
In the absence of a NO synthase inhibitor, the thromboxane mimetic U46619 evoked concentration dependent constriction (pEC50 = 7.69±0.08, n = 5; Figure 2A). The selective Rho kinase inhibitor Y27632 concentration-dependently inhibited this response (pEC50 = 7.45±0.15 and 7.02±0.14, in presence of 1 and 10 µM Y27632, respectively, n = 5, P<0.05, Figure 2A). Y27632 (10 µM) also significantly reduced the maximum constriction produced by U46619 (control, 96.1±4.6%; 10 µM Y27632, 26.4±5.7%, n = 5, P<0.05; Figure 2A).
Figure 2. Effect of inhibition of Rho Kinase on TP induced constriction and EDH responses obtained in the presence of TP stimulation in the rat middle cerebral artery.
(A) Concentration response curve showing the vasoconstrictor response produced by the thromboxane A2 mimetic U46619 (1 nM-1 µM; n = 5) in rat middle cerebral arteries in the presence and absence of the selective Rho kinase inhibitor Y27632 (1 and 10 µM; n = 5). Inhibition of Rho kinase significantly and concentration dependently reduced the maximum constriction produced by U46619 and significantly shifted the concentration response curve to the right. (B) Histogram showing SLIGRL (20 µM) induced EDH evoked in the presence of U46619 (50–100 nM) in vessels able to synthesise NO. Also shown are EDH in the presence of KCa3.1 blockade (1 µM, TRAM-34), blockade of both KCa2.3 and 3.1 (100 nM apamin+TRAM-34) and blockade of KCa1.1, 2.3 and 3.1 (100 nM Iberiotoxin+apamin+TRAM-34). Block of KCa3.1 alone was sufficient to significantly reduce EDH; subsequent block of KCa2.3 had no further effect indicating this channel was not functional. Residual EDH was inhibited by further blockade of KCa1.1. (C) Histogram showing SLIGRL induced EDH in the presence of U46619 and the Rho Kinase inhibitor Y27632 in vessels able to synthesise NO. EDH was only significantly reduced following combined blockade of both KCa3.1 and 2.3, indicating that the KCa2.3 channel was now functional. *P<0.05 indicates a significant difference from control (U46619 alone) using one-way ANOVA with Tukey’s post-test, n = 5–6) φP<0.05 indicates a significant difference from Y27632 as determined by one-way ANOVA with Tukey’s post-test, n = 5–6.
When MCAs are able to synthesise NO, EDH is mediated by both KCa2.3 and KCa3.1 with a small NO mediated KCa1.1 component [6], [10]. Under similar conditions in the present study, excepting that 100 nM U46619 was present to stimulate TP receptors, SLIGRL-evoked EDH (25.4±2.5 mV, n = 5) was partially blocked by the KCa3.1 inhibitor TRAM-34 (1 µM; hyperpolarization of 11.6±0.9 mV, n = 5, P<0.05) and the subsequent application of the KCa2.3 blocker apamin (100 nM) had no further effect (11.0±1.1 mV, n = 5). However, the residual EDH was suppressed by the KCa1.1 (BKCa) blocker, iberiotoxin (100 nM; 4.0±0.5 mV, n = 5; Figure 2B). In the presence of U46619 if arteries were exposed to the Rho kinase inhibitor, Y27632 (10µM: control hyperpolarization to SLIGRL, 26.2±3.14 mV, plus Y27632 26.5±4.2 mV, n = 8), despite a trend toward block, TRAM-34 did not significantly inhibit EDH (16.2±1.9 mV, n = 8). However, the subsequent addition of apamin significantly inhibited EDH (to 6.9±1.0 mV, n = 6, P<0.05). The residual hyperpolarization was again inhibited further by iberiotoxin, as in the absence of Y27632 (3.4±0.2 mV, n = 5; Figure 2C). These results indicate that stimulation of TP receptors with U46619 inhibits KCa2.3 function and that subsequent inhibition of Rho kinase restores or protects this component of hyperpolarization.
Inhibiting Rho Kinase Restores the Input of KCa2.3 to EDH in the Presence of a NO Synthase Inhibitor
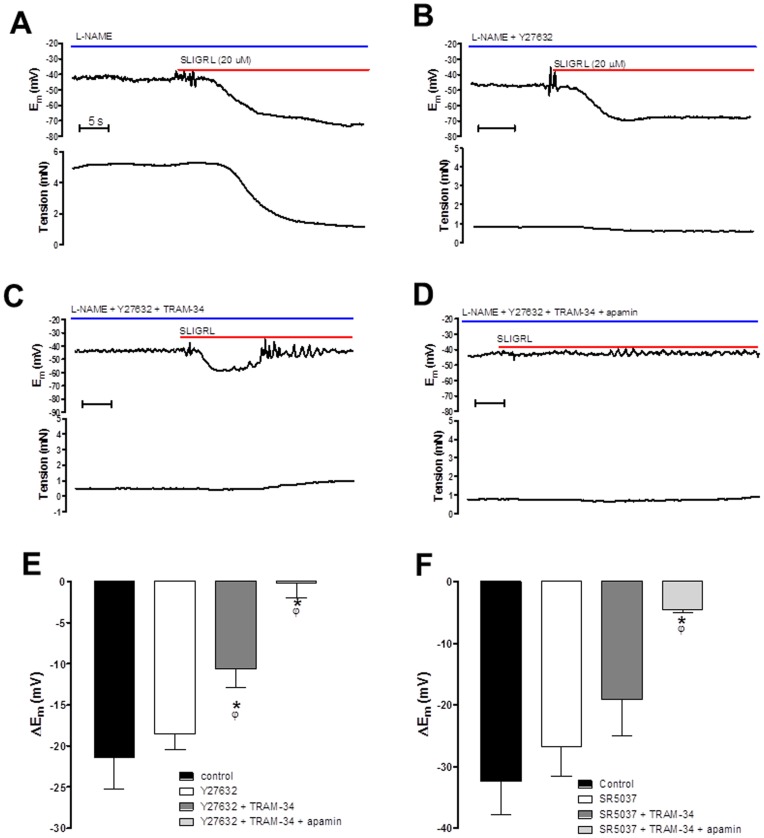
Previous studies demonstrate that in the presence of the NOS inhibitor L-NAME (100 µM) the MCA constricts and EDH is blocked by TRAM-34 alone indicating a critical role for KCa3.1 [1]. In this study 100 µM L-NAME contracted cerebral arteries and a higher concentration of L-NAME (300 µM) had no additional constrictor effect (data not shown). In the presence of L-NAME the EDH responses were completely inhibited by TRAM-34 alone (Figure S1) confirming that under these conditions only KCa3.1 contributes to EDH. The contraction was reversed by SLIGRL (to stimulate EDH and associated relaxation of 21.4±3.9 mV and 67.9±6.1%, respectively n = 6; Figure 3A, E). Addition of the Rho kinase inhibitor Y27632 (10 µM) also reversed the L-NAME induced constriction (relaxation of 86.0±2.4%, n = 6) and the subsequent relaxation to SLIGRL became too small to assess accurately (Figure 3B). In contrast, EDH was unaffected by Y27632 (18.5±1.9 mV to 20 µM SLIGRL, n = 5), and TRAM-34 now only partially reduced this hyperpolarization (10.6±2.2 mV, n = 4, P<0.05; Figure 3C,E). The residual hyperpolarization was abolished by the additional presence of apamin (0.2±1.8 mV, n = 4, P<0.05; Figure 3D, E). A structurally distinct and extremely selective Rho kinase inhibitor SR5037 [20] had a similar effects to Y27632. This novel agent reversed vasoconstriction to L-NAME (relaxation of 85.2±3.7% n = 4) and the remaining tension was too small to assess accurately. SR5037 also restored the KCa2.3 component to EDH, as TRAM-34 failed to completely block hyperpolarization and the combination of TRAM-34 and apamin were both required to block hyperpolarization (Figure 3F). Thus, in the presence of L-NAME block of KCa3.1 with TRAM-34 alone inhibits the EDH response. Inhibition of Rho kinase with selective and structurally distinct inhibitors reveals a KCa2.3 input to EDH responses indicating that Rho kinase normally supresses the function of these channels in the presence of L-NAME.
Figure 3. Original traces showing the isolated EDH response evoked by SLIGRL (20 µM) in rat MCAs treated with the NOS inhibitor L-NAME (100 µM).
Upper panels show Em, lower panels tension. (A) Control EDH response. (B) EDH response in the presence of the Rho kinase inhibitor Y27632 (10 µM). (C) EDH response in the presence of Y27632 and the KCa3.1 blocker, TRAM-34 (1 µM). (D) EDH response in the presence of Y27632, TRAM-34 and the KCa2.3 blocker apamin (100 nM). (E) Histogram showing SLIGRL-induced EDH in the presence of Y27632 (10 µM), Y27632 and TRAM-34 and the combination of Y27632, TRAM-34 and apamin. (F) Histogram showing SLIGRL-induced EDH mediated hyperpolarization in the presence of SR5037 (1 µM), SR5037 and TRAM-34 and the combination of SR5037, TRAM-34 and apamin. Both Y27632 and SR5037 fully relaxed L-NAME induced tone, hyperpolarization was unaffected. Normally in the presence of L-NAME blockade of KCa3.1 is sufficient to block the EDH response, however following inhibition of Rho kinase subsequent inhibition of KCa2.3 is required to fully block the EDH response. *P<0.05 indicates a significant difference from control, one-way ANOVA with Tukey’s post-test, n = 4–6. φP<0.05 indicates a significant difference from Rho kinase inhibitor alone (Y27632 or SR5037) as determined by one-way ANOVA with Tukey’s post-test, n = 4–6.
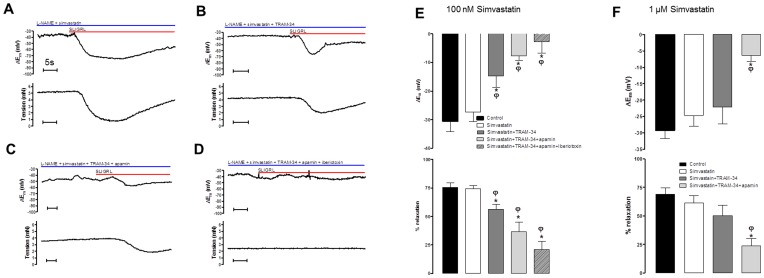
Simvastatin Prevented Loss of KCa2.3 Input to EDH Following TP Receptor Stimulation
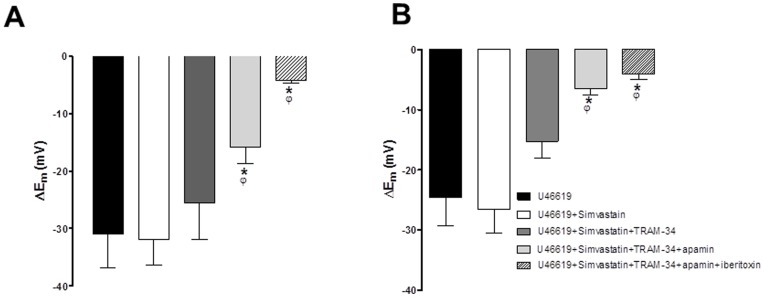
In MCAs able to synthesise NO inhibition of Rho kinase with a selective inhibitor protected KCa2.3 function against TP receptor stimulation produced by U46619. In a separate group of experiments in vessels able to synthesise NO and stimulated with U46619 (50–100 nM) we assessed if statins shared this effect of Rho kinase inhibitors. SLIGRL (20 µM) evoked EDH of 30.9±5.9 mV (n = 7). This response was not modified by 100 nM simvastatin (31.8±4.4 mV, n = 7). Subsequently, EDH was not reduced with TRAM-34 (25.4±6.4 mV, n = 4), but was progressively and significantly reduced by adding apamin and then iberiotoxin EDH (to 15.8±2.9 mV, n = 4, P<0.05 then 4.1±0.5 mV, n = 3, P<0.05; Figure 4A). At a higher concentration simvastatin (1 µM) again did not modify EDH evoked in the presence of U46619 (at 24.6±4.6 mV and 26.6±4.0 mV, pre and post simvastatin respectively, n = 8), and a similar inhibitory profile was observed with KCa channel blockers, EDH of 15.2±2.9 mV, n = 8 with TRAM-34, 6.5±1.0 mV, n = 5, P<0.05 with apamin and 4.0±0.9 mV, n = 5, P<0.05 with iberiotoxin (Figure 4B). Simvastatin (100 nM or 1 µM) did not significantly alter U46619 induced tone; however a higher concentration of simvastatin (10 µM) fully relaxed tone (data not shown). Normally there is no KCa2.3 component to hyperpolarization in the presence of U46619 but in the presence of simvastatin, at either concentration, blockade of this channel was required to significantly inhibit hyperpolarization. Thus a statin, like the Rho kinase inhibitor, protects the KCa2.3 component to EDH from TP receptor stimulation.
Figure 4. Histograms showing EDH evoked by SLIGRL (20 µM) in rat MCAs that are able to synthesise NO but treated with the TP receptor agonist, U46619 (50–100 nM).
Also shown is the effect of the HMG-CoA reductase inhibitor simvastatin at either 100 nM (A) or 1 µM (B) on EDH and the effect of blocking KCa3.1 alone (TRAM-34, 1 µM), in the subsequent presence of blockade of both KCa2.3 (apamin 100 nM) and KCa3.1 as well as the combined of blockade of KCa1.1 (iberiotoxin 100 nM), KCa2.3 and KCa3.1. *P<0.05 indicates a significant difference from control using one way ANOVA with Tukey’s post-test, n = 4–7. φP<0.05 indicates a significant difference from simvastatin (100 nM or 1 µM) as determined by one-way ANOVA with Tukey’s post-test, n = 4–7.
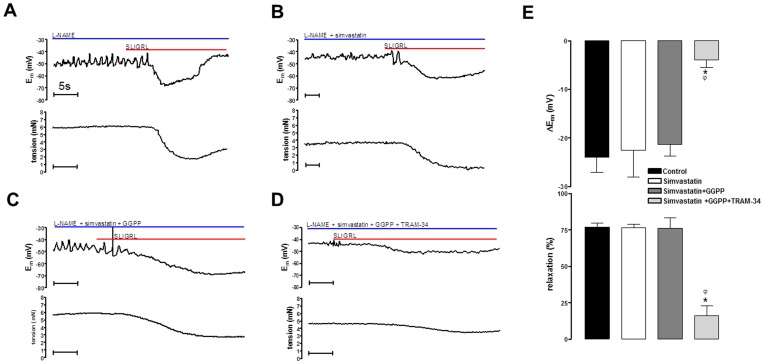
Simvastatin and Lovastatin Restore the Input of KCa2.3 to EDH in the Presence of a NO Synthase Inhibitor while Geranylgeranyl-pyrophosphate Circumvents this Protection
In rat MCAs treated with L-NAME we assessed if statins shared the effect of Rho kinase inhibitors to restore the KCa2.3 component of EDH. EDH and relaxation to SLIGRL was 30.7±3.5 mV and 75.5±4.1%, respectively (n = 8 Figure 5E). In the presence of 100 nM simvastatin this was unchanged (28.2±3.8 mV, n = 7, and 74.2±3.0%, n = 8; Figure 5A, E). TRAM-34 then significantly attenuated hyperpolarization and relaxation (to 14.8±4.0 mV and 56.4±4.2%, respectively, n = 8, P<0.05; Figure 5B, E). The subsequent addition of apamin further attenuated these responses (to 7.7±1.6 mV and 36.5±8.5%, n = 6, P<0.05; Figure 5C,E) as did iberiotoxin (to 2.7±3.9 mV and 21.1±7.0%, n = 4, P<0.05; Figure 5D, E). Both 1 µM simvastatin (figure 5F) or 100 nM Lovastatin (Figure S2) had a similar influence i.e. a combination of TRAM-34 and apamin was required to significantly to block the EDH response (Figure 5F and Figure S2). Normally in the presence of L-NAME, TRAM-34 alone can block the EDH response (Figure S1). In the presence of statins the additional requirement of apamin to fully inhibit EDH indicate these agents share the ability of Rho kinase inhibitors to protect KCa2.3 function.
Figure 5. Effect of simvastatin on isolated EDH responses obtained in the presence of a NO synthase inhibitor.
(A–D) Original traces showing the effect of 100 nM simvastatin (A) on isolated SLIGRL-induced EDH-mediated responses (hyperpolarization, upper panels; relaxation, lower panels) obtained in rat MCAs treated with the NOS inhibitor L-NAME (100 µM). Also shown is the effect of block of KCa3.1 (TRAM-34; B); combined block of KCa2.3 and 3.1 with apamin and TRAM-34 (C) and the further blockade of KCa1.1, 2.3 and 3.1 with iberiotoxin, apamin and TRAM-34 (D). Also shown (E–G) are histograms of the mean data for SLIGRL-induced EDH mediated responses (hyperpolarization, upper panels; relaxation, lower panels) in the presence of 100 nM simvastatin (E), 1 µM simvastatin (F). Normally in the presence of L-NAME inhibition of KCa3.1 alone is sufficient to block the EDH response. However, statins revealed a KCa2.3 component to the EDH response. *P<0.05 indicates a difference from control, φP<0.05 indicates a significant difference from simvastatin (100 nM or 1 µM) as determined by one-way ANOVA with Tukey’s post-test, n = 5–8.
Further experiments were conducted to assess the potential mechanisms by which statins protect KCa2.3 function in arteries treated with L-NAME. EDH and relaxation to SLIGRL (23.9±3.2 mV and 76.9±2.9%, n = 4) was not altered by simvastatin (100 nM; 22.5±5.5 mV and 76.6±2.3%, n = 4) or by the combination of simvastatin and isoprenoid GGPP (1 µM; 21.3±2.4 mV and 76.2±7.1%, n = 4). However, with GGPP present EDH responses were virtually abolished by the addition of TRAM-34 alone (3.9±1.6 mV and 16.3±6.5%, n = 4, P<0.05; Figure 6). As GGPP only restores the isoprenylation pathway and not cholesterol synthesis [28] the ability of statins to protect KCa2.3 function is likely to be a pleiotropic effect independent of effects on cholesterol synthesis.
Figure 6. Effect of restoring the isoprenoid signalling pathway on EDH responses obtained in the presence of simvastatin and a NO synthase inhibitor.
(A–D) original traces showing isolated SLIGRL-induced, EDH-mediated hyperpolarizations (upper panels) and relaxations (lower panels) obtained from rat MCAs treated with the NOS inhibitor L-NAME (100 µM; A). Also shown is the additional effect of simvastatin (100 nM), addition of GGPP (1 µM; C) and the combination of simvastatin and GGPP with the KCa3.1 inhibitor TRAM-34 (1 µM; D). (E) Histogram showing the mean data for isolated EDH-mediated responses (hyperpolarization, upper panel; relaxation, lower panel). While GGPP did not alter the total EDH mediated response it reversed the ability of simvastatin to protect KCa2.3 function as inhibition of KCa3.1 with TRAM-34 alone was sufficient to significantly inhibit the EDH response. *P<0.05 indicates a significant difference from control using one-way ANOVA with Tukey’s post-test, n = 4. φP<0.05 indicates a significant difference from simvastatin, as determined by one-way ANOVA with Tukey’s post-test, n = 4.
Discussion
These data implicate a novel role for Rho kinase in mediating TP receptor-dependent regulation of KCa2.3 channel function in EDH and associated smooth muscle cell relaxation in the rat MCA. They provide a mechanistic explanation for our previous observation that NO can protect KCa2.3 function in this artery by suppressing TP receptor signalling [6] and that stimulation of TP receptors can inhibit KCa2.3 function [6], [7], [29]. Our data also show that statins [21] restore KCa2.3 input to EDH and that this action is independent of effects on synthesis of cholesterol, possibly reflecting inhibition of Rho-mediated signalling.
To record and quantify the EDH response it is necessary to block NOS. In most arteries inhibition of EDH requires combined block endothelial cell KCa2.3 and 3.1 channels [3] Unusually, the rat MCA displays an EDH response that is solely due to KCa3.1 activation, as inhibition of KCa3.1 abolished EDH regardless of the agonist used to stimulate the EDH response [1], [2]. However, MCAs express both KCa2.3 and KCa3.1 channel protein [4], [5]. An explanation for this apparent paradox is that KCa2.3 can contribute to EDH, but only when the artery is able to synthesize NO, i.e. in the absence of a NOS inhibitor [5]. KCa2.3 also contributes to EDH in the presence of a NOS inhibitor, provided that TP receptors are antagonized [6]. In each case, inhibition of KCa2.3 alone had no discernible effect, but inhibition of EDH was now only significant following inhibition of both KCa2.3 and KCa3.1. The mechanism by which TP receptors selectively suppressed KCa2.3 function was not clear.
TP receptors couple to several second messenger systems, particularly PLC [30], and small GTPases such as Rho [31], [32]. In vascular smooth muscle cells, both these pathways contribute to constriction, but Rho-mediated pathways predominate [13], block of U46619-mediated constriction confirms this in the MCA. We demonstrate that TP receptors are also located on endothelial cells of the MCA (Figure 1). As KCa2.3 are only expressed in the endothelium of this artery [5], TP receptors could potentially selectively regulate the function of this channel by Rho kinase-dependent mechanisms.
We assessed the role of Rho kinase in regulation of KCa2.3 channels in MCAs able to synthesise NO. Under these conditions stimulation of TP receptors causes a loss of function of KCa2.3 channel [6]. We now show that inhibiting Rho kinase with Y27632 can “protect” or restore the KCa2.3 input to EDH that is blocked in the presence of the TP receptor agonist, U46619 (Figure 2C). It is important to note that the residual KCa1.1 component of EDH seen in these conditions is NO mediated [10]. In the absence of NO synthesis (i.e. in the presence of L-NAME), EDH was blocked with TRAM-34 (Figure S2), indicating that KCa3.1 channels alone underpinned hyperpolarization, as previously reported [1]. In the presence of either Y27632 or a structurally distinct and highly selective Rho kinase inhibitor, SR5037 [20], TRAM-34 now failed to fully block EDH (Figure 3); EDH was only fully blocked if KCa2.3 channels were subsequently inhibited with apamin (Figure 3D–F). It was impossible to measure effect on EDH mediated relaxation as Rho kinase inhibition fully relaxed tone (Figure 3B). Thus, inhibition of Rho kinase restores the KCa2.3 component of EDH, indicating that TP signalling through Rho kinase negatively and selectively modulates KCa2.3 (Figure 7). Inhibition of Rho kinase also reduced the constriction produced by blockade of NOS implying that Rho kinase is involved in this response, which is perhaps unsurprising since TP receptor stimulation is implicated in the constriction produced by NOS inhibitors in the MCA [33], [34].
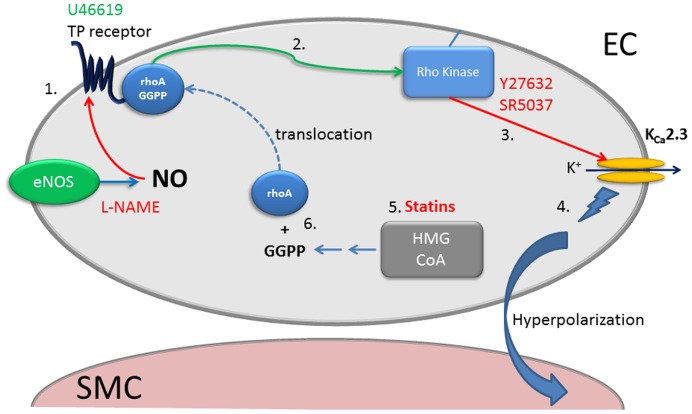
Figure 7. Schematic diagram showing potential regulatory mechanisms of KCa2.3 channels by TP receptor/rho mediated signalling.
Increased stimulation of the TP receptor with the agonist U46619 or following inhibition of NOS (eNOS) with L-NAME (NO can supress the action of TP receptors or synthesis of metabolites that activate TP) (1) results in activation of rhoA and stimulation of Rho kinase (2). Rho kinase (or associated signalling) inhibits KCa2.3 function (3) and inhibitors of Rho kinase (Y27632 or SR5037) restore or protect the KCa2.3 component of EDH (4). Statins by inhibiting HMG-CoA prevent formation of the isoprenoid GGPP (5). This reduces Rho mediated signalling by preventing GGPP dependent translocation of rhoA to the plasma membrane (6). Therefore statins protect KCa2.3 function by inhibiting Rho-mediated signalling via the TP receptor (1–3.). Red arrows/text indicates an inhibitory mechanism. Green text/arrows represent a stimulatory mechanism. Blue arrows indicate synthetic pathways; dashed blue line indicates translocation to the plasma membrane.
As Rho kinase appears to inhibit endothelial cell KCa2.3 function, we postulated statins might act to ‘protect’ the input of these channels in a similar manner to the Rho kinase inhibitors. As a consequence of inhibiting HMG-CoA reductase, statins also inhibit Rho kinase [35]. This “pleiotropic” effect is explained by an ability to prevent isoprenylation and subsequent translocation to the membrane of GTPases, such as Rho, an effect reversed by addition of exogenous isoprenoids [15], [23]. This appeared to be the case, in arteries able to synthesise NO and exposed to the TP receptor agonist U46619 (to inhibit KCa2.3) the statin, simvastatin (100 nM and 1 µM) restored the input of KCa2.3 to EDH (Figure 4). Simvastatin also restored KCa2.3 input to EDH-mediated responses once NOS was blocked (Figure 5A–F); a response that normally reflects only the activity of KCa3.1 (Figure S1) [1]. A structurally distinct statin, lovastatin had similar effects (Figure S2). At 100 nM simvastatin seemed to reveal a KCa1.1 component to EDH that was absent at a higher concentrations or with lovastatin. NO synthesis was inhibited in these experiments so this probably reflects an ability of this lower concentration of simvastatin to directly stimulate this channel [36].
The restorative action of the statins on KCa2.3 function is unlikely to reflect a reduction in cholesterol synthesis as this mainly occurs in the liver [28]. However, arteries do express HMG-CoA reductase [37], inhibition of which reduces production of isoprenoids including geranylgeranylpyrophosphate (GGPP) [28]. GGPP acts as a post-translational lipid attachment essential for the function of many proteins including Rho, rac and cd42 [28], [38]. Exogenous GGPP circumvented the ability of statins to restore KCa2.3 function (Figure 6). Therefore, statins protect KCa2.3 channel function by inhibiting isoprenylation. As reductions in isoprenylation reduce Rho signalling we speculate this is the mechanism by which statins protect KCa2.3 function. Indeed, inhibition of Rho is the mechanism by which statins augment NOS function in endothelial cells [15], [23]. Although the concentrations of statins used in this study did not produce powerful vasodilator responses seen with the inhibitors of Rho kinase, higher concentrations of statins (>1 µM) do cause relaxation (data not shown). Perhaps this indicates that the lower, clinically relevant concentrations [21] used in this study are not sufficient to fully inhibit Rho signalling in smooth muscle cells.
It is important to note we used the closed ring (lactone) form of simvastatin (and lovastatin) in this study. These require hydrolysis to the open ring Na+ salt (acid) form to inhibit HMG-CoA. [39] a process that occurs rapidly in rodent plasma [40]. While it is unknown if it this can also occur in arterial tissues CYP450 enzymes can dehydrogenate lovastatin (and simvastatin) [41] to the open acid form and the CYP3A4 subtype involved has been shown to be expressed in monkey endothelial cells [42]. Regardless, in this study GGPP reversed the effects of the lactone version of simvastatin indicating that the effects were mediated via inhibition of HMG Co-A. This is supported by a previous report in porcine vascular smooth muscle cells where the lactone form of simvastatin mediated effects dependent on inhibition of HMG Co-A [36].
Although our data strongly suggest a regulatory role for rho kinase on endothelial cell KCa2.3 channel input to EDH, it remains to be determined how Rho/Rho kinase inhibits KCa2.3. It is possible regulation involves direct phosphorylation of the channel by Rho kinase, or an associated kinase, similar to the regulation of KCa3.1 channels by kinases in immune cells [43]. Alternatively, Rho kinase might indirectly modulate KCa2.3 by remodelling the cytoskeleton; delayed rectifier channels in cerebral artery smooth muscle cells are inhibited by actin polymerization that is Rho kinase dependent [44].
Whatever the precise mechanism, vasoconstrictors such as TP, endothelin-1 and angiotensin II signal via the Rho kinase pathway and receptors for these vasoconstrictors are present on endothelial cells [14], [45], [46]. As a number of models of hypertension and diabetes are associated with an increased production/function of these vasoconstrictors it is perhaps not surprising that EDH-mediated signalling is suppressed [47]. The ability of statins to protect endothelial cell KCa function appears to involve isoprenoids and Rho-mediated signalling; such protection may help to explain the beneficial effect of statin treatment after stroke [48]. As Rho signalling inhibits a vital component of EDH, KCa2.3 channels located on the endothelium, our data support the rationale for treating endothelial dysfunction and related disorders by targeting Rho signalling.
Supporting Information
Histograms of the mean data for SLIGRL-induced EDH (hyperpolarization, upper panels; relaxation, lower panels) in the presence of the NOS inhibitor L-NAME (100 and 300 µM). The EDH responses were completely abolished by the KCa3.1 blocker TRAM-34 (1 µM). *P<0.05 indicates a significant difference from the 100 µM L-NAME control group; n = 3.
(TIF)
Histograms of the mean data for SLIGRL-induced EDH mediated (hyperpolarization, upper panels; relaxation, lower panels) in the presence of the NOS inhibitor L-NAME (100 µM) and the subsequent effect of the statin lovastatin (100 nM). Normally in the presence of L-NAME inhibition of KCa3.1 alone is sufficient to block the EDH response. However, lovastatin revealed a KCa2.3 component to the EDH response. *P<0.05 indicates a difference from control, φP<0.05 indicates a significant difference from lovastatin as determined by one-way ANOVA with Tukey’s post-test, n = 5.
(TIF)
Acknowledgments
SR5037 was a kind gift from Professor Phil LoGrasso (Scripps Research Institute, Jupiter, Florida, USA).
Funding Statement
This work was supported by the British Heart Foundation (http://www.bhf.org.uk) (Alister McNeish - FS/06/076/21988 and Graeme Cottrell - FS/08/017/25027) and a Wellcome Trust (www.wellcome.ac.uk) programme grant (Christopher Garland). Christopher Garland holds a Royal Society-Wolfson Research Merit Award and Francesc Jimenez-Altayo was supported by the Ministerio de Educación y Ciencia (España). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McNeish AJ, Dora KA, Garland CJ (2005) Possible Role for K+ in Endothelium-Derived Hyperpolarizing Factor-Linked Dilatation in Rat Middle Cerebral Artery. Stroke 36: 1526–1532. [DOI] [PubMed] [Google Scholar]
- 2. Marrelli SP, Eckmann MS, Hunte MS (2003) Role of endothelial intermediate conductance KCa channels in cerebral EDHF-mediated dilations. Am J Physiol Heart Circ Physiol 285: H1590–H1599. [DOI] [PubMed] [Google Scholar]
- 3. Garland CJ, Hiley CR, Dora KA (2011) EDHF: spreading the influence of the endothelium. Br J Pharmacol 164: 839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA (2009) SKCa and IKCa Channels, Myogenic Tone, and Vasodilator Responses in Middle Cerebral Arteries and Parenchymal Arterioles: Effect of Ischemia and Reperfusion. Stroke 40: 1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, et al. (2006) Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke 37: 1277–1282. [DOI] [PubMed] [Google Scholar]
- 6. McNeish AJ, Garland CJ (2007) Thromboxane A2 inhibition of SKCa after NO synthase block in rat middle cerebral artery. Br J Pharmacol 151: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crane GJ, Garland CJ (2004) Thromboxane receptor stimulation associated with loss of SKCa activity and reduced EDHF responses in the rat isolated mesenteric artery. Br J Pharmacol 142: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geeson J, Larsson K, Hourani SM, Toms NJ (2002) Sodium nitroprusside-induced rat fundus relaxation is ryanodine-sensitive and involves L-type Ca2+ channel and small conductance Ca(2+)-sensitive K+ channel components. Auton Autacoid Pharmacol 22: 297–301. [DOI] [PubMed] [Google Scholar]
- 9. Randriamboavonjy V, Kiss L, Falck JR, Busse R, Fleming I (2005) The synthesis of 20-HETE in small porcine coronary arteries antagonizes EDHF-mediated relaxation. Cardiovasc Res 65: 487–494. [DOI] [PubMed] [Google Scholar]
- 10. Yuill KH, McNeish AJ, Kansui Y, Garland CJ, Dora KA (2010) Nitric Oxide Suppresses Cerebral Vasomotion by sGC-Independent Effects on Ryanodine Receptors and Voltage-Gated Calcium Channels. J Vasc Res 47: 93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reid HM, Kinsella BT (2003) The {alpha}, but Not the {beta}, Isoform of the Human Thromboxane A2 Receptor Is a Target for Nitric Oxide-mediated Desensitization: Independent Modulation of TP{alpha} Signaling by Nitric Oxide and Prostacyclin. J Biol Chem 278: 51190–51202. [DOI] [PubMed] [Google Scholar]
- 12. Minamiyama Y, Takemura S, Imaoka S, Funae Y, Tanimoto Y, et al. (1997) Irreversible Inhibition of Cytochrome P450 by Nitric Oxide. J Pharmacol Exp Ther 283: 1479–1485. [PubMed] [Google Scholar]
- 13. Huang J-S, Ramamurthy SK, Lin X, Le Breton GC (2004) Cell signalling through thromboxane A2 receptors. Cellular Signalling 16: 521–533. [DOI] [PubMed] [Google Scholar]
- 14. Kent K, Collins L, Schwerin F, Raychowdhury M, Ware J (1993) Identification of functional PGH2/TxA2 receptors on human endothelial cells. Circ Res 72: 958–965. [DOI] [PubMed] [Google Scholar]
- 15. Laufs U, La Fata V, Plutzky J, Liao JK (1998) Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation 97: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 16. Stocker M, Krause M, Pedarzani P (1999) An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci 96: 4662–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, et al. (2000) Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proc Natl Acad Sci 97: 8151–8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galvez A, Gimenez-Gallego G, Reuben JP, Roy-Contancin L, Feigenbaum P, et al. (1990) Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-activated potassium channel from venom of the scorpion Buthus tamulus. J Biol Chem 265: 11083–11090. [PubMed] [Google Scholar]
- 19. Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, et al. (1997) Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389: 990–994. [DOI] [PubMed] [Google Scholar]
- 20. Fang X, Yin Y, Chen YT, Yao L, Wang B, et al. (2010) Tetrahydroisoquinoline Derivatives As Highly Selective and Potent Rho Kinase Inhibitors. Journal Med Chem 53: 5727–5737. [DOI] [PubMed] [Google Scholar]
- 21. Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, et al. (1999) New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Thera 84: 413–428. [DOI] [PubMed] [Google Scholar]
- 22. Corsini A, Maggi FM, Catapano AL (1995) Pharmacology of competitive inhibitors of HMg-CoA reductase. Pharmacol Res 31: 9–27. [DOI] [PubMed] [Google Scholar]
- 23. Laufs U, Liao JK (1998) Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem 273: 24266–24271. [DOI] [PubMed] [Google Scholar]
- 24. McNeish AJ, Altayo FJ, Garland CJ (2010) Evidence both L-type and non-L-type voltage-dependent calcium channels contribute to cerebral artery vasospasm following loss of NO in the rat. Vasc Pharmacol 53: 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wickman G, Lan C, Vollrath B (2003) Functional Roles of the Rho/Rho Kinase Pathway and Protein Kinase C in the Regulation of Cerebrovascular Constriction Mediated by Hemoglobin: Relevance to Subarachnoid Hemorrhage and Vasospasm. Circ Res 92: 809–816. [DOI] [PubMed] [Google Scholar]
- 26. Garland CJ, McPherson GA (1992) Evidence that nitric oxide does not mediate the hyperpolarisation and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol 105: 429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander SP, Mathie A, Peters JA (2011) Guide to Receptors and Channels (GRAC), 5th edition. British J Pharmacol 164: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liao JK, Laufs U (2005) Pleiotropic Effects of Statins. Ann Rev Pharmacol Toxicol 45: 89–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Plane F, Garland CJ (1996) Influence of contractile agonists on the mechanism of endothelium-dependent relaxation in rat isolated mesenteric artery. Br J Pharmacol 119: 191–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baldassare JJ, Tarver AP, Henderson PA, Mackin WM, Sahagan B, et al. (1993) Reconstitution of thromboxane A2 receptor-stimulated phosphoinositide hydrolysis in isolated platelet membranes: involvement of phosphoinositide-specific phospholipase C-beta and GTP-binding protein Gq. Biochem J 291 (Pt 1): 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP (1998) The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTP[gamma]S-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Letters 440: 183–187. [DOI] [PubMed] [Google Scholar]
- 32. Sakurada S, Okamoto H, Takuwa N, Sugimoto N, Takuwa Y (2001) Rho activation in excitatory agonist-stimulated vascular smooth muscle. Am J Physiol Cell Physiol 281: C571–578. [DOI] [PubMed] [Google Scholar]
- 33. Lacza Z, Herman P, Gorlach C, Hortobagyi T, Sandor P, et al. (2001) NO Synthase Blockade Induces Chaotic Cerebral Vasomotion via Activation of Thromboxane Receptors. Stroke 32: 2609–2614. [DOI] [PubMed] [Google Scholar]
- 34. Benyo Z, Gorlach C, Wahl M (1998) Involvement of Thromboxane A2 in the Mediation of the Contractile Effect Induced by Inhibition of Nitric Oxide Synthesis in Isolated Rat Middle Cerebral Arteries. J Cereb Blood Flow Metab 18: 616–618. [DOI] [PubMed] [Google Scholar]
- 35. Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, et al. (1995) Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 333: 1301–1307. [DOI] [PubMed] [Google Scholar]
- 36. Seto SW, Au ALS, Lam TY, Chim SSC, Lee SMY, et al. (2007) Modulation by simvastatin of iberiotoxin-sensitive, Ca2+-activated K+ channels of porcine coronary artery smooth muscle cells. Br J Pharmacol 151: 987–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corsini A, Mazzotti M, Raiteri M, Soma MR, Gabbiani G, et al. (1993) Relationship between mevalonate pathway and arterial myocyte proliferation: in vitro studies with inhibitors of HMG-CoA reductase. Atherosclerosis 101: 117–125. [DOI] [PubMed] [Google Scholar]
- 38. Van Aelst L, D’Souza-Schorey C (1997) Rho GTPases and signaling networks. Genes & Development 11: 2295–2322. [DOI] [PubMed] [Google Scholar]
- 39. Alberts AW, Chen J, Kuron G, Hunt V, Huff J, et al. (1980) Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proceedings of the Natl Acad Sci 77: 3957–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blum CB (1994) Comparison of properties of four inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Am J Cardiol 73: 3D–11D. [DOI] [PubMed] [Google Scholar]
- 41. Vyas KP, Kari PH, Prakash SR, Duggan DE (1990) Biotransformation of lovastatin. II. In vitro metabolism by rat and mouse liver microsomes and involvement of cytochrome P-450 in dehydrogenation of lovastatin. Drug Metabol Disp 18: 218–222. [PubMed] [Google Scholar]
- 42. Ayajiki K, Fujioka H, Toda N, Okada S, Minamiyama Y, et al. (2003) Mediation of Arachidonic Acid Metabolite(s) Produced by Endothelial Cytochrome P-450 3A4 in Monkey Arterial Relaxation. Hypertension Res 26: 237–243. [DOI] [PubMed] [Google Scholar]
- 43. Srivastava S, Li Z, Ko K, Choudhury P, Albaqumi M, et al. (2006) Histidine Phosphorylation of the Potassium Channel KCa3.1 by Nucleoside Diphosphate Kinase B Is Required for Activation of KCa3.1 and CD4 T Cells. Molecular Cell 24: 665–675. [DOI] [PubMed] [Google Scholar]
- 44. Luykenaar KD, El-Rahman RA, Walsh MP, Welsh DG (2009) Rho-kinase-mediated suppression of KDR current in cerebral arteries requires an intact actin cytoskeleton. Am J Physiol Heart Circ Physiol 296: H917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, et al. (1995) The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest 95: 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Masaki T, Kimura S, Yanagisawa M, Goto K (1991) Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation 84: 1457–1468. [DOI] [PubMed] [Google Scholar]
- 47. Hilgers RH, Webb RC (2007) Reduced expression of SKCa and IKCa channel proteins in rat small mesenteric arteries during angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol 292: H2275–2284. [DOI] [PubMed] [Google Scholar]
- 48. Welch KMA (2006) High-Dose Atorvastatin after Stroke or Transient Ischemic Attack. N Engl J Med 355: 549–559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histograms of the mean data for SLIGRL-induced EDH (hyperpolarization, upper panels; relaxation, lower panels) in the presence of the NOS inhibitor L-NAME (100 and 300 µM). The EDH responses were completely abolished by the KCa3.1 blocker TRAM-34 (1 µM). *P<0.05 indicates a significant difference from the 100 µM L-NAME control group; n = 3.
(TIF)
Histograms of the mean data for SLIGRL-induced EDH mediated (hyperpolarization, upper panels; relaxation, lower panels) in the presence of the NOS inhibitor L-NAME (100 µM) and the subsequent effect of the statin lovastatin (100 nM). Normally in the presence of L-NAME inhibition of KCa3.1 alone is sufficient to block the EDH response. However, lovastatin revealed a KCa2.3 component to the EDH response. *P<0.05 indicates a difference from control, φP<0.05 indicates a significant difference from lovastatin as determined by one-way ANOVA with Tukey’s post-test, n = 5.
(TIF)