Introduction
Glioblastoma multiforme is the most common and aggressive form of CNS tumors. Though current therapies including surgical removal, chemotherapy and radiation seem to extend the survival of the affected patients, the current prognosis still has a median survival of less than 1 year. Surgical removal and radiation therapy crudely target large regions of the brain while chemotherapy targets all dividing cells of the body, thus these methods tend to leave the patient with considerable side effects (Sughrue et al., 2009). Moreover, the lack of effectiveness of these treatments has left researchers looking for alternative forms of treatment. One significant alternative is the use of the immune system, with its high specificity, it has the potential to target and eliminate the tumor cells directly; though knowledge of the immune environment within and around gliomas is lacking. Furthermore, the markers unique to glioma cells and the extent of immunosuppression in the glioma milieu remain largely unknown.
Matricellular proteins are secreted into the extracellular space and interact with cell surface receptors, proteases and structural proteins such as collagen (Bornstein, 2009). SPARC (secreted protein acidic and rich in cysteine, osteonectin) is a matricellular protein which promotes cell migration as it facilitates an intermediate stage of adhesion as opposed to the strong adhesion of most matricellular proteins (Bornstein, 2002). The role of SPARC in cell migration makes it an important factor during normal development, wound healing and tissue remodeling (Workman and Sage, 2011; Framson and Sage, 2004; Brekken and Sage, 2001). SPARC is upregulated by glioma cells (Rempel, 1998), and is associated with increased tumor metastasis and proliferation (Golembieski, 1999; Schultz, 2002; Rich, 2003; Schittenhelm, 2006). This may be partially due to the increase of several proteases, which leads to tissue degradation and allows room for infiltrative tumor cells to migrate (Golembieski, 2008). Targeting and decreasing SPARC expression with siRNA has proven to decrease tumor invasiveness and tumor cell survival (Seno et al., 2009; Shi et al., 2007).
Recently, the scavenger receptor stabilin-1 was identified as the first known receptor for SPARC. When bound, SPARC is internalized by stabilin-1 and rendered to the endosomal pathway where it is subsequently degraded (Kzhyshkowska, 2006). Stabilin-1 is expressed on the surface of alternatively activated macrophages (AAMø) which participate in wound healing and in the anti-inflammatory process (Kzhyshkowska, 2004; Park et al., 2009; Palani et al, 2011; Mosser and Edwards, 2008). Although SPARC is known to exist in the tumor environment, the presence or location of its receptors are not yet known. The capacity of stabilin-1 expressing macrophages to clear SPARC has the potential to be significant in the control of glioma growth and proliferation.
Macrophages display a wide spectrum of activation states which are generalized as either classically activated (M1) or alternatively activated (M2) (Mosser and Edwards, 2008). In contrast to the microbicidal, inflammatory classically activated macrophages; M2 macrophages are anti-inflammatory and are involved in tissue repair through upregulation of CXCR3, IL-10, arginase-1, the mannose receptor MMR and the scavenger receptor stabilin-1 (Mosser and Edwards, 2008; Kzhyshkowska, 2006; Kzhyshkowska, 2004). During glioblastomas, a population of macrophages associates with the tumor: tumor associated macrophages (TAMs). This population of macrophages comprises a significant proportion of the tumor, accounting for up to 50% of the tumor mass (Solinas, 2009). The phenotype of TAMs is not yet well defined, though it is generally accepted that TAMs are primarily M2 activated. Furthermore, TAMs display pro-tumor functions: promoting tumor cell survival, proliferation and metastasis (Mantovani, 2002; Luo, 2006; Talmadge, 2007; Solinas, 2009; Gordon, 2010; Zhang, 2011). Indeed, high levels of TAMs are often associated with a worse prognosis (Bingle, 2002; Zhang, 2011).
In this study, we investigated the phenotype of tumor associated macrophages in an attempt to clarify their activation status and whether or not they expressed stabilin-1. Here we demonstrate a significant increase of total lymphocyte and macrophage infiltration in the glioma injected hemisphere (GL) two weeks post injection, a two fold increase in stabilin-1 and MMR expression compared to the naïve hemisphere at day 7 post injection and reduction of stabilin-1 expression in the glioma hemisphere by day 14 post injection.
These data demonstrate the presence of stabilin-1 at the site of initial glioma growth but then a downregulation of stabilin-1 as the tumor progresses. It is well known that tumors can cause phenotypic changes in infiltrating immune cells and thus may be responsible for the change in stabilin-1 expression. Increasing this receptor expression on infiltrating macrophages may help in the control of glioma growth.
Results
Scavenger receptor stabilin-1 is expressed in early glioma
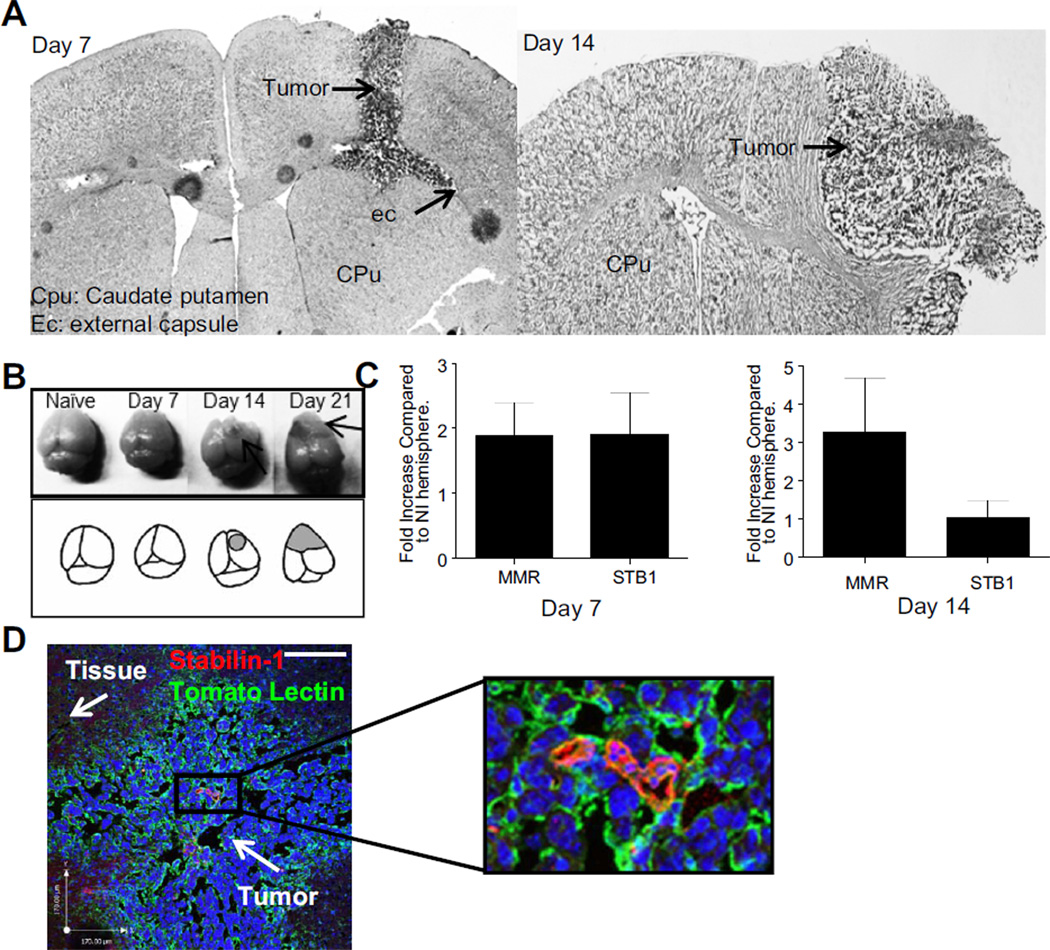
A model of glioma was used in this study where GL-26 cells were injected intra-cranially and allowed to grow for either 7, 14 or 21 days (Fig1A, B). Injection of 90,000 GL-26 cells in the caudate putamen (CPu) leads to a stable tumor which initially grows in the needle tract before expanding in the border between the striatum and the external capsule (ec). By day 14, the needle tract is mostly closed and the tumor expands from the dorsal portion of the caudate putamen to the exterior of the cortex (Fig1A) and by day 21, the tumor has expanded to both hemispheres (Fig1B).
Figure 1. Stabilin-1 expression in the tumor environment.
12 µm brain coronal sections of day 7 or 14 post injection with GL-26 were stained with haematoxylin followed by eosin to confirm tumor presence and growth (A). Naïve, day 7, 14 and 21 post GL-26 injected brains are photographed prior to sectioning, shading and arrows represents tumor area (B). Brains were taken at day 7 or 14 post injection with GL-26 and separated by hemisphere (GL-26 injected and non-injected). RNA was extracted for real time PCR analysis and the differential CT method was used to plot stabilin-1 (STB1) and MMR expression in the injected hemisphere relative to the naïve hemisphere (NI) (C). Immunofluorescent staining for tomato lectin (green) and stabilin-1 (red) was performed on 18 µm thick section from brains extracted 7 days post glioma injection (D). Scale bar is 170 µm.
To determine if stabilin-1 is present in the tumor environment, expression of stabilin-1 was measured using RT-qPCR at day 7 post injection in the right striatum. Furthermore, as stabilin-1 has been shown to be predominantly expressed on AAMø, expression of MMR was also measured to test if AAMø were present in our tumor model. Both stabilin-1 and MMR transcripts are up-regulated 1.912 ± 0.6391 and 1.881 ± 0.5023 times (Fig1C) respectively in the injected hemisphere (GL) when compared to the non-injected hemisphere (NI).
At day 14, although high levels of MMR are maintained on the injected hemisphere, the expression of stabilin-1 was reduced (Fig1C). Indeed, at day 14 post glioma injection, MMR expression increased 3.265 ± 1.423 fold higher in the injected hemisphere whereas stabilin-1 expression was reduced to equal levels in both hemispheres (1.031 ± 0.4456 fold higher in the injected hemisphere compared to non-injected hemisphere).
Immunofluorescent staining at day 7 post injection for stabilin-1 and tomato lectin at the site of the tumor reveals both membrane and cytoplasmic stabilin-1 within the tumor tissue (Fig1D). Tomato lectin is known to stain for macrophages, blood vessels and microglia. These images suggest a small population of infiltrating macrophages which express stabilin-1 within gliomas.
These data demonstrate the presence of a growing glioma at day 7 and 14 post injection which is associated with increased MMR expression and early (day 7) stabilin-1 upregulation in the injected hemisphere.
Stabilin-1 expression on macrophages decreases with tumor growth
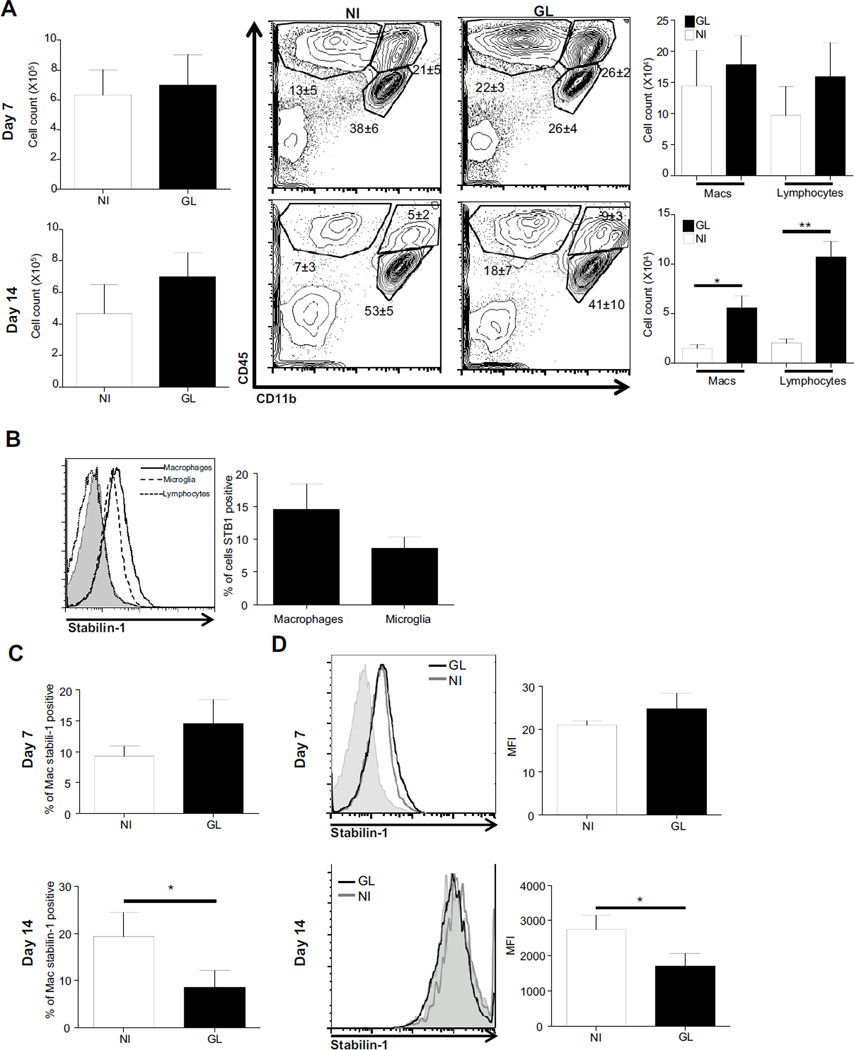
To determine the cell types which express stabilin-1 in the tumor environment, infiltrating macrophages, lymphocytes and local microglial populations were analyzed at day 7 and 14 post glioma injection by flow cytometry. At day 7 post injection, there were no differences in total mononuclear cell numbers between the non-injected and injected hemisphere (Fig2A). Furthermore, no differences in macrophage, lymphocyte or microglial populations either in composition or cell numbers between the non-injected hemisphere and the injected hemisphere were apparent (Fig2A). Of the cells analyzed, stabilin-1 expression was detected on macrophages and microglia but not lymphocytes (Fig2B). The proportion of stabilin-1 expressing macrophages (Fig2C) and the levels of expression (Fig2D) were not significantly different between the injected and the non-injected hemisphere at day 7 post injection.
Figure 2. Stabilin-1 expression on macrophages.
Brain mononuclear cells were extracted, counted and stained for CD45 and CD11b allowing for differentiation of lymphocyte, macrophage and microglial populations (A). Cells were also stained for stabilin-1, and cells expressing stabilin-1 were identified (B). Proportion of total macrophages at day 7 and 14 post injection which are positive for stabilin-1 (C), and expression levels measured with mean fluorescent intensity for stabilin-1 at day 7 and day 14 post injection (D) were determined. NI: Non-injected hemisphere, GL: glioma injected hemisphere.
By day 14, although there were no significant differences observed in the total number of mononuclear cells recovered from the injected and non-injected hemisphere, the increase in glioma size was accompanied by an increase in infiltrating macrophage (p= 0.0371) and lymphocyte (p= 0.0056) numbers in the injected hemisphere (Fig2A). This indicates a growing adaptive T-cell associated response. The proportion of stabilin-1 expressing macrophages were significantly lower (p= 0.0322) in the injected compared to the non-injected hemisphere (Fig2C). Experiments were conducted on separate days and thus a direct comparison cannot be made between expression levels (MFI) between day 7 and day 14. However, the level of stabilin-1 expression on infiltrating macrophages at day 14 was significantly lower (p=0.0329) in the tumor associated hemisphere when compared to the non-injected hemisphere (Fig2D).
These data suggest that stabilin-1 is expressed on AAMø and that this expression is less likely to be associated with the tumor environment as the glioma progresses.
Stabilin-1 expressing macrophages also express CD40, MMR
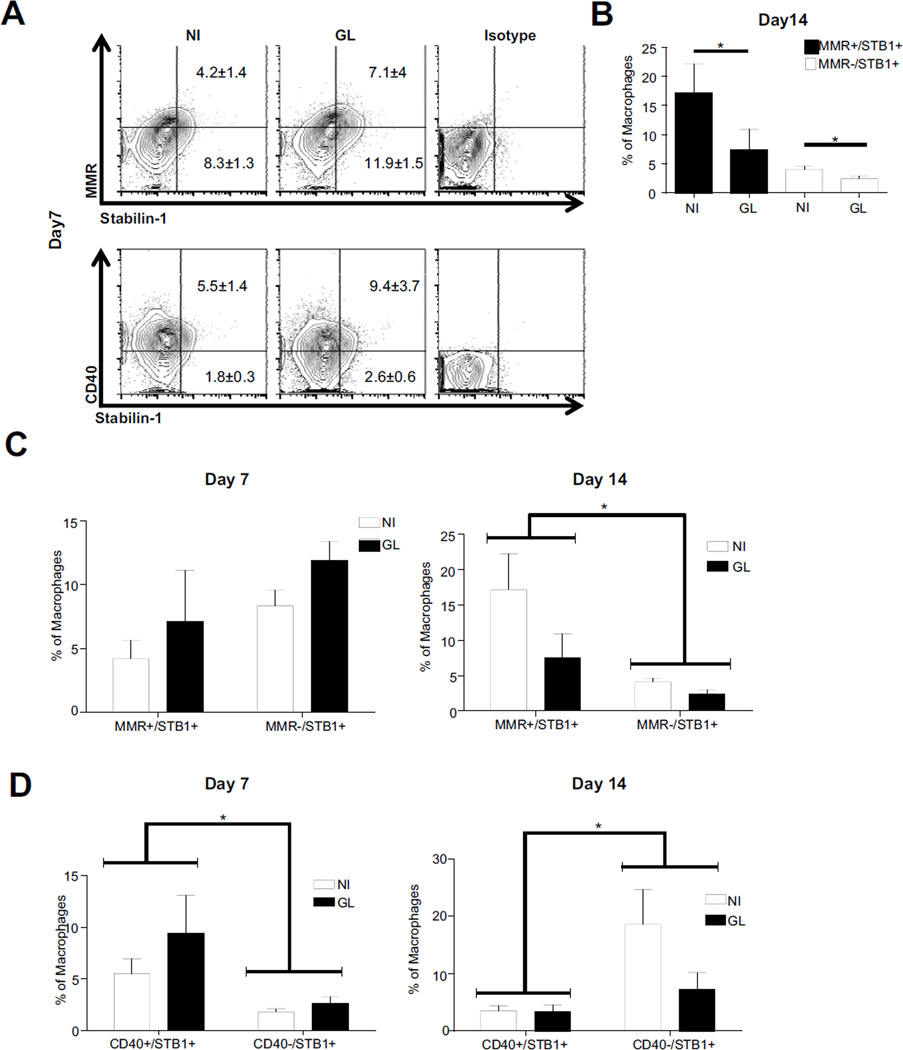
In order to define the phenotype of stabilin-1 expressing macrophages, CD40 and MMR expression was measured as representation of classical and alternative activation respectively. At day 7, stabilin-1 was present on both alternative and CD40+ macrophages (Fig3A). As the tumor progresses, there is a loss of stabilin-1 expression on AAMø in the injected compared to the non-injected hemisphere by day 14 (p=0.0443) (Fig3B). Proportions of CD40 expressing macrophages also expressing stabilin-1 are not significantly different between hemispheres.
Figure 3. Stabilin-1 expressing macrophages change as the tumor progresses.
Stabilin-1 expressing macrophage populations were analyzed for co-expression of MMR and CD40 at day 7 post injection (A). Analysis of proportions of stabilin-1 (STB1+) macrophages for co-expression of MMR at day 14 (B). Macrophage populations in glioma brains at day 7 and 14 post injection were analyzed for M1 and M2 phenotypes using MMR+/STB1+ or MMR−/STB1+ (C) and CD40+/STB1+ or CD40−/STB1+ (D).
A group analysis at day 7 post injection reveals no differences in stabilin-1 expression on AAMø population and MMR− macrophages. As the tumor progresses, stabilin-1 is preferentially expressed on AAMø by day 14 (Fig3C) (two-way ANOVA p=0.0192). Furthermore, there is a significantly larger population of CD40+ macrophages expressing stabilin-1 at day 7 (two-way ANOVA p=0.0312) compared to CD40− macrophages. This expression dramatically shifts at day 14 resulting in preferential stabilin-1 expression on CD40− macrophage populations as opposed to M1 (Fig3D) (two-way ANOVA p=0.0279).
This data suggests a shift in the cell phenotype which expresses stabilin-1 between day 7 and 14 post injection. Initially, stabilin-1 is expressed on both CD40+ and MMR+ macrophage phenotypes. However, as the tumor progresses and stabilin-1 is downregulated, it is mostly expressed on the MMR+ macrophages and is dramatically reduced on CD40+ macrophages.
Discussion
In this report, we stereotactically injected GL-26 tumor cells in the right hemisphere of mice brains to determine the phenotype and progression of the macrophage response during the first two weeks of tumor growth. GL-26 cells are very invasive, proliferate fast and form a glioblastoma in the mouse with similar properties to the glioblastomas seen in humans.
The role of SPARC in tumor proliferation and metastasis has been described since the late 1990’s, but besides recent siRNA gene silencing of SPARC, no study has addressed a mechanism for blocking or degrading SPARC as a potentially therapeutic target in the control of gliomas (Rempel, 1998; Golembieski, 1999; Schultz, 2002; Rich, 2003; Seno, 2009; Shi, 2007). stabilin-1 is the only known receptor of SPARC and works as a scavenger receptor, internalizing and degrading bound SPARC (Kzhyshkowska, 2006; Kzhyshkowska, 2012). Being expressed on macrophages, stabilin-1 has the potential of being expressed in the tumor microenvironment and slow the tumor proliferation by decreasing local SPARC levels.
It is well known, however, that TAMs change phenotypes with the progression of the tumor (Ruffell, 2012; Mantovani, 2002; Luo, 2006; Talmadge, 2007; Solinas, 2009; Gordon, 2010; Zhang, 2011). Indeed, as the tumor progresses, factors that promote the pro-tumorigenic polarization of TAMs can be divided into those derived from the immune system, from the tumor cells or from tissue stress. TAMs receive IL-10, IL-4 and IL-13 signaling from T-regs and Th2 cells, IL-10 and CCL2 signals from the tumor cells and sense hypoxia and ECM degradation in the tumor microenvironment. These signals cause the TAMs to in turn suppress the immune system with CCL22 and ROS secretion, promote tumor invasion through ECM remodeling and EGF secretion and finally, promote angiogenesis at the tumor site via VEGFA secretion (Ruffell et al, 2012; Mantovani, 2011). Though macrophages have the potential of expressing the SPARC scavenger receptor stabilin-1, it is unknown whether TAMs express stabilin-1 and whether this expression is maintained when TAMs become pro-tumorigenic. It should be noted that TAMS are known to also secrete SPARC. Whether SPARC expressing TAMS are also expressing stabilin-1 in a self-regulatory process or whether these are two distinct macrophage populations is not known and warrants further investigation. Our studies show a time dependent shift in the stabilin-1 expressing population. The disappearance of this phenotype as the tumor progresses may be due to tumor derived signals or intrinsic to the function of these macrophages leading to the dominant presence of SPARC.
In this report, we examined the macrophages in the tumor microenvironment for stabilin-1 expression during the initial progression of the tumor. We show an initial upregulation of stabilin-1 transcripts in the hemisphere injected with GL-26 cells which is not maintained two weeks post injection. Furthermore, we show an initial population of stabilin-1 expressing macrophages which is significantly reduced at day 14 post injection. At day 7, the stabilin-1 expressing macrophages are activated as demonstrated by their high co-expression of CD40. At day 14 however, the macrophages still expressing stabilin-1 are more polarized towards the M2 phenotype, as seen by the co-expression of MMR. These results suggest an initial anti-tumor attempt by the activated macrophage to express stabilin-1 in the tumor environment. Possibly due to signals from the tumor cells and tissue disruption, TAMs stop expressing stabilin-1 as they switch phenotypes to one that promotes tissue remodeling and angiogenesis.
The question that remains is obviously whether or not stabilin-1 expressing TAMs could possibly slow tumor progression or change the tumor microenvironment. Though we have not conducted these studies, overexpressing stabilin-1 in macrophages in glioma bearing mice would be a crucial next step in characterizing the role of this subset of macrophages in the tumor environment. In addition, although our model uses the GL-26 line due to its similarity in aggressiveness and proliferative capabilities to human glioblastomas, the tumor in human glioblastomas differs from the mouse GL-26 line. Indeed, human glioblastomas arise from several diverse cell types which can originate from neural, myeloid and glial origins (Seyfried et al., 2001; Yuan et al., 2004; Huysentruyt et al., 2011). What role stabilin-1 could play in this situation remains to be investigated.
It has been shown through siRNA targeting of SPARC that depletion of SPARC slows tumor progression and metastasis (Seno et al., 2009; Shi et al., 2007). Though these results are exciting, they are not therapeutically relevant to humans. However, degradation of SPARC through stabilin-1 in our own macrophages deserves more attention as a potential target for glioblastoma therapy.
Materials and Methods
Mice and Injections
Female C57BL/6 were obtained from Jackson Laboratories and maintained in a pathogen free environment under IACUC established protocols at the University of California Riverside. The murine (C57BL/6) glioma cell line, GL-26, which is highly tumorigenic in the C57BL/6 mice, was obtained as a generous gift from Dr. Pedro Lowenstein. GL26 cells were cultured in DMEM/F12 supplemented with 10% FCS, 1% penicillin/streptomycin, 1% L-glutamine and 1% non-essential amino acids. Cultured GL-26 cells were then harvested by trypsinization and 90,000 GL-26 cells in 3µL of sterile PBS was injected intracranially 1mm anterior and 2 mm lateral to the junction of the coronal and sagittal sutures (bregma) at a depth of 2mm using a stereotactic mouse frame. Mice were anesthetized with ketamine (1:5) and xylazine (1:50) in sterile PBS with volume injected determined by individual mouse weight.
Histology
Mice were perfused intracardially with 4% paraforlmaldehyde (PFA) in PBS and brains were extracted, incubated in 4% PFA overnight and then 30% sucrose in PBS until brains equalized. Next, brains were flash frozen in isopentane, embedded in OCT and cryosectioned in coronal sections (12 µm). Sections were stained with eosin and haematoxylin.
Real Time PCR
Stabilin-1 specific primers (forward 5′-GCTTTGAACCTCAGCCACTC-3′ and reverse 5′-GGCAATTACACTGCCCACTT-3′) and macrophage mannose receptor specific primers (forward5′- CTCTGTTCAGCTATTGGACGC-3′ and reverse 5′- TGGCACTCCCAAACATAATTT-3′) for Real Time PCR were purchased from IDT's primer Quest (http://www.idtdna.com/Scitools/Applications/Primerquest/). cDNA synthesis and Real-time PCR was performed using the Bioline One-step Kit with the CFX-96 real-time PCR Detection System (Bio-Rad). The reaction total was a 20µl mixture with 10µl SYBR Green/SensiFAST qPCR Master Mix (2×) and 400nM primer. The reaction conditions were as follows: 10 min at 45°C, followed by 2 min at 95°C and then 40 cycles of 5s at 95°C and 20s at 60°C. The HPRT (Hypoxine Phosphoribosyl- Transferase) forward primer (5′-CCCTCTGGTAGATTGTCGCTTA-3′) and reverse primer (5′AGATGCTGTTACTGATAGGAAATTGA -3′) were used as an endogenous control. Quantified results represent the fold induction of target gene expression using the differential CT method. NTC, no-template control (reagent alone without template) was included in each assay to detect any possible contamination of the PCR reagents.
Preparation of Brain Mononuclear Cells (BMNCs) suspension
For BMNCs, harvested brain tissues were minced with a razor blade and passed multiple times through an 18-gauge needle. The suspension was then incubated with 100µl of collagenase/dispase (1mg/ml) (Roche Diagnostics, Indianapolis, IN) for 45 min at 37°C and then a further 45 min at 37°C with 300µl DNAse (10 mg/ml) (Sigma). Following enzymatic digestion, the cell suspension was passed through a 70µm cell strainer, resuspended in 40 ml of RPMI complete and centrifuged at 1200rpm for 10 min at 4°C. The pellet was resuspended in 60% isotonic Percoll (GE Healthcare Bioscience, Uppsala, Sweden) solution (in RPMI complete) and overlaid with 30% percoll solution made in 1× sterile PBS. The Percoll gradient was centrifuged at 2000rpm for 25 min at 25°C without brakes. After centrifugation, the myelin layer on top of the gradient was removed. BMNCs (lymphocytes, macrophages, dendritic cells and microglia) were harvested from the 30%–60% percoll interphase and washed twice in complete RPMI medium for further analysis.
Immunofluorescent staining
Brains from C57BL/6 mice injected with gliomas were extracted 7 days post injection and frozen in OCT. Serial section (18 microns) were cut and incubated with primary antibodies for stabilin-1 (Santa Cruz antibodies) and biotinylated tomato lectin for 3 hours at room temperature. Secondary antibodies (streptavidin 488 and donkey anti goat 568) were incubated for 2 hours in room temperature. Slides were then mounted with ProLong Gold anti fade reagent with DAPI (Life Technologies).
Flow Cytometry
1×10^6 BMNCs were placed in FACS tubes (BD Falcon™, MA, USA), centrifuged at 1200 rpm for 5 minutes at 4°C and resuspended in FACS buffer (1X PBS containing 4%BSA, 0.01%EDTA). After a further round of centrifugation the cells were pre-incubated with a saturating solution of FC block (eBioscience, San Diego, USA) for 5 minutes on ice and stained with various conjugated antibodies against CD11b, CD45, and CD40 (purchased from eBioscience), MMR (biolegend, San Diego, CA) as well as a non-conjugated antibody against rabbit anti Stabilin-1 (Santa Cruz antibodies, Santa Cruz, CA) for 30min on ice. Samples stained with Stabilin-1 went through a secondary incubation with anti-rabbit Alexa Fluor 647 for 30 minutes on ice. Cells were washed with FACS buffer and analyzed using the BD FACSCantoTM II flowcytometer and FlowJo analysis software v.8.7.3.
Highlights.
Stabilin-1 expressed on macrophages, microglia but not T-cells
Stabilin-1 expressed on alternatively activated macrophages
Stabilin-1 is expressed within the tumor environment
Expression of stabilin-1 decreases as the tumor progresses
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 2.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Current opinion in cell biology. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 3.Brekken RA, Sage EH, U EHS. Mini review SPARC, a matricellular protein : at the crossroads of cell matrix SPARC, a matricellular protein : at the crossroads of cell matrix communication. Matrix Biology. 2001:815–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 4.Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? Journal of cellular biochemistry. 2004;92:679–690. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- 5.Golembieski Wa, Ge S, Nelson K, Mikkelsen T, Rempel S. Increased SPARC expression promotes U87 glioblastoma invasion in vitro. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 1999;17:463–472. doi: 10.1016/s0736-5748(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 6.Golembieski Wa, et al. HSP27 mediates SPARC-induced changes in glioma morphology, migration, and invasion. Glia. 2008;56:1061–1075. doi: 10.1002/glia.20679. [DOI] [PubMed] [Google Scholar]
- 7.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Huysentruyt LC, Akgoc Z, Seyfried TN. Hypothesis: are neoplastic macrophages/microglia present in glioblastoma multiforme? ASN neuro. 2011;3:183–193. doi: 10.1042/AN20110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kzhyshkowska J. Stabilin-1, a homeostatic scavenger receptor with multiple functions. Journal of Cellular and Molecular Medicine. 2006:10. doi: 10.1111/j.1582-4934.2006.tb00425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kzhyshkowska J, et al. Stabilin-1 localizes to endosomes and the trans-Golgi network in human macrophages and interacts with GGA adaptors. Journal of Leukocyte Biology. 2004;76:1151–1161. doi: 10.1189/jlb.0504300. [DOI] [PubMed] [Google Scholar]
- 11.Kzhyshkowska J, et al. Novel Function of Alternatively Activated Macrophages : Stabilin-1-Mediated Clearance of SPARC 1. The Journal of Immunology. 2012 doi: 10.4049/jimmunol.176.10.5825. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. The journal of clinical investigation. 2006:116. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: New vistas and open questions. European journal of immunology. 2011;41:2522–2525. doi: 10.1002/eji.201141894. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 15.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Genetics. 2009;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palani S, et al. Stabilin-1/CLEVER-1, a type 2 macrophage marker, is an adhesion and scavenging molecule on human placental macrophages. European journal of immunology. 2011;41:2052–2063. doi: 10.1002/eji.201041376. [DOI] [PubMed] [Google Scholar]
- 17.Park S-Y, et al. Stabilin-1 mediates phosphatidylserine-dependent clearance of cell corpses in alternatively activated macrophages. Journal of cell science. 2009;122:3365–3373. doi: 10.1242/jcs.049569. [DOI] [PubMed] [Google Scholar]
- 18.Rich JN, et al. Bone-related genes expressed in advanced malignancies induce invasion and metastasis in a genetically defined human cancer model. The Journal of biological chemistry. 2003;278:15951–15957. doi: 10.1074/jbc.M211498200. [DOI] [PubMed] [Google Scholar]
- 19.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends in immunology. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schittenhelm J, et al. Patterns of SPARC expression and basement membrane intactness at the tumour-brain border of invasive meningiomas. Neuropathology and applied neurobiology. 2006;32:525–531. doi: 10.1111/j.1365-2990.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 21.Schultz C, Lemke N, Ge S, Golembieski WA, Rempel SA. Secreted Protein Acidic and Rich in Cysteine Promotes Glioma Invasion and Delays Tumor Growth in Vivo Secreted Protein Acidic and Rich in Cysteine Promotes Glioma Invasion and Delays Tumor Growth in Vivo 1. Animals. 2002:6270–6277. [PubMed] [Google Scholar]
- 22.Seno T, et al. Downregulation of SPARC expression inhibits cell migration and invasion in malignant gliomas. International Journal of Oncology. 2009:707–715. doi: 10.3892/ijo_00000197. [DOI] [PubMed] [Google Scholar]
- 23.Seyfried TN. Perspectives on Brain Tumor Formation Involving Macrophages, Glia, and Neural Stem. Perspectives in Biology and Medicine. 2001;44:263–282. doi: 10.1353/pbm.2001.0035. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q, et al. Targeting SPARC expression decreases glioma cellular survival and invasion associated with reduced activities of FAK and ILK kinases. Oncogene. 2007;26:4084–4094. doi: 10.1038/sj.onc.1210181. [DOI] [PubMed] [Google Scholar]
- 25.Solinas G, Germano G, Mantovani a, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. Journal of leukocyte biology. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 26.Sughrue ME, et al. Immunological considerations of modern animal models of malignant primary brain tumors. Journal of translational medicine. 2009;7:84. doi: 10.1186/1479-5876-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasievich Ea, Huang L. The suppressive tumor microenvironment: a challenge in cancer immunotherapy. Molecular pharmaceutics. 2011;8:635–641. doi: 10.1021/mp1004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Workman G, Sage EH. Identification of a sequence in the matricellular protein SPARC that interacts with the scavenger receptor stabilin-1. Journal of cellular biochemistry. 2011;112:1003–1008. doi: 10.1002/jcb.23015. [DOI] [PubMed] [Google Scholar]
- 29.Yuan X, et al. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, et al. M2-Polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics. 2011;66:1879–1886. doi: 10.1590/S1807-59322011001100006. [DOI] [PMC free article] [PubMed] [Google Scholar]