Summary
Asymmetric cell divisions are a fundamental feature of neural development, and misregulation can lead to brain abnormalities or tumor formation. During an asymmetric cell division, molecular determinants are segregated preferentially into one daughter cell to specify its fate. An important goal is to identify the asymmetric determinants in neural progenitor cells, which could be tumor suppressors or inducers of specific neural fates. Here we show that the double-stranded RNA-binding protein Stau2 is distributed asymmetrically during progenitor divisions in the developing mouse cortex, preferentially segregating into the Tbr2+ neuroblast daughter, taking with it a sub-set of RNAs. Knockdown of Stau2 stimulates differentiation and over-expression produces periventricular neuronal masses, demonstrating its functional importance for normal cortical development. We immunoprecipitated Stau2 to examine its cargo mRNAs, and found enrichment for known asymmetric and basal cell determinants, such as Trim32, and identified novel candidates, including a subset involved in primary cilium function.
Introduction
The ability to undergo asymmetric cell divisions is considered a cardinal feature of many stem and progenitor cells and a fundamental mechanism by which central nervous system (CNS) progenitor cells generate diverse types of progeny. During an asymmetric cell division, stem cells generate a copy of themselves (self-renewal) and a differentiated daughter cell. The balance between self-renewal and differentiation governs the behavior of stem cells during embryonic growth and development and in adult homeostasis (Huttner and Kosodo, 2005; Knoblich, 2008; Roegiers and Jan, 2004). Perturbations in this balance can lead to excessive or diminished tissue development and contribute to benign or malignant overgrowth leading to tumors. Consequently, understanding the molecular mechanisms underlying asymmetric cell division is a central goal of stem cell biology.
The developing mouse cerebral cortex is an excellent model for studying asymmetric cell divisions and the generation of diverse neural cell fates. Cortical progenitor cells undergo repeated asymmetric cell divisions to produce diverse neurons, a process that we have shown, through time-lapse microscopy and lineage tracing, to be recapitulated in isolated cells in vitro (Shen et al., 2006). In the early mouse neuroepithelium around embryonic day 10 (E10), the dividing precursor cells undergo largely symmetric cell divisions that expand the progenitor pool. Later, these neuroepithelial cells transform into elongated radial glial cells (RGCs) with cell bodies in the apical ventricular zone (VZ); these are the principal progenitor cells for cortical pyramidal neurons and glia (Kriegstein and Alvarez-Buylla, 2009). During neurogenesis, RGCs frequently divide asymmetrically to produce another RGC and either a postmitotic neuron or an intermediate progenitor cell (IPC), also termed a basal progenitor cell. RGCs and IPCs can be distinguished by marker expression, for instance by Pax6+ and Tbr2+ respectively. IPCs delaminate into a second germinal zone, the subventricular zone (SVZ), where they divide a limited number of times to produce neurons early in development and glial cells later. The newborn neurons migrate along the radial process of RGCs into the cortical plate, arriving in layer-specific order from deep (layer 6) to superficial (layer 2).
Our understanding of the mechanism of asymmetric cell division in mammalian cells owes much to pioneering experiments in invertebrates. While the essential machinery for segregating molecules into one daughter cell versus another appears evolutionarily conserved, substantial differences exist and fundamental aspects of the mammalian process remain unknown. One important gap in our knowledge concerns identification of the cytoplasmic determinants that are shepherded into one daughter cell versus the other during asymmetric cell divisions. A few of the key players have been revealed, including Numb, EGFR, Dyrk1a, MALS-3 and Trim32 (Ferron et al., 2010; Schwamborn et al., 2009; Shen et al., 2002; Srinivasan et al., 2008; Sun et al., 2005; Zhong et al., 1996), but given the complexity of the mammalian CNS and known regional and temporal differences in progenitor cell sub-types, more asymmetric determinants are anticipated.
In Drosophila, one of the molecules that is segregated asymmetrically during neuroblast (NB) divisions is the double-stranded RNA-binding protein (dsRBP) Staufen. As the stem-like NB divides, Staufen binds mRNAs of cell fate determinants segregating them into the basal ganglion mother cell (GMC) to promote differentiation and suppress the stem cell state (Broadus et al., 1998; Doe et al., 1991). The possible role of Staufen in mammalian asymmetric neural progenitor (NPC) cell divisions has not yet been investigated. In mammals, there are two distinct Staufen genes: Staufen1 (Stau1) and Staufen2 (Stau2). Based on conservation of the RNA binding domains, Stau2 is most similar to Drosophila Staufen and while Stau1 is expressed in most tissues, Stau2 is predominantly expressed in the brain, (Duchaine et al., 2002) indicating a special role in neural cells. Previous studies in rodent hippocampal neurons show that Stau1 does not co-localize with Stau2 in ribonucleoprotein particles (RNPs) (Duchaine et al., 2002), but whether this indicates different functions is not yet clear.
Stau2 is enriched in the somatodendritic compartment of mature hippocampal neurons where it has been implicated in mRNA localization, transport and translation (Goetze et al., 2006; Kiebler et al., 1999). It has been suggested that Stau2 transports RNAs from the nucleus into neuronal dendrites, holding them under the dendritic spine until stimulated to be released by activity (Goetze et al., 2006). It also is present in the cell nucleus and functions as a nucleo-cytoplasmic shuttle protein (Macchi et al., 2004; Miki et al., 2005).
To date, mammalian Staufen proteins have only been characterized in postmitotic neurons, and asymmetric localization and segregation of Staufen proteins in CNS progenitor cell divisions has not been described. Moreover, specific Stau2-associated RNAs in dividing NPCs have yet to be identified. Hence, fundamental questions regarding Stau2 function during neural development remain to be addressed. Here we examined the role of Stau2 in cortical progenitor cell divisions. We show that Stau2 expression is polarized in dividing cells and asymmetrically segregated during mitosis, preferentially into the Tbr2+ IPC daughter cell. Manipulation of Stau2 levels in vivo or in vitro critically alters cortical NPC behavior. We performed RNA immunoprecipitation and microarray analysis (RIP-Chip) to identify Stau2 associated transcripts, which include putative asymmetric determinants.
Results
Stau2 Protein is Apically Localized in the Embryonic Cortical Ventricular Zone
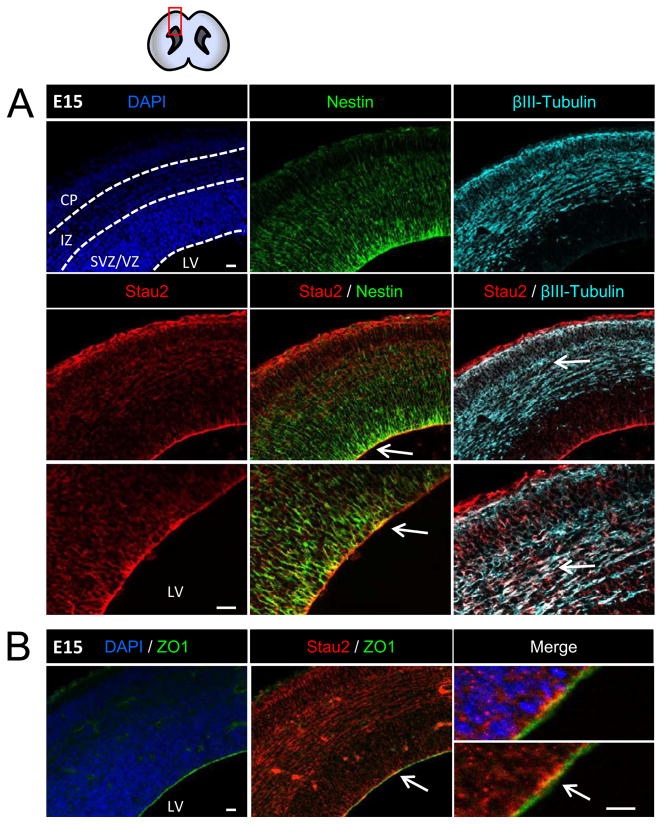
We performed immunostaining with an extensively characterized antibody specific for Stau2 (Goetze et al., 2006) on cryostat sections of developing mouse forebrains. We focused on the cerebral cortex from E13 (mid-gestation), when the RGCs increase asymmetric cell division events concomitant with active neurogenesis, through to late embryogenesis, when neurogenesis declines prior to birth. At E13, 15, and 17, immunolabeling for Stau2 is seen throughout the cortex, with strong expression near the apical edge (Fig. 1, and Supplemental Fig. 1). At E15, there is strong coexpression of the progenitor marker Nestin with Stau2 protein in cell bodies residing in the VZ (Fig. 1A), and apical enrichment of Stau2 is further confirmed by co-staining for the apically localized tight junction protein ZO1 (Fig. 1B). Co-staining with the neuronal marker β-tubulin III reveals that differentiating neurons located in the cortical plate also express Stau2, but this is noticeably reduced by E17 (Supplemental Fig. 1), while Stau2 remains strongly expressed throughout the later embryonic VZ.
Figure 1. Stau2 Expression in Embryonic Mouse Cortex.
Immunohistochemistry with a Stau2-specific antibody at E15 revealed staining throughout the cortex that is enriched in the radial glial neural progenitor cells at the apical VZ border and at the cortical surface where RGC endfeet are located. A) Strong co-localization with the progenitor cell marker Nestin (green) in the VZ. Stau2 is also present in β-tubulin III+ neurons (cyan) in the cortical plate. B) Co-localization of Stau2 (red) and the apically localized tight junction protein ZO1(green). Arrows indicate area enlarged in lower panel. Scale bar is 20 μm for all panels. VZ/SVZ, Ventricular Zone/Subventricular Zone; IZ, Intermediate Zone; CP, Cortical Plate; LV, lateral ventricle.
Stau2 Distribution is Polarized in Mitotic NPCs
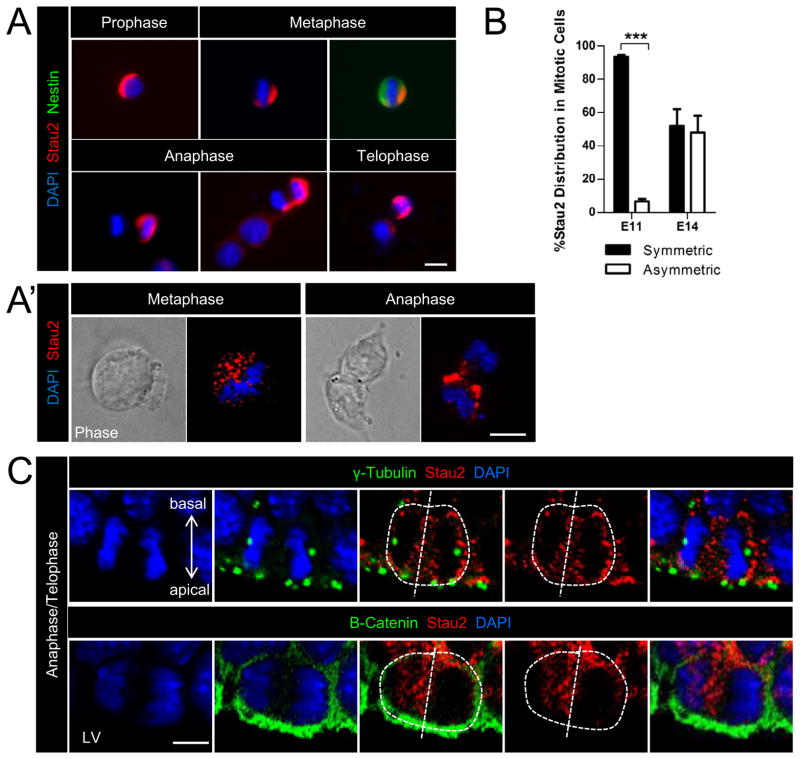
At midgestation, RGCs typically divide asymmetrically to generate two daughter cells with different fates, an RGC and an IPC. To examine Stau2 expression and localization during mitosis, we carried out immunostaining in isolated cell cultures of mouse primary cortical progenitor cells. E13-14 cortical cells were plated at clonal density and fixed 20–24 hours later to capture cells in different stages of division. Stau2 immunostaining with two different antibodies revealed polarized expression in cells undergoing mitosis, identified by DNA staining with DAPI, which shows condensed chromosomes (Fig. 2A,A′). Stau2 is polarized in prophase and metaphase, and staining can be seen in a crescent over half of the cell, a visible hallmark of segregating determinants (Jan and Jan, 2001; Zhong et al., 1996). During later stages of mitosis, Stau2 staining is frequently strong in one newly segregating daughter cell during anaphase and telophase, but weak or absent in the other daughter (Fig. 2A,A′).
Figure 2. Stau2 is Asymmetrically Distributed During Cortical Progenitor Cell Divisions.
A) Polarized distribution and asymmetric segregation of Stau2 (red) is observed in cultured cortical progenitor cells at different stages of mitosis, identified by condensed DAPI nuclear staining (blue). Nestin stained (green) E14 NPC in metaphase has a Stau2 crescent. A′) High power images of cells in metaphase and anaphase show asymmetric Stau2 localization. B) Quantification of Stau2 distribution in cells undergoing mitosis in culture. C) E15 cortex cryostat sections: VZ cells undergoing anaphase/telophase were identified by condensed DAPI staining (blue) and show punctuate asymmetric localization of Stau2 (red) in these mitotic cells. γ-tubulin reveals the centrosomes and β-catenin the cell junctions, helping to visualize the cell division plane. High power images show that in dividing cells with a vertical division plane typical for asymmetric cell divisions at this stage, Stau2 can be segregated predominantly into one daughter cell in vivo (mean ± SEM Student’s t-test; ***p<.001). Scale bar is 10 μm for all panels. LV, lateral ventricle.
Quantification of asymmetric segregation of Stau2 in mitotic cultured NPCs is shown in Fig. 2B, identifying cells in mitosis with anti-phosphohistone H3 and DAPI staining. At E10-11, divisions are predominantly symmetric, and consistent with this, primary NPCs plated at E10-11 and fixed 24 hours later show symmetric Stau2 distribution in 93.3% of Stau-expressing mitotic cells, with only 6.7% of cells having a polarized or asymmetrically segregating Stau2 expression pattern. In contrast, primary cells taken from E13-14 cortex and fixed 24 hours later show significantly more asymmetric Stau2 distribution: in 48.1% of the Stau2-expressing cells undergoing mitosis while symmetric staining is observed in the remainder. The same result was obtained with two different antibodies specific for Stau2. This finding is consistent with the increase in asymmetric cell divisions occurring as cortical neurogenesis progresses.
Asymmetric segregation of Stau2 is also observed in cells undergoing mitosis in vivo in the cortical VZ. At E15, the majority of asymmetric divisions have a vertical division plane (Konno et al., 2008; Kosodo et al., 2004; Noctor et al., 2008). Sections were immunostained for Stau2 and γ-tubulin, which localizes to the centrosomes, or with β-catenin to reveal cell junctions, allowing visualization of spindle pole orientation and thus cleavage plane during the later stages of mitosis. Inspecting E15 cortex sections at high power, Stau2 protein can be seen asymmetrically distributed in dividing cells with a vertical cleavage plane (Fig. 2C). At high power, Stau2 staining often appears punctate, consistent with its distribution in RNA granules, although staining with additional markers will verify this. Thus, both in vitro and in vivo, Stau2 is expressed by NPCs and can be asymmetrically localized and segregated during mitosis.
Stau2 is Asymmetrically Distributed into Daughter Cell Pairs
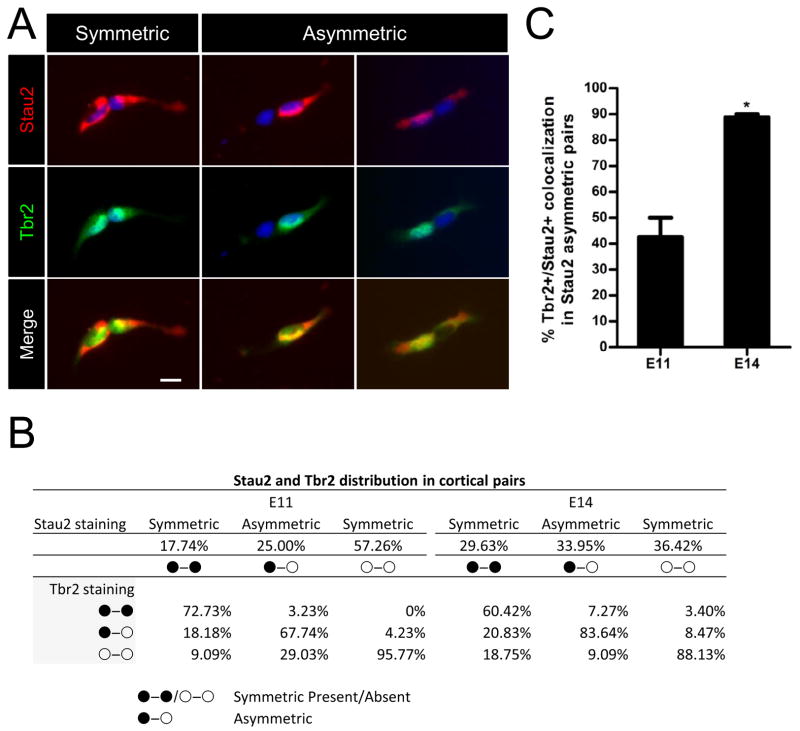
The asymmetric localization and segregation of Stau2 at different stages of mitosis in cultured Nestin+ cortical NPCs predicts differential segregation of Stau2 into daughter cell pairs. We tested this using the cell pair assay that we developed previously (Shen et al., 2002). Primary E10-11 or E13-14 cortical cells were plated at clonal density (40–80 cells/well) in Terasaki plates, then imaged at low power to create a comprehensive map of the location of each single cell. Cells were fixed 20–24 hours later and each of the single cells that had divided was identified by comparing the location of cell pairs to the original single cells in the mapped images. Cells were stained for Stau2 and its distribution in daughter cell pairs was quantified (Fig. 3). Cytoplasmic staining for Stau2 was typically weak and diffuse, so we scored strong nuclear/perinuclear localization as Stau2+ and weak or negative nuclear/perinuclear staining as Stau2-. In Drosophila neuroblasts, Stau2 is asymmetrically segregated into the basal GMC, equivalent to the IPC, hence we co-stained the cell pairs with the IPC marker Tbr2 (Fig. 3A) to determine whether the segregation pattern is conserved between flies and mammals. As summarized in Fig. 3B, we found that cell pairs that were asymmetric for Stau2 were predominantly asymmetric for Tbr2 at both E11 and E14. Cell pairs that were symmetric positive for Stau2 were also usually symmetric for Tbr2 expression, either positive or negative. Cell pairs that were negative for Stau2 were largely negative for Tbr2. When we examined daughter cell pairs asymmetric for Stau2 and quantified the percentage of Tbr2 colocalization with the Stau2+ daughter, we found that this occurred 42.5% of the time at E10-11 when neurogenesis is just beginning, but in 88.8% of cases at E13-14 during peak neurogenesis (Fig. 3C). These findings are consistent with Stau2 playing a role in IPC determination during active neurogenesis.
Figure 3. Stau2 Preferentially Distributes into the IPC at Midgestation.
A) Stau2 (red) shows both symmetric and asymmetric distribution in E11 and E14 cultured progenitor daughter cell pairs. Single cortical progenitor cells were plated at clonal density, mapped to record their initial position and fixed at 20–24 hours. Staining for Stau2 (strong red perinuclear/nuclear staining identified as Stau2+) and IPC marker Tbr2 (green) reveals Stau2 preferentially segregates into Tbr2+ cells (green, nuclear). Scale bar 10 μm. B) Summary of Stau2 and Tbr2 distribution patterns. C) Percentage of Tbr2 colocalization with the Stau2+ daughter in cell pairs asymmetric for Stau2. At E11, of the 67.7% of cells showing asymmetric localization of Stau2 and Tbr2 (in B), 42.5% have Stau2 and Tbr2 in the same daughter cell. This is increased at E14: of the 83.6% of cells showing asymmetric localization of Stau2 and Tbr2 (in B), 88.8% have Stau2 and Tbr2 in the same daughter cell (mean ± SEM Student’s t-test; *p<0.5). n=124 pairs at E11 and n=164 pairs at E14. Scale bar is 10 μm
Stau2 Knockdown Stimulates the RGC to IPC Transition
To address whether Stau2 influences cell fate, we analyzed the consequences of Stau2 depletion or overexpression during mouse cortical development. We utilized a previously characterized shRNA construct (Goetze et al., 2006), confirmed to knockdown Stau2 levels in mouse, and also generated a new shRNA Stau2 construct compatible with lentiviral packaging, which significantly decreased Stau2 mRNA levels to less than 15% of control vector, while Stau1 levels were unaffected (Supplemental Fig. 2). We generated a full-length 62KD Stau2 overexpression lentiviral vector and confirmed overexpression and the activity of the knockdown construct by western blot (Supplemental Fig. 2).
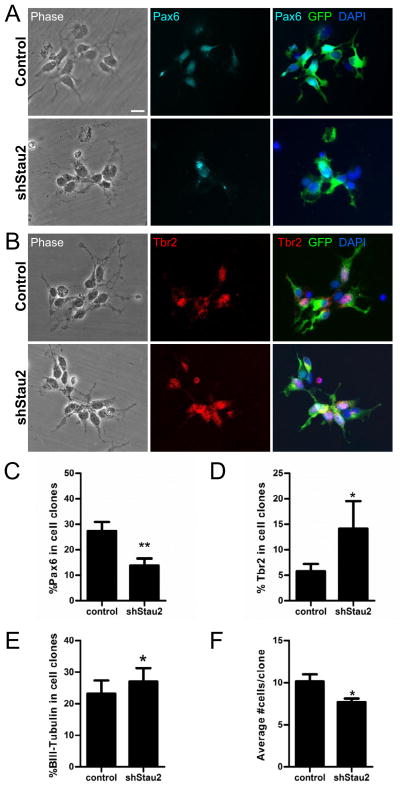
We examined Stau2 function in isolated embryonic cortical cells using lentiviral vector delivered shRNA constructs to maximize transduction efficiency (>95% cells were transduced, seen by GFP+ labeling). Isolated E11-12 NPCs were transduced with Stau2 shRNA and control vectors and plated at clonal density. After 3 days in culture, clones were fixed and immunostained with markers to assess cell fate. Treatment with Stau2 shRNA resulted in a significant decrease in the percentage of cells positive for the apical progenitor cell marker Pax6 (control = 27.28% ± 3.62%; shRNA Stau2 = 13.77% ± 2.79%, p<0.01) (Fig. 4A,C) and a significant increase in the percentage of cells positive for the IPC marker Tbr2+ (control = 5.78% ± 1.44%; shRNA Stau2 = 14.13% ± 5.40%, p<0.05) (Fig. 4B,D). Immunostaining for β-tubulin III revealed a slight increase in the percentage of neurons after shRNA Stau2 treatment after 3 days in culture (control = 23.12% ± 4.18%; shRNA Stau2 = 26.98% ± 4.27%, p<0.05) (Fig. 4E). Furthermore, Stau2 shRNA knockdown resulted in an overall decrease in clone size (control = 10.13% ± 0.85%; shRNA Stau2 = 7.68% ± 0.43%, p<0.05) (Fig. 4F), supporting the concept that Stau2 plays a role in promoting differentiation.
Figure 4. Stau2 Knockdown Promotes Differentiation in vitro.
A) Stau2 knockdown in E10-11 cortical cultures fixed after 3 days in culture. A) Clones show fewer Pax6+ cells (cyan) after shRNA Stau2 knockdown compared with control, quantified in C). B) Clones show more Tbr2+ cells (Red) after shRNA Stau2 compared with control, quantified in D). E) Quantification of β-tubulin III staining reveals more neurons after shRNA Stau2 knockdown. F) The average number of cells per clone is reduced after shRNA Stau2 knockdown consistent with premature differentiation of progenitor cells. (mean ± SEM Student’s t-test; *p<0.05, **p<0.01; In vitro counts for β-tubulin III (in E) showed a binomial distribution and were analyzed with a two-sample binomial test using R statistical software). Scale bar is 10 μm.
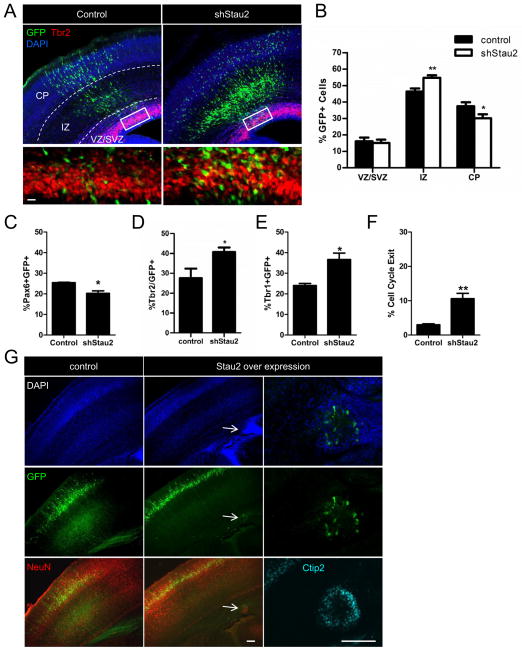
To address whether Stau2 determines cell fate in vivo, we analyzed the consequences of Stau2 depletion or overexpression during mouse cortical development using in utero electroporation. Stau2 shRNA or control plasmids were injected into the lateral ventricles at E13-E15 and electroporated into the cortical VZ. The results of in vivo knockdown were analyzed 72 hours later (at E16-E18). Stau2 knockdown resulted in a significant increase in GFP+ cells in the intermediate zone (IZ) at 72 hours post injection compared to control vector (control = 46.36% ± 1.91; shRNA Stau2 = 54.71% ± 1.59, p<0.01) (Fig. 5A,B) and significantly fewer GFP+ cells had reached the cortical plate (control = 37.48% ± 2.40; shRNA Stau2 = 30.15% ± 2.42, p<0.05). We did not detect a difference in the percentage of GFP+ cells located in the VZ or SVZ, however, there was a change in progenitor sub-type composition: After Stau2 knockdown, there were fewer Pax6+GFP+ cells (control = 25.43% ± 0.21%; shRNA Stau2 = 20.22% ± 1.24%, p<0.05), and more GFP+Tbr2+ cells (control = 27.60% ± 4.74; shRNA Stau2 = 40.75% ± 2.19, p<0.05) (Fig. 5C,D), indicating that the progenitor population was skewed towards the IPC fate. In addition, immunostaining with the neuronal marker Tbr1 showed an increase in the percentage of Tbr1+GFP+ cells within the cortical plate and IZ after Stau2 knockdown (control = 24.03% ± 1.00%; shRNA Stau2 = 36.67% ± 3.10%, p<0.05) (Fig. 5E), consistent with premature neuronal differentiation. It is possible that knocking down Stau2 in vivo alters the balance between symmetric and asymmetric cell division events. Thus, when Stau2 levels are reduced, the transport of cell fate determining RNAs is no longer appropriately polarized into one daughter cell, instead these mRNAs are present in both daughter cells so that they both acquire an IPC fate. This would lead to early depletion of apical progenitor cells with an early increase in basal progenitor cells and differentiating neurons. This interpretation is also supported by analysis of cell cycle exit rates, performed as described (Srinivasan et al., 2008). Pregnant animals that had been electroporated in utero with Stau2 shRNA or control plasmids at E13.5 were injected with BrdU at 70 hours and sacrificed 2 hours later. After sectioning, the germinal zones were assessed for cells that stained with BrdU alone or BrdU plus Ki67, indicating cells that had exited the cell cycle or were continuing in the cell cycle, respectively. We found the percentage of GFP+ cells that were BrdU+ but Ki67− was significantly increased in shRNA Stau2 brains (control = 2.99% ± 0.27%; shRNA Stau2 = 10.59% ± 1.56%, p<0.01) (Fig. 5F), showing that more cells had exited the cell cycle.
Figure 5. Stau2 shRNA Knockdown Stimulates Differentiation in vivo.
A) In utero electroporation of Stau2 shRNA at E13-15 then harvested at E16-18 shows more GFP+ cells (green) in the IZ, and fewer in the CP compared to control. Box indicates area enlarged in panels beneath showing more GFP+ (green) and Tbr2+ (red) IPCs cells after Stau2 knockdown. B) Quantification of GFP+ cell location. C–F) Quantification of sections shows fewer Pax6+GFP+ cells, more Tbr2+GFP+ cells, more Tbr1+GFP+ and increased cell cycle exit.(mean ± SEM Student’s t-test; * p<0.05, **p<0.01). G) Stau2 overexpression leads to periventricular heterotopias. After electroporation at E13-14 of Stau2 overexpression or control constructs (green), examination at P4 shows periventricular heterotopias (arrows) after Stau2 overexpression. The abnormal foci include cells positive for the mature neuronal marker NeuN (red) and the deep layer marker Ctip2+ (cyan). Scale bar is 20 μm in A and 100 μm in the rightmost panels in G.
We then investigated the effects of overexpressing Stau2. The full length Stau2 overexpression plasmid with a GFP reporter was electroporated into the developing cortex at around E14 and animals were sacrificed 3 or 10 days later at E17 or postnatal day 4 (P4). When the cortices were sectioned, we discovered abnormal periventricular cell masses containing GFP+ cells in approximately 25% of the Stau2 overexpression animals in both embryonic (not shown) and postnatal stages (Fig. 5G); these were not detected after electroporation of control vector. The heterotopias stained for the neuronal marker NeuN and varied in size from 100 to 250μm. Some of the neurons expressed Ctip2, showing that they had differentiated into deep layer neurons. These findings suggest that overexpression of Stau2 could lead to incomplete segregation of asymmetric determinants, perhaps due to overloading of the segregation machinery, such that both daughter cells inherit Stau2 cargo RNAs and undergo premature differentiation and disruption of neuronal migration. Taken together, these data suggest that the amount of Stau2 expressed in cortical progenitor cells is critical, as reduced levels leads to premature differentiation, and overexpression can disrupt normal development, revealing an important role for Stau2 and associated cargo RNAs in cell fate determination in the developing brain.
Genome-Wide Identification of mRNAs in Stau2 Containing RNPs
Given that Stau2 is preferentially segregated into the IPC daughter cell at asymmetric cell divisions, we are particularly interested in the RNAs transported in Stau2-containing RNPs, as these could include important determinants that suppress the RGC and enhance the IPC fate. We prepared extracts from E13-14 cortex then immunoprecipitated endogenous Stau2 using a monoclonal Stau2 antibody and an anti-T7 phage protein antibody in parallel samples as a control. Immunoprecipitated total RNAs were processed and hybridized to Affymetrix GeneChip Mouse Gene 1.0 ST arrays. Following normalization, expression values in the top 80th percentile across all samples (n=24,558 probe sets) (the E13-14 cortex transcriptome list) were retained for further analysis. To identify targets enriched in the Stau2 IP, three datasets were created. The first included transcripts with a 1.5 or higher fold change in Stau2 RIP versus total input RNA samples. The second included transcripts showing a 1.5 or higher fold change in T7 negative control versus input. To account for off-target binding and minimize non-specific RNAs, the third dataset was generated, consisting of the Stau2 enrichment set (first set) minus its overlap with the T7 negative control set (second set). This third dataset was compared against the input to determine statistically significant enrichment compared to random sampling, which resulted in a final Stau2 RIP enriched list of 1566 probe sets (Supplemental Table S1). The final 1566 list was compared to the E13-14 cortex transcriptome list, to look for statistically relevant Gene Ontology (GO) term associations (Supplemental Table S2).
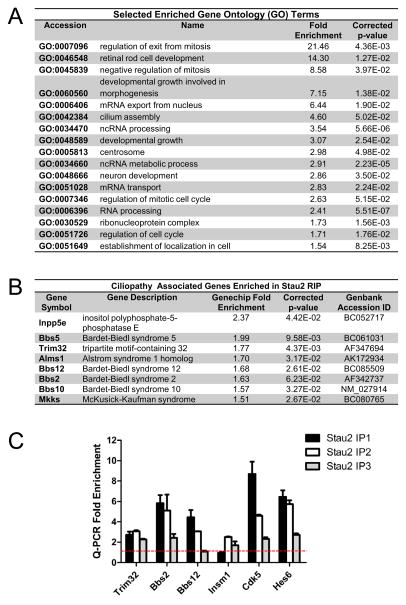
Figure 6A shows selected enriched GO terms in the final Stau2 RIP enriched list. There was a large fold enrichment for the following GO terms: regulation of exit from mitosis, negative regulation of mitosis, and regulation of cell cycle, which fits well with the premature differentiation we observed after Stau2 knockdown. Also not surprisingly, the list was enriched in RNA-associated terms including processing, metabolism, transport, and non-coding RNA (ncRNA) processing. Intriguingly, we noted that centrosome and cilium assembly, which is centrosome-related, are enriched sectors. Furthermore, the Stau2 enriched list included a surprising number of the centrosome-associated Bardet-Biedl Syndrome (BBS) genes. Bbs genes encode for proteins that form a complex called the BBSome which contributes to primary cilium formation and function, and mutations in Bbs proteins can produce ciliopathies (Quinlan et al., 2008). Figure 6B shows ciliopathy associated genes and their fold enrichment in the Stau2 RIP enriched list. This gene list includes 6 Bbs genes including Bbs 2,5,10,12,Mkks (also known as Bbs6), and Trim32 (Bbs11). To confirm Stau2 target RNA enrichment, we performed q-PCR for several putative targets on independent RIP samples and saw consistent results (Fig. 6C).
Figure 6. Identification of Stau2 Associated mRNAs.
A) Selected enriched GO terms and corresponding p-values. B) A number of enriched genes are associated with human ciliopathies. C) q-PCR validation on 3 separate independent RIP samples of selected Stau2 enriched targets identified by Genechip analysis. Graph shows relative fold enrichment compared to input RNA (red line) and normalized to non-enriched control. Note for sample Stau2 IP3, RIP was carried out with a separate Stau2 specific antibody from IP1 and IP2, but still shows enrichment for most mRNAs.
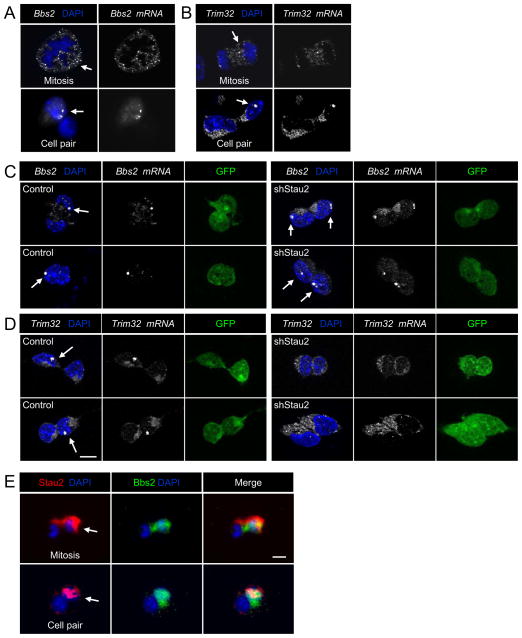
If Stau2 protein is indeed segregating mRNAs asymmetrically during cortical divisions, we should be able to visualize this using fluorescent in situ hybridization (FISH). Binding of multiple oligonucleotide probes each labeled with a fluorophore allows specific detection of single mRNA molecules within a cell. Probes generated to Trim32 and Bbs2 showed polarized localization in dividing NPCs and asymmetric localization in daughter cell pairs (Fig. 7A,B). We often observed one or two brightly concentrated spots of Bbs2 and Trim32 mRNA localization in daughter cell pairs (arrows in Fig. 7A,B) and it is tempting to speculate that these mRNAs could be localizing to the Bbsome or centrosome.
Figure 7. Fluorescent In Situ Hybridization Reveals Asymmetric Localization of Stau2 mRNA Targets in Cultured Neural Progenitors.
Bright concentrated dots of mRNA staining are often observed for both Bbs2 and Trim32 mRNAs (arrows). A) Localization of Bbs2 mRNA in a cell entering metaphase (top, arrow). Asymmetric expression of Bbs2 in daughter cell pairs (bottom, arrow). B) Asymmetric localization of Trim32 mRNA in a cell in anaphase/telophase (top, arrow). Asymmetric expression of Trim32 in daughter cell pairs (bottom, arrow). C,D) Asymmetric distribution of Bbs2 and Trim32 mRNAs in daughter cell pairs is altered after Stau2 knockdown. Bright dots for Bbs2 are asymmetric in control and symmetric in shRNA Stau2 treated panels (arrows). Bright dots for Trim32 (arrows) are asymmetric in control and not visible in shRNA Stau2 treated panels. E) Bbs2 protein (green) is asymmetrically localized in dividing cortical progenitors and in daughter cell pairs, and co-localizes with Stau2 protein (red). Scale bar is 10 μm.
We assessed whether asymmetric localization of Bbs2 andTrim32 mRNAs is altered in daughter cell pairs after Stau2 knockdown. E11 embryonic cortical cells were infected with control or shRNA Stau2 expressing lentiviral vectors and cultured for three days as neurospheres to allow viral expression, then the neurospheres were dissociated to single cells and plated at clonal density for the pair assay. Cells were fixed 20 hours later and FISH was carried out. In control conditions, Bbs2 and Trim32 mRNAs were typically distributed in large bright localized spots as well as in a less intense, diffuse pattern, and the distribution was approximately 50% symmetric and 50% asymmetric in cell pairs (Fig. 7C,D), consistent with our prior cell pair analysis. After Stau2 knockdown, Bbs2 mRNA was found symmetrically in approximately 70% of pairs, with bright dots in both daughter cells (Fig. 7C), and the Trim32 mRNA was seen symmetrically in 80% of daughter cell pairs, which often lacked the bright spots seen in controls (Fig. 7D).
Asymmetric segregation of the Trim32 protein can occur during asymmetric NPC divisions (Schwamborn et al., 2009) but the distribution of other Bbs proteins has not been examined. Staining with a polyclonal antibody against Bbs2 protein reveals asymmetric localization in the same daughter cell that is also Stau2 positive (Fig. 7E). While to date we found that Stau2 immunochemistry is not compatible with the single molecule FISH, it seems most plausible from the accumulated evidence that Bbs2 mRNA and protein are both moving into the Stau2+ daughter cell. Hence redundant segregation of protein and the corresponding mRNA, seen in Drosophila for prospero asymmetric segregation (Hirata et al., 1995; Knoblich et al., 1995), and which appears to be the case for Trim32 and Bbs2, might be a recurring theme in asymmetric cell divisions, assuring that segregation of these important molecules occurs appropriately.
Discussion
In this study, we examined the expression and function of the dsRBP Stau2 during development of the mouse cerebral cortex. We provide evidence that Stau2 protein is strongly expressed in the embryonic VZ and focused in the apical progenitor cells, the RGCs. Perturbation in Stau2 levels has a significant impact on cortical progenitor cell maintenance and differentiation, demonstrating its importance for normal development. Further, we identified Stau2 cargo mRNAs that are segregated during asymmetric NPC divisions, which include known and putative novel asymmetric determinants.
Our findings and those of a companion paper (Vessey et al.) indicate that knockdown of Stau2 in vivo during forebrain development speeds differentiation, with an early production of more Tbr2+ IPCs and a reduction in Pax6+ apical RGCs. This fits with the concept that Stau2 normally ensures preferential distribution of molecules that suppress the RGC state and promote the IPC fate. With reduced Stau2, these molecules could be distributed symmetrically and thus act on both progenitor daughters to lead to RGC depletion, IPC production and premature differentiation. We note that there are normally Stau2 negative pairs that nevertheless do not differentiate into Tbr2+ daughters. It is possible that in some divisions, IPC determinants are not expressed, so that even in the absence of Stau2 the daughter cells remain Tbr2−.
Interestingly, overexpression of Stau2 also perturbs cortical development, in some cases leading to formation of periventricular heterotopias. Heterotopias can form due to premature differentiation of progenitor cells, for example, early depletion of RGCs prevents normal neuronal guidance so that cells are trapped near the ventricle. Whether Stau2 overexpression could be the cause of some heterotopias in patients is an intriguing possibility. Stau2 targets are enriched for BBSome proteins, which are important for primary cilia formation and function (Zaghloul and Katsanis, 2009), and perturbation in cilia formation can cause cortical heterotopias (Hsiao et al., 2009).
Prior studies identified cargo RNAs of Stau2 in differentiated neurons in rat brain, and in immortal cultured cell lines (Furic et al., 2008; Maher-Laporte and DesGroseillers 2010) and here we add information on cargo in NPCs. Stau2 has several dsRNA binding domains (St Johnston et al., 1992), a tubulin-binding domain and possible protein interaction domains, so it could interact with several sets of RNA molecules, either directly or via interaction with other proteins. Consistent with this, we observed a large number of Stau2 enriched transcripts. Another explanation for this breadth of enriched genes is that we immunoprecipitated endogenous Stau2 from whole E13-14 cortex, including progenitor cells and differentiating/differentiated neurons. We chose to use the entire cortex rather than a purified NPC population because cell sorting could change the target mRNA profile, although this would be a worthwhile next step. Nonetheless, this approach has provided an overview of the Stau2 enriched sets of transcripts in the developing cortex.
We were encouraged to see that the Stau2 associated transcripts included Trim32, given that Trim32 protein can be asymmetrically segregated in cortical progenitor cells and important for differentiation. Intriguingly, in addition to Trim32 (Bbs11), the Stau2 enriched list includes a number of other BBSome genes. Bbs proteins are important for assembly and vesicular transport in the primary cilium and mutations result in Bardet-Biedl syndrome (Zaghloul and Katsanis, 2009). The GO term for cilium assembly was close to significance with a p=0.0502, and we found additional cilia-associated genes in the enriched database, e.g. tetratricopeptide repeat domain proteins Ttc30a1, Ttc30a2, and Ttc30b, that were not included in the GO analysis, further strengthening the enriched cilium terms.
Stau2 could regulate BBSome formation by asymmetrically segregating specific assembly components into one daughter cell during mitosis. This could produce differential signaling between the two daughter cells by altering their ability to respond to ligands such as hedgehog or Wnt family proteins (Goetz and Anderson, 2010; Quinlan et al., 2008). This possibility is interesting in light of other known asymmetric cell division mechanisms, in which the environment has control over the cell division outcome: for example, in the case of Numb segregation, the two daughter cells show differential sensitivity to Notch ligands, and in the case of asymmetric EGFR, the two daughter cells show differential sensitivity to EGF ligands (Sun et al., 2005).
In a recent gene profiling study (Kawaguchi et al., 2008), mouse cortical progenitor gene expression was classified, progressing from RGCs (class 1) through an early IPC (class 2/3) to more mature IPCs (class 3) and neurons (class 4). Interestingly, most of the Stau2 enriched genes, were in the 2/3 class (approx. 50%) and do not include later IPC markers such as Tbr2 or Tis21 (Kowalczyk et al., 2009); it appears that Stau2 preferentially binds a subset of transcripts that are expressed in the early transition from RGC to IPCs. The Stau2 enriched list includes several other genes known to be important in the apical-basal cell decision, for example, Hes6 (Gratton et al., 2003), Cdk5 (Buchman et al.; Jessberger et al., 2009; Lizarraga et al., 2010), and Insulinoma-associated 1 (Insm1) (Farkas et al., 2008). The Stau2 enriched transcripts will be valuable to mine for novel regulators of self-renewal and differentiation. Given that cortical progenitor cells change over time, we expect that some of the RNA cargo molecules might change over time, contributing to the temporal specification of IPCs, which will be a point for future studies. Our findings on Stau2 further underline the evolutionary conservation of mechanisms that control asymmetric cell divisions and generate diverse neural progeny.
Experimental Procedures
Animals
The care and use animal protocols were approved by the University at Albany (UA) Institutional Animal Care and Use Committee. We are committed to comply with the Principles for Use of Animals, the provisions of the Animal Welfare Acts and other applicable laws and regulations. Swiss Webster mice obtained from Taconic Farms were used in all experiments.
Cortical Cell Culture
For adherent cultures, cerebral cortices of timed pregnant Swiss Webster mouse embryos (Taconic Farms) were dissociated and cultured as described previously in serum-free DMEM with 10 ng/mL FGF2 (Invitrogen) in PLL-coated Terasaki plates (Shen, 2006). For clonal analysis, 40–80 cells were plated in Terasaki plate microwells, and clonal development was monitored by daily microscopic inspection (Shen et al., 2002). Knockdown experiments were performed as described previously (Fasano, 2007) Quantification: 12–15 random fields per condition were imaged and counted in each experiment.
Immunostaining
Cryostat sections and cell cultures were fixed and immunostained as described (Shen et al., 2006). Primary antibodies used were: β-tubulin III, mouse IgG2b 1:800 (Sigma); Pax6, mouse 1:20 (Developmental Studies Hybridoma Bank); Nestin, mouse, 1:25 (Developmental Studies Hybridoma Bank); NeuN, mouse 1:100 (Chemicon); Ctip2, rat 1:400 (Abcam); Tbr2, rabbit 1:250 (Abcam); Stau2, rabbit 1:1,000 (gift M. Kiebler); Stau2, mouse 1:250 (Abcam); Bbs2, rabbit 1:100 (Protein Tech Group); Tbr1, rabbit 1:200 (Abcam); γ-Tubulin, mouse 1:1000 (Abcam); β-Catenin, mouse, 1:200 (BD); ZO1, mouse 1:250 (Invitrogen), BrdU, rat 1:100 (Abcam). Antigen retrieval: 10mM citrate buffer for 15 minutes at 95 °C. Immunoreactivity was visualized using Alexa Fluor-conjugated secondary antibodies (1:1,000; Invitrogen). Phase and fluorescent images were acquired using a Zeiss Z-1 apotome inverted microscope and a Zeiss AxioCam MRm digital camera with Axiovision 4.6 software.
Lentivial Vector Production and Viral Packaging
shRNA and overexpression constructs for Stau2 were constructed, harvested, and titered as described previously (Fasano et al., 2007). Oligonucleotides containing a 19mer targeting Stau2, followed by a loop sequence and then reverse complement, were synthesized and cloned into the H1 lentiviral vector using XbaI/SmaI. The 19mer sequences are as follows: Stau2 shRNA1, 5′-ACCTCTGGCACAACTCTAA -3′; Stau2 shRNA2, 5′-TGCCACTGCGTCCGTATAA -3′. Previously published shRNA sequences targeting Stau2 were also used (Goetze et al., 2006). For overexpression constructs, the ORF of Stau2 was PCR-amplified, sequence-verified, and cloned into a modified version of FUGW (Fasano et al., 2007). An IRES-eGFP was also included for visualization in cells. For viral transduction, lentiviral vectors were added to dissociated cortical cultures just before plating at a multiplicity of infection of 10.
In utero Electroporation
Plasmids were transfected by in utero electroporation as described previously (Fasano et al., 2007). Dissected brains were fixed in 4% paraformadehyde overnight at 4°C, cyroprotected in 30% sucrose, and then cryosectioned coronally at 12 μm onto slides or, for post-natal brains at 40 μm for staining as floating sections. Sections were imaged on a Zeiss Z1 apotome inverted microscope. For analysis, 2–4 sections from each embryo were used to calculate the average GFP+ cells in each condition. 4–8 embryos were used to calculate final averages. Proliferating cells were labeled with an intraperitoneal injection of BrdU 100mg/kg (Sigma) 2 hr prior to sacrifice. We calculated cell-cycle-exit as the fraction of BrdU-labeled, electroporated cells that exited the cell cycle (GFP+, BrdU+, and Ki67 ), over the total number of electroporated cells that had been labeled with BrdU (GFP+ and BrdU+).
RNA Immunoprecipitation (RIP)
Immunoprecipitation of Stau2-containing RNPs was carried out on cell lysates from dissected E13-E14 cortices. Stau2 was immunoprecipitated using a monoclonal Stau2 antibody, and an anti-T7 phage protein antibody as control. For RIP, 5ug of Stau2 antibody (ab60724, Abcam) or T7 antibody (69522, Novagen) was mixed with Dynabeads Protein G (Invitrogen) and tumbled at room temperature for 25 minutes. RIP buffers and procedures were carried out as described previously (Baroni et al., 2008; Tenenbaum et al., 2002). All buffers were made using nuclease-free solutions and water.
Microarray Hybridization
Approximately 10ng of total RNA (input) and immunoprecipitated RNA (Stau IP and T7 IP) were amplified using the NuGEN pico protocol (NuGEN) to generate sense target cDNA that was hybridized to Affymetrix Mouse Gene 1.0 ST arrays. All experiments were done in triplicate, with Stau2 RIP, negative control T7 RIP and total (input) RNA samples generated for a total of 9 microarrays. Raw data was normalized using the iterative PLIER16 algorithm and quantile normalization, and quality controlled for hybridization efficiency. Probe sets that showed signal values in the top 80th percentile across all samples (n=24,558 probe sets) were retained. The CEL files were further analyzed using GeneSpring GX v10. Validation of select identified targets was carried out by q-PCR (see supplemental methods). The microarray data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (GEO) and are accessible through the GEO series accession number GSE38222 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE38222).
Single molecule Fluorescent In Situ Hybridization (FISH)
Mouse cortical progenitor cells were isolated and cultured on Lab-Tek chambered #1.0 Borosilicate coverglass system. Stellaris FISH probes (Biosearch Technologies) for Bbs2 and Trim32 open reading frames were generated using probe designer (http://singlemoleculefish.com/probe-designer/), and labeled with the reporter dye Cal Fluor Red 590. FISH was carried out on primary neural progenitor cultures using published protocols (Raj and Tyagi, 2010; Zenklusen and Singer, 2010). Probes were hybridized for 18 hours at 37°C.
Statistics
All statistical tests, unpaired t-tests, and one-way ANOVAs with Bonferroni post-hoc tests were carried out with GraphPad Prism version 5. Welch’s correction was used with the unpaired t-test if needed to account for differences in variance. In vitro counts for β-tubulin III showed a binomial distribution and were analyzed with a two-sample binomial test using R statistical software. For in vitro cell counts, the percentage of cells staining for cell-type specific makers were determined per clone (n= at least 24 clones for all conditions counted and experiments were independently repeated at least once).
Supplementary Material
Highlights.
The RNA binding protein Stau2 is asymmetrically distributed in neural progenitors.
Perturbation of Stau2 levels causes abnormal CNS progenitor behavior.
Stau2 associated transcripts include known and potentially novel fate determinants.
Asymmetric segregation of RNA networks via Stau2 contributes to cell diversification.
Acknowledgments
This work was supported by NIH grant R01NS074047 (to S.T.), F32 NS061461 (to G.K.) and the Empire State Stem Cell Fund through New York State Department of Health contract # C024352. We greatly appreciate the assistance of Dr. Sridar Chittur and the services provided by the Center for Functional Genomics Microarray Core Facility. We are also very grateful to Rachel Wurster (Neural Stem Cell Institute) for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baroni TE, Chittur SV, George AD, Tenenbaum SA. Advances in RIP-chip analysis : RNA-binding protein immunoprecipitation-microarray profiling. Methods in molecular biology (Clifton, NJ. 2008;419:93–108. doi: 10.1007/978-1-59745-033-1_6. [DOI] [PubMed] [Google Scholar]
- Broadus J, Fuerstenberg S, Doe CQ. Staufen-dependent localization of prospero mRNA contributes to neuroblast daughter-cell fate. Nature. 1998;391:792–795. doi: 10.1038/35861. [DOI] [PubMed] [Google Scholar]
- Buchman JJ, Tseng HC, Zhou Y, Frank CL, Xie Z, Tsai LH. Cdk5rap2 interacts with pericentrin to maintain the neural progenitor pool in the developing neocortex. Neuron. 2010;66:386–402. doi: 10.1016/j.neuron.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Doe CQ, Chu-LaGraff Q, Wright DM, Scott MP. The prospero gene specifies cell fates in the Drosophila central nervous system. Cell. 1991;65:451–464. doi: 10.1016/0092-8674(91)90463-9. [DOI] [PubMed] [Google Scholar]
- Duchaine TF, Hemraj I, Furic L, Deitinghoff A, Kiebler MA, DesGroseillers L. Staufen2 isoforms localize to the somatodendritic domain of neurons and interact with different organelles. Journal of cell science. 2002;115:3285–3295. doi: 10.1242/jcs.115.16.3285. [DOI] [PubMed] [Google Scholar]
- Farkas LM, Haffner C, Giger T, Khaitovich P, Nowick K, Birchmeier C, Paabo S, Huttner WB. Insulinoma-associated 1 has a panneurogenic role and promotes the generation and expansion of basal progenitors in the developing mouse neocortex. Neuron. 2008;60:40–55. doi: 10.1016/j.neuron.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Fasano CA, Dimos JT, Ivanova NB, Lowry N, Lemischka IR, Temple S. shRNA knockdown of Bmi-1 reveals a critical role for p21-Rb pathway in NSC self-renewal during development. Cell Stem Cell. 2007;1:87–99. doi: 10.1016/j.stem.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Ferron SR, Pozo N, Laguna A, Aranda S, Porlan E, Moreno M, Fillat C, de la Luna S, Sanchez P, Arbones ML, et al. Regulated segregation of kinase Dyrk1A during asymmetric neural stem cell division is critical for EGFR-mediated biased signaling. Cell stem cell. 2010;7:367–379. doi: 10.1016/j.stem.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Furic L, Maher-Laporte M, DesGroseillers L. A genome-wide approach identifies distinct but overlapping subsets of cellular mRNAs associated with Staufen1- and Staufen2-containing ribonucleoprotein complexes. RNA (New York, NY. 2008;14:324–335. doi: 10.1261/rna.720308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetze B, Tuebing F, Xie Y, Dorostkar MM, Thomas S, Pehl U, Boehm S, Macchi P, Kiebler MA. The brain-specific double-stranded RNA-binding protein Staufen2 is required for dendritic spine morphogenesis. The Journal of cell biology. 2006;172:221–231. doi: 10.1083/jcb.200509035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton MO, Torban E, Jasmin SB, Theriault FM, German MS, Stifani S. Hes6 promotes cortical neurogenesis and inhibits Hes1 transcription repression activity by multiple mechanisms. Molecular and cellular biology. 2003;23:6922–6935. doi: 10.1128/MCB.23.19.6922-6935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata J, Nakagoshi H, Nabeshima Y, Matsuzaki F. Asymmetric segregation of the homeodomain protein Prospero during Drosophila development. Nature. 1995;377:627–630. doi: 10.1038/377627a0. [DOI] [PubMed] [Google Scholar]
- Hsiao YC, Tong ZJ, Westfall JE, Ault JG, Page-McCaw PS, Ferland RJ. Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking. Human molecular genetics. 2009;18:3926–3941. doi: 10.1093/hmg/ddp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner WB, Kosodo Y. Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Current opinion in cell biology. 2005;17:648–657. doi: 10.1016/j.ceb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY. Asymmetric cell division in the Drosophila nervous system. Nature reviews. 2001;2:772–779. doi: 10.1038/35097516. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Gage FH, Eisch AJ, Lagace DC. Making a neuron: Cdk5 in embryonic and adult neurogenesis. Trends in neurosciences. 2009;32:575–582. doi: 10.1016/j.tins.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi A, Ikawa T, Kasukawa T, Ueda HR, Kurimoto K, Saitou M, Matsuzaki F. Single-cell gene profiling defines differential progenitor subclasses in mammalian neurogenesis. Development (Cambridge, England) 2008;135:3113–3124. doi: 10.1242/dev.022616. [DOI] [PubMed] [Google Scholar]
- Kiebler MA, Hemraj I, Verkade P, Kohrmann M, Fortes P, Marion RM, Ortin J, Dotti CG. The mammalian staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: implications for its involvement in mRNA transport. J Neurosci. 1999;19:288–297. doi: 10.1523/JNEUROSCI.19-01-00288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN. Asymmetric segregation of Numb and Prospero during cell division. Nature. 1995;377:624–627. doi: 10.1038/377624a0. [DOI] [PubMed] [Google Scholar]
- Konno D, Shioi G, Shitamukai A, Mori A, Kiyonari H, Miyata T, Matsuzaki F. Neuroepithelial progenitors undergo LGN-dependent planar divisions to maintain self-renewability during mammalian neurogenesis. Nature cell biology. 2008;10:93–101. doi: 10.1038/ncb1673. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Roper K, Haubensak W, Marzesco AM, Corbeil D, Huttner WB. Asymmetric distribution of the apical plasma membrane during neurogenic divisions of mammalian neuroepithelial cells. The EMBO journal. 2004;23:2314–2324. doi: 10.1038/sj.emboj.7600223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19:2439–2450. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annual review of neuroscience. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga SB, Margossian SP, Harris MH, Campagna DR, Han AP, Blevins S, Mudbhary R, Barker JE, Walsh CA, Fleming MD. Cdk5rap2 regulates centrosome function and chromosome segregation in neuronal progenitors. Development (Cambridge, England) 2010;137:1907–1917. doi: 10.1242/dev.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchi P, Brownawell AM, Grunewald B, DesGroseillers L, Macara IG, Kiebler MA. The brain-specific double-stranded RNA-binding protein Staufen2: nucleolar accumulation and isoform-specific exportin-5-dependent export. The Journal of biological chemistry. 2004;279:31440–31444. doi: 10.1074/jbc.C400226200. [DOI] [PubMed] [Google Scholar]
- Maher-Laporte M, DesGroseillers L. Genome wide identification of Staufen2-bound mRNAs in embryonic rat brains. BMB reports. 2010;43:344–348. doi: 10.5483/bmbrep.2010.43.5.344. [DOI] [PubMed] [Google Scholar]
- Miki T, Takano K, Yoneda Y. The role of mammalian Staufen on mRNA traffic: a view from its nucleocytoplasmic shuttling function. Cell structure and function. 2005;30:51–56. doi: 10.1247/csf.30.51. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Kriegstein AR. Distinct behaviors of neural stem and progenitor cells underlie cortical neurogenesis. The Journal of comparative neurology. 2008;508:28–44. doi: 10.1002/cne.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RJ, Tobin JL, Beales PL. Modeling ciliopathies: Primary cilia in development and disease. Current topics in developmental biology. 2008;84:249–310. doi: 10.1016/S0070-2153(08)00605-4. [DOI] [PubMed] [Google Scholar]
- Raj A, Tyagi S. Detection of individual endogenous RNA transcripts in situ using multiple singly labeled probes. Methods in enzymology. 2010;472:365–386. doi: 10.1016/S0076-6879(10)72004-8. [DOI] [PubMed] [Google Scholar]
- Roegiers F, Jan YN. Asymmetric cell division. Current opinion in cell biology. 2004;16:195–205. doi: 10.1016/j.ceb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Schwamborn JC, Berezikov E, Knoblich JA. The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell. 2009;136:913–925. doi: 10.1016/j.cell.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nature neuroscience. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- Shen Q, Zhong W, Jan YN, Temple S. Asymmetric Numb distribution is critical for asymmetric cell division of mouse cerebral cortical stem cells and neuroblasts. Development (Cambridge, England) 2002;129:4843–4853. doi: 10.1242/dev.129.20.4843. [DOI] [PubMed] [Google Scholar]
- Srinivasan K, Roosa J, Olsen O, Lee SH, Bredt DS, McConnell SK. MALS-3 regulates polarity and early neurogenesis in the developing cerebral cortex. Development (Cambridge, England) 2008;135:1781–1790. doi: 10.1242/dev.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Johnston D, Brown NH, Gall JG, Jantsch M. A conserved double-stranded RNA-binding domain. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10979–10983. doi: 10.1073/pnas.89.22.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Goderie SK, Temple S. Asymmetric distribution of EGFR receptor during mitosis generates diverse CNS progenitor cells. Neuron. 2005;45:873–886. doi: 10.1016/j.neuron.2005.01.045. [DOI] [PubMed] [Google Scholar]
- Tenenbaum SA, Lager PJ, Carson CC, Keene JD. Ribonomics: identifying mRNA subsets in mRNP complexes using antibodies to RNA-binding proteins and genomic arrays. Methods (San Diego, Calif. 2002;26:191–198. doi: 10.1016/S1046-2023(02)00022-1. [DOI] [PubMed] [Google Scholar]
- Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. The Journal of clinical investigation. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenklusen D, Singer RH. Analyzing mRNA expression using single mRNA resolution fluorescent in situ hybridization. Methods in enzymology. 2010;470:641–659. doi: 10.1016/S0076-6879(10)70026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Feder JN, Jiang MM, Jan LY, Jan YN. Asymmetric localization of a mammalian numb homolog during mouse cortical neurogenesis. Neuron. 1996;17:43–53. doi: 10.1016/s0896-6273(00)80279-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.