Abstract
Human papillomaviruses (HPV) are small DNA tumor viruses. HPV infection requires entry of virions into epithelial host cells that support the viral life cycle. Here, we used an in vivo mouse model, in which HPV pseudoviruses (PVs) are scored for their ability to transduce reporter genes, to test the role of various cellular proteins in entry. We initially investigated the role of integrin α6β4 in mediating early steps of HPV infection. Deficiency of integrin α6β4 modestly but significantly suppressed reporter-gene transduction by PVs in conditional integrin β4 knockout mice. We also investigated the role of syndecan 1, a heparin sulfate proteoglycan (HSPG) for its role in HPV infection. We didn’t see a significant reduction in reporter-gene transduction by PVs in syndecan-1 null mice. This indicates that this HSPG is not essential for early steps in HPV infection, but does not discount a need of other HSPGs in mediating HPV infection.
Keywords: Human Papillomavirus (HPV), Integrin α6β4 (Int α6β4), Heparin Sulfate Proteoglycans (HSPGs), Syndecan-1 (Sdc-1)
Introduction
Human papillomaviruses (HPVs) are the most common sexually transmitted (STD) pathogens. They mainly cause benign lesions called papillomas or warts on skin and mucosa. Approximately a dozen mucosotropic HPV genotypes (the ‘high risk’ HPVs), including HPV16, are etiologically linked to the development of multiple malignancies, such as cervical, vaginal, anal, penile and a subset of head and neck cancers (zur Hausen, 2009). In the currently held model for the HPV life cycle, HPVs enter cells within the basal layer of stratified squamous epithelia at sites of wounding, establish their genomes as nuclear plasmids in these cells, and only upon differentiation of daughter cells, produce progeny virus that are released into the environment to infect the next host (Sapp and Bienkowska-Haba, 2009; Schiller et al., 2010). Despite considerable research regarding the biology and oncogenicity of HPVs, the earliest steps of HPV infection responsible for the binding/entry of virus particles to the natural host cells, their internalization and delivery of the HPV genomes to the host nucleus are not yet well defined. In this study, we examined the cellular requirements for binding and entry.
The current literature on the cellular requirements for HPV infection is confusing. Different proteins have been identified to mediate HPV infection by different groups, including integrin α6β4 (Evander et al., 1997; McMillan et al., 1999) and its natural ligand laminin 5 located in the extracellular matrix that forms the basement membrane to which basal epithelial cells bind (Culp et al., 2006a; Culp et al., 2006b), HSPGs on cell surface (Giroglou et al., 2001; Joyce et al., 1999; Knappe et al., 2007; Shafti-Keramat et al., 2003; Surviladze et al., 2012) and in extracellular matrix (Selinka et al., 2007), as well as cyclophilins (Bienkowska-Haba et al., 2009; Bienkowska-Haba et al., 2012). While integrin α6β4 is debated as to its role in binding and entry of HPVs, there has arisen equally inconclusive data regarding the role of alternative cellular receptors in mediating HPV entry. Because papillomaviral VLPs have long been known to bind to a broad range of cell lines (Muller et al., 1995), this has led to the hypothesis that papillomaviral receptors must be highly conserved in their expression amongst different cell types. Several laboratories have described studies in tissue culture that support a role of HSPGs in HPV infection. A fairly well conserved motif in the carboxyl region of the major capsid protein, L1, which is conserved among different HPV genotypes, is similar to known heparin binding motifs (Joyce et al., 1999). HSPGs are reported in mediating entry of HPV16L1/L2 and HPV33L1/L2 PVs in COS-7 and HeLa cells (Giroglou et al., 2001); whereas, other studies refute a role of integrin α6β4 in entry of HPV33L1/L2 PVs to these same cells (Selinka et al., 2002). The amino acid residues of HPV16 L1 that mediate binding to HSPGs have been further defined (Knappe et al., 2007), and this binding was found to cause a conformational change in L1 that exposes a furin cleavage site in the minor capsid protein, L2, that was found to be critical for infection (Richards et al., 2006). Carrageenan, a class of sulfated polysaccharides extracted from algae, was found to inhibit HPV PVs infections in vitro (Buck et al., 2006) and in vivo (Roberts et al., 2007). Syndecan-1, a predominant HSPG in epithelial cells has a high binding affinity for HPV16 VLPs and increases susceptibility to infection by HPV11 (Shafti-Keramat et al., 2003), suggesting it may function as a HPV receptor. However, HPV31 infection of HaCaT cells was not inhibited by heparin or heparinase, indicating that the role of HSPGs might be cell type if not HPV genotype specific (Patterson et al., 2005). In contrast, syndecan-1 was argued to complex with HPV16 and may play a crucial role in infection (Surviladze et al., 2012). The loss of the activity of cyclophilins (CyP), especially Cyclophilin B (CyPB) which colocalizes with syndecan-1, blocks HPV16/18 infection in cells (Bienkowska-Haba et al., 2009). An interesting finding indicates that integrin α6β4 interacts and colocalizes with syndecan-1 (Wang et al., 2010). These findings complicate the interpretation of a role of integrin α6β4 and/or HSPGs in HPV infection process. In sum, there is considerable confusion as to what cellular proteins are important for HPV infection.
We posit that the differing and in many cases mutually exclusive conclusions reported in the literature at least in part reflect the choice of cells and culturing conditions used in past studies. We hypothesize that infection by HPV is mediated by multiple cellular proteins including integrin α6β4 and syndecan-1. To avoid the confusion rendered by cell culture studies, we tested our hypothesis in an animal model that provides us the means for monitoring early steps of binding and entry by HPV pseudoviruses (PVs) in a relevant tissue. If multiple proteins facilitate HPV infection in vivo, we may not observe a complete disruption of HPV infection when only a single cellular gene is deleted. In such circumstances, the efficiency of transduction by an HPV PS must be accessed in a dose-dependent manner to evaluate the relative importance of candidate proteins. To achieve the goal, we adapted a mouse model that permits one to monitor transduction by HPV PVs in a relevant tissue, the female reproductive tract (Roberts et al., 2007). We modified this in vivo model to allow us to quantify transduction by HPV16 pseudoviruses carrying the reporter gene Luciferase. This refined mouse model permitted us to quantify in vivo reporter gene transduction by HPV pseudoviruses in a dose-dependent manner, thereby providing us the means to access cellular requirements using genetically engineered mouse strains deficient for individual cellular genes and their gene products that have been previously implicated in HPV entry in tissue culture studies. Mice conditionally deficient for integrin α6β4, or nulligenic for syndecan-1 were evaluated for their susceptibility to reporter gene transduction by HPV pseudoviruses.
Results
Modification of a mouse model for quantifying the efficiency of reporter gene transduction by HPV PVs: a model for assessing genetic requirements for early steps in papillomavirus infection
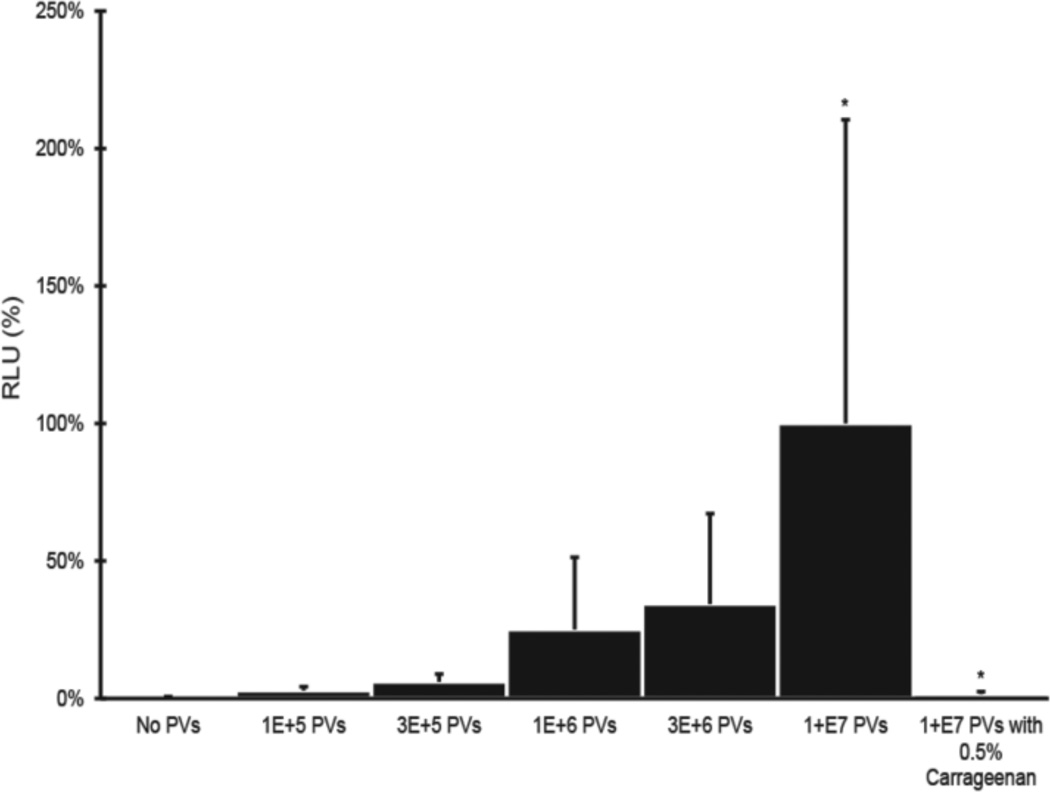
To test our hypothesis that multiple cellular proteins mediate HPV binding/entry together or independently, we first modified a previously described mouse model for in vivo transduction of reporter genes by HPV PVs (Roberts et al., 2007). HPV16 PVs (HPV16:LucF) containing both firefly luciferase and green fluorescent protein genes were generated, the titers of these stocks measured by flow cytometric measurement of GFP-positive cells exposed to serial dilutions of the PV stock, and then known titers of PV used to infect reproductive tracts of experimental mice. The luminescence (luciferase assay) from vaginocervical tissue lysates were subsequently measured quantitatively as readout for HPV infection. The relative luminescence units (RLU) per µg total protein (Bradford protein assay) corresponded to the amount of HPV16:LucF challenged in mice reproductive tracts (Fig.1). Our refined mouse model provides an alternative means to quantify the efficiency of HPV PV gene transduction in vivo, as a means for assessing genetic requirements for early steps in papillomavirus infection.
Fig. 1. HPV16 infection in vivo in a dose dependent.
Each group of mice reproductive tracts (N=6) were challenged with different amount of PVs (HPV16:LucF) as indicated. An additional group of mice were challenged with 1E+07 PVs and 0.5% carrageenan. 48 hours after infection, mouse female reproductive tracts were harvested, protein lysates made, and luciferase assays were carried out to quantify the infectivity. Concentrations of total proteins were measure by Bradford protein assay. The infectivity level (relative luminescence units, RLU / µg) of mice treated with 1E+07 PVs was set as 100%. * P = 0.004 (Wilcoxon rank sum test, 2 sided).
Infectivity of HPV, as scored by PV gene transduction, is significant reduced in K14Cre/Intβ4f/f mice
Integrin α6β4, restricted primarily to the basal layer of epithelia (Wilhelmsen et al., 2006), has been argued by some but not other investigators to mediate binding to and possibly the uptake of papillomavirus particles by cells (Evander et al., 1997; McMillan et al., 1999). The finding that laminin 5, the ECM partner to integrin α6β4, may serve to trap HPV particles to the ECM (Culp et al., 2006a; Culp et al., 2006b), provides another potential link to this integrin. This led us to hypothesize that integrin α6β4 in the relevant tissue may behave differently from what has been indicated in monolayer tissue culture. It has been proposed that a non-HSPG receptor is essential for HPV infection (Kines et al., 2009; Surviladze et al., 2012). That there are interactions between integrin α6β4 and syndecan-1 and others HSPGs (Wang et al., 2010) raises the possibility that integrin α6β4 may act independently or in conjunction with other cellular proteins in facilitating HPV infection in vivo. We applied our mouse model to access the role of integrin α6β4 in HPV infection in vivo by testing whether the efficiency of reporter gene transduction by HPV PVs is diminished significantly in mice deficient for integrin α6β4.
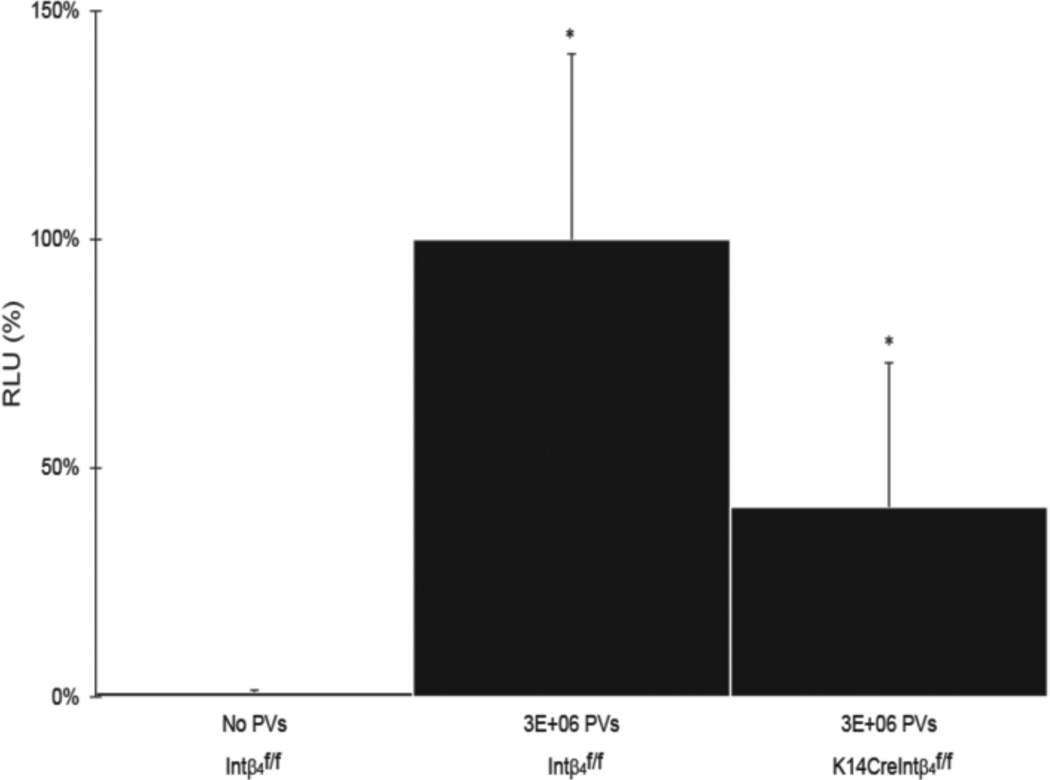
Because of the perinatal lethality resulting from loss of both alleles of integrin α6 or integrin β4 in the germ line (Raymond et al., 2005), we examine the requirement of integrin α6β4 for HPV PV gene transduction in vivo, making use of genetically engineered mice conditionally deficient for integrin β4 (K14CreIntβ4f/f) (Raymond et al., 2005) (integrin α6 conditional mice were not available for study). Cells of skin epithelia from K14CreIntβ4f/f mice that have undergone Cre-loxP mediated disruption of the conditional allele for integrin β4 also have undetectable levels of integrin α6. (Raymond et al., 2005). Importantly, there was no compensatory increase in expression of other integrins in the integrin β4 deficient cells in vivo (Raymond et al., 2005). We observed that the efficiency of HPV16 PVs gene transduction was modestly but significantly reduced in K14CreIntβ4f/f mice 48 hours post infection when compared to that in Intβ4f/f mice (Fig.2). This same reduction in infection was observed over 96 hours indicating that the reduction in infection was not simply due to a delay in infection (Fig.S1). In both the wild type and the integrin conditional null mice, the luciferase signal decreased over the time course with maximal expression seen at 48 hours. This was as expected because the rapid turnover of epithelial cells in the female reproductive tract results in sloughing off of infected cells by 72–96 hours. By 120 hours a difference in luciferase signal between the wild type and integrin conditional null mice was no longer observed and the level of luciferase activity was low in both strains, presumably reflective of the epithelial cell turnover. These data support the hypothesis that integrin α6β4 contributes quantitatively, but perhaps not qualitatively, to the efficiency of HPV infection in vivo.
Fig. 2. HPV16 infectivity in integrin α6β4 sufficient verse deficient mice.
Intβ4f/f and K14CreIntβ4f/f mice reproductive tracts (N=6) were challenged with or without 3E+06 PVs (HPV16:LucF) as indicated. 48 hours after infection, mouse female reproductive tracts were harvested, protein lysates made, and luciferase assays were carried out to quantify the infectivity. Concentrations of total proteins were measure by Bradford protein assay. The infectivity level (relative luminescence units, RLU / µg) of Intβ4f/f mice treated with 3E+06 PVs was set as 100%. * P = 0.04 (Wilcoxon rank sum test, 2 sided).
HPV PVs transduce reporter genes in portions of mouse female reproductive tract epithelia having little-to-no detectable levels of integrin α6
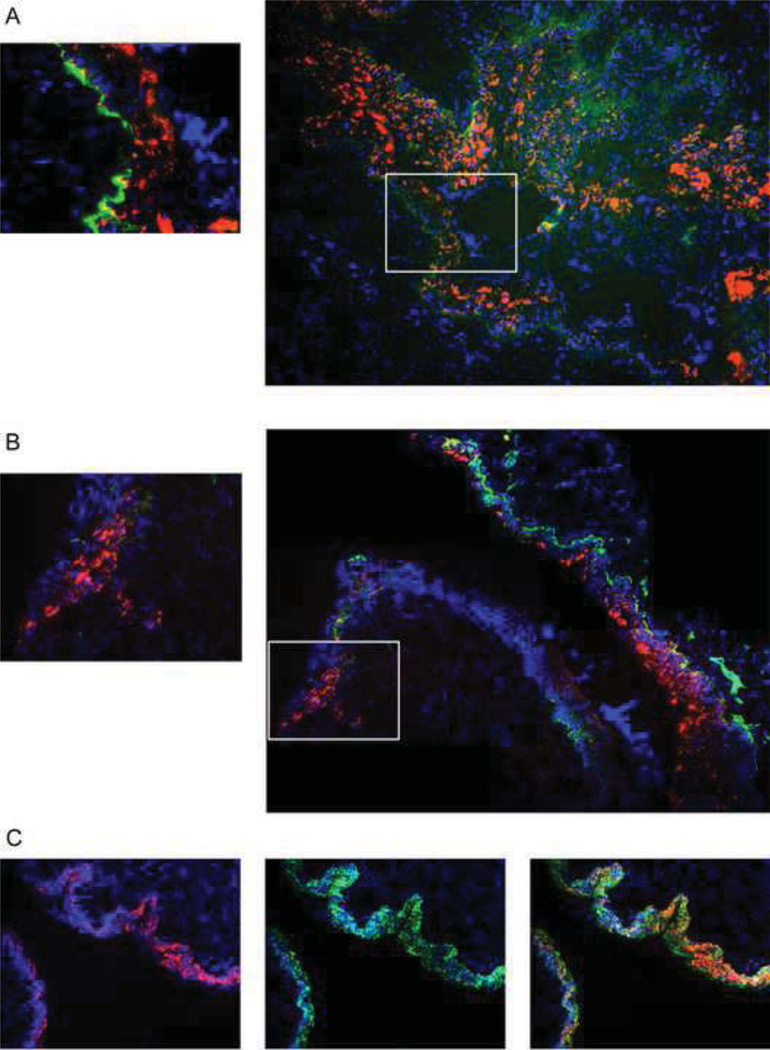
In the original study describing the phenotypes of the K14creIntβ4f/f mice in skin epithelia, it was observed that not all epithelial cells lost expression of integrin β4, and this correlated with retention of expression of integrin α6 (Raymond et al., 2005). To learn whether this is also the case in the female reproductive tract and to learn if HPV pseudoviruses selectively transducer those portions of the reproductive tract deficient for integrin expression, we performed integrin α6-specific immunofluorescence on K14creIntβ4f/f mice. In the female reproductive tract epithelia, Intβ4f/f mice express integrin α6 consistently in basal cells (Fig.3A); whereas, K14creIntβ4f/f mice lose expression of integrin α6 with patches of integrin α6-negative tissue interdigitated between patches of integrin α6-positive tissue (Fig.3B). This is consistent with what was observed in the skin of this same mouse genotype (Raymond et al., 2005). To look at susceptibility of integrin α6-positive versus integrin α6-negative tissue to HPV PV-mediated gene transduction, K14creIntβ4f/f and Intβ4f/f mice were exposed to HPV16:TWB PVs carrying the tdTomato gene and 72 hours post-exposure reproductive tracts were snap frozen, sectioned, stained with antibodies to integrin α6 and analyzed by fluorescence microscopy. We observed that most epithelial cells in the reproductive tract of the Intβ4f/f mice express integrin α6 in basement membrane and displayed robust tdTomato signal indicative of infection (Fig.3A). The tdTomato-positive cells were confirmed to be of epithelial origin by staining for Keratin 14, a marker for epithelial cells (Fig.3C). In the K14CreIntβ4f/f mice, we observed that most of the tdTomato positive epithelia accompany positive staining for integrin α6; however, there were clearly tdTomoato red positive cells in regions without detectable levels of integrin α6. From these observations, we conclude that cellular integrin α6β4 is not absolutely required for HPV infection in vivo, though it appears to influence the efficiency of infection.
Fig. 3. HPV16 PVs infection in the reproductive tract epithelia of integrin α6β4 sufficient verse deficient mice.
Intβ4f/f and K14CreIntβ4f/f mice reproductive tracts were challenged with 1E+07 PVs (HPV16:TWB). 72 hours after infection, mouse female reproductive tracts were harvested, snap frozen, cryosection made, DAPI staining (blue) for cell nuclei and tdTomato red fluorescence (red) indicated as viral infection were monitored by microscopy. (A) HPV16 PVs infection in Intβ4f/f mice; FITC (green) indicates the immunohistochemistry staining for integrin α6 (B) HPV16 PVs infection in K14CreIntβ4f/f mice; FITC (green) indicates the immunohistochemistry staining for integrin α6 (C) HPV16 PVs infection in Intβ4f/f mice; FITC (green) indicates the immunohistochemistry staining for Keratin14.
Syndecan-1, the predominant HSPG in epithelial cells, is not necessary for HPV infection in vivo, based upon PV gene transduction studies
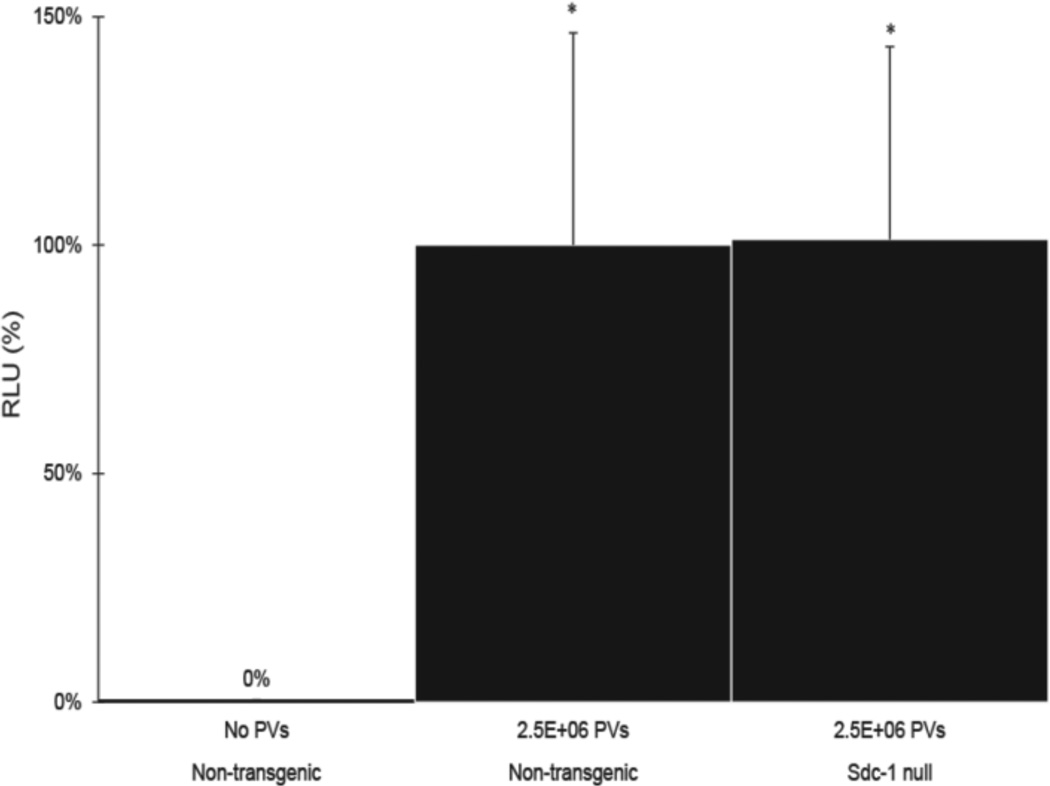
To address whether syndecan-1 is required for HPV infection, we first confirmed the binding of HPV16 PVs to mouse syndecan-1 by immunoprecipitation in mouse C127 cells (Fig. S2), and then monitored the efficiency of HPV PV –mediated gene transduction in syndecan-1 null mice. The efficiency of HPV PV-mediated gene transduction was not significantly different in syndecan-1-sufficient versus -null mice by monitoring the level of luciferase expression in extracts of female reproductive tracts of mice exposed to HPV16:Lucf PVs (Fig. 4). We also observed the same results when using HPV31 and HPV45 PVs (Fig. S3). These results argue that syndecan-1 is not essential for HPV infection in this mouse model. However, because carrageenan did inhibit in vivo HPV PV-mediated gene transduction in our hands (Fig.1) as reported by others (Roberts et al., 2007), this raises the likelihood that other HSPGs can mediate infection in the absence of syndecan-1.
Fig. 4. HPV16 infectivity in int-1 -sufficient versus -null mice.
Non-transgenic (syndecan-1-sufficient) and syndecan-1 null mice reproductive tracts (N=6) were challenged with or without 2.5E+06 PVs (HPV16:LucF) as indicated. 48 hours after infection, mouse female reproductive tracts were harvested, protein lysates made, and luciferase assays were carried out to quantify the infectivity. Concentrations of total proteins were measure by Bradford protein assay. The infectivity level (relative luminescence units, RLU / µg) of non-transgenic mice treated with 2.5E+06 PVs was set as 100%. * P = 0.87 (Wilcoxon rank sum test, 2 sided).
Discussion
Based upon our in vivo studies, we conclude that neither integrin α6β4 nor syndecan-1, two putative cell surface receptors for papillomaviruses are absolutely required for gene transduction of the mouse female reproductive tracts by HPV pseudoviruses. These results provide a strong rationale for pursuing the identification of alternative cell surface receptors for HPVs. One obvious possibility, in the case of syndecan-1 null mice, is that other HSPGs can serve as replacement receptors for syndecan-1. Earlier studies documented compensatory increases in other HS-modified proteins (Liu et al., 2003), and the surface expression of integrins (Stepp et al., 2007) in syndecan-1 null keratinocytes. Thus an obvious route of further study is to look at the possible role of these proteins that might also be increased in their expression in the female reproductive tracts of syndecan-1 null mice. That carrageenan is an effective inhibitor of HPV pseudovirus infection in the mouse female reproductive tract argues that HSPGs are important for HPV infection in this mouse model (Fig.1) (Roberts et al., 2007).
On the other hand, compensation is not likely to be an explanation for the retention of infectivity in areas of the female reproductive tract of K14CreIntβ4f/f mice that fail to express detectable integrin α6, as no compensatory increases in other integrins have been detected in these mice (Raymond et al., 2005). Also, the fact that integrin α6 was lost in the female reproductive tract of K14CreIntβ4f/f mice would argue that no alternative integrin α6 partner is induced, for, were a partner induced in its expression, then levels of integrin α6 would not have decreased. This leaves us to question the absolute necessity for any integrin in mediating HPV infection in vivo in this mouse model, even though there clearly is a quantitative reduction in infection in the K14CreIntβ4f/f mice.
Our study demonstrates the utility of mice in monitoring the cellular requirements for HPV infection, and points to their use in assessing the role of other cellular factors in mediating HPV infection. Our and others’ previous studies (Huang et al., 2010; Karanam et al., 2010) demonstrated that the strong antiviral activity of gamma secretase inhibitors both in vitro and in vivo. Gamma secretase is known to cleave a number of cell surface molecules including growth factor receptors (Kopan and Ilagan, 2004). One or more of these cell surface targets of gamma secretase may prove to be essential cell surface receptors for HPV infection. The availability of genetically engineered strains of mice deficient in these cellular genes provides a basis for definitive future studies.
Material and methods
Plasmids
p16SheLL, p31SheLL, and p45SheLL (~10.8 kilo base pair plasmids and too large to be packaged in HPV VLPs) that express the HPV major capsid protein, L1, and minor capsid protein, L2. pLucF, that encodes b oth firefly luciferase (Luc) and green fluorescent protein (GFP). pTWB, that encodes red fluorescent protein (tdTomato). These plasmids are gifts from Dr. Christopher Buck in National Cancer Institute.
Cell cultures
293FT cells expressing enhanced SV40 large T antigen (Invitrogen) were maintained in DMEM medium (high glucose) (Invitrogen) containing 10% fetal bovine serum (Harlan) supplemented with 0.1 mM MEM non-essential amino acids (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 2 mM L-glutamine (Invitrogen) and 500 ug/ml Geneticin. Mouse C127 cells were cultured in DMEM medium containing 10% fetal bovine serum.
Antibodies
Rat anti-human integrin α6 antibody conjugated with fluorescein isothiocyanate (FITC), GoH3 (Serotec). Rabbitt-anti mouse keratin14 antibody conjugated with FITC (Covance/BAbCO). Mouse anti-HPV16 L1 antibody (Abcam, ab69). Mouse anti-syndecan-1 antibody (Santa Cruz). Goat anti-mouse HRP light chain (Jackson Immunoresearch)
Generation of HPV16 pseudoviruses (PVs)
HPV virus-like particles (VLPs) carrying encapsidated reporter plasmids are referred in the papilomavirus field as HPV pseudoviruses (PVs). Reporter pseudovirions were produced using previously-described methods (Buck et al., 2004; Buck and Thompson, 2007). Briefly, two plasmids expressing HPV16 L1/L2 capsids proteins and reporter genes respectively were cotransfected into 293FT cells to generate HPV16 pseudovirions, HPV16:LucF or HPV16:TWB. Pseudovirions were harvested by detergent lysis of the cells 48 hours after transfection. Particles were allowed to mature overnight (Buck et al., 2005) and were purified by ultracentrifugation through OptiPrep Density Gradient Medium (Sigma). The titers of HPV16 PVs were determined by flow cytometric analysis of 293FT cells treated with various dilutions of purified pseudovirion stock. Alternatively, HPV31 and HPV45 PVs were generated as described as above.
Mouse model for quantifying the in vivo efficiency of HPV infection
HPV:LucF pseudoviruses were used to establish a quantitative mouse infection model for HPV in the female reproductive tract. The method is a modified version of a previously published model (Roberts et al., 2007). Indicated numbers of HPV:LucF PVs were delivered intravaginally in 4% carboxyl methyl cellulose (Sigama Cat. #C4888) using 6 ~ 8 weeks old virgin female mice. The mice were prepared by subcutaneous injection with 3 mg medroxyprogesterone acetate (Sicor, Depo-Provera ) 4 days prior pseudovirus challenge to induce diestrus. 6 hours prior to PVs instillation, the mice were pre-treated vaginally with with Conceptrol (Caldwell Consumer Health), an over the counter spermicide product containing 4% nonoxynol-9. Administration of nonoxynol-9 has previously been shown to potentiate HPV PVs infection due to chemical injury of the vaginal/cervical epithelium (Roberts et al., 2007). 48 hours after delivery of the PVs, mouse female reproductive tracts were harvested, homogenized in lysis buffer for luciferase assay by a tissue homogenizer (PowerGen 125, Fisher Scientific), and soluble tissue lysates were used to quantify the efficiency of infection by luciferase assay as per the manufacturer (Promega, Cat. #1501 and #1531).
Bradford protein assay
The amount of total proteins in reproductive tissue lysates used for luciferase assay was measured by Bradford protein assay (Bio-Rad Protein Assay Dye reagent Concentrate, Cat. #500-0006). The infectivity of PVs is indicated as relative luminescence units (RLU) per µg.
A time course of HPV16 infection in integrin α6 sufficient verse deficient mice
Intβ4f/f and K14CreIntβ4f/f mice reproductive tracts were challenged as described as above with or without 3E+06 HPV16:LucF PVs. Mouse female reproductive tracts were harvested at 48, 72, 96, and 120 hour post infection, protein lysates made, and luciferase assays were carried out to quantify the infectivity. Concentrations of total proteins were measure by Bradford protein assay.
Cell susceptibility for HPV16 infection in integrin α6β4 sufficient verse deficient mice
Alternatively, experiments describe above were repeated using 1E+07 HPV16:TWB PVs in Intβ4f/f and K14CreIntβ4f/f mice. 72 hours after delivery of the PVs, mouse female reproductive tracts were harvested, snap frozen, cryosection made, and tdTomato red fluorescence expression indicated as viral infection were monitored by microscopy (Axiovert 200M, Carl Zeiss Microimiaging).
Immunohistochemistry staining for integrin and Keratin 14
Mouse female reproductive tracts infected with HPV16:TWB were collected, embedded in cryoprotectant (Tissue-Tek O.C.T., Sakura Finetek Europ, Zoeterwoude, The Netherlands). Cryosections were prepared, fixed in ice-cold acetone, and blocked in PBS with 5% Bovine Serum Albumin (BSA, A2153, Sigma) or 5% horse serum (H0146, Sigma) for one hour at room temperature. Cryosections are then incubated with 1:100 dilution of primary Ab conjugated with FITC in blocking solution at 4 °C overnight. Slides mounting medium with 4'-6-Diamidino-2-phenylindole (DAPI) (Vectashield, hard set, H-1500, Vector Laboratories) was used for cell nuclei staining. Fluorescence images were taken by Carl Zeiss microscopy.
Immunoprecipitation (IP)/Westerns
1E+07 mouse C127 cells were exposed to 1E+08 HPV16 PVs (10 M.O.I.) for 4 hours, and then treated or untreated (Mock Infection) with water-soluble cross-linker (1 mM DTSSP, Thermo Scientific) in cold PBS and incubated for 30 min at 4°C. Cells were washed three times with cold PBS and quenched with 50 mM Tris pH 7.5 for 15 min at 4°C. Cell were washed three times with cold PBS and solubilized with 1ml cold lysis buffer (1% TX100, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM PMSF, 10 ng/ml leupeptin, 10 ng/ml aprotinin). Save soluble proteins in supernatants by centrifugation at 13,000 rpm for 10 min. Add mouse anti HPV16 L1 antibody with protein A-agarose (Thermo Scientific, Pierce) beads for 1h at 4°C. Beads were washed twice with cold lysis buffer, and resuspended in 20 ul Laemmli sample buffer and boiled for 5 min. Solubilized proteins were resolved by 12 % SDS-PAGE (Boi-Rad) and were electroblotted onto PVDF membranes. Membranes were blocked, and probed with primary antibodies (mouse anti-HPV16 L1 or anti-syndecan-1) and anti-mouse secondary antibody. Antibody/protein complexes on blots were detected using enhanced chemiluminescence (Amersham Biosciences).
HPV infections in syndecan-1 sufficient verse null mice
Alternatively, experiments describe above were repeated using 2.5E+06 HPV16:LucF/HPV31:LucF/HPV45:LucF PVs in non-transgenic (syndecan-1 sufficient) and syndecan-1 null mice. Mouse female reproductive tracts were harvested at 48 hour post infection, protein lysates made, and luciferase assays were carried out to quantify the infectivity. Concentrations of total proteins were measure by Bradford protein assay.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bienkowska-Haba M, Patel HD, Sapp M. Target cell cyclophilins facilitate human papillomavirus type 16 infection. PLoS Pathog. 2009;5:e1000524. doi: 10.1371/journal.ppat.1000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowska-Haba M, Williams C, Kim SM, Garcea RL, Sapp M. Cyclophilins Facilitate Dissociation of the HPV16 Capsid Protein L1 from the L2/DNA Complex Following Virus Entry. J Virol. 2012 doi: 10.1128/JVI.00980-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Thompson CD. Production of papillomavirus-based gene transfer vectors. Chapter 26, Unit 26 21. Curr Protoc Cell Biol. 2007 doi: 10.1002/0471143030.cb2601s37. [DOI] [PubMed] [Google Scholar]
- Buck CB, Thompson CD, Pang YY, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. J Virol. 2005;79:2839–2846. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Thompson CD, Roberts JN, Muller M, Lowy DR, Schiller JT. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp TD, Budgeon LR, Christensen ND. Human papillomaviruses bind a basal extracellular matrix component secreted by keratinocytes which is distinct from a membrane-associated receptor. Virology. 2006a;347:147–159. doi: 10.1016/j.virol.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Culp TD, Budgeon LR, Marinkovich MP, Meneguzzi G, Christensen ND. Keratinocyte-secreted laminin 5 can function as a transient receptor for human papillomaviruses by binding virions and transferring them to adjacent cells. J Virol. 2006b;80:8940–8950. doi: 10.1128/JVI.00724-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evander M, Frazer IH, Payne E, Qi YM, Hengst K, McMillan NA. Identification of the alpha6 integrin as a candidate receptor for papillomaviruses. J Virol. 1997;71:2449–2456. doi: 10.1128/jvi.71.3.2449-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroglou T, Florin L, Schafer F, Streeck RE, Sapp M. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75:1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HS, Buck CB, Lambert PF. Inhibition of gamma secretase blocks HPV infection. Virology. 2010;407:391–396. doi: 10.1016/j.virol.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, Sands JA, Jansen KU, Keller PM. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. The Journal of biological chemistry. 1999;274:5810–5822. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]
- Karanam B, Peng S, Li T, Buck C, Day PM, Roden RBS. Papillomavirus infection requires gamma secretase. Journal of virology. 2010;84:10661–10670. doi: 10.1128/JVI.01081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci U S A. 2009;106:20458–20463. doi: 10.1073/pnas.0908502106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe M, Bodevin S, Selinka HC, Spillmann D, Streeck RE, Chen XS, Lindahl U, Sapp M. Surface-exposed amino acid residues of HPV16 L1 protein mediating interaction with cell surface heparan sulfate. The Journal of biological chemistry. 2007;282:27913–27922. doi: 10.1074/jbc.M705127200. [DOI] [PubMed] [Google Scholar]
- Kopan R, Ilagan MX. Gamma-secretase: proteasome of the membrane? Nature reviews. Molecular cell biology. 2004;5:499–504. doi: 10.1038/nrm1406. [DOI] [PubMed] [Google Scholar]
- Liu BY, Kim YC, Leatherberry V, Cowin P, Alexander CM. Mammary gland development requires syndecan-1 to create a beta-catenin/TCF-responsive mammary epithelial subpopulation. Oncogene. 2003;22:9243–9253. doi: 10.1038/sj.onc.1207217. [DOI] [PubMed] [Google Scholar]
- McMillan NA, Payne E, Frazer IH, Evander M. Expression of the alpha6 integrin confers papillomavirus binding upon receptor-negative B-cells. Virology. 1999;261:271–279. doi: 10.1006/viro.1999.9825. [DOI] [PubMed] [Google Scholar]
- Muller M, Gissmann L, Cristiano RJ, Sun XY, Frazer IH, Jenson AB, Alonso A, Zentgraf H, Zhou J. Papillomavirus capsid binding and uptake by cells from different tissues and species. J Virol. 1995;69:948–954. doi: 10.1128/jvi.69.2.948-954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson NA, Smith JL, Ozbun MA. Human papillomavirus type 31b infection of human keratinocytes does not require heparan sulfate. J Virol. 2005;79:6838–6847. doi: 10.1128/JVI.79.11.6838-6847.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond K, Kreft M, Janssen H, Calafat J, Sonnenberg A. Keratinocytes display normal proliferation, survival and differentiation in conditional beta4-integrin knockout mice. Journal of cell science. 2005;118:1045–1060. doi: 10.1242/jcs.01689. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Sapp M, Bienkowska-Haba M. Viral entry mechanisms: human papillomavirus and a long journey from extracellular matrix to the nucleus. The FEBS journal. 2009;276:7206–7216. doi: 10.1111/j.1742-4658.2009.07400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller JT, Day PM, Kines RC. Current understanding of the mechanism of HPV infection. Gynecologic oncology. 2010;118:S12–S17. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinka HC, Florin L, Patel HD, Freitag K, Schmidtke M, Makarov VA, Sapp M. Inhibition of transfer to secondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papillomavirus. J Virol. 2007;81:10970–10980. doi: 10.1128/JVI.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinka HC, Giroglou T, Sapp M. Analysis of the infectious entry pathway of human papillomavirus type 33 pseudovirions. Virology. 2002;299:279–287. doi: 10.1006/viro.2001.1493. [DOI] [PubMed] [Google Scholar]
- Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77:13125–13135. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Liu Y, Pal-Ghosh S, Jurjus RA, Tadvalkar G, Sekaran A, Losicco K, Jiang L, Larsen M, Li L, Yuspa SH. Reduced migration, altered matrix and enhanced TGFbeta1 signaling are signatures of mouse keratinocytes lacking Sdc1. Journal of cell science. 2007;120:2851–2863. doi: 10.1242/jcs.03480. [DOI] [PubMed] [Google Scholar]
- Surviladze Z, Dziduszko A, Ozbun MA. Essential roles for soluble virion-associated heparan sulfonated proteoglycans and growth factors in human papillomavirus infections. PLoS Pathog. 2012;8:e1002519. doi: 10.1371/journal.ppat.1002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Leavitt L, Ramaswamy R, Rapraeger AC. Interaction of syndecan and alpha6beta4 integrin cytoplasmic domains: regulation of ErbB2-mediated integrin activation. The Journal of biological chemistry. 2010;285:13569–13579. doi: 10.1074/jbc.M110.102137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, Sonnenberg A. Multiple functions of the integrin alpha6beta4 in epidermal homeostasis and tumorigenesis. Molecular and cellular biology. 2006;26:2877–2886. doi: 10.1128/MCB.26.8.2877-2886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in the causation of human cancers - a brief historical account. Virology. 2009;384:260–265. doi: 10.1016/j.virol.2008.11.046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.