Abstract
Background
Selected contact allergens are known to induce phenotypic and functional maturation of dendritic cells (DCs). Such changes occurring in DCs have been employed as assay readouts to predict skin-sensitizing potentials of small chemicals.
Objective
To respond to the urgent needs for reliable in vitro tests to identify contact allergens, we sought to develop a DC-based assay designed to detect early change(s) induced by sensitizers.
Methods
Signature gene expression profiles of skin sensitization were determined by GeneChip and quantitative RT-PCR analyses of RNA samples harvested from mouse skin and XS106 DC line after exposure to dinitrofluorobenzene (DNFB). Production of reactive oxygen species (ROS) was examined indirectly by measuring the level of oxidative stress – XS106 DCs were labeled with a fluorescent dye, CM-H2DCFDA, exposed to test chemicals, and then examined for fluorescence signals by flow cytometer.
Results
DNFB induced abundant mRNA expression of several redox regulatory genes in both mouse skin and XS106DCs. Expression of these genes was inducible by hydrogen peroxide and blocked by a ROS inhibitor, diphenyleneiodonium. Rapid and significant ROS production was induced by 25 of the 28 tested skin sensitizers, but only by 3 of the 21 tested skin irritants.
Conclusions
Our small-scale validation study demonstrates the practical utility of our DC-based ROS production assay to detect structurally diverse contact allergens with varying sensitizing potencies. It is tempting to speculate that ROS production in DCs may represent an early event during the sensitization phase.
1. INTRODUCTION
Allergic contact dermatitis represents T cell-mediated adaptive immune responses to skin sensitizers, in which epidermal Langerhans cells and/or dermal dendritic cells (DCs) are believed to play pathogenic roles during the sensitization phase [1,2]. In mouse models of contact hypersensitivity response, Langerhans cells were found to undergo morphological, phenotypic, and functional maturation during the sensitization phase [3–5]. Subsequent studies have unveiled a variety of changes inducible by skin sensitizers in DCs in culture, including activation of several signal transduction pathways, elevated surface expression of MHC class II molecule, co-stimulatory molecules, and adhesion molecules, and production of selected cytokines and chemokines [6–8]. More recent studies have further defined diverse sets of signature genes expressed by DCs after in vitro exposure to skin sensitizers [9–11]. However, initial and universal responses of DCs to sensitizers still remain relatively unknown, because most of the above changes became detectable 4–48 hours after the treatment, and because many of these studies analyzed the impacts of prototypic haptens with strong sensitizing potencies. Thus, the primary purpose of this study was to identify an early change occurring in DCs after exposure to structurally and functionally diverse contact allergens.
Virtually all toxicology tests have long been performed using animals, primarily rodents, to meet with regulatory requirements. The local lymph node assay represents a gold standard in vivo assay to assess skin sensitizing potentials [12–14]. Briefly, mice receive repeated applications of a test chemical on the ear skin, followed by intravenous injection of 3H-thymidine – a stimulation index is then calculated based on 3H-thymidine incorporation into the draining auricular lymph nodes. However, owing to rapid and dramatic changes in public views, regulatory requirements, scientific knowledge, and increasing costs associated with animal testing, concerted efforts are now being made toward development, validation, and use of alternative in vivo tests [15]. The peptide reactivity assay represents the first alternative test developed to assess skin sensitizing potentials [16]. Because most sensitizers are electrophilic and, thus, react with nucleophilic amino acids, this assay is designed to evaluate the ability of a test chemical to bind to synthetic peptides containing cysteine residues. Based on the observations that strong sensitizers induce DC maturation, various DC-based in vitro assays have been developed – they are designed to detect hapten-induced changes in signaling, gene expression, surface phenotype, and cytokine/chemokines production [17]. Unfortunately, none of the currently available DC-based assays has been fully validated for sensitivity and specificity or has actually replaced the local lymph node assay. We reasoned that identification of the initial event(s) taking place in DCs upon exposure to contact allergens might allow us to design and construct a novel assay platform. Thus, our second purpose was to develop a new conceptual framework for the next generation of DC-based assays for skin sensitizers.
2. MATERIALS AND METHODS
2.1. Animal experiments
C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). For global gene expression profiling, mice received topical application of 0.5% DNFB or 1% benzalkonium chloride (BAC) on the right ear and vehicle alone on the left ear, as described elsewhere [18]. All animal experiments were approved by the Institutional Animal Care and Use Committee at University of Toledo College of Medicine and conducted according to the guidelines of the National Institutes of Health (NIH).
2.2. Cell preparations
The XS106 DC line was established from the epidermis of newborn A/J mice and its phenotypic and functional features are described elsewhere [19]. XS106 DCs were maintained in complete RPMI 1640 supplemented with 0.5 ng/mL of GM-CSF and 5% NS47 fibroblast culture supernatant. The Pam 212 KC line established from BALB/c mice [20] was maintained in complete RPMI in the absence of added growth factors. Bone marrow-derived murine DC cultures and monocyte-derived human DC cultures were generated by using standard protocols [21,22]. The resulting DC cultures were >95% pure assessed by surface expression of I-Aβ and CD11c for mouse DCs or HLR-DA and CD11c for human DCs.
2.3. Gene expression analyses
Total RNAs extracted from mouse skin samples or cell cultures using the TRIzol (Invitrogen, San Diego, CA) and RNeasy Plus Mini Kit (Qiagen, Valencia, CA) were examined for gene expression profiles using the GeneChip® Mouse Genome 430 2.0 Array (Affymetrix, Santa Clara, CA). The data were normalized with Microarray Suite 5.0 (Affymetrix) and filtered with GeneMaths XT (Applied Maths, Austin TX). HO-1, Srxn1, Txnrd1, and Gclm mRNA expression was examined by quantitative RT-PCR by using the corresponding primer sets purchased from SA Biosciences (Frederick, MD), LightCycler (Roche Applied Science, Indianapolis, IN), and QuantiTect SYBR Green PCR Master Mix (Qiagen). The expression level was normalized based on the GAPDH mRNA.
2.4. ROS production assay
Test chemicals were purchased from Sigma-Aldrich (St. Louis, MO), MP Biomedicals (Solon, OH), or Alfa Aesar (Ward Hill, MA) and dissolved in 0.1% DMSO in HBSS. XS106 DCs or Pam 212 KCs (1.5 × 106 cells/mL) were labeled with 2 µM CM-H2DCFDA in HBSS for 15 minutes at 37°C, washed extensively, and then exposed to test compounds in the above buffer for 5–60 minutes. In some experiments, CM-H2DCFDA-loaded cells were incubated with test compounds in HBSS for 30 minutes, washed extensively, and then cultured for extended periods in complete RPMI medium in the continuous presence of the test compounds. The samples were analyzed by FACSCalibur (BD Biosciences, San Jose, CA) for propidium iodide (PI) uptake and for MFI within the PI-negative populations. Regression curves were generated from the dose-response dataset for cell viability and ROS production and using the Regression Wizard (Sigmoidal dose-response) function in the Sigma Plot program to calculate 5% killing doses and ROSmax values.
2.5. Statistical analyses
Comparisons between two groups were performed with a two-tailed Student t-test, and more than two-groups were assessed using an analysis of variance and Dunnett’s test. All experiments were repeated at least three times to assess reproducibility.
3. RESULTS
3.1. Identification of DNFB-inducible gene expression profiles
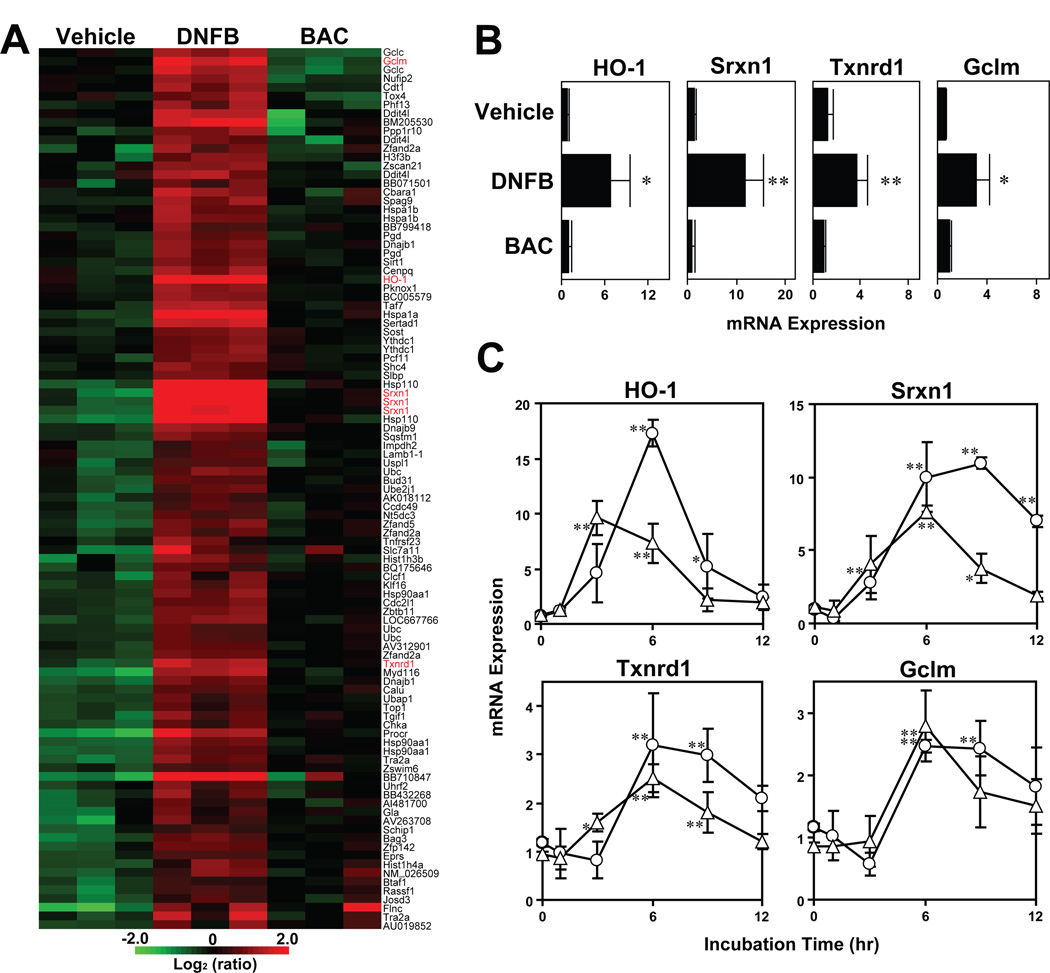
To identify gene expression profiles observed in the skin during the sensitization phase, we topically applied a prototypic sensitizer DNFB, a skin irritant BAC, or vehicle alone to C57BL/6 mice and examined the gene expression profiles 6 hours later by GeneChip® Mouse 430 2.0 Array (Fig. 1A). It should be stated that the tested concentration of BAC (1%) was selected based on our preliminary observation that it caused only modest skin inflammation in the level comparable to that induced by 0.5% DNFB in naïve mice. From the clustering analysis, we identified 83 genes that were expressed at significantly (>2-fold, P < 0.05, n = 3) higher levels in DNFB-painted skin, but not in BAC-painted skin, compared to vehicle-treated control skin. The whole-gene-array datasets have been deposited in Gene Expression Omnibus (GEO) with accession number GSE20726. The DNFB-inducible genes included a unique set of genes encoding redox regulatory enzymes, such as heme oxygenase 1 (HO-1), sulfiredoxin 1 (Srxn1), thioredoxin reductase 1 (Txnrd1), and glutamate-cysteine ligase modifier subunit (Gclm). To confirm these observations, we examined mRNA expression of those redox regulatory genes by quantitative RT-PCR (Fig. 1B). Again, all the four genes were expressed at significantly (3 to 10-fold, P < 0.05, n = 3) elevated levels in DNFB-painted skin, but not in BAC-painted skin. To determine whether DCs might respond to DNFB in a similar manner, we exposed our skin-derived mouse DC line, XS106, to DNFB or vehicle alone. DNFB elevated HO-1, Srxn1, Txnrd1, and Gclm mRNA expression in a dose- and time-dependent manner, with peak responses observed after 6 hours (Fig. 1C). Because HO-1, Srxn1, Txnrd1, and Gclm are all involved in the metabolism of ROS and/or the production of anti-oxidants, we interpreted our gene expression data to suggest that skin sensitizers may trigger rapid ROS generation of DCs and that DCs may express those redox regulatory genes in response to the excessive oxidative stress. If both hypotheses are correct, ROS production would then represent an early signature event taking place in DCs after exposure to skin sensitizers.
Fig. 1. Gene expression profiles in DNFB-painted skin and in DNFB-treated DCs.
(A) Ear skin samples harvested 6 hours after topical application of DNFB, BAC, or vehicle alone were analyzed for global gene expression profiles. The heat map was created from three independent skin samples/group to show the genes that were significantly (>2-fold, P < 0.05, n = 3) upregulated in the skin painted with DNFB (but not BAC), as compared to the steady-state levels observed in vehicle-painted skin. Redox regulatory genes are indicated in red. (B) The same skin samples were analyzed for mRNA expression of the indicated redox regulatory genes by quantitative RT-PCR. Data shown are relative expression levels (means ± SD, 3 mice/group) compared to the baseline observed in vehicle-painted skin. * P < 0.05; ** P < 0.01. (C) XS106 DCs were incubated with DNFB at 4 µM (triangles) or 8 µM (circles) for the indicated periods and examined for mRNA expression of the indicated genes by quantitative RT-PCR. Data shown are relative expression levels (means ± SD, triplicate cultures/group) compared to the baseline observed in control samples treated with vehicle alone. * P < 0.05; ** P < 0.01.
3.2. Rapid ROS generation in DCs after in vitro exposure to skin sensitizers
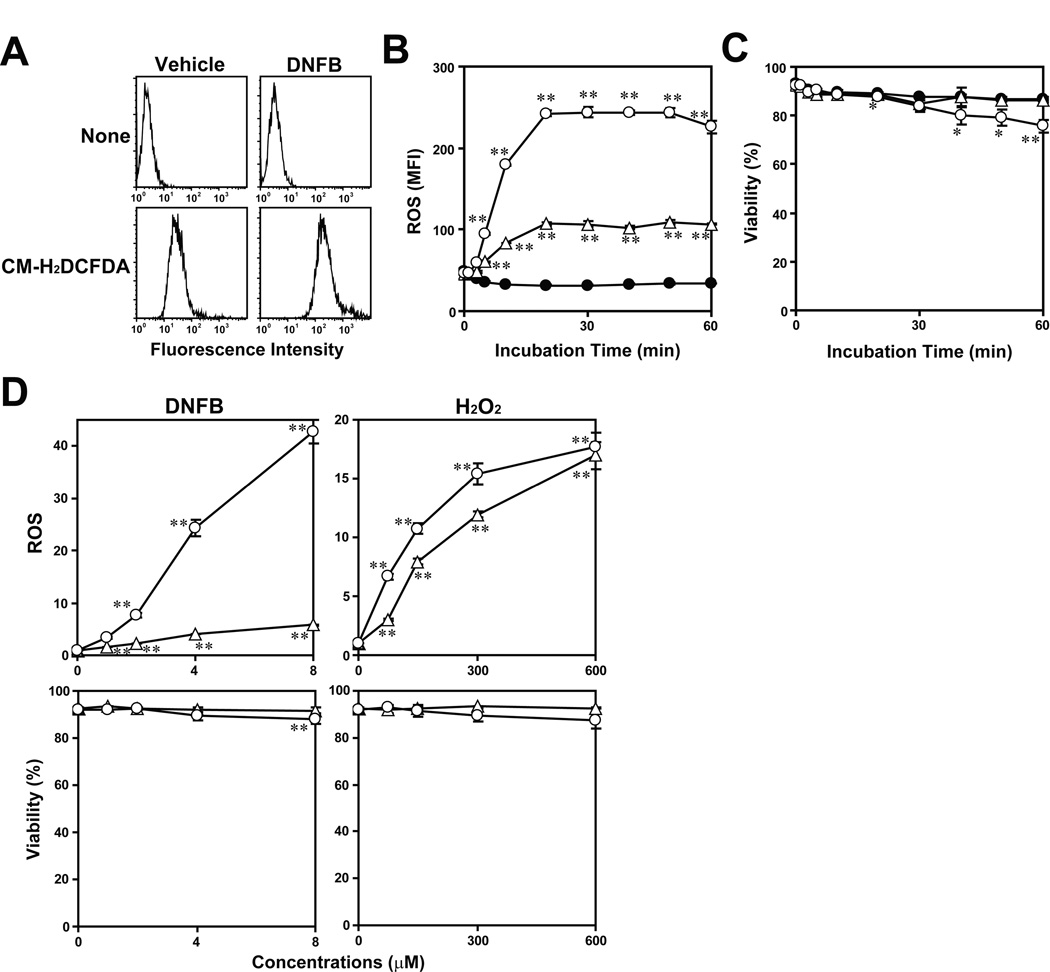
To test the first part of the above hypothesis, we examined ROS production in DCs indirectly by measuring the level of oxidative stress with a ROS sensitive dye CM-H2DCFDA, which is converted into a green fluorescent form by peroxides, peroxynitrite, or hydroxyl radical. Briefly, XS106 DCs were pre-loaded with CM-H2DCFDA for 15 minutes, washed extensively, and then incubated with 8 µM DNFB or vehicle alone. Brief (20 minutes) exposure to DNFB induced a marked increase in the fluorescence intensity (Fig. 2A). Using the mean fluorescence intensity (MFI) as a parameter, we next quantified the level of oxidative stress – treatment with 4 µM DNFB elevated MFI levels in a time-dependent manner, with significant (P < 0.01) elevation already detectable after 5 minutes (Fig. 2B, open triangles). It should be stated that DNFB at this concentration caused no significant cell death at any tested time points as measured by PI uptake (Fig. 2C, open triangles). DNFB at 8 µM induced more robust oxidative stress, although modest cell death was observed at later time points (open circles).
Fig. 2. DNFB treatment triggers rapid ROS production in XS106 DCs.
(A) XS106 DCs pre-loaded with HBSS alone or CM-H2DCFDA were incubated for 20minutes with 8 µM DNFB or vehicle alone. The samples were then examined for fluorescence profiles within the PI-negative populations. (B,C) CM-H2DCFDA-loaded XS106 DCs were incubated for the indicated periods with vehicle alone (closed circles), 4 µM DNFB (triangles), or 8 µM DNFB (circles) and then examined for ROS production by measuring the level of oxidative stress (B) and for cell viability by PI uptake (C). (D) XS106 DCs (circles) or Pam 212 KCs (triangles) were pre-loaded with CM-H2DCFDA and then incubated for 15 minutes with DNFB and H2O2 at the indicated concentrations. The samples were examined for ROS production and cell viability. Data shown are the means ± SD from triplicate cultures. * P < 0.05; ** P < 0.01.
To test cell type-specificity, we next exposed the XS106 DC line and the Pam 212 keratinocyte (KC) line in parallel to DNFB at graded concentrations for 15 minutes. Once again, DNFB triggered ROS generation in XS106 DCs in a dose-dependent manner (Fig. 2D, open circles). DNFB also induced ROS production in Pam 212 KCs, albeit in much smaller magnitudes. Interestingly, the two cell types were comparable to each other in the levels of ROS production after exposure to hydrogen peroxide (Fig. 2D, right panels). DNFB-induced ROS production in XS106 DCs was also confirmed with a second ROS-sensitive dye, dihydrorhodamine-123 (Supplementary Fig. S1). Furthermore, DNFB treatment also triggered ROS production in primary murine DC cultures generated from bone marrow cells (Supplementary Fig. S2), as well as in primary human DC cultures generated from peripheral blood monocytes (Supplementary Fig. S3). Importantly, DNFB at the tested concentrations did not significantly affect the viability of either murine or human DC populations. Based on these observations, we have concluded that rapid ROS generation, indeed, represents an early response of DCs to DNFB treatment.
Causative relationship between DNFB-induced ROS generation and redox regulatory gene expression
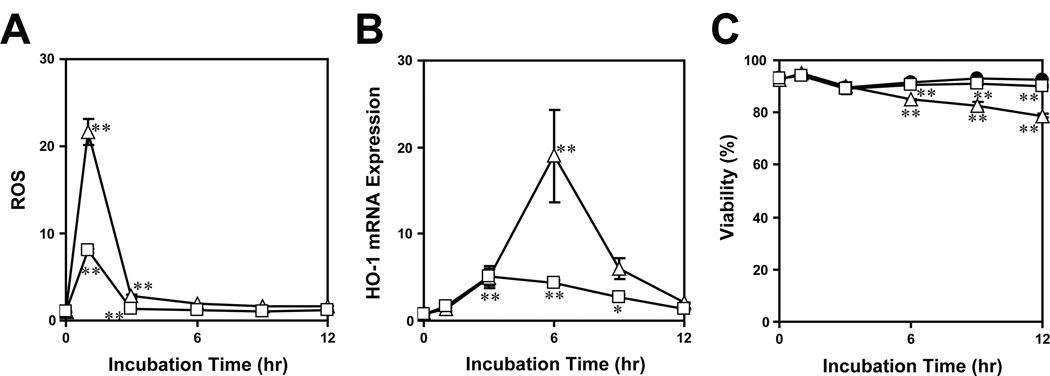
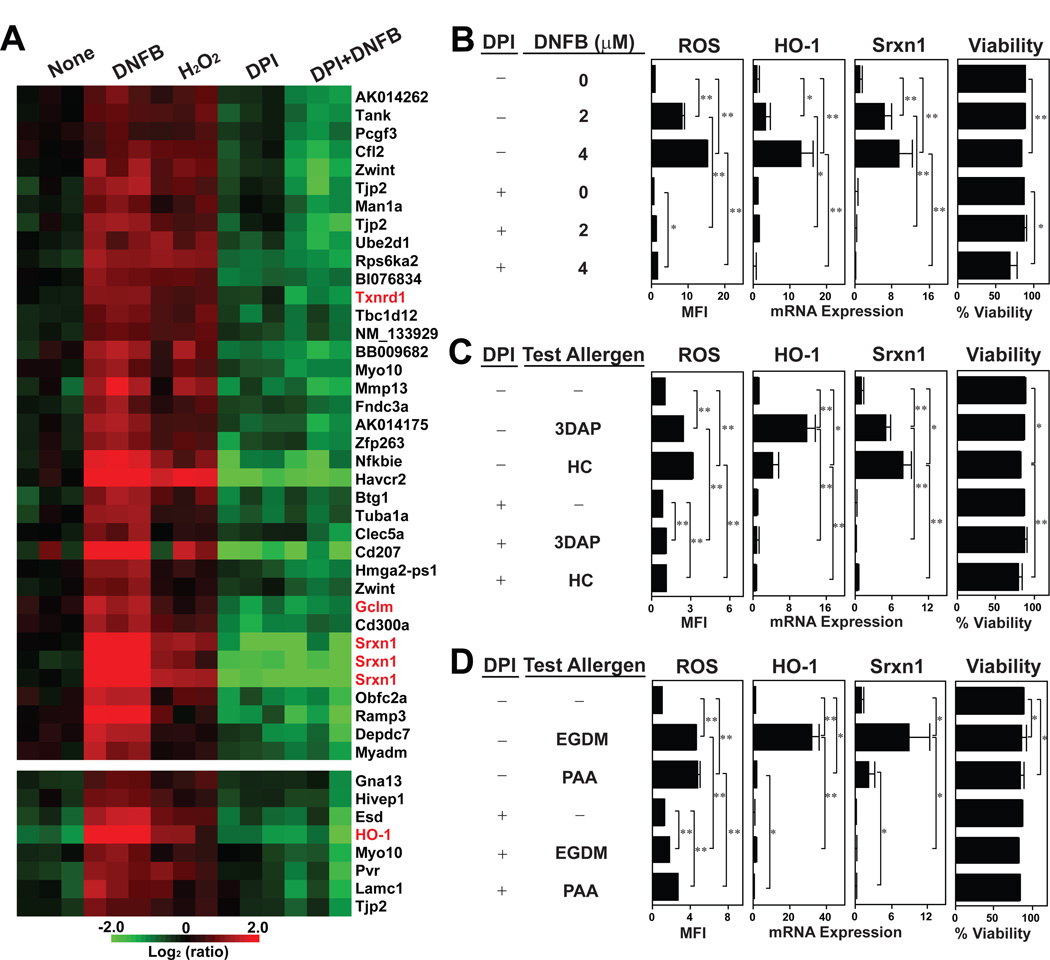
A key question concerned the functional outcome. In this regard, the time-course for DNFB-induced HO-1 mRNA expression peaking at 6 hours lagged behind that for DNFB-triggered ROS production (Fig. 3). We interpreted this to suggest a causative relationship between the two events taking place in DNFB-treated DCs. Thus, we next sought to determine whether some of the redox regulatory genes are also inducible by exposure to hydrogen peroxide, and whether such DNFB-induced expression of those genes can be prevented by blocking ROS generation. To answer to both questions, we examined global gene expression profiles in XS106 DCs after brief exposure to: a) vehicle alone, b) DNFB, c) hydrogen peroxide, d) a ROS inhibitor, diphenyleneiodonium (DPI), or e) DNFB plus DPI (Fig. 4A, GEO accession number: GSE20727). Hydrogen peroxide mimicked DNFB by inducing the expression of HO-1, Srxn1, Txnrd1, and Gclm mRNAs. Conversely, DNFB-induced expression of these genes was prevented by DPI almost completely.
Fig. 3. Time-kinetics for DNFB-induced ROS production and HO-1 mRNA expression.
XS106 DCs were cultured for the indicated periods in the presence of vehicle alone (closed circles), 2 µM DNFB (open squares), or 4 µM DNFB (open triangles). The samples were then cultured in examined for squares) of ROS production (A), HO-1 mRNA expression (B), and cell viability (C. Data shown are relative expression levels (means ± SD, triplicate cultures/group) compared to the baseline observed in control samples cultured with vehicle alone. * P < 0.05; ** P < 0.01.
Fig. 4. Causative relationship between DNFB-induced ROS production and redox regulatory gene expression.
(A) XS106 DCs were cultured for 6 hours with 6 µM DNFB, 300 µM H2O2 or vehicle alone. To some samples, 25 µM DPI was added from 30 minutes before the above culturing period. All samples were examined for global gene expression profiles. The heat map was created from three independent samples/group to show the genes that were significantly (>2-fold, P < 0.05) upregulated by DNFB as well as by H2O2. Redox regulatory genes are indicated in red. (B–D) XS106 DCs were cultured with DNFB at the indicated concentrations (B) or with the indicated skin sensitizers (C and D) and then examined for ROS production (after 15 minutes) and for HO-1 and Srxn1 mRNA expression and cell viability (after 6 hours). To some samples, 25 µM DPI was added from 30 minutes before the culturing periods. Data shown are the means ± SD from triplicate cultures. * P < 0.05; ** P < 0.01.
To confirm these observations, we measured ROS production with CM-H2DCFDA and redox regulatory gene expression by quantitative RT-PCR in the next set of experiments. Again, DNFB triggered robust ROS production and elevated HO-1 and Srxn1 mRNA in XS106 DCs (Fig. 4B). Importantly, DPI inhibited these DNFB-inducible changes almost completely without affecting cell viability. We employed the same assay systems to examine the impacts of four structurally diverse skin sensitizers: 3-dimethylaminopropylamine (3DAP), hydroxycitronellal (HC), ethyleneglycol dimethacrylate (EGDM), and phenylacetaldehyde (PAA). These allergens also induced significant ROS production and HO-1 and Srxn1 mRNA expression, and DIP efficiently prevented both outcomes (Fig. 4C and 4D). These data support our hypothesis that skin sensitizers induce the expression of redox regulatory genes by triggering rapid ROS production.
Detection of skin sensitizers with a newly developed DC-based assay designed to measure oxidative stress
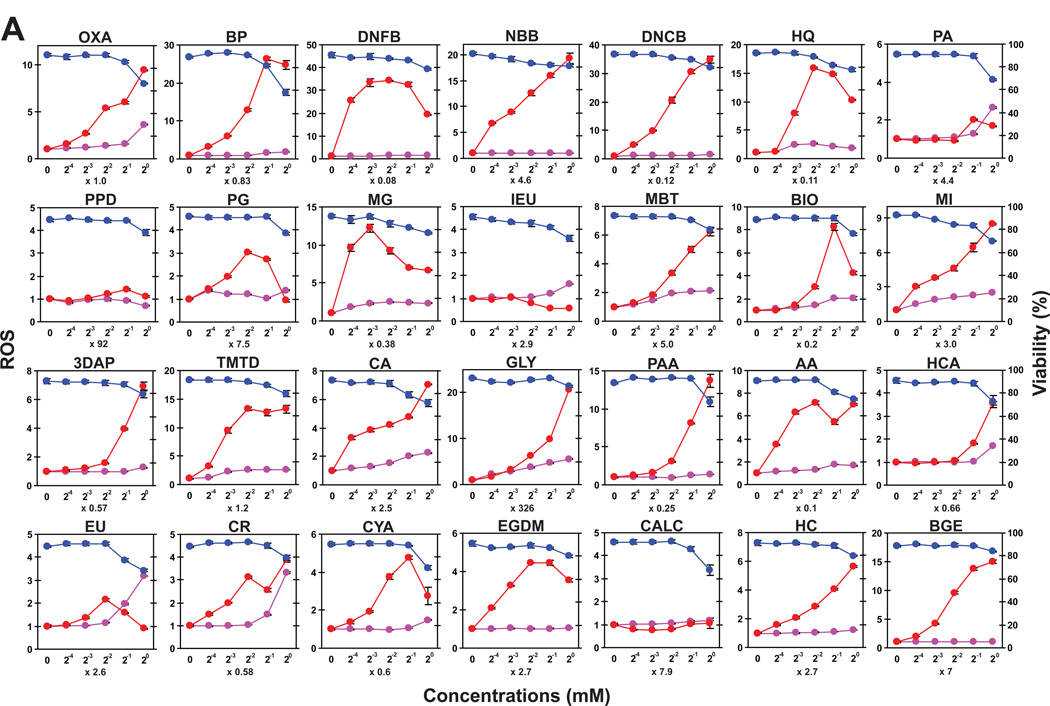
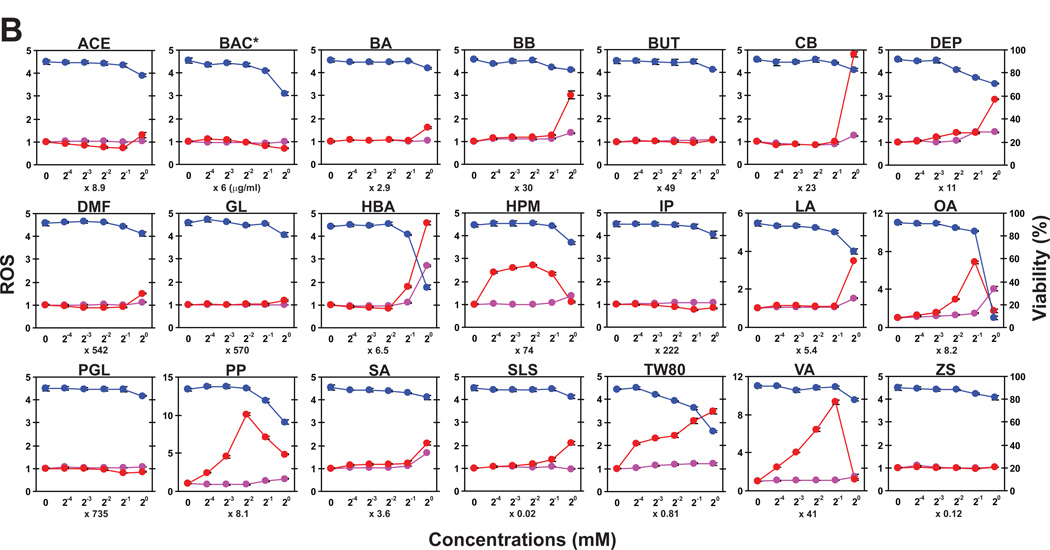
Our observations with DNFB and four additional sensitizers implied that one might be able to detect skin-sensitizing potentials of test chemicals by measuring their abilities to trigger oxidative stress in DCs. To test this, we selected 28 allergens, based on their skin sensitizing potentials measured by the murine local lymph node assay [12,13,23,24,25,26,27], as well as their structural diversity, availability from commercial sources, and solubility in our standard buffer. We also included 21 skin irritants for comparison. All the test compounds are listed in Supplementary Table S1 with abbreviations, functional properties, and references. Each of these 49 compounds was added at five different concentrations in triplicates to XS106 DCs pre-loaded with CM-H2DCFDA. After 15 minutes, we measured MFI (Fig. 5, red lines) and cell viability (blue lines). Because some test compounds may exhibit auto-fluorescence signals, unloaded XS106 DCs were incubated with all test compounds in parallel to serve as additional controls (purple lines). Most of the tested skin sensitizers induced significant ROS production at sub-toxic concentrations (Fig. 5A). By contrast, only a small number of the tested irritants, such as propyl paraben (PP), vanillin (VA), and octanoic acid (OA), induced significant ROS production (Fig. 5B). BAC, which was employed as a prototypic irritant in our GeneChip analyses, failed to induce significant ROS production at any tested concentration. As expected, many compounds showed significant cytotoxicity at high concentrations. Although modest auto-fluorescence signals were detected with some chemicals, they did not affect the measurement of ROS production.
Fig. 5. Small-scale validation study of DC-based ROS production assay.
XS106 DCs pre-loaded with CM-H2DCFDA (red and blue lines) or with HBSS alone (purple lines) were incubated for 15 minutes with each of the 28 skin sensitizers (A) or 21 skin irritants (B) at five different concentrations. The samples were then examined for ROS production (red lines), auto-fluorescence signals (purple lines), and cell viability (blue lines). Abbreviations and functional properties of all tested chemicals are described in Supplementary Table S1.
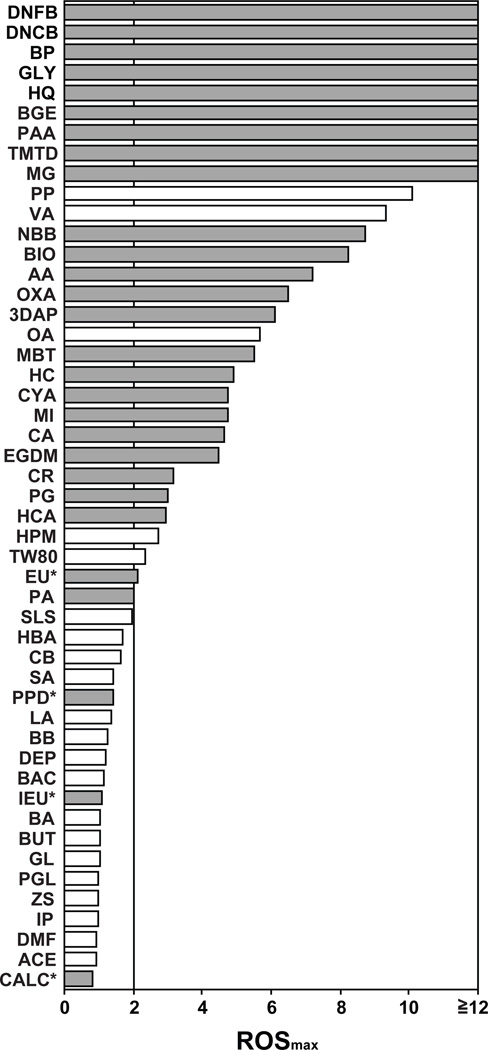
To analyze the above dataset in a more systematic manner, we calculated the 5% killing dose, which was defined as the concentration of each compound causing 5% reduction in cell viability. We then calculated the ROSmax value, which was defined as the maximal magnitude of ROS generation induced by each compound at the dose lower than its 5% killing doses, compared to the baseline MFI level. The actual 5% killing doses and ROSmax values for all tested chemicals are shown in Supplementary Table S1. Finally, we scored the compounds showing ROSmax values higher than 2.0 as “positive” (Fig 6). Strikingly, 25 of the 28 tested skin sensitizers were scored as positive, indicating relatively high sensitivity (89.3%). The sensitizers that failed to induce positive ROS generation were 1,4-phenylenediamine (PPD), isoeugenol (ISO), and cinnamic alcohol (CALC). Interestingly, they have all been categorized as pre/pro-haptens [28]. Conversely, only 5 of the 21 tested irritants showed positive responses, indicating relatively high specificity (76.2%) as well. Taken together, our data demonstrate that ROS production in DCs represents an early signature event inducible by structurally diverse sensitizers with varying potencies.
Fig. 6. Sensitivity and specificity of DC-based ROS production assay.
The ROSmax values of all tested sensitizers (closed bars) and irritants (open bars) are shown. Raw data for 5% killing doses and ROSmax values can be found in Supplementary Table S1. The asterisks indicate the compounds known as pre/pro-haptens.
DISCUSSION
Our GeneChip and quantitative RT-PCR analyses have unveiled that several redox regulatory genes (e.g., HO-1, Srxn1, Txnrd1, and Gclm) are expressed in mouse skin after DNFB application, as well as in DCs after in vitro exposure to DNFB. Interestingly, all these genes are known to be regulated by the Keap1/Nrf2 pathway [29–31]. Nrf2 is the key transcription factor binding to the antioxidant response element (ARE) and, thereby, initiating the expression of a broad spectrum of ARE-regulated genes encoding enzymes and proteins involved in the metabolism of elecrophiles and oxidants. Keap1 is a cytosolic repressor protein that retains Nrf2 in the cytoplasm and promotes its proteosomal degradation. Thus, disruption of the association of Nrf2 with Keap1 is required for nuclear translocation of Nrf2 and for the subsequent activation of ARE-regulated genes [31,32]. Our observations are in complete agreement with the recent in vitro observations that contact sensitizers induce ARE-dependent gene transactivation. By using a human breast cancer cell line engineered to express the luciferase reporter gene under the control of tandem repeats of the ARE sequence [33], Natsch and Emter screened 70 chemicals with varying skin sensitizing potencies – strikingly, 57 chemicals (81.4%) were found to induce significant (≥ 1.5-fold) induction of luciferase activities above the baseline level [34]. Ade et al. demonstrated that all four tested sensitizers induce mRNA expression of two ARE-responsive genes, HO-1 and NAD(P)H quinine oxidoreductase 1 [35].
With regard to mechanisms for the activation of the ARE/Nrf2 pathway, one may suggest that electrophilicity of skin sensitizers is responsible, because covalent modification of highly reactive cysteine residues of Keap1 protein can lead to its dissociation from Nrf2 [31,36]. On the other hand, oxidative stress can also directly modulate the stability of Keap1 [37]. As the most abundant small intracellular bio-thiol, glutathione prevents oxidation of cysteine residues of Keap1. Oxidation of glutathione, thus, potentiates Keap1 modification, leading to the activation of the Keap1/Nrf2 pathway [32]. Our data support the latter mechanism. Not only did DPI prevent ROS production in DNFB-treated DCs, it also inhibited DNFB-induced expression of HO-1, Srxn1, Txnrd1, and Gclm mRNAs. Moreover, these genes were inducible in DCs by exposure to hydrogen peroxide. Thus, we propose that contact allergens can trigger the expression of ARE-regulated genes by a ROS-mediated mechanism. It is tempting to speculate further that rapid ROS production in DCs may represent an initial event ruling the sensitization phase of contact hypersensitivity response. In fact, Aiba and his colleagues have demonstrated a causative relationship between sensitizer-induced oxidative stress in DCs and their subsequent activation/maturation [38,39]. Phenotypic maturation of DCs has been induced by in vitro exposure to hydrogen peroxide [40]. Furthermore, transgenic mice over-producing extracellular superoxide dismutase showed impaired Langerhans cell migration from sensitizer-painted skin and severely diminished contact hypersensitivity responses [41].
In an attempt to translate our findings, we have developed a DC-based ROS assay using our skin-derived DC line, XS106. In a small-scale validation study, our assay showed relatively high sensitivity (with a false negative rate of 3/28) and high specificity (with a false positive rate of 5/21). Not only is our assay robust in the magnitude of responses, it is also simple and fast – a fully trained investigator can analyze up to 300 samples/day single-handedly in the current protocol. Our assay is also compatible to high-throughput screening with minor modifications. We believe that the ROS production assay can be combined with other in vivo tests, such as the peptide reactivity assay and DC maturation assays, to form a new platform for predicting skin sensitizing potentials of industrial and environmental chemicals.
It is important to point out major limitations of the present study. First, we assessed ROS production only indirectly by measuring the level of oxidative stress with ROS-sensitive fluorescent dyes, because our primary objective was to develop a simple in vitro assay. Thus, molecular identifies of the excessive oxidative stress detected in hapten-treated DCs and underlying mechanisms for hapten-induced ROS production remain to be determined. Second, our assay failed to detect pre/pro-haptens, i.e., PPD, ISO, and CALC. In this regard, pro-haptens have been successfully converted into active forms by in vitro incubation with cytochrome p450 enzymes [42] or with horseradish peroxidase and hydrogen peroxide [28]. Such pre-treatments may improve the sensitivity of our assay. Likewise, some of the tested skin irritants, e.g., PP, VA, and OA, caused significant ROS production. It is conceivable that some of these irritants showing false positive signals may not be recognized by effector T cells expressing the relevant T cell receptors. This limitation must be overcome by combining our assay with other in vitro methods designed to assess different features required for a given chemical to serve as a contact allergen. Our assay was not designed to identify photo-sensitizing compounds. Since ultraviolet light exposure causes ROS production, it is tempting to speculate that combination of such compounds and ultraviolet light irradiation may produce robust ROS production in a synergistic manner. Finally, although our screening capability has been markedly improved by the use of the XS106 DC line, its mouse origin may raise a concern as an assay to detect human allergens. In this regard, DNFB induced significant ROS production in both mouse and human primary DC cultures as well, and the XS106 DC line was, indeed, capable of respond to many of the tested chemicals known to cause allergic contact dermatitis in human. Despite these and other limitations, we believe that the present study forms both conceptual and technical frameworks for the use of our DC-based ROS assays for rapid detection of skin sensitizing chemicals.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Hironori Matsushima for his scientific inputs and Ms. Traci McDaniel for her secretarial assistance. This project was funded by grants (to A.T.) from the NIH, COLIPA (European Cosmetics Association), and Kao Corporation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
M.M. is an employee of Kao Corporation, which has supported the present study.
REFERENCES
- 1.Kaplan DH, Kissenpfennig A, Clausen BE. Insights into Langerhans cell function from Langerhans cell ablation models. Eur J Immunol. 2008;38:2369–2376. doi: 10.1002/eji.200838397. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan DH. In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol. 2010;31:446–451. doi: 10.1016/j.it.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiba S, Katz SI. Phenotypic and functional characteristics of in vivo-activated Langerhans cells. J Immunol. 1990;145:2791–2796. [PubMed] [Google Scholar]
- 4.Cumberbatch M, Gould SJ, Peters SW, Kimber I. MHC class II expression by Langerhans' cells and lymph node dendritic cells: possible evidence for maturation of Langerhans' cells following contact sensitization. Immunology. 1991;74:414–419. [PMC free article] [PubMed] [Google Scholar]
- 5.Enk AH, Katz SI. Early molecular events in the induction-phase of contact sensitivity. Proc Natl Acad Sci USA. 1992;89:1398–1402. doi: 10.1073/pnas.89.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuhn U, Brand P, Willemsen J, Jonuleit H, Enk AH, Brandwijk-Petershans R, et al. Induction of tyrosine phosphorylation in human MHC class II-positive antigen-presenting cells by stimulation with contact sensitizers. J Immunol. 1998;160:667–673. [PubMed] [Google Scholar]
- 7.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- 8.Aiba S, Manome H, Nakagawa S, Mollah ZU, Mizuashi M, Ohtani T, et al. p38 Mitogen-activated protein kinase and extracellular signal-regulated kinases play distinct roles in the activation of dendritic cells by two representative haptens, NiCl2 and 2,4-dinitrochlorobenzene. J Invest Dermatol. 2003;120:390–399. doi: 10.1046/j.1523-1747.2003.12065.x. [DOI] [PubMed] [Google Scholar]
- 9.Ryan CA, Gildea LA, Hulette BC, Dearman RJ, Kimber I, Gerberick GF. Gene expression changes in peripheral blood-derived dendritic cells following exposure to a contact allergen. Toxicol Lett. 2004;150:301–316. doi: 10.1016/j.toxlet.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Gildea LA, Ryan CA, Foertsch LM, Kennedy JM, Dearman RJ, Kimber I, et al. Identification of gene expression changes induced by chemical allergens in dendritic cells: opportunities for skin sensitization testing. J Invest Dermatol. 2006;126:1813–1822. doi: 10.1038/sj.jid.5700319. [DOI] [PubMed] [Google Scholar]
- 11.Schoeters E, Verheyen GR, Nelissen I, Van Rompay AR, Hooyberghs J, Van Den Heuvel RL, et al. Microarray analyses in dendritic cells reveal potential biomarkers for chemical-induced skin sensitization. Mol Immunol. 2007;44:3222–3233. doi: 10.1016/j.molimm.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Gerberick GF, Ryan CA, Dearman RJ, Kimber I. Local lymph node assay (LLNA) for detection of sensitization capacity of chemicals. Methods. 2007;41:54–60. doi: 10.1016/j.ymeth.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Gerberick GF, Ryan CA, Kern PS, Schlatter H, Dearman RJ, Kimber I, et al. Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis. 2005;16:157–202. [PubMed] [Google Scholar]
- 14.Basketter DA, Evans P, Fielder RJ, Gerberick GF, Dearman RJ, Kimber I. Local lymph node assay - validation, conduct and use in practice. Food Chem Toxicol. 2002;40:593–598. doi: 10.1016/s0278-6915(01)00130-2. [DOI] [PubMed] [Google Scholar]
- 15.Hartung T. Toxicology for the twenty-first century. Nature. 2009;460:208–212. doi: 10.1038/460208a. [DOI] [PubMed] [Google Scholar]
- 16.Gerberick GF, Vassallo JD, Bailey RE, Chaney JG, Morrall SW, Lepoittevin JP. Development of a peptide reactivity assay for screening contact allergens. Toxicol Sci. 2004;81:332–343. doi: 10.1093/toxsci/kfh213. [DOI] [PubMed] [Google Scholar]
- 17.Ryan CA, Kimber I, Basketter DA, Pallardy M, Gildea LA, Gerberick GF. Dendritic cells and skin sensitization: biological roles and uses in hazard identification. Toxicol Appl Pharmacol. 2007;221:384–394. doi: 10.1016/j.taap.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Nishibu A, Ward BR, Jester JV, Ploegh HL, Boes M, Takashima A. Behavioral responses of epidermal Langerhans cells in situ to local pathological stimuli. J Invest Dermatol. 2006;126:787–796. doi: 10.1038/sj.jid.5700107. [DOI] [PubMed] [Google Scholar]
- 19.Matsue H, Matsue K, Walters M, Okumura K, Yagita H, Takashima A. Induction of antigen-specific immunosuppression by CD95L cDNA-transfected "killer" dendritic cells. Nat Med. 1999;5:930–937. doi: 10.1038/11375. [DOI] [PubMed] [Google Scholar]
- 20.Yuspa SH, Hawley-Nelson P, Koehler B, Stanley JR. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980;40:4694–4703. [PubMed] [Google Scholar]
- 21.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basketter DA, Dearman RJ, Hilton J, Kimber I. Dinitrohalobenzenes: evaluation of relative skin sensitization potential using the local lymph node assay. Contact Dermatitis. 1997;36:97–100. doi: 10.1111/j.1600-0536.1997.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 24.Basketter DA, Gerberick GF, Kimber I. Strategies for identifying false positive responses in predictive skin sensitization tests. Food Chem Toxicol. 1998;36:327–333. doi: 10.1016/s0278-6915(97)00158-0. [DOI] [PubMed] [Google Scholar]
- 25.Basketter DA, Gerberick GF, Kimber I, Loveless SE. The local lymph node assay: a viable alternative to currently accepted skin sensitization tests. Food Chem Toxicol. 1996;34:985–997. doi: 10.1016/s0278-6915(96)00059-2. [DOI] [PubMed] [Google Scholar]
- 26.Basketter DA, Scholes EW, Chamberlain M, Barratt MD. An alternative strategy to the use of guinea pigs for the identification of skin sensitization hazard. Food Chem Toxicol. 1995;33:1051–1056. doi: 10.1016/0278-6915(95)00073-9. [DOI] [PubMed] [Google Scholar]
- 27.Gerberick GF, Vassallo JD, Foertsch LM, Price BB, Chaney JG, Lepoittevin JP. Quantification of chemical peptide reactivity for screening contact allergens: a classification tree model approach. Toxicol Sci. 2007;97:417–427. doi: 10.1093/toxsci/kfm064. [DOI] [PubMed] [Google Scholar]
- 28.Gerberick GF, Troutman JA, Foertsch LM, Vassallo JD, Quijano M, Dobson RL, et al. Investigation of peptide reactivity of pro-hapten skin sensitizers using a peroxidase-peroxide oxidation system. Toxicol Sci. 2009;112:164–174. doi: 10.1093/toxsci/kfp192. [DOI] [PubMed] [Google Scholar]
- 29.Soriano FX, Baxter P, Murray LM, Sporn MB, Gillingwater TH, Hardingham GE. Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin. Mol Cells. 2009;27:279–282. doi: 10.1007/s10059-009-0050-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prestera T, Talalay P, Alam J, Ahn YI, Lee PJ, Choi AM. Parallel induction of heme oxygenase-1 and chemoprotective phase 2 enzymes by electrophiles and antioxidants: regulation by upstream antioxidant-responsive elements (ARE) Mol Med. 1995;1:827–837. [PMC free article] [PubMed] [Google Scholar]
- 31.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 32.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–234. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 33.Wang XJ, Hayes JD, Wolf CR. Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of nrf2 by cancer chemotherapeutic agents. Cancer Res. 2006;66:10983–10994. doi: 10.1158/0008-5472.CAN-06-2298. [DOI] [PubMed] [Google Scholar]
- 34.Natsch A, Emter R. Skin sensitizers induce antioxidant response element dependent genes: application to the in vitro testing of the sensitization potential of chemicals. Toxicol Sci. 2008;102:110–119. doi: 10.1093/toxsci/kfm259. [DOI] [PubMed] [Google Scholar]
- 35.Ade N, Leon F, Pallardy M, Peiffer JL, Kerdine-Romer S, Tissier MH, et al. HMOX1 and NQO1 genes are upregulated in response to contact sensitizers in dendritic cells and THP-1 cell line: role of the Keap1/Nrf2 pathway. Toxicol Sci. 2009;107:451–460. doi: 10.1093/toxsci/kfn243. [DOI] [PubMed] [Google Scholar]
- 36.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuashi M, Ohtani T, Nakagawa S, Aiba S. Redox imbalance induced by contact sensitizers triggers the maturation of dendritic cells. J Invest Dermatol. 2005;124:579–586. doi: 10.1111/j.0022-202X.2005.23624.x. [DOI] [PubMed] [Google Scholar]
- 39.Kagatani S, Sasaki Y, Hirota M, Mizuashi M, Suzuki M, Ohtani T, et al. Oxidation of cell surface thiol groups by contact sensitizers triggers the maturation of dendritic cells. J Invest Dermatol. 2010;130:175–183. doi: 10.1038/jid.2009.229. [DOI] [PubMed] [Google Scholar]
- 40.Rutault K, Alderman C, Chain BM, Katz DR. Reactive oxygen species activate human peripheral blood dendritic cells. Free Radic Biol Med. 1999;26:232–238. doi: 10.1016/s0891-5849(98)00194-4. [DOI] [PubMed] [Google Scholar]
- 41.Na K, Kim KE, Park ST, Kim TY. EC-SOD suppresses contact hypersensitivity in mouse skin by impairing Langerhans cell migration. J Invest Dermatol. 2007;127:1930–1937. doi: 10.1038/sj.jid.5700802. [DOI] [PubMed] [Google Scholar]
- 42.Bergstrom MA, Ott H, Carlsson A, Neis M, Zwadlo-Klarwasser G, Jonsson CA, et al. A skin-like cytochrome P450 cocktail activates prohaptens to contact allergenic metabolites. J Invest Dermatol. 2007;127:1145–1153. doi: 10.1038/sj.jid.5700638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.