Abstract
Antibodies to brain antigens are present in stroke survivors. In this study, we assessed autoantibody responses to white matter antigens, their correlation to white matter disease and stroke outcome. Antibody titers (immunoglobulin G [igG]) to myelin basic protein (MBP), proteolipid protein (PLP) and tetanus toxoid (TT) were available at one or more time points for 112 subjects with ischemic stroke. In comparison to the control subjects (N=40), there was a global decrease in IgG titers to TT early after stroke. Patients with white matter disease on magnetic resonance imaging had elevated titers of antibodies to both MBP and PLP at 30 days after stroke, and anti-MBP antibodies were associated with worse outcome. The potential pathologic consequences of antibodies to white matter, especially MBP, is deserving of further investigation.
Keywords: MBP, stroke, antibodies, white matter, Fazekas
The presence of autoimmune responses to central nervous system (CNS) antigens in patients with stroke has been appreciated since the early 1970’s. In fact, early studies showed that the T cell response to myelin associated proteins was more robust in stroke survivors than in patients with multiple sclerosis (Kallen et al., 1977; Youngchaiyud et al., 1974). We recently showed that Th1 type cellular immune responses to brain antigens occur following stroke and the likelihood such responses is enhanced by the occurrence of systemic infection (Becker et al., 2011). Further, we found that the Th1 response to myelin basic protein (MBP) was an independent predictor of stroke outcome – more robust cellular responses to MBP were associated with a decreased likelihood of good outcome at 90 days after stroke onset.
Recent studies have identified antibodies to neurofilament and portions of the N-methyl- D-aspartate (NMDA) receptor in patients with stroke (Bornstein et al., 2001; Dambinova et al., 2003). The relevance of these autoantibodies to stroke outcome is unknown. Using the same cohort of patients with ischemic stroke in which we assessed the cellular immune response to brain antigens, we now characterize the humoral immune response to brain antigens by measuring the titers of antibodies to MBP and proteolipid protein (PLP).
Materials and Methods
Research Subjects
We prospectively enrolled patients with ischemic stroke admitted to Harborview Medical Center from 9/2005 through 5/2009 who were at least 18 years of age, could be enrolled within 72 hours of symptom onset and were felt not likely to die from their stroke. Patients with ongoing therapy for malignancy, known history of HIV, Hepatitis B or C, history of brain tumor, anemia (hematocrit<35 on admission), and those taking immunomodulatory drugs were excluded. Blood was drawn as soon as possible after stroke onset and at 3, 7, 30, 90, 180 and 365 days after stroke onset. Plasma and serum were frozen at −80°until use. The study was approved by the Institutional Review Board and all patients or their surrogates provided informed consent.
Clinical and Infection Data
Demographic and clinical data were collected on all patients. Stroke severity was determined by the National Institutes of Health Stroke Scale (NIHSS) score and outcome by the stroke impact scale (SIS) (Duncan et al., 2003). In hospital infection was defined as clinical symptoms of an infection (fever and/or pyuria for urinary tract infection [UTI] and fever and/or productive cough and radiographic evidence of consolidation for pneumonia [PNA]) and positive culture data (for both PNA and UTI). Total infarct volume on initial diffusion weighted MRI imaging was calculated by the ABC/2 method (Sims et al., 2009). Assessment was also made for chronic infarcts. The degree of white matter disease on the axial FLAIR images was graded using the Fazekas scale by an independent neuroradiologist; the periventricular and deep white matter scores were summed for analysis (Fazekas et al., 1987).
Laboratory Studies
Serum antibody titers (immunoglobulin G [IgG]) to tetanus toxoid (TT) were determined using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (IBL International) and results presented as IU/mL. To determine relative antibody titers to MBP and PLP, 96 well plates (NUNC MaxiSorp™) were coated with either human MBP (Sigma; 0.10 µg/well) or PLP (Biogenesis; 0.10 µg/well) and incubated overnight at 4°C. Following extensive washing, the plates were incubated overnight at 4°C with serum (diluted 1:10) samples (100 µl/well). After washing, antigen bound human IgG was detected with peroxidase conjugated goat anti-human IgG antibodies (Pierce) and the plates developed with tetramethyl benzidine (TMB; Pierce). The absorbance was assessed at 450 nm (BioTek®). Results are presented as relative absorbance. All experiments were performed in duplicate. Control wells included (1) those with serum but no secondary antibody, (2) those without serum but with secondary antibody and (3) and those without serum or secondary antibody. Serum samples were additionally screened for the presence of anti-phospholipid antibodies (anticardiolipin IgM [MPL] and IgG [GPL] and β2glycoprotein-1 IgG SGU]) by the hospital clinical laboratory using ELISA; titers <15 for MPL, GPL or SGU were assigned a value of 0 for analyses.
Leukocyte counts and differential were performed by the clinical hematology laboratory. The concentration of interleukin (IL)-10 was determined (along with a panel of additional cytokines) using a cytometric bead-based system (Luminex). For the purpose of analysis, concentrations below the limit of detection (0.30 pg/mL) were assigned a value of 0.30 pg/mL. Plasma cortisol was determined by the hospital laboratory using standard methods.
Lymphocyte Responses
Lymphocytes were isolated over a ficoll gradient and frozen in liquid nitrogen until use. Enzyme linked immunoSPOT (ELISPOT) assays were done to detect the antigen specific secretion of interferon (IFN)-γ and transforming growth factor (TGF)-β1 (R&D Systems). Cells were cultured for 24 hours in 96-well plates (MultiScreen®-IP; Millipore) at a concentration of 1×106 per mL in media alone or with human MBP (25 µg/mL; Sigma-Aldrich), human PLP (5 µg/mL; ABD Serotec), or TT (5 µg/mL; Sigma- Aldrich) and incubated for 24 hours. Experiments were performed in triplicate; spots were counted using a semi-automated system (MetaMorph®). The ratio of the relative increase in the number of cells secreting IFN-γ to the relative increase in the number of cells secreting TGF-β to a given antigen is used to represent the degree of Th1 response to each antigen.
Statistics
Descriptive data are presented as the median and interquartile range (IQR) for continuous variables and percents for categorical variables. Group comparisons were performed using the Mann-Whitney U test or the χ2 test statistic as appropriate. Logistic regression was used to test the association between serum antibody titers and outcome (using the SIS) at days 30, 90, 180, and 365 after stroke onset. To test the correlations between serum antibody titers and clinical/laboratory variables, data were log transformed and either unadjusted or adjusted for stroke severity (using the NIHSS); data are presented as Pearson’s rho. Significance was set at P<0.05. No formal adjustments were made to P values to account for multiple comparisons; results should therefore be interpreted cautiously.
Results
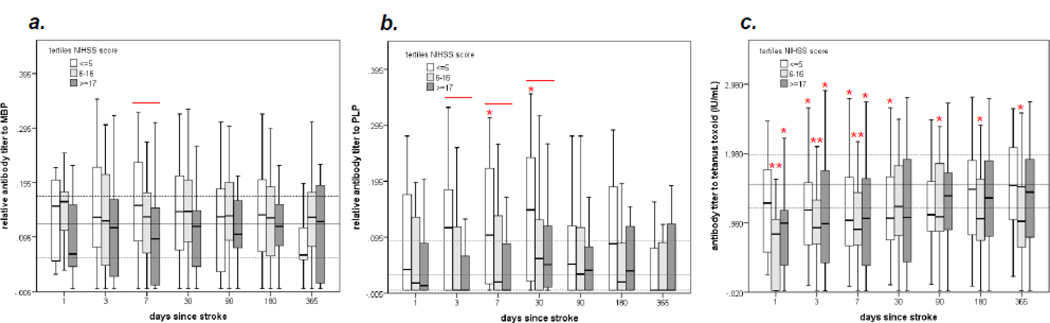
The parent study enrolled 114 patients with acute ischemic stroke from 9/2005 through 5/2009; antibody titers were available for 112 of these patients at one or more time points. The characteristics of this study population have been described elsewhere (Becker et al., 2011; Tanzi et al., 2011; Zierath et al., 2011). Consistent with these previous papers, patients were categorized according to baseline stroke severity (mild = NIHSS ≤5, moderate = NIHSS 6–16, severe = NIHSS≥17). Figure 1 shows the changes in the titer of antibody to MBP, PLP and TT over the course of time following stroke as a function of stroke severity. Anti-MBP antibody titers were decreased among patients with more severe strokes at day 7 after stroke onset; the titers of anti-MBP antibodies among stroke patients, however, did not differ from that of controls. Patients with more severe stroke had lower titers of anti-PLP antibodies than patients with less severe stroke from day 3 to 30 after stroke while patients with mild stroke (NIHSS≤5) had higher titers of anti-PLP antibodies than control subjects at day 7 and 30 after stroke. In comparison to controls, patients with stroke, irrespective of stroke severity, had markedly lower titers of anti-TT antibodies until day 30 after stroke onset; there were, however, no differences in the titers of anti-TT antibodies among patients with mild, moderate and severe stroke.
Figure 1.
Comparison of antibody titers to MBP (a), PLP (b) and TT (c) over the course of 365 days after stroke onset based on initial stroke severity. Differences (P<0.05) between patients are indicated by the solid line above the box plots at the given time point (Kruskal-Wallis H test). The solid horizontal gray line indicates the median value of the control population and the dotted lines the interquartile range. Differences between each tertile of stroke severity and the control population are indicated by *P<0.05 or **P<0.001 (Mann-Whitney U test).
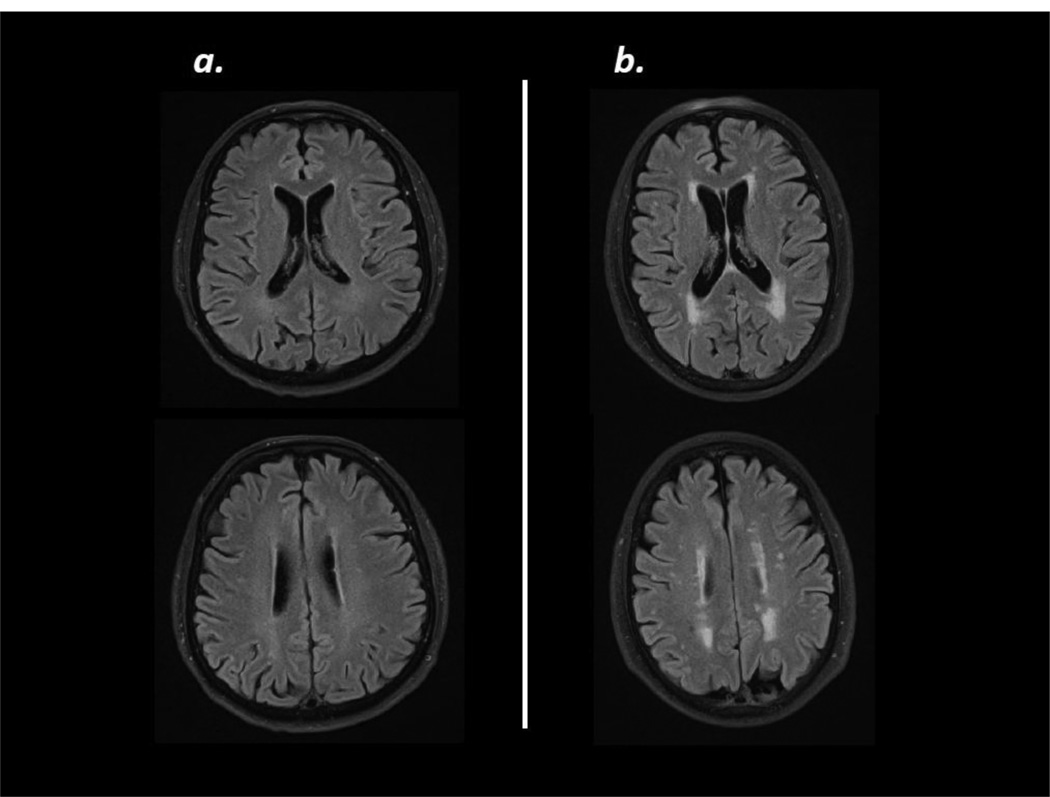
Serum antibody titers to MBP, PLP and TT at all time points after stroke were similar among patients who developed early post-stroke infection (by day 15) and those who did not (data not shown). Based on an a priori hypothesis that antibodies to MBP and PLP might be associated with white matter disease, initial MRI scans (available for 110 patients) were graded using the Fazekas Score (Fazekas et al., 1987). Scores for deep white matter hyperintensities and periventricular white matter hyperintensities were summed; the distribution of scores is seen in Table 1. The median score was 2; patients were thus considered to have white matter disease if the Fazekas score was greater than 2 (ie. ≥3). Representative MRI scans are seen in Figure 2. The characteristics of these patients are presented in Table 2. Patients with Fazekas scores ≥3 were older, were more likely to be hypertensive, more likely to have an old stroke on imaging and more likely to have a lacunar stroke at presentation than patients with scores <3. Both stroke severity (NIHSS score) and infarct volume were similar among patients with and without white matter disease.
Table 1.
Distribution of summed Fazekas scores on initial MRI (N=110).
| Total Fazekas Score: | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| N= | 26 | 28 | 34 | 13 | 4 | 5 |
Figure 2.
Example MRIs (5mm axial FLAIR) showing the typical range of white matter disease in this study. The scan on the left (a) shows minimal white matter disease corresponding to a combined Fazekas score of 1 and the scan on the right (b) shows a moderate amount of white matter disease corresponding to a combined score of 3.
Table 2.
Characteristics of patients with and without white matter disease. Data are presented at the proportion or the median (interquartile range). Statistics are by Mann-Whitney U test or χ2 as appropriate.
| Fazekas ≤2 N=88 |
Fazekas ≥3 N=22 |
P | |
|---|---|---|---|
| demographics | |||
| age | 52 (42, 64) | 68 (61–73) | <0.001 |
| female | 31/88 (35%) | 6/22 (27%) | NS |
| Caucasian | 78/88 (89%) | 21/22 (95%) | NS |
| atrial fibrillation | 11/88 (12%) | 4/22 (18%) | NS |
| hypertension | 42/88 (48%) | 16/22 (73%) | 0.036 |
| smoker | 38/88 (43%) | 5/22 (23%) | 0.079 |
| coronary heart disease | 19/88 (22%) | 6/22 (27%) | NS |
| diabetes | 21/88 (24%) | 6/22 (27%) | NS |
| characteristics of presenting stroke | |||
| NIHSS | 10 (3, 19) | 10 (4, 18) | NS |
| total infarct volume (cc) | 15.2 (1.5, 94.8) | 5.2 (0.5, 55.5) | NS |
| hemorrhagic conversion (any) | 18/88 (20%) | 4/22 (18%) | NS |
| prior infarct on imaging* | 16/88 (18%) | 10/22 (45%) | 0.007 |
| treatment | |||
| endovascular intervention | 13/88 (15%) | 1/22 (4%) | 0.198 |
| IV tPA | 19/88 (22%) | 7/22 (32%) | NS |
| hemicraniectomy | 7/88 (8%) | 1/22 (4%) | NS |
| stroke etiology | |||
| lacunar | 6/88 (7%) | 5/22 (23%) | 0.026 |
| cardioembolic | 23/88 (26%) | 6/22 (27%) | NS |
| large vessel disease | 15/88 (17%) | 2/22 (9%) | NS |
| dissection | 6/88 (7%) | 0/22 | NS |
| other | 25/88 (28%) | 4/22 (18%) | NS |
| unknown | 19/88 (22%) | 5/22 (23%) | NS |
| stroke related complications | |||
| infection by day 15 | 20/87 (23%) | 6/22 (27%) | NS |
| pneumonia by day 15 | 8/87 (9%) | 3/22 (14%) | NS |
NIHSS = National Institutes of Health Stroke Scale Score, IV tPA = intravenous tissue plasminogen activator,
signifies a radiological infarct, NS= not significant and signifies P≥0.200.
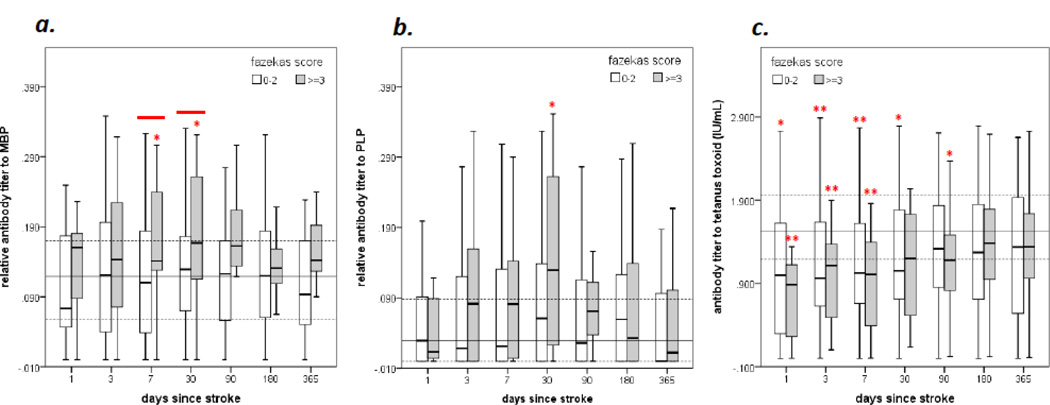
The differences in antibody titers to MBP, PLP and TT among patients with (combined Fazekas score ≥3) and without (combined Fazekas score <3) white matter disease are displayed in Figure 3. Because stroke severity is inversely related to the antibody response (Figure 1c and Table 4), data are adjusted for the NIHSS score. In comparison to the control population, patients with a combined Fazekas score ≥3 had higher titers of antibodies to both MBP and PLP by 30 days after stroke onset while the titers of antibodies to TT were lower than that seen in the control population. Patients with white matter disease also had higher anti-MBP antibody titers than patients without white matter disease at days 7 and 30 after stroke onset (Figure 3a). Patients who experienced a hemorrhagic transformation of their infarct had lower antibody titers to MBP and PLP at days 3, 7 and 30 after stroke (data not shown). Patients with hemorrhagic transformation also had more severe strokes than those without (NIHSS scores 21 [15, 28] versus 8 [3, 18], P<0.001). Serum samples were additionally screened for the presence of anti-phospholipid antibodies (anticardiolipin IgM and IgG and β2glycoprotein-1 IgG) at these same time points and no differences in antibody titers or in the proportion of patients with detectable anti-phospholipid antibodies were seen between patients with and without white matter disease (data not shown).
Figure 3.
Comparison of antibody titers to MBP (a), PLP (b) and TT (c) over the course of 180 days after stroke onset based on the degree of white matter disease on the initial MRI. Differences (P<0.05) between patients are indicated by the solid line above the box plots at the given time point and are adjusted for initial stroke severity. The solid horizontal gray line indicates the median value of the control population and the dotted lines the interquartile range. Differences between patients with and without white matter disease and the control population are indicated by *P<0.05 or **P<0.001 (Mann- Whitney U test).
Table 4.
Correlates of the antibody titers to MBP, PLP and TT at multiple different time points after stroke. Data are log transformed and presented as Pearson’s rho.
| abMBP* | NIHSS | infarct volume | IL-10 | cortisol | lymphs | Th1 MBP | Th1 PLP | Th1 TT |
|---|---|---|---|---|---|---|---|---|
| Day 2 | −0.266 P=0.163 |
−0.263 P=0.127 |
−0.337 P=0.146 |
0.312 NS |
0.352 P=0.151 |
0.253 NS |
−0.058 NS |
0.157 NS |
| Day 4 |
−0.223 P=0.022 |
−0.171 NS |
−0.203 P=0.049 |
−0.072 NS |
0.047 NS |
−0.207 P=0.050 |
−0.098 NS |
−0.160 P=0.120 |
| Day 7 |
−0.271 P=0.008 |
−0.274 P=0.008 |
−0.331 P=0.001 |
−0.030 NS |
0.215 P=0.050 |
0.032 NS |
−0.033 NS |
−0.238 P=0.026 |
| Day 30 | −0.094 NS |
−0.186 P=0.084 |
−0.193 P=0.079 |
0.103 NS |
0.149 P=0.174 |
−0.188 P=0.105 |
−0.128 NS |
0.008 NS |
| Day 90 | 0.056 NS |
−0.112 NS |
0.009 NS |
0.123 NS |
−0.002 NS |
0.010 NS |
−0.091 NS |
−0.013 NS |
| Day 180 | −0.091 NS |
−0.072 NS |
−0.247 P=0.047 |
0.074 NS |
0.163 P=0.188 |
−0.194 P=0.148 |
0.042 NS |
0.038 NS |
| Day 365 | 0.035 NS |
−0.005 NS |
−0.057 NS |
−0.006 NS |
0.099 NS |
−0.061 NS |
−0.087 NS |
−0.233 P=0.138 |
| abPLP* | NIHSS | infarct volume | IL-10 | cortisol | lymphs | TH1 MBP | TH1 PLP | TH1 TT |
| Day 2 | −0.255 P=0.182 |
−0.150 NS |
−0.448 P=0.036 |
0.088 NS |
0.486 P=0.041 |
0.229 NS |
−0.073 NS |
−0.064 NS |
| Day 4 | −0.301 P=0.002 |
−0.238 P=0.014 |
−0.288 P=0.004 |
−0.262 P=0.008 |
0.065 NS |
−0.134 NS |
−0.065 NS |
−0.119 NS |
| Day 7 | −0.292 P=0.004 |
−0.173 P=0.097 |
−0.309 P=0.002 |
−0.172 P=0.103 |
0.251 P=0.021 |
−0.035 NS |
−0.075 NS |
−0.192 P=0.072 |
| Day 30 | −0.129 NS |
−0.214 P=0.046 |
−0.049 NS |
0.036 NS |
0.225 P=0.038 |
−0.035 NS |
0.041 NS |
−0.144 NS |
| Day 90 | −0.010 NS |
−0.104 NS |
−0.140 NS |
0.001 NS |
0.063 NS |
−0.027 NS |
−0.282 P=0.029 |
−0.305 P=0.015 |
| Day 180 | −0.149 NS |
−0.098 NS |
−0.064 NS |
0.149 NS |
0.209 P=0.092 |
0.050 NS |
0.245 P=0.083 |
0.118 NS |
| Day 365 | 0.069 NS |
0.162 NS |
−0.077 NS |
−0.154 NS |
0.125 NS |
0.177 NS |
0.018 NS |
0.131 NS |
| abTT** | NIHSS | infarct volume | IL-10 | cortisol | lymphs | TH1 MBP | TH1 PLP | TH1 TT |
| Day 2 | −0.182 NS |
−0.020 NS |
−0.489 P=0.024 |
0.197 NS |
−0.040 NS |
0.203 NS |
−0.133 NS |
−0.107 NS |
| Day 4 | −0.059 NS |
−0.062 NS |
−0.046 NS |
−0.010 NS |
−0.013 NS |
−0.179 0.092 |
−0.041 NS |
0.122 NS |
| Day 7 | −0.072 NS |
−0.044 NS |
−0.111 NS |
−0.161 P=0.128 |
−0.013 NS |
0.058 NS |
0.063 NS |
0.007 NS |
| Day 30 | −0.050 NS |
−0.080 NS |
0.135 NS |
−0.075 NS |
0.217 P=0.048 |
−0.001 NS |
0.041 NS |
−0.092 NS |
| Day 90 | −0.086 NS |
−0.013 NS |
−0.044 NS |
0.044 NS |
0.009 NS |
−0.115 NS |
−0.135 NS |
−0.136 NS |
| Day 180 | −0.106 NS |
−0.081 NS |
−0.033 NS |
0.178 P−0.143 |
0.177 P=0.155 |
0.079 NS |
−0.160 NS |
−0.108 NS |
| Day 365 | −0.154 NS |
−0.076 NS |
−0.087 NS |
0.075 NS |
0.222 P=0.121 |
−0.315 P=0.042 |
−0.103 NS |
−0.132 NS |
antibody titers to MBP and PLP are quantified by relative absorbance,
antibody titers to TT are quantified by IU/mL, abMBP=antibody titer to myelin basic protein, abPLP=antibody titer to proteolipid protein, abTT=antibody titer to tetanus toxoid, NIHSS=National Institutes of Health Stroke Scale Score, IL=interleukin, lymphs=lymphocytes, NS=not significant (P≥0.200).
Table 3 shows the effects of white matter disease (combined Fazekas score ≥3) as well antibody titers to MBP, PLP and TT on stroke outcome. At 365 days after stroke, the median SIS for patients in whom antibody titers were available was 92 (70, 100). For the purpose of these analyses, a poor outcome was considered to be an SIS less than or equal to the 25th percentile of the entire cohort (ie. ≤70). There was a trend towards worse outcome in patients with white matter disease. Patients who had anti-MBP antibody titers greater than the 75th percentile of that seen in the entire cohort of stroke patients at 365 days after stroke onset were 5–6 times more likely to have poor outcome than those with lower antibody titers. There was also a trend towards worse outcome with elevated anti-MBP titers at earlier time points. (Because anti-TT antibody titers were decreased after stroke, analyses were also controlled for the titer of anti-TT antibodies.) High titers of anti-PLP and anti-TT antibodies were not associated with worse outcome from stroke.
Table 3.
Predictors of poor outcome (SIS≤70) at days 30, 90, 180 and 365 after stroke. Data are either unadjusted or adjusted for NIHSS, age, and titer of anti-TT antibodies.
| Fazekas≥3 | abMBP*75% | abPLP*75% | abTT**75% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR (CI) | P | OR (CI) | P | OR (CI) | P | OR (CI) | P | ||
| day 30 | 1.654 (0.582–4.700) | NS | 1.054 (0.388–2.862) | NS | 0.688 (0.253–1.866) | NS | 1.084 (0.399–2.940) | NS | |
| Adj. for: |
NIHSS | 2.064 (0.590–7.224) | NS | 1.595 (0.476–5.345) | NS | 2.103 (0.565–7.831) | NS | 0.608 (0.174–2.128) | NS |
| NIHSS, age | 1.808 (0.438–6.657) | NS | 1.459 (0.430–4.949) | NS | 2.352 (0.599–9.229) | NS | 0.690 (0.191–2.486) | NS | |
| NIHSS, age, abTT | 1.801 (0.426–7.620) | NS | 1.448 (0.421–4.980) | NS | 2.356 (0.586–9.467) | NS | --- | --- | |
| day 90 | 1.980 (0.674–5.813) | NS | 1.524 (0.495–4.687) | NS | 0.471 (0.136–1.633) | NS | 0.635 (0.197–2.048) | NS | |
| Adj. for: |
NIHSS | 2.560 (0.672–9.750) | 0.168 | 4.135 (0.951–17.990) | 0.058 | 0.754 (0.184–3.095) | NS | 0.579 (0.149–2.248) | NS |
| NIHSS, age | 1.995 (0.466–8.547) | NS | 3.578 (0.811–15.786) | 0.092 | 0.760 (0.179–3.231) | NS | 0.783 (0.188–3.266) | NS | |
| NIHSS, age, abTT | 1.920 (0.385–9.583) | NS | 3.533 (0.767–16.261) | 0.105 | 0.727 (0.169–3.118) | NS | --- | --- | |
| day 180 | 2.061 (0.683–6.217) | 0.199 | 0.771 (0.215–2.757) | NS | 0.750 (0.209–2.686) | NS | 1.121 (0.333–3.781) | NS | |
| Adj. for: |
NIHSS | 2.448 (0.658–9.105) | 0.182 | 3.938 (0.696–22.292) | 0.121 | 1.086 (0.234–5.047) | NS | 1.371 (0.322–5.845) | NS |
| NIHSS, age | 1.662 (0.392–7.045) | NS | 4.184 (0.741–23.625) | 0.105 | 1.559 (0.303–8.019) | NS | 1.760 (0.393–7.879) | NS | |
| NIHSS, age, abTT | 1.968 (0.325–11.904) | NS | 4.509 (0.793–25.632) | 0.089 | 1.531 (0.296–7.926) | NS | --- | --- | |
| day 365 | 2.519 (0.0844–7.517) | 0.098 | 4.500 (1.133–17.878) | 0.033 | 1.095 (0.293–4.097) | NS | 0.958 (0.263–3.492) | NS | |
| Adj. for: |
NIHSS | 3.190 (0.866–11.743) | 0.081 | 4.957 (1.039–23.659) | 0.045 | 1.011 (0.225–4.550) | NS | 1.203 (0.267–5.419) | NS |
| NIHSS, age | 2.523 (0.613–10.390) | 0.200 | 5.189 (1.034–26.052) | 0.045 | 1.052 (0.226–4.890) | NS | 1.267 (0.268–5.990) | NS | |
| NIHSS, age, abTT | 3.147 (0.513–19.296) | NS | 6.077 (1.090–33.888) | 0.040 | 1.026 (0.217–4.846) | NS | --- | --- | |
SIS=stroke impact scale,
antibody titers to MBP and PLP are quantified by relative absorbance,
antibody titers to TT are quantified by IU/mL, abMBP 75%=antibody titer to myelin basic protein >75th percentile for stroke patients at that time point; abPLP 75%=antibody titer to proteolipid protein >75th percentile for stroke patients at that time point, abTT 75%=antibody titer to tetanus toxoid >75th percentile for stroke patients at that time point, abTT=antibody titer to tetanus toxoid, NIHSS=National Institutes of Health Stroke Scare, NS=not significant (P>0.200)
Finally, correlates of the humoral immune response to MBP, PLP and TT were explored and presented in Table 4. Early after stroke onset (within the first week), the titer of anti-MBP and anti-PLP antibodies were inversely correlated to stroke severity and infarct volume. Plasma IL-10 was also independently associated with decreased titers of antibodies to MBP and PLP. In general, there was little correlation between plasma cortisol and antibody titers. Of note, there was also little relationship between the titers of antibodies to MBP, PLP, or TT and the cellular response to the same antigens; when a relationship was seen, higher titers of antibodies were associated with less robust cellular responses.
Discussion
To our knowledge, this study is the first to systematically and longitudinally evaluate immunoglobulin titers in patients after ischemic stroke, and there are several novel and noteworthy observations. Firstly, stroke is associated with a rapid decrease in the titer of anti-TT IgG antibodies. Secondly, patients with white matter disease have higher titers of IgG antibodies to MBP and PLP than patients without. Thirdly, elevated antibody titers to MBP are associated with worse long term outcome from stroke. These observations are of potential clinical importance and warrant further consideration.
The antibody titer to TT was assessed as a control response to an “irrelevant” antigen; specifically, we wanted to be certain that any potential increase in the antibody titer to MBP and PLP did not merely reflect a non-specific acute phase response with increased immunoglobulin synthesis. Implicit in this choice of a control response was the presumption that most individuals would have been vaccinated to TT at some point in their life. Following immunization with an antigen like TT, the serum titer of antibodies is maintained by a pool of long-lived plasma cells (Amanna et al., 2007; Gatto et al., 2007; Manz et al., 1998). That stroke should lead to an immediate decrease in the titer of these antibodies is thus difficult to explain, especially when considering the fact that complete eradication of memory B cells does not affect antibody titers for some period of time (Ahuja et al., 2008; Amanna et al., 2007; DiLillo et al., 2008; Manz et al., 1998). Further, the half-life of IgG in circulation is approximately 21 days (Morell et al., 1970). Review of the literature, however, shows that a similar rapid decrease in immunoglobulin concentrations is seen in patients with a variety of traumatic injuries, especially burns (Kagan et al., 1989; Kohn and Cort, 1969; Munster et al., 1970; Pileri et al., 2009). In animals studies, the response to antigens following burn injury appears to be intact ex vivo, although these ex vivo response are not reflected by antibody titers in vivo (Gadd et al., 1988; Molloy et al., 1994). Experimental studies actually show that there is a decrease in the total amount of IgG in circulation following burn injury and that this decrement in IgG may be as much as 30% in comparison to control animals and lasts up to 3 weeks (Gadd et al., 1988; Molloy et al., 1994). The global decrease in immunoglobulins is attributed, at least in part, to increased catabolism/clearance of proteins from the circulation (Davies et al., 1971; Gadd et al., 1988).
Given that the overall concentration of immunoglobulin in circulation is decreased in the current study (at least as reflected by the concentration of anti-TT IgG), the elevation of anti-MBP and anti-PLP IgG antibodies in patients with white matter disease is especially noteworthy. The pathogenesis of white matter disease in the elderly is assumed to be chronic microvascular ischemia, and progression of white matter disease is more common in patients with a higher baseline lesion load (Gouw et al., 2008; Sachdev et al., 2007). The contribution of traditional vascular risk factors to the progression of white matter injury, however, is more debatable, with some studies showing an association (Gouw et al., 2008; van Dijk et al., 2008) and others showing no association (Sachdev et al., 2007). Alternative risk factors for progression of white matter disease include the apolipoprotein (APO)E4 genotype (Godin et al., 2009). APOE has a potent role in modulating the immune response, and the APOE4 genotype is associated with increased inflammation and worse outcome from a variety of neurological insults, including stroke (Gromadzka et al., 2007; Ost et al., 2008; Vitek et al., 2009). The association of APOE4 with progression of white matter disease, as well as the observation that neuroinflammation contributes to white matter damage in experimental stroke, suggest that immune mechanisms might contribute to the pathogenesis of white matter disease (Jalal et al., 2012). That white matter disease is associated with an active immune response is suggested by the observation that among patients with lung cancer, those white matter disease appear to be somewhat protected against the development of brain metastases (Mazzone et al., 2009).
The presence of autoantibodies in patients with stroke was initially demonstrated years ago (Bornstein et al., 2001; Dambinova et al., 2003). Specific association of antibodies to heat shock protein (HSP)60 and white matter disease was shown in a recent study (Kimura et al., 2012). Whether antibodies to HSP60, MBP or PLP are an epiphenomenon of white matter injury or contribute is not known. For antibodies to contribute to CNS pathology, they must first gain access to the CNS. This access could occur during discrete and obvious episodes of blood brain barrier (BBB) dysfunction (ie. recurrent stroke) or at other times when the impairment of the BBB is less obvious, like with episodes of extreme hypertension or in patients with lacunar stroke. For instance, recent data show that patients with lacunar stroke and white matter disease have mild diffuse impairment of the BBB (Taheri et al., 2011; Wardlaw et al., 2009). And while quite speculative, it is possible to imagine that chronic or even intermittent leakage of antibodies into the brain could lead to an inflammatory response and myelin damage contributing to the progression of white matter disease. The association between elevated titers of anti-MBP antibodies and poor long term outcome from stroke in our study suggests the possibility that some autoantibodies may have pathological consequences. A major limitation of this study, however, is that antibody titers were only assessed to three antigens, and there is a wide array of proteins to which an antibody response could be generated after stroke. Future studies should incorporate new technologies that allow for autoantibody screening to a wide array of antigens simultaneously.
In a previous study we found that patients who developed infection in the post-stroke period were more likely to develop a Th1 response to brain antigens, presumably through the phenomenon known as bystander activation (Becker et al., 2011). In this study we did not find a relationship between post-stroke infection and serum antibody titers to MBP, PLP or TT at later time points – that is, low titers of antibodies early after stroke onset did not appear to predispose to infection, and infection did not seem to predispose to higher titers of antibodies at later time points. Further, we did not find a robust or persistent correlation between the cellular immune response (ie. the Th1 response) and humoral immune responses (ie. the antibody titer) to MBP, PLP or TT. The type of immune response that develops to a particular antigen is dependent upon the microenvironment at the site of antigen encounter. A Th1 type response is favored by an inflammatory microenvironment where IFN-γ is present, such as might occur during a systemic infection; a Th2 response, which is classically associated with humoral immunity and the secretion of antibodies, is favored by the presence of cytokines such as IL-4 (Weaver et al., 2006; Zhou et al., 2009). The lack of correlation between the Th1 response and the antibody titer to a specific antigen is thus not surprising.
In the first week after stroke onset there is an inverse relationship between stroke severity and titers of anti-MBP and anti-PLP antibodies. Both plasma IL-10 and cortisol are elevated after stroke, and the degree of elevation is related to stroke severity (Chang et al., 2010; Christensen et al., 2004; Tanzi et al., 2011). Further, both IL-10 and cortisol are known to modulate the immune response and are felt to be markers of post-stroke immunodepression (Urra et al., 2009). Importantly, the inverse relationship between IL-10 and antibody titers in the week after stroke onset persists after controlling for stroke severity, while the relationship between cortisol and anti-MBP and anti-PLP antibody titers does not. And the fact that IL-10 is associated with decreased anti-TT antibody titers at only one time point after stroke (day 2) suggests that this cytokine is not responsible for the global and persistent decrease in anti-TT titers. IL-10, however, does appear to play an important role in the systemic immunodepression seen in many critically ill patients, including stroke (Albaiceta et al., 2007; Miller et al., 2007). An important implication of these findings pertains to the mandate from Center for Medicare and Medicaid Services that all hospitalized patients over the age of 65 be immunized to influenza and pneumococcus. While the current study did not assess the response to immunization in the immediate aftermath of stroke, the growing body of literature documenting the presence of post-stroke “immunodepression” should raise concern about routine immunizations in these patients. The decrease in anti-TT immunoglobulins after stroke in this study further suggest that there should be an informed conversation about the wisdom of immunizing patients in the immediate poststroke period.
The strengths of this study include the wide array of patients included, including those with mild and severe strokes and those with and those without white matter disease. Further, each patient was extensively characterized from a clinical, radiological and immunological point of view. One of the key finding of this study is the fact that patients with MRI defined white matter disease at admission had elevated titers of antibodies to both MBP and PLP at 30 days after stroke while there was a general decline in CNS non-specific (TT) immunoglobulins in most patients after stroke. The elevated titers of IgG antibodies to white matter antigens in patients with white matter disease thus suggests, but does not prove, that these immune responses form as a result of white matter injury and/or contribute to white matter injury. It is important to note, however, that no formal adjustments were made to P values to account for multiple comparisons; results should therefore be interpreted cautiously.
In summary, this study shows that there is an immediate decrease in anti-TT IgG following stroke, which likely reflects an increase in catabolism/clearance of these antibodies. On the other hand, patients with white matter disease have elevated titers of anti-MBP and anti-PLP antibodies, and elevated titers of anti-MBP antibodies are associated worse long-term outcome. Future studies should address the possibility that CNS autoimmune responses seen in conjunction with ischemic brain injury contribute to ongoing pathology and affect long term outcome.
Acknowledgments
Acknowledgements/Funding
This work was funded by National Institute of Neurological Disorders and Stroke R01NS049197.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflicts of Interest
None.
References
- Ahuja A, Anderson SM, Khalil A, Shlomchik MJ. Maintenance of the plasma cell pool is independent of memory B cells. Proc Natl Acad Sci U S A. 2008;105:4802–4807. doi: 10.1073/pnas.0800555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaiceta GM, Pedreira PR, Garcia-Prieto E, Taboada F. Therapeutic implications of immunoparalysis in critically ill patients. Inflamm Allergy Drug Targets. 2007;6:191–196. doi: 10.2174/187152807783334337. [DOI] [PubMed] [Google Scholar]
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- Becker KJ, Kalil AJ, Tanzi P, Zierath DK, Savos AV, Gee JM, Hadwin J, Carter KT, Shibata D, Cain KC. Autoimmune Responses to the Brain After Stroke Are Associated With Worse Outcome. Stroke. 2011;42:2763–2769. doi: 10.1161/STROKEAHA.111.619593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56:529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- Chang LT, Yuen CM, Liou CW, Lu CH, Chang WN, Youssef AA, Yip HK. Link between interleukin-10 level and outcome after ischemic stroke. Neuroimmunomodulation. 2010;17:223–228. doi: 10.1159/000290038. [DOI] [PubMed] [Google Scholar]
- Christensen H, Boysen G, Johannesen HH. Serum-cortisol reflects severity and mortality in acute stroke. J Neurol Sci. 2004;217:175–180. doi: 10.1016/j.jns.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-Daspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin Chem. 2003;49:1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- Davies JW, Bull JP, Ricketts CR. Catabolic response to injury. Lancet. 1971;2:320. doi: 10.1016/s0140-6736(71)91370-5. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Hamaguchi Y, Ueda Y, Yang K, Uchida J, Haas KM, Kelsoe G, Tedder TF. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol. 2008;180:361–371. doi: 10.4049/jimmunol.180.1.361. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Lai SM, Bode RK, Perera S, DeRosa J. Stroke Impact Scale-16: A brief assessment of physical function. Neurology. 2003;60:291–296. doi: 10.1212/01.wnl.0000041493.65665.d6. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Gadd MA, Hansbrough JF, Soderberg CS, Field TO. Antibody formation after murine injury. J Surg Res. 1988;44:649–657. doi: 10.1016/0022-4804(88)90096-0. [DOI] [PubMed] [Google Scholar]
- Gatto D, Martin SW, Bessa J, Pellicioli E, Saudan P, Hinton HJ, Bachmann MF. Regulation of memory antibody levels: the role of persisting antigen versus plasma cell life span. J Immunol. 2007;178:67–76. doi: 10.4049/jimmunol.178.1.67. [DOI] [PubMed] [Google Scholar]
- Godin O, Tzourio C, Maillard P, Alperovitch A, Mazoyer B, Dufouil C. Apolipoprotein E genotype is related to progression of white matter lesion load. Stroke. 2009;40:3186–3190. doi: 10.1161/STROKEAHA.109.555839. [DOI] [PubMed] [Google Scholar]
- Gouw AA, van der Flier WM, Fazekas F, van Straaten EC, Pantoni L, Poggesi A, Inzitari D, Erkinjuntti T, Wahlund LO, Waldemar G, Schmidt R, Scheltens P, Barkhof F. Progression of white matter hyperintensities 19 and incidence of new lacunes over a 3-year period: the Leukoaraiosis and Disability study. Stroke. 2008;39:1414–1420. doi: 10.1161/STROKEAHA.107.498535. [DOI] [PubMed] [Google Scholar]
- Gromadzka G, Baranska-Gieruszczak M, Sarzynska-Dlugosz I, Ciesielska A, Czlonkowska A. The APOE polymorphism and 1-year outcome in ischemic stroke: genotype-gender interaction. Acta Neurol Scand. 2007;116:392–398. doi: 10.1111/j.1600-0404.2007.00880.x. [DOI] [PubMed] [Google Scholar]
- Jalal FY, Yang Y, Thompson J, Lopez AC, Rosenberg GA. Myelin Loss Associated With Neuroinflammation in Hypertensive Rats. Stroke. 2012;43:1115–1122. doi: 10.1161/STROKEAHA.111.643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan RJ, Bratescu A, Jonasson O, Matsuda T, Teodorescu M. The relationship between the percentage of circulating B cells, corticosteroid levels, and other immunologic parameters in thermally injured patients. J Trauma. 1989;29:208–213. doi: 10.1097/00005373-198902000-00010. [DOI] [PubMed] [Google Scholar]
- Kallen B, Nilsson O, Thelin C. Effect of encephalitogenic protein on migration in agarose of leukoytes from patients with multiple sclerosis. A longitudinal study of patients with relapsing multiple sclerosis or with cerebral infarction. Acta Neurol Scand. 1977;55:47–56. [PubMed] [Google Scholar]
- Kimura A, Sakurai T, Yamada M, Koumura A, Hayashi Y, Tanaka Y, Hozumi I, Takemura M, Seishima M, Inuzuka T. Elevated Anti-Heat Shock Protein 60 Antibody Titer is Related to White Matter Hyperintensities. J Stroke Cerebrovasc Dis. 2012;21:305–309. doi: 10.1016/j.jstrokecerebrovasdis.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Kohn J, Cort DF. Immunoglobulins in burned patients. Lancet. 1969;1:836–837. doi: 10.1016/s0140-6736(69)92092-3. [DOI] [PubMed] [Google Scholar]
- Manz RA, Lohning M, Cassese G, Thiel A, Radbruch A. Survival of longlived plasma cells is independent of antigen. Int Immunol. 1998;10:1703–1711. doi: 10.1093/intimm/10.11.1703. [DOI] [PubMed] [Google Scholar]
- Mazzone PJ, Marchi N, Fazio V, Taylor JM, Masaryk T, Bury L, Mekhail T, Janigro D. Small vessel ischemic disease of the brain and brain metastases in lung cancer patients. PLoS ONE. 2009;4:e7242. doi: 10.1371/journal.pone.0007242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AC, Rashid RM, Elamin EM. The "T" in trauma: the helper T-cell response and the role of immunomodulation in trauma and burn patients. J Trauma. 2007;63:1407–1417. doi: 10.1097/TA.0b013e31815b839e. [DOI] [PubMed] [Google Scholar]
- Molloy RG, Nestor M, Collins KH, Holzheimer RG, Mannick JA, Rodrick ML. The humoral immune response after thermal injury: an experimental model. Surgery. 1994;115:341–348. [PubMed] [Google Scholar]
- Morell A, Terry WD, Waldmann TA. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970;49:673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster AM, Hoagland HC, Pruitt BA., Jr The effect of thermal injury on serum immunoglobulins. Ann Surg. 1970;172:965–969. doi: 10.1097/00000658-197012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost M, Nylen K, Csajbok L, Blennow K, Rosengren L, Nellgard B. Apolipoprotein E polymorphism and gender difference in outcome after severe traumatic brain injury. Acta Anaesthesiol Scand. 2008;52:1364–1369. doi: 10.1111/j.1399-6576.2008.01675.x. [DOI] [PubMed] [Google Scholar]
- Pileri D, Accardo-Palumbo A, D’Amelio L, D’Arpa N, Arnone G, Grisaffi C, Amico M, Brancato R, Lombardo C, Conte F. Serum Levels of Cortisol, Immunoglobulin, and C-reactive Protein in Burn Patients. Ann Burns Fire Disasters. 2009;22:3–5. [PMC free article] [PubMed] [Google Scholar]
- Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68:214–222. doi: 10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed] [Google Scholar]
- Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, Schwamm LH. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taheri S, Gasparovic C, Huisa BN, Adair JC, Edmonds E, Prestopnik J, Grossetete M, Shah NJ, Wills J, Qualls C, Rosenberg GA. Bloodbrain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–2163. doi: 10.1161/STROKEAHA.110.611731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzi P, Cain K, Kalil A, Zierath D, Savos A, Gee JM, Shibata D, Hadwin J, Carter K, Becker K. Post-stroke infection: a role for IL-1ra? Neurocrit Care. 2011;14:244–252. doi: 10.1007/s12028-010-9490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40:1262–1268. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- van Dijk EJ, Prins ND, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MM. Progression of cerebral small vessel disease in relation to risk factors and cognitive consequences: Rotterdam Scan study. Stroke. 2008;39:2712–2719. doi: 10.1161/STROKEAHA.107.513176. [DOI] [PubMed] [Google Scholar]
- Vitek MP, Brown CM, Colton CA. APOE genotype-specific differences in the innate immune response. Neurobiol Aging. 2009;30:1350–1360. doi: 10.1016/j.neurobiolaging.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Doubal F, Armitage P, Chappell F, Carpenter T, Munoz Maniega S, Farrall A, Sudlow C, Dennis M, Dhillon B. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Youngchaiyud U, Coates AS, Whittingham S, Mackay IR. Cellular-immune response to myelin protein: absence in multiple sclerosis and presence in cerebrovascular accidents. Aust N Z J Med. 1974;4:535–538. doi: 10.1111/j.1445-5994.1974.tb03233.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zierath D, Tanzi P, Cain K, Shibata D, Becker K. Plasma {alpha}-Melanocyte Stimulating Hormone Predicts Outcome in Ischemic Stroke. Stroke. 2011;42:3415–3420. doi: 10.1161/STROKEAHA.111.627331. [DOI] [PMC free article] [PubMed] [Google Scholar]