Abstract
The generation of polyfunctional CD8+ T cells, in response to vaccination or natural infection, has been associated with improved protective immunity. However, it is unclear whether the maintenance of polyfunctionality is related to particular cellular phenotypic characteristics. To determine whether the cytokine expression profile is linked to the memory differentiation stage, we analyzed the degree of polyfunctionality of HIV-specific CD8+ T cells within different memory subpopulations in 20 ART-naïve HIV-1 infected individuals at approximately 34 weeks post infection. These profiles were compared with CMV-specific CD8+ T cell responses in HIV-uninfected controls and in individuals chronically infected with HIV. Our results showed that the polyfunctional abilities of HIV-specific CD8+ T cells differed according to their memory phenotype. Early-differentiated cells (CD45RO+CD27+) exhibited a higher proportion of cells positive for three or four functions (p<0.001), and a lower proportion of mono-functional cells (p<0.001) compared to terminally-differentiated (CD45RO−CD27−) HIV-specific CD8+ T cells. The majority of terminally-differentiated HIV-specific CD8+ T cells were mono-functional (median 69% [IQR: 57–83]), producing predominantly CD107a or MIP1β. Moreover, proportions of HIV-specific mono-functional CD8+ T cells positively associated with proportions of terminally-differentiated HIV-specific CD8+ T cells (p=0.019, r=0.54). In contrast, CMV-specific CD8+ T cell polyfunctional capacities were similar across all memory subpopulations, with terminally- and early-differentiated cells endowed with comparable polyfunctionality. Overall, these data show that the polyfunctional abilities of HIV-specific CD8+ T cells are influenced by the stage of memory differentiation, which is not the case for CMV-specific responses.
INTRODUCTION
An increasing body of evidence suggests that HIV-1 replication can be partially controlled by T cell immune responses (1–3). However, progression to AIDS occurs in almost all untreated individuals, reflecting the inability of the immune system to mount effective, sustained responses. It has been clearly demonstrated that the overall magnitude of IFNγ-producing HIV-specific CD8+ T cells does not associate with viral control or the establishment of the viral set point (4–8). However, Betts and others have reported that long term non-progressors (LTNPs) were characterized by having higher frequencies of polyfunctional HIV-specific CD8+ T cells compared to non-controllers (9–12), introducing the concept that the quality, rather than the quantity, of antigen-specific T cell responses may dictate T-cell antiviral capacity and play a role in controlling viral replication.
In addition to the range of functional abilities that antigen-specific T cells exhibit, there is enormous phenotypic heterogeneity within the memory T cell compartment. Tremendous effort has been deployed to decipher the phenotypic characteristics of HIV-specific T cells that correlate with viral control. HIV-specific CD8+ T cells possess a distinct memory differentiation profile when compared to other virus-specific CD8+ T cells such as EBV, CMV and HCV (13), exhibiting mainly a transitional memory phenotype (i.e. CD45RO+CD27+CCR7−) (14–16). Moreover, the distribution of HIV-specific CD8+ memory T cells in early infection influences the subsequent viral set point, where proportions of terminally-differentiated HIV-specific CD8+ T cells (defined as CD45RA−CCR7− or CD45RO+CCR7−CD27−) associated with high viral set points (15, 17, 18). This would suggests that excessive maturation of HIV-specific memory CD8+ T cells to the late stage of differentiation could impact on the ability to control virus.
Distinct memory T cell subsets have different proliferation, survival and homing capacities (19–21), so it is therefore plausible that cell maturation could impact on the functional abilities of antigen-specific CD8+ T cells. Indeed, different CD8+ T cell memory subsets have variable capacities to produce cytokines such as IFNγ or IL-2 in response to stimulation with PMA/ionomycin (22), establishing that distinct memory subpopulations have differing inherent functional abilities. However, the relationship between the differentiation stage of a T cell and its polyfunctional profile appears to be more complex, since antigen-specific CD8+ T cells exhibit unique memory maturation profiles depending on their viral specificity (13, 23). Thus, it is likely that antigen load, antigen persistence, quality of co-stimulation as well as the cytokine milieu are all contributing factors controlling both cell differentiation and the polyfunctional profile of antigen-specific CD8+ T cells (24–27). However, it is still unclear whether the stage of memory maturation defines the polyfunctional capacity of CD8+T cell after antigen re-stimulation. In this study, we sought to examine the link between memory phenotype and the polyfunctional ability of antigen-specific CD8+ T cells, by assessing the degree of polyfunctionality of HIV-specific CD8+ T cells within distinct memory subsets in 20 ART-naïve HIV-1 infected individuals and comparing them to CMV-specific CD8+ T cell responses.
MATERIALS AND METHODS
Study participants
For HIV-specific CD8+ T cell responses, a subset of 20 individuals, from the Centre for AIDS Programme of Research in South Africa (CAPRISA) HIV-1 acute infection cohort were analyzed. This cohort, located in Durban, South Africa, has been described previously (28, 29). The time post infection was estimated either by a prospective RNA positive/antibody negative measurement or taken as the midpoint between the last antibody negative test and first antibody positive enzyme-linked immunosorbent assay test. Study participants were followed for 12 months, and follow-up is ongoing. Data from samples collected at approximately 8 months post infection (median 34 weeks, ranging from 22 to 42), are reported herein. All studied subject were antiretroviral therapy (ART) naïve. The University of KwaZulu-Natal, University of Witwatersrand and University of Cape Town Research Ethics Committees approved the study, and all participants provided written informed consent for participation in the study.
Since no data were collected on CMV-specific responses in the CAPRISA cohort, we were obliged to use a different cohort to compare HIV and CMV-specific CD8+ T cell responses. Hence for CMV-specific CD8+ T cell responses, 12 individuals from another cohort (Canadian/African Prevention Trial, CAPT) recruited from the Perinatal HIV Research Unit, Johannesburg, South Africa, were analyzed. Six individuals were HIV-uninfected and the remaining six were chronically infected with HIV-1 and were ART-untreated. The University of Witwatersrand Research Ethics Committee approved the study, and all participants provided written informed consent for participation in the study.
Plasma viral load and CD4 T cell count determination
Plasma HIV-1 RNA levels were quantified using the COBAS AMPLICOR™ HIV-1 monitor test version 1.5 (Roche Diagnostics). Absolute blood CD4+ and CD8+ T cell counts were measured using a FACSCalibur flow cytometer and expressed as cells/mm3. For the CAPRISA cohort (n=20), the median plasma viral load, at the time point studied, was 26,550 HIV RNA copies/ml (ranging from 400 to 425,000) and the median CD4+ count was 484 cells/mm3 (ranging from 342 to 1411). For the HIV-infected subjects (n=6) from the CAPT cohort, the median viral load was 35,625 HIV RNA copies/ml (ranging from 440 to 281,000) and the median CD4 count was 542 cells/mm3 (ranging from 391 to 713). Of note, there were no statistical differences in the absolute CD4 count and viral load between the two HIV-infected groups.
Synthetic peptides
A set of 432 synthetic overlapping peptides spanning the entire expressed HIV-1 clade C proteome corresponding to gene products from the HIV-1 consensus C (Gag), isolate Du151 (Pol and Nef) and isolate Du179 (gp160 Env) were synthesized using 9-Fluorenylmethoxy carbonyl chemistry and standard based solid phase techniques (Natural and Medical Sciences Institute, University of Tubingen, Tubingen, Germany). The non-consensus synthesized peptides were based on sequences from isolates used for manufacture of a clade C vaccine (30). The CMV peptide pool, consisting of a set of 138 peptides (15mers overlapping by 11 amino acids) corresponding to HCMV pp65, was obtained from the NIH AIDS Research and Reference Reagent Program. Pooled peptides were used at a final concentration of 1 μg/ml.
In three individuals, HIV-specific CD8+ T cell polyfunctional profiles were also measured in response to autologous 9mer single peptides. For CAP210, p24-Gag164-172 (Gag-YL9: 164YVDRFFKTL172) and Vif79-88 (Vif-WI9: 79WHLGHGVSI88) peptides were used. For CAP228, Env56-64 (Env-DV9: 56DAKAYEREV64) peptide was used, and for CAP239, Nef82-90 (Nef-KF9: 82KAAVDLSFF90) was used. Each peptide was used at a final concentration of 2μg/ml. Viral sequencing was carried out as previously described (31).
The estimated purity of peptides was >80% as measured by high performance liquid chromatography and mass spectrometry. Individual peptides were diluted in dimethyl sulfoxide (DMSO, Sigma-Aldrich) and prepared as previously described (6). All prepared individual peptides or peptide pools were stored at −80°C prior to use.
Cell preparation
Peripheral blood mononuclear cells (PBMC) were isolated by standard Ficoll-Hypaque density gradient centrifugation (Amersham Pharmacia) and cryopreserved in 90% heat inactivated fetal bovine serum (FBS, Invitrogen) with 10% DMSO in liquid nitrogen until needed. Frozen PBMC were thawed and rested in RPMI 1640 (Invitrogen) containing 10% heat-inactivated FBS and 50 IU of Gentamycin (Invitrogen) at 37°C and 5% CO2 for 18 hours prior to use in intracellular cytokine staining assays.
Surface phenotypic and intracellular cytokine staining using flow cytometry
Flow cytometric detection of phenotypic and functional markers was performed as described in (32). The following antibodies and fluorophores were used: CD3-APCcy7, CD45RO-PE TexasRed, CD27-PEcy5, IFNγ-FITC and TNFα-PEcy7 (all Beckman Coulter), CD4-PEcy5.5 (Caltag), CD8-QD705 (Invitrogen), IL-2-APC and CD107a-Alexa680 (ebioscience), MIP1β-PE (R&D), CD14-Pacific Blue and CD19-Pacific Blue (both Biolegend), and the violet amine reactive dye “Vivid” (Molecular Probes). All antibodies were pre-titered to optimal concentrations. Briefly, PBMC were stimulated with anti-CD28 and anti-CD49d (1 μg/ml) and one to four peptide pools (Gag, Pol, Nef and Env), autologous peptides or the CMV peptide pool for 6 hours in the presence of brefeldin-A (10 μg/ml, Sigma), monensin (0.7 μg/ml, BD Biosciences) and CD107a antibodies. Cells were first stained with Vivid for 10 min and washed once with PBS. Cells were then surface-stained with CD4, CD8, CD45RO, CD27, CD14 and CD19 antibodies. Cells were fixed and permeabilized using Cytofix/Cytoperm buffer (BD Biosciences) and stained intracellularly with CD3, MIP1β, TNFα and IFNγ and IL-2. After washing, cells were resuspended in 1% paraformaldeyde (Electron Microscopy Solutions). Approximately 500 000 events were acquired per stimulation on an LSRII flow cytometer (BD Biosciences) and analysis was performed using FlowJo (v9.4.3, Treestar). Dead cells (ViVid+), monocytes (CD14+) and B cells (CD19+) were excluded from the analysis. Cells were gated on singlets, live CD3+, small lymphocytes and CD8+ cells. Naïve CD8+ T cells (CD45RO−CD27+) were excluded to identify total CD8+ antigen-experienced T cells (hereafter referred to as ‘memory’ cells). Different memory subsets were identified using CD45RO and CD27 expression, and HIV− or CMV-specific CD8+ T cells were identified based on CD107a, MIP1β, TNFα and/or IFNγ expression. We do not report on IL-2 production as it was absent or below our cutoff for a positive response (see below) for HIV and CMV-specific CD8+ T cells. Memory subsets and cytokine producing CD8+ T cells are expressed as a percentage of total CD8+ memory cells. The gating strategy is provided in Supplementary Figure S1. A positive cytokine response was defined as at least twice the background (no antigen, only co-stimulatory antibodies), greater than 0.05% of total memory T cells and at least 40 events. The latter criterion was introduced so as to minimize the possibility of error due to a low number of events when further subdividing these cells into the four memory subsets. Polyfunctionality of antigen-specific cells was analyzed using a boolean gating strategy and represented visually using Pestle (v1.6.2) and Spice (v5.1) software (provided by the NIH) (33).
Statistical analysis
Statistical analysis and graphical presentation were performed using InStat and Prism software (v5, GraphPad). Data are expressed as median values with interquartile ranges and analyzed by the use of nonparametric statistics. Statistical analysis of significance was calculated using either Mann-Whitney or Kruskal-Wallis ANOVA using Dunn’s test for multiple comparisons. All tests were two-tailed and a value of p<0.05 was considered statistically significant. The relationship between memory populations was analyzed using Spearman rank correlations.
RESULTS
Polyfunctional and memory maturation profiles of HIV-specific CD8+ T cell responses at 8 months post-infection
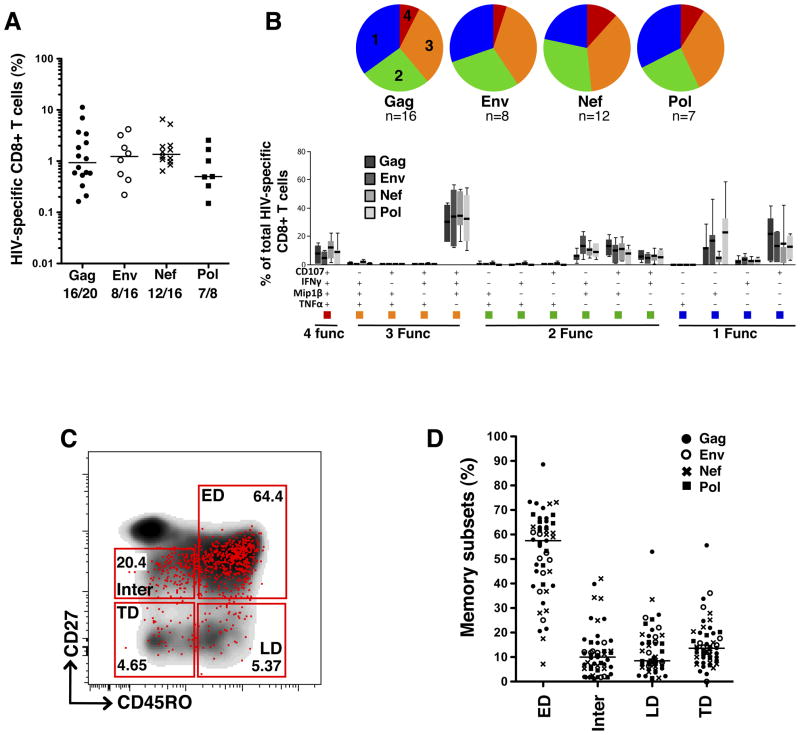
To investigate the relationship between the polyfunctional repertoire of HIV-specific CD8+ T cells and their maturation profiles, we selected positive responders to Gag, Env, Nef and Pol peptide pools amongst HIV-1 subtype C infected individuals. Twenty individuals were tested for Gag responses, 16 for Env and Nef responses and 8 for Pol responses. Clinical characteristics of study participants are reported in the Methods section. PBMCs, collected at a median of 34 weeks post infection were stimulated with the relevant peptide pools and labeled with a panel of antibodies to assess four different functions and four distinct memory subsets. The functions measured were the cytokines IFNγ and TNFα, the chemokine MIP1β, and CD107a, used as a surrogate marker of degranulation. The four memory subsets were early-differentiated cells (ED: CD45RO+CD27+), late-differentiated cells (LD: CD45RO+CD27−), intermediate cells (Inter: CD45RO−CD27dim) and terminally-differentiated cells (TD: CD45RO−CD27−). The average magnitude of total HIV-specific CD8+ T cells in responding individuals was 1.8% (ranging from 0.15% to 11.2% of total memory CD8+ T cells, Figure 1A). No statistically significant differences were observed between the frequencies of Gag, Env, Nef or Pol responses. Of note, the frequency of HIV-specific CD8+ T cells strongly associated with the absolute number of HIV-specific CD8+ T cells (calculated based on absolute CD8+ count, r=0.84, p<0.0001, data not shown). Further assessment of the combination of functions exhibited by HIV-specific CD8+ T cell responses revealed that approximately 40% of the total responses consisted of cells positive for three or four functions, with no significant differences in the polyfunctional abilities of cells between the different HIV peptide pools tested (Figure 1B). Regardless of the specificity of the CD8+ T cell responses against the HIV peptide pools tested, the most prevalent population observed consisted of cells simultaneously producing IFNγ, MIP1β, and degranulating (IFNγ+MIP1β+CD107a+; median 25% of total response [IQR: 11–34]). HIV-specific CD8+ T cells endowed with two functions were mostly distributed amongst the CD107a+MIP1β+, IFNγ+MIP1β+ and CD107a+IFNγ+ cells (median 11% [IQR: 7–17], 8% [IQR: 4–9] and 6% [IQR: 1.6–7] of total response, respectively). Mono-functional HIV-specific CD8+ T cells were predominantly CD107a+ or MIP1β+ (median 18% [IQR: 10–29] and 14% [IQR: 8–23] of total response, respectively). Of note, the proportion of cells producing only IFNγ was marginal, representing less than 3% of the total measured response, and TNFα was detected only in cells endowed with 4 functions (Figure 1B). These profiles are consistent with previous reports on the functional characteristics of HIV-specific CD8+ T cells (9, 34–36).
Figure 1. Polyfunctional and memory maturation profiles of HIV-specific CD8+ T cells.
(A) Magnitude of total cytokine production (CD107a, MIP1β, IFNγ or TNFα) in CD8+ T cells in response to HIV Gag, Env, Nef and Pol peptide pools. Results are expressed as % of total memory CD8+ T cells. Numbers correspond to the number of positive responses/number of tested individuals. (B) Polyfunctional profiles of HIV-specific CD8+ T cells. All possible combinations of four functions (CD107a, MIP1β, IFNγ and TNFα) produced by Gag, Env, Nef and Pol-specific CD8+ T cells are shown on the x-axis. Box and whiskers indicate the median percentage and range of the total response contributed by CD8+ T cells. Functional profiles are grouped and color-coded according to number of functions and summarized in the pie charts. Each slice of the pie corresponds to the median production of 4 (red), 3 (orange), 2 (green) or 1 (blue) function. (C) Representative dot plot of the memory maturation profile of total CD8+ T cells (density) and Gag-specific total cytokine+ CD8+ T cells (red dots) from one participant. ED: Early-differentiated memory cells (CD45RO+CD27+), LD: Late-differentiated memory cells (CD45RO+CD27−), Inter: Intermediate memory cells (CD45RO−CD27dim) and TD: Terminally-differentiated memory cells (CD45RO−CD27−). (D) Distribution of HIV-specific CD8+ T cells amongst memory subsets, showing 43 HIV-specific responses measured in 20 participants. Closed circles (●) correspond to Gag responses, open circles (○) represent Env responses, crosses (X) correspond to Nef responses and black squares (■) represent Pol responses. Horizontal lines indicate median values.
We next assessed the memory maturation profiles of HIV-specific CD8+ T cells based on the expression of CD45RO and CD27. A representative dot plot of the memory profile of HIV-specific CD8+ T cells is presented in Figure 1C. Total HIV-specific CD8+ T cells exhibited predominantly an early-differentiated (ED) memory phenotype (CD45RO+CD27+); however, the frequencies of ED HIV-specific CD8+ T cells were variable amongst the individuals analyzed (median 57%, ranging from 7% to 88%, Figure 1D). The percentage of ED HIV-specific CD8+ T cells was negatively correlated with viral load (p<0.0001, r=−0.6, data not shown), suggesting that these variations likely reflect the differences in viral replication levels in these individuals. This is in accordance with data previously reported from the same cohort (15). Of note, the memory maturation profiles did not differ significantly between Gag, Env, Nef and Pol-specific responses (Supplementary Figure S2A).
In summary, our results show that Gag-, Env-, Nef- and Pol-responsive CD8+ T cells were quantitatively and qualitatively similar in our cohort, and HIV-s pecific CD8+ T cells exhibited mainly an ED memory phenotype and moderate polyfunctionality.
Functionality of HIV-specific CD8+ T cells within distinct memory subsets
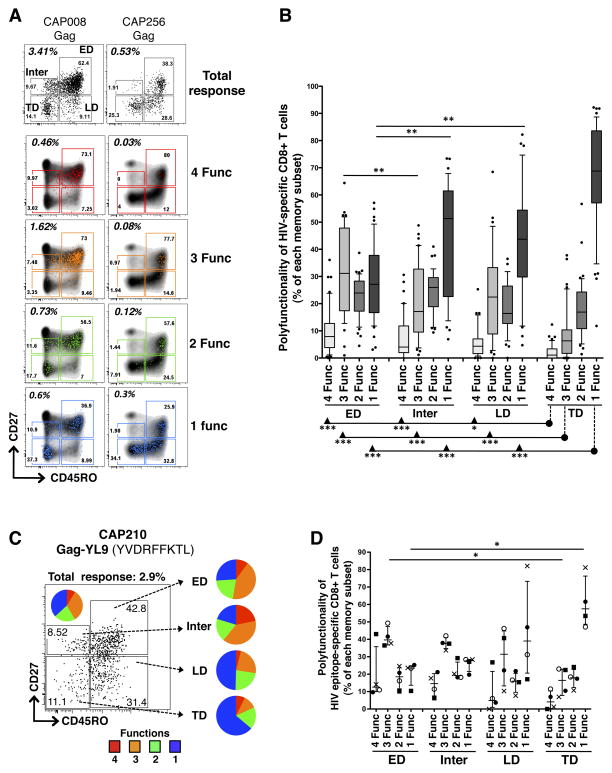
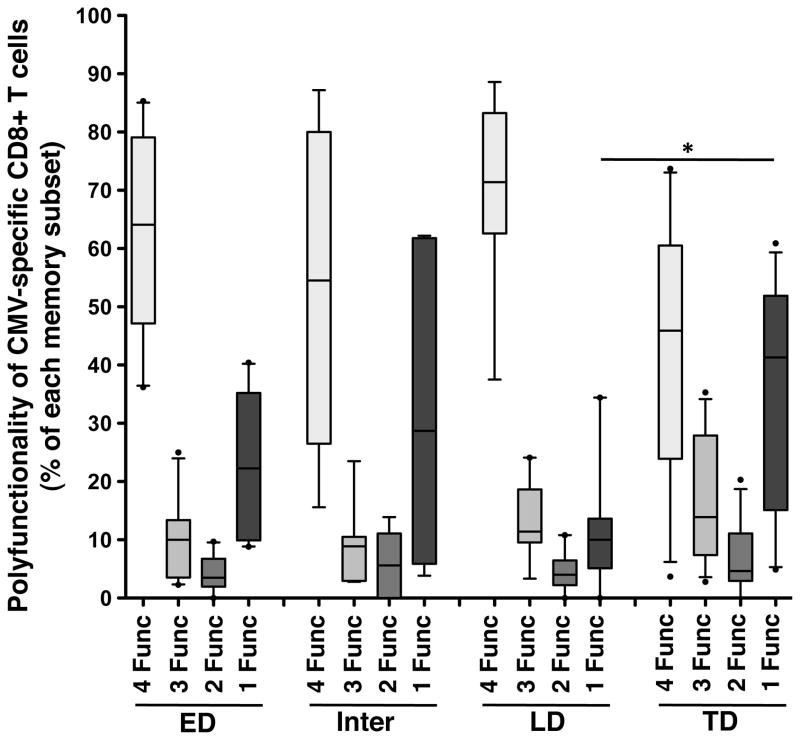
To determine whether the polyfunctional properties exhibited by antigen-specific CD8+ T cells were dictated by their differentiation stage, we next compared the functional profiles of HIV-specific CD8+ T cells within different memory subsets. Since the functions and phenotypes of CD8+ T cells specific for Gag, Env, Nef and Pol were similar (Supplementary Figure S2B), we combined all HIV-specific responses for these analyses, and here report on 43 peptide pool responses in 20 individuals. Briefly, total HIV-specific CD8+ T cells were gated on distinct memory subsets (ED, Inter, LD and ED), and we then measured the distribution of HIV-specific CD8+ T cells expressing 4, 3, 2 or 1 function in each memory subpopulation. Figure 2A shows representative dot plots of memory profiles of HIV-specific T cells positive for 4, 3, 2 or 1 function, from two individuals with a high and a low magnitude Gag-specific CD8+ T cell response. Depending on their memory profiles, HIV-specific CD8+ T cells had distinct polyfunctional abilities, with decreasing polyfunctionality coinciding with an increase in differentiation from ED to TD memory subsets (Figure 2B). HIV-specific CD8+ T cells with a TD phenotype were primarily mono-functional (median 69% [IQR: 57–83]), whilst ED CD8+ T cells had a significantly lower proportion of mono-functional cells (median 27% [IQR: 16–38], p<0.001) and a significantly higher proportion of cells positive for 4 and 3 functions (p<0.001) compared to TD CD8+ T cells. Inter and LD HIV-specific CD8+ T cells showed an intermediate polyfunctional profile consisting of: 1) significantly more mono-functional cells compared to the ED subset (median 51% [IQR: 22–61], p<0.01 and 43% [IQR: 30–54], p<0.01, respectively), and 2) significantly more cells positive for 4 and 3 functions compared to the TD subset (Figure 2B). It is worth noting that although the TD subset had the highest proportion of mono-functional cells (Figure 2B), they contributed only approximately 20% to the absolute number of HIV-specific mono-functional CD8+ T cells (data not shown), since the TD subset itself represents only a median of 14% of all HIV-specific CD8+ T cells (as shown in Figure 1D). Consequently, even though there was a lower proportion of mono-functional CD8+ HIV-specific cells within the ED subset compared to the TD subset, in absolute numbers there were more circulating mono-functional ED cells than mono-functional TD cells, since ED cells make up almost 60% of total HIV-specific CD8+ T cells.
Figure 2. Polyfunctional profile of HIV-specific CD8+ T cells in defined memory subsets.
(A) Representative density and overlay dot plots of memory maturation in total CD8+ T cells (density) and Gag-specific CD8+ T cells endowed with 4 (red), 3 (orange), 2 (green) or 1 (blue) functions for two study individuals with a high and low Gag response. (B) Proportions of HIV-specific CD8+ T cells, exhibiting 4, 3, 2 or 1 functions, across distinct memory subsets. Responses to Gag, Env, Nef and Pol responses have been pooled (n=43 in 20 individuals). Results are shown as box and whisker (10–90 percentile) plots, with outliers depicted with black dots. (C) Representative example of memory profile of total Gag-YL9-specific CD8+ T cells (producing CD107a, MIP1β, IFNγ or TNFα). The pie chart inlaid within the dot plot corresponds to the polyfunctional profile of total Gag-YL9-specific CD8+ T cells. Adjacent pies represent the degree of polyfunctionality in Gag-YL9-specific CD8+ T cells according to their maturation phenotype. (D) Proportion of HIV-specific CD8+ T cells expressing 4, 3, 2 and 1 functions across distinct memory subsets (n=4 responses, in 3 individuals). Each symbol corresponds to an autologous peptide response. ●: Gag (YL9)-specific response in CAP210; ○ : Vif (WI9)-response in CAP210;X: Env (DV9)-specific response in CAP228 and ■: Nef (KF9)-specific response in CAP239. The median and interquartile ranges are shown for each group. The p-values were calculated using one-way ANOVA non-parametric Kruskal-Wallis test; ***: p<0.001, **: p<0.01, *: p<0.05.
To determine whether differences in HIV-specific CD8+ T cell polyfunctional profiles observed within each memory subset could also be identified using single peptides, and were not an artifact of heterogeneous specificities in the pools, we assessed the degree of polyfunctionality in each memory subpopulation in response to stimulation with optimal autologous 9mer peptides. Figure 2C shows the polyfunctional profile of Gag-VL9-specific CD8+ T cells within the different memory subsets in one individual (CAP210). We found that increasing cell differentiation from ED to TD subsets resulted in a decreased proportion of epitope-specific cells exhibiting 3 and 4 functions, and an elevated proportion of mono-functional epitope-specific cells. Four autologous peptide responses (Gag-VL9, Vif-WI9, Env-DV9 and Nef-KF9) were measured in three individuals (CAP210, CAP228 and CAP239). As demonstrated with peptide pools, epitope-specific CD8+ responses displayed greater polyfunctionality in the ED subset when compared to the TD subset (p<0.05; Figure 2D).
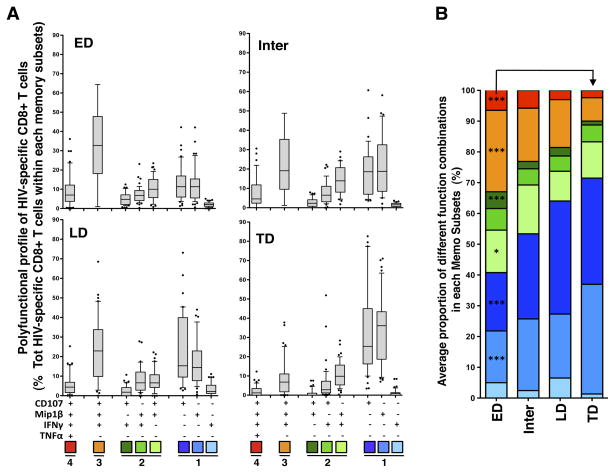
To assess the specific cytokines that made up the different polyfunctional subsets, we next compared the proportions of the eight most prevalent combinations of functions detected within each HIV-specific CD8+ T cell memory subset (Figure 3A). Both CD107a+ and MIP1β+ cells accounted for the increased proportion of mono-functional cells in the TD memory subset; the proportion of CD107a+ cells were almost three-fold higher in ED compared to TD subsets, from a median of 13% (IQR: 6–17) to 33% (IQR: 16–45; p<0.0001), whilst the proportion of CD8+ T cells producing MIP1β alone was a median of 13% (IQR: 5–15) in ED HIV-specific cells compared to 34% (IQR: 19–43) in TD HIV-specific cells (p<0.0001; Figure 3A). Interestingly, no significant change in the proportion of cells expressing IFNγ only was observed between the different memory subpopulations. Figure 3B summarizes the mean distribution of detectable cytokine combinations within each HIV-specific CD8+ T cell memory subset, illustrating the progressive increase in the proportion of CD107a+ and MIP1β+ mono-functional cells and the decrease in the proportion of cells endowed with 3 functions (CD107a+MIP1β+IFNγ+) and 4 functions in HIV-specific cells from early to terminal differentiation. Importantly, the degree of polyfunctionality of HIV-specific CD8+ T cells in each memory subset was similar between individuals with low (VL<3,000 copies/ml) or high viral load (VL>100,000 copies/ml; Supplementary Figure S3A), indicating that the distribution of polyfunctional cells within each memory subset was independent of viraemia.
Figure 3. Cytokine combinations produced by HIV-specific CD8+ T cells in defined memory subsets.
(A) Proportion of HIV-specific CD8+ T cells producing eight distinct combinations of four functions, shown on the x-axis, across distinct memory subsets. Gag, Env, Nef and Pol responses have been pooled (n=43 in 20 individuals). Results are shown as box and whisker (10–90 percentile) plots, and outliers are depicted with black dots. (B) Mean production of different cytokine combinations in HIV-specific ED, Inter, LD and TD subsets. The p-values depicted on the graph correspond to the comparison between ED and TD subsets, and were calculated using a one-way ANOVA non-parametric Kruskal-Wallis test; ***: p<0.001, *: p<0.05.
Overall, these data suggest that in HIV infection the polyfunctional profile of HIV-specific cells is related to their differentiation stage, where early-differentiated cells exhibit a greater degree of polyfunctionality, and terminally-differentiated cells are more mono-functional.
Maturation and functional characteristics of CMV-specific CD8+ T cells
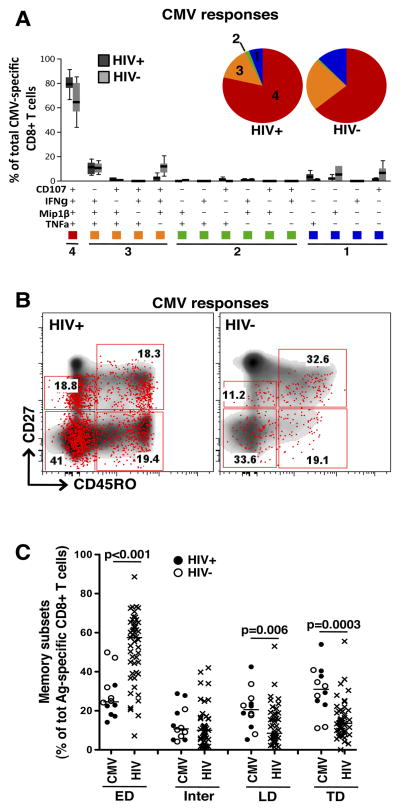
To investigate whether our observations linking the degree of polyfunctionality with the memory maturation profile in HIV infection holds true for another pathogen, we measured the polyfunctional attributes of distinct CMV-specific CD8+ T cell subsets in 12 participants, six of whom were HIV-uninfected and six chronically infected with HIV. All HIV-infected participants were ART-naïve. CMV-specific CD8+ T cells were highly polyfunctional, with approximately 75% (IQR: 63–85) of the total response consisting of cells positive for 4 functions (i.e. CD107a, MIP1β, IFNγ and TNFα; Figure 4A). No significant differences were detected between HIV-infected and uninfected individuals. Interestingly, the prevalent cytokine combinations observed in CMV-specific CD8+ T cells were distinct from HIV-specific responses, with CMV-specific cells positive for 3 functions consisting mostly of MIP1β+IFNγ+TNFα+ cells. CMV-specific cells endowed with 2 functions were present at low levels or were undetectable, and mono-functional CMV-specific cells expressing TNFα only were detectable (Figure 4A), a subset that was absent for HIV-specific responses. Representative examples of CMV-specific CD8+ T cells and their memory subset distribution are depicted in Figure 4B.
Figure 4. Polyfunctional and memory maturation profiles of CMV-specific CD8+ T cell responses.
(A) Polyfunctional profiles of CMV-specific CD8+ T cells. All possible combinations of four functions (CD107a, MIP1β, IFNγ and TNFα) produced by HIV-infected (n=6) and HIV-uninfected (n=6) individuals are shown on the x-axis. Box and whiskers indicates the median percentage and interquartile range of the total response contributed by CD8+ T cells. Functional profiles are grouped and color-coded according to number of functions and summarized in the pie charts. Each slice of the pie corresponds to the median production of 4 (red), 3 (orange), 2 (green) or 1 (blue) function. (B) Representative dot plots of the memory maturation profile of total CD8+ T cells (density) and CMV-specific total cytokine+ CD8+ T cells (red dots) in one HIV-infected and one HIV-uninfected individual. (C) Comparison of memory maturation profiles of CMV− and HIV-specific CD8+ T cells. Closed circles (●) correspond to HIV-infected individuals (n=6) and open circles (○) represent HIV-uninfected individuals (n=6). Horizontal lines indicate median values. The p-values were calculated using a one-way ANOVA non-parametric Kruskal-Wallis test; ***: p<0.001, **: p<0.01, *: p<0.05.
When we compared the cell memory distribution for each antigen, we found that the proportion of TD cells was significantly higher in CMV-specific CD8+ T cells when compared to HIV-specific CD8+ T cells (median 31% [IQR: 24–40] and 14% [IQR: 9–20], respectively, p=0.003), and this was true regardless of HIV infection status (Figure 4C). Thus, CMV-specific responses exhibited a high degree of polyfunctionality and a differentiated phenotype, as previously described (13, 16, 34).
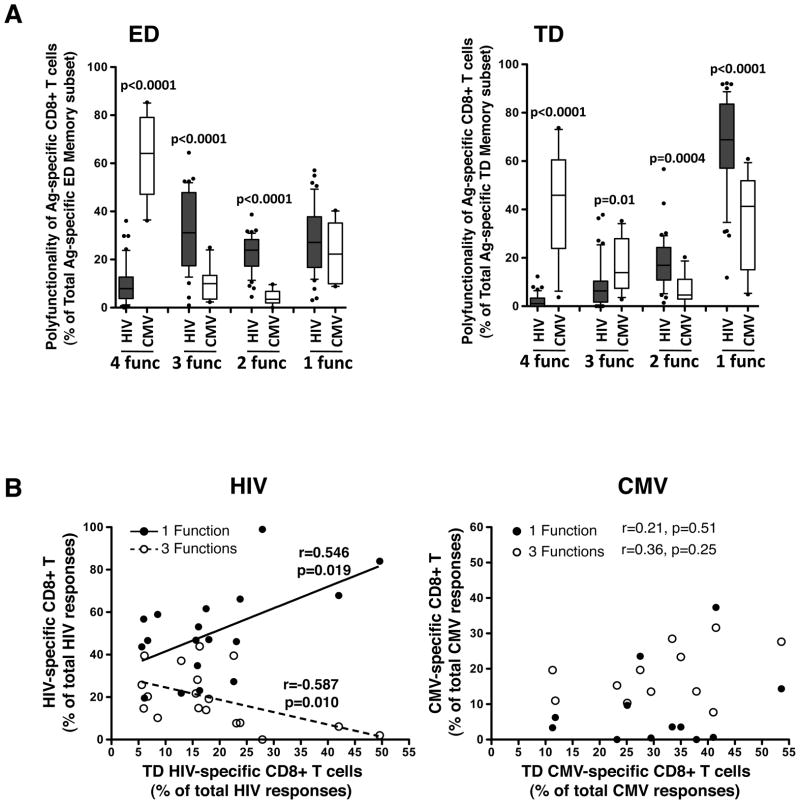
We next examined the distribution of polyfunctionality between different CMV-specific CD8+ T cell memory subsets, as we had done for HIV. Interestingly, and in contrast to HIV-specific CD8+ T cells, the polyfunctional capacity of CMV-specific CD8+ T cells was similar across all memory subsets (Figure 5), suggesting that for CMV-specific CD8+ T cell responses, polyfunctional profiles are neither linked to specific memory subsets nor enriched in particular memory subsets. Of note, the degree of polyfunctionality of CMV-specific CD8+ T cells within each subset was comparable in HIV-infected and uninfected individuals (Supplementary Figure S3B). The difference between HIV− and CMV-specific CD8+ T cells was further emphasized when we compared the polyfunctional properties of each memory subset. Figure 6A shows that ED HIV-specific CD8+ T cells exhibited a significantly higher proportion of cells positive for 2 or 3 functions and a lower proportion of 4-function cells (p<0.0001) compared to ED CMV-specific CD8+ T cells. Moreover, TD HIV-specific CD8+ T cells exhibited a significantly higher proportion of cells positive for 1 or 2 functions (p<0.0001 and p=0.0004, respectively) and a lower proportion of cells positive for 3 or 4 functions (p=0.01 and p<0.0001, respectively) compared to CMV-specific responses. Furthermore, there was a significant positive association between the proportion of HIV-specific CD8+ T cells endowed with one function and the proportion of TD HIV-specific CD8+ T cells (p=0.019, r=0.546), whilst the proportion of HIV-specific CD8+ T cells positive for 3 functions was inversely correlated with the proportion of TD HIV-specific CD8+ T cells (p=0.01, r=−0.587; Figure 6B, left panel). No such associations were observed for CMV-specific CD8+ T cells (Figure 6B, right panel). Overall, these results show that for HIV-specific CD8+ T cells, polyfunctional properties were linked to differentiation levels, in contrast to CMV-specific CD8+ T cells, where polyfunctional capacities were similar across all memory subsets.
Figure 5. Polyfunctional profiles of CMV-specific CD8+ T cells in defined memory subsets.
Proportion of CMV-specific CD8+ T cells exhibiting 4, 3, 2 or 1 functions across the different memory subsets. HIV-infected and uninfected individuals have been pooled (n=12). Results are shown as box and whisker (10–90 percentile) plots, and outliers are depicted with black dots. The p-values were calculated using a one-way ANOVA non-parametric Kruskal-Wallis test; *: p<0.05.
Figure 6. Comparison of the polyfunctional profiles of HIV− and CMV-specific CD8+ T cells in defined memory subsets.
(A) Proportion of HIV-specific (n=43 responses in 20 individuals) and CMV-specific (n=12) CD8+ T cells producing 4, 3, 2 and 1 function across different memory subsets (ED, Inter, LD and TD). Results are shown as box and whisker (10–90 percentile) plots, with outliers depicted with black dots. Statistical comparisons where determined by a Mann-Whitney non-parametric t-test. (B) Associations between the proportions of antigen-specific CD8+ T cells endowed with 1 or 3 functions (% of total antigen-specific CD8+ T cells) and the proportions of TD antigen-specific CD8+ T cells (% of total antigen-specific CD8+ T cells). HIV-specific responses are shown on the left (n=20 individuals) and CMV-specific responses (n=12) on the right. Statistical associations were performed by a two-tailed non-parametric Spearman rank correlation.
DISCUSSION
Despite abundant literature on both the memory differentiation profiles and polyfunctional capacities of HIV-specific CD8+ T cells at different stages of infection (9–11, 13–15), less is known about how these T cell characteristics are associated with each other. In this study, we examined the relationship between the memory phenotype of CD8+ T cells and the polyfunctional responses of these cells, as measured by IFNγ, TNFα, MIP1β and CD107a expression. We studied HIV-specific CD8+ T cell responses in 20 HIV-1 infected individuals at approximately 34 weeks post infection, and compared them to CMV-specific T responses. We detected a higher proportion of polyfunctional CD8+ T cells specific for CMV compared to HIV, which agrees with previous observations (34). HIV-specific responses were enriched for cells with an early-differentiated phenotype, whilst CMV-specific cells were mainly of a terminally-differentiated phenotype, consistent with earlier findings (14, 37). Our novel observation was a distinct pattern of polyfunctionality between HIV− and CMV-specific CD8+ T cell memory subsets. For HIV-specific CD8+ T cells, early-differentiated memory cells (CD45RO+CD27+) were enriched for a polyfunctional response, and there was a significant reduction in the number of functions in terminally-differentiated cells (CD45RO−CD27−). In contrast, CMV-specific CD8+ T cells exhibited a polyfunctional profile that was similar across early, late or terminally-differentiated memory subsets. Moreover, the CMV response was highly polyfunctional (80–90% of cells expressing 3 or 4 functions) in both HIV-infected and uninfected individuals, compared to HIV-specific CD8+ T cells (40–50%). This implies that there is not a global decrease in CD8+ T cell polyfunctionality in the background of HIV infection. Differences in antigen load or recurrence or CD4+ help may account for differential (poly)function between HIV-specific CD8+ T cells and cells of other specificities. Overall, this suggests that the hierarchical loss in the number of functions by an antigen-specific CD8+ T cell as memory differentiation proceeds is dependent on the infecting pathogen, and that memory phenotype and polyfunctional characteristics of CD8+ T cells can differ significantly.
Seminal work from Sallusto and colleagues (20) introduced the concept of antigen-specific cells being divided into memory subsets expressing different phenotypic markers with distinct homing and survival abilities, and also alluded to a functional distinction between different memory subsets. A number of factors have been shown to shape this functional and phenotypic heterogeneity of antigen-specific T cells. Co-stimulatory signals engaged during T cell priming, as well as cytokines such as IL-2 and IL-21, can modulate T cell functionality (38–41). Major determinants of CD8+ T cell polyfunctionality are antigen concentration (26, 42, 43) and TCR affinity (44). Work from murine models has proposed that the clonal expansion process can modulate the profile of secreted cytokines (45, 46). With regard to memory differentiation of CD8+ T cells, the degree of cell maturation is dependent on similar factors, namely antigen load, co-stimulation signals and the cytokine environment (reviewed in (47)). Hence, being regulated by similar factors, it can be speculated that cell differentiation and polyfunctional potential could be co-dependent phenomena, where the generation and maintenance of late-stage differentiated effector cells, endowed with a rapid response to pathogens and a high degree of polyfunction, would be favorable to ensure viral control. Indeed, in certain well-controlled infections or successful vaccinations (such as CMV, vaccinia or yellow fever vaccine), antigen-specific CD8+ T cells that are generated are highly differentiated and highly polyfunctional (23, 48). In contrast, during uncontrolled HIV infection, HIV-specific CD8+ T cells exhibit mainly an early-differentiated memory phenotype, often regarded as an immature stage (14, 37). However, the relationship between maturation and polyfunction appears to be more complex, as antigen-experienced cells exhibit heterogeneous memory and cytokine secretion profiles dependent upon different antigen specificities (reviewed in (21)). Our data are in agreement with the latter observation, where we show that differentiation towards late memory for HIV-specific CD8+ T cells is accompanied by a progressive loss of polyfunctional capacities, whilst CMV-specific CD8+ T cells retain their polyfunctional capacities regardless of memory differentiation.
Although sustained HIV replication appears to play a predominant role in driving CD8+ T cells towards a late stage of memory maturation (15, 17, 18), we found no difference in the memory-polyfunction association between those with high and low HIV viral loads, i.e. those controlling viral replication did not resemble the stable ‘polyfunctionality regardless of memory phenotype’ pattern of CMV-specific CD8+ T cells. It would be of interest to determine whether this is also the case for HIV-specific cells from individuals on long-term, successful ART, where antigen load is reduced.
Our starting point for this study was that polyfunctional T cells, capable of carrying out a range of functions simultaneously, exhibit superior protective immunity (9, 49). We can speculate that measuring multiple functions on a per cell basis may more closely reflect the ability of CD8+ T cells to impart antiviral effects and may be more relevant than focusing only on one function, such as IFNγ. However, the combination of specific functions rather the number of functions per se may offer a more refined and accurate assessment of protective immunity (50). Upregulation of perforin has recently been highlighted as an important indicator of cytotoxic potential for control of HIV (51, 52). This is also a consideration in the question of how maturation profiles of CD8+ T cells and functional abilities are linked, since specific functions such as perforin and IL-2 may indeed be associated with particular memory phenotypes, whilst other functions may not be as tightly regulated. Loss of CD28 expression appears to be coupled to loss of IL-2 production, whilst T-bet expression correlates with perforin upregulation (53). In our study, we did not measure perforin and nor could we detect IL-2 responses. Indeed, CMV-specific CD8+ T cells rarely produce IL-2 (53) and IL-2 production in the context of HIV infection is confined to long-term non-progressors (54, 55).
Excessive activation of the immune system can influence both the function and phenotype of CD8+ T cells. Several studies have described the upregulation of PD-1 and other inhibitory receptors such as CD160, 2B4 and LAG3 on CD8+ T cells in chronic viral infections, including HIV, which results in defective cytokine production and lack of polyfunctional responses. This can be partially reversed by blocking the interaction of these receptors with their ligands (56–58). Yamamoto and colleagues (37) recently described elevated levels and co-expression of several negative regulators on HIV-specific CD8+ T cells compared to CMV-specific cells. Co-expression of these inhibitory receptors correlated inversely with polyfunctionality, and PD-1 blockade restored cytokine production. It would thus be of interest to determine whether, in HIV infection, the memory maturation profile of HIV-specific CD8+ T cells coincides with their degree of functional inhibition.
In conclusion, our data show that the polyfunctional abilities of HIV-specific CD8+ T cells are influenced by the stage of memory differentiation, which is not the case for CMV-specific responses. This emphasizes that different pathogens generate CD8+ T cell responses with distinct polyfunctional-memory subset profiles; this may reflect the distinct life histories of pathogens and their interactions with the immune system. A better understanding of which memory-function combinations lead to superior and durable protective immunity against HIV is needed.
Supplementary Material
Acknowledgments
We thank the participants, clinical and laboratory staff at CAPRISA for the specimens. We thank Laurie Lamoreaux and Jennifer Fischer for technical help, and Steve Perfetto for LSRII training.
This work was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, U.S. Department of Health and Human Services Grant U19 A151794 (to S.A.K.) and R01 AI084387 (to W.A.B.); the Canada-Africa Prevention Trials Network (CAPTN); and the Bill and Melinda Gates Comprehensive T Cell Vaccine Immune Monitoring Consortium award. W.A.B. was supported by a Columbia University Fogarty AIDS International Training Research Fellowship, and is a Wellcome Trust Intermediate Fellow in Public Health and Tropical Medicine.
Footnotes
Disclosure: The authors have no conflict of interest
CONTRIBUTION:
Conceived and designed the experiments: KM, RK, MR, SAK, MRA, CW, CMG and WAB. Performed the experiments: WAB. Analyzed the data: CR, WAB. Contributed reagents/materials/analysis tools: ML, NG, KM, SAK, GdB. Wrote the paper: CR, WAB.
References
- 1.Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. Journal of virology. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science (New York, NY. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 3.Streeck H, Nixon DF. T cell immunity in acute HIV-1 infection. The Journal of infectious diseases. 2010;202(Suppl 2):S302–308. doi: 10.1086/655652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, Koup RA, Picker LJ. Analysis of total human immunodeficiency virus (HIV)-specific CD4(+) and CD8(+) T-cell responses: relationship to viral load in untreated HIV infection. Journal of virology. 2001;75:11983–11991. doi: 10.1128/JVI.75.24.11983-11991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addo MM, Yu XG, Rathod A, Cohen D, Eldridge RL, Strick D, Johnston MN, Corcoran C, Wurcel AG, Fitzpatrick CA, Feeney ME, Rodriguez WR, Basgoz N, Draenert R, Stone DR, Brander C, Goulder PJ, Rosenberg ES, Altfeld M, Walker BD. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. Journal of virology. 2003;77:2081–2092. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masemola A, Mashishi T, Khoury G, Mohube P, Mokgotho P, Vardas E, Colvin M, Zijenah L, Katzenstein D, Musonda R, Allen S, Kumwenda N, Taha T, Gray G, McIntyre J, Karim SA, Sheppard HW, Gray CM. Hierarchical targeting of subtype C human immunodeficiency virus type 1 proteins by CD8+ T cells: correlation with viral load. Journal of virology. 2004;78:3233–3243. doi: 10.1128/JVI.78.7.3233-3243.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez WR, Addo MM, Rathod A, Fitzpatrick CA, Yu XG, Perkins B, Rosenberg ES, Altfeld M, Walker BD. CD8+ T lymphocyte responses target functionally important regions of Protease and Integrase in HIV-1 infected subjects. Journal of translational medicine. 2004;2:15. doi: 10.1186/1479-5876-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foxall RB, Cortesao CS, Albuquerque AS, Soares RS, Victorino RM, Sousa AE. Gag-specific CD4+ T-cell frequency is inversely correlated with proviral load and directly correlated with immune activation in infection with human immunodeficiency virus type 2 (HIV-2) but not HIV-1. Journal of virology. 2008;82:9795–9799. doi: 10.1128/JVI.01217-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. Journal of virology. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I, Costagliola D, Rouzioux C, Agut H, Marcelin AG, Douek D, Autran B, Appay V. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. The Journal of experimental medicine. 2007;204:2473–2485. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owen RE, Heitman JW, Hirschkorn DF, Lanteri MC, Biswas HH, Martin JN, Krone MR, Deeks SG, Norris PJ. HIV+ elite controllers have low HIV-specific T-cell activation yet maintain strong, polyfunctional T-cell responses. AIDS (London, England) 2010;24:1095–1105. doi: 10.1097/QAD.0b013e3283377a1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, Richman DD, Gruener N, Pape G, Waters A, Easterbrook P, Salio M, Cerundolo V, McMichael AJ, Rowland-Jones SL. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nature medicine. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 14.Champagne P, Ogg GS, King AS, Knabenhans C, Ellefsen K, Nobile M, Appay V, Rizzardi GP, Fleury S, Lipp M, Forster R, Rowland-Jones S, Sekaly RP, McMichael AJ, Pantaleo G. Skewed maturation of memory HIV-specific CD8 T lymphocytes. Nature. 2001;410:106–111. doi: 10.1038/35065118. [DOI] [PubMed] [Google Scholar]
- 15.Burgers WA, Riou C, Mlotshwa M, Maenetje P, de Assis Rosa D, Brenchley J, Mlisana K, Douek DC, Koup R, Roederer M, de Bruyn G, Karim SA, Williamson C, Gray CM. Association of HIV-specific and total CD8+ T memory phenotypes in subtype C HIV-1 infection with viral set point. J Immunol. 2009;182:4751–4761. doi: 10.4049/jimmunol.0803801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagannathan P, Osborne CM, Royce C, Manion MM, Tilton JC, Li L, Fischer S, Hallahan CW, Metcalf JA, McLaughlin M, Pipeling M, McDyer JF, Manley TJ, Meier JL, Altman JD, Hertel L, Davey RT, Jr, Connors M, Migueles SA. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. Journal of virology. 2009;83:2728–2742. doi: 10.1128/JVI.02128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Northfield JW, Loo CP, Barbour JD, Spotts G, Hecht FM, Klenerman P, Nixon DF, Michaelsson J. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T(EMRA) cells in early infection are linked to control of HIV-1 viremia and predict the subsequent viral load set point. Journal of virology. 2007;81:5759–5765. doi: 10.1128/JVI.00045-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Addo MM, Draenert R, Rathod A, Verrill CL, Davis BT, Gandhi RT, Robbins GK, Basgoz NO, Stone DR, Cohen DE, Johnston MN, Flynn T, Wurcel AG, Rosenberg ES, Altfeld M, Walker BD. Fully differentiated HIV-1 specific CD8+ T effector cells are more frequently detectable in controlled than in progressive HIV-1 infection. PloS one. 2007;2:e321. doi: 10.1371/journal.pone.0000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Current opinion in immunology. 2005;17:326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 21.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–983. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 22.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. The Journal of experimental medicine. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akondy RS, Monson ND, Miller JD, Edupuganti S, Teuwen D, Wu H, Quyyumi F, Garg S, Altman JD, Del Rio C, Keyserling HL, Ploss A, Rice CM, Orenstein WA, Mulligan MJ, Ahmed R. The yellow fever virus vaccine induces a broad and polyfunctional human memory CD8+ T cell response. J Immunol. 2009;183:7919–7930. doi: 10.4049/jimmunol.0803903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamadia LE, van Leeuwen EM, Remmerswaal EB, Yong SL, Surachno S, Wertheim-van Dillen PM, Ten Berge IJ, Van Lier RA. The size and phenotype of virus-specific T cell populations is determined by repetitive antigenic stimulation and environmental cytokines. J Immunol. 2004;172:6107–6114. doi: 10.4049/jimmunol.172.10.6107. [DOI] [PubMed] [Google Scholar]
- 25.Papagno L, Spina CA, Marchant A, Salio M, Rufer N, Little S, Dong T, Chesney G, Waters A, Easterbrook P, Dunbar PR, Shepherd D, Cerundolo V, Emery V, Griffiths P, Conlon C, McMichael AJ, Richman DD, Rowland-Jones SL, Appay V. Immune activation and CD8+ T-cell differentiation towards senescence in HIV-1 infection. PLoS biology. 2004;2:E20. doi: 10.1371/journal.pbio.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Streeck H, Brumme ZL, Anastario M, Cohen KW, Jolin JS, Meier A, Brumme CJ, Rosenberg ES, Alter G, Allen TM, Walker BD, Altfeld M. Antigen load and viral sequence diversification determine the functional profile of HIV-1-specific CD8+ T cells. PLoS medicine. 2008;5:e100. doi: 10.1371/journal.pmed.0050100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Loggerenberg F, Mlisana K, Williamson C, Auld SC, Morris L, Gray CM, Abdool Karim Q, Grobler A, Barnabas N, Iriogbe I, Abdool Karim SS. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PloS one. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mlisana K, Auld SC, Grobler A, van Loggerenberg F, Williamson C, Iriogbe I, Sobieszczyk ME, Abdool Karim SS. Anaemia in acute HIV-1 subtype C infection. PloS one. 2008;3:e1626. doi: 10.1371/journal.pone.0001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson C, Morris L, Maughan MF, Ping LH, Dryga SA, Thomas R, Reap EA, Cilliers T, van Harmelen J, Pascual A, Ramjee G, Gray G, Johnston R, Karim SA, Swanstrom R. Characterization and selection of HIV-1 subtype C isolates for use in vaccine development. AIDS research and human retroviruses. 2003;19:133–144. doi: 10.1089/088922203762688649. [DOI] [PubMed] [Google Scholar]
- 31.Chopera DR, Woodman Z, Mlisana K, Mlotshwa M, Martin DP, Seoighe C, Treurnicht F, Assisde Rosa D, Hide W, Abdool Karim S, Gray CM, Williamson C t. C. s. team. Transmission of HIV-1 CTL escape variants provides HLA-mismatched recipients with a survival advantage. PLoS Pathogens. 2008;4:e1000033. doi: 10.1371/journal.ppat.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamoreaux L, Roederer M, Koup R. Intracellular cytokine optimization and standard operating procedure. Nature protocols. 2006;1:1507–1516. doi: 10.1038/nprot.2006.268. [DOI] [PubMed] [Google Scholar]
- 33.Roederer M, Nozzi JL, Nason MC. SPICE: Exploration and Analysis of Post-Cytometric Complex Multivariate Datasets. Cytometry A. 2011;79A:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nature reviews. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 35.Freel SA, Lamoreaux L, Chattopadhyay PK, Saunders K, Zarkowsky D, Overman RG, Ochsenbauer C, Edmonds TG, Kappes JC, Cunningham CK, Denny TN, Weinhold KJ, Ferrari G, Haynes BF, Koup RA, Graham BS, Roederer M, Tomaras GD. Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. Journal of virology. 2010;84:4998–5006. doi: 10.1128/JVI.00138-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duvall MG, Precopio ML, Ambrozak DA, Jaye A, McMichael AJ, Whittle HC, Roederer M, Rowland-Jones SL, Koup RA. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38:350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T, Price DA, Casazza JP, Ferrari G, Nason M, Chattopadhyay PK, Roederer M, Gostick E, Katsikis PD, Douek DC, Haubrich R, Petrovas C, Koup RA. Surface expression patterns of negative regulatory molecules identify determinants of virus-specific CD8+ T-cell exhaustion in HIV infection. Blood. 2011;117:4805–4815. doi: 10.1182/blood-2010-11-317297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi N, Matsumoto K, Saito H, Nanki T, Miyasaka N, Kobata T, Azuma M, Lee SK, Mizutani S, Morio T. Impaired CD4 and CD8 effector function and decreased memory T cell populations in ICOS-deficient patients. J Immunol. 2009;182:5515–5527. doi: 10.4049/jimmunol.0803256. [DOI] [PubMed] [Google Scholar]
- 39.Rajasekaran K, Xiong V, Fong L, Gorski J, Malarkannan S. Functional dichotomy between NKG2D and CD28-mediated co-stimulation in human CD8+ T cells. PloS one. 2010;5 doi: 10.1371/journal.pone.0012635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chevalier MF, Julg B, Pyo A, Flanders M, Ranasinghe S, Soghoian DZ, Kwon DS, Rychert J, Lian J, Muller MI, Cutler S, McAndrew E, Jessen H, Pereyra F, Rosenberg ES, Altfeld M, Walker BD, Streeck H. HIV-1-specific interleukin-21+ CD4+ T cell responses contribute to durable viral control through the modulation of HIV-specific CD8+ T cell function. Journal of virology. 2011;85:733–741. doi: 10.1128/JVI.02030-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams LD, Bansal A, Sabbaj S, Heath SL, Song W, Tang J, Zajac AJ, Goepfert PA. Interleukin-21-producing HIV-1-specific CD8 T cells are preferentially seen in elite controllers. Journal of virology. 2011;85:2316–2324. doi: 10.1128/JVI.01476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rehr M, Cahenzli J, Haas A, Price DA, Gostick E, Huber M, Karrer U, Oxenius A. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. Journal of virology. 2008;82:3391–3404. doi: 10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betts MR, Price DA, Brenchley JM, Lore K, Guenaga FJ, Smed-Sorensen A, Ambrozak DR, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J Immunol. 2004;172:6407–6417. doi: 10.4049/jimmunol.172.10.6407. [DOI] [PubMed] [Google Scholar]
- 44.Almeida JR, Sauce D, Price DA, Papagno L, Shin SY, Moris A, Larsen M, Pancino G, Douek DC, Autran B, Saez-Cirion A, Appay V. Antigen sensitivity is a major determinant of CD8+ T-cell polyfunctionality and HIV-suppressive activity. Blood. 2009;113:6351–6360. doi: 10.1182/blood-2009-02-206557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gett AV, Hodgkin PD. Cell division regulates the T cell cytokine repertoire, revealing a mechanism underlying immune class regulation. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9488–9493. doi: 10.1073/pnas.95.16.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denton AE, Russ BE, Doherty PC, Rao S, Turner SJ. Differentiation-dependent functional and epigenetic landscapes for cytokine genes in virus-specific CD8+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15306–15311. doi: 10.1073/pnas.1112520108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmed R, Bevan MJ, Reiner SL, Fearon DT. The precursors of memory: models and controversies. Nature reviews. 2009;9:662–668. doi: 10.1038/nri2619. [DOI] [PubMed] [Google Scholar]
- 48.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, Bailer R, Graham BS, Roederer M, Koup RA. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. The Journal of experimental medicine. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature medicine. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 50.Makedonas G, Betts MR. Living in a house of cards: re-evaluating CD8+ T-cell immune correlates against HIV. Immunological reviews. 2011;239:109–124. doi: 10.1111/j.1600-065X.2010.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, Gudonis D, Goepfert PA, Lederman MM, Frank I, Makedonas G, Kaul R, Walker BD, Betts MR. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hersperger AR, Martin JN, Shin LY, Sheth PM, Kovacs CM, Cosma GL, Makedonas G, Pereyra F, Walker BD, Kaul R, Deeks SG, Betts MR. Increased HIV-specific CD8+ T-cell cytotoxic potential in HIV elite controllers is associated with T-bet expression. Blood. 2011;117:3799–3808. doi: 10.1182/blood-2010-12-322727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makedonas G, Hutnick N, Haney D, Amick AC, Gardner J, Cosma G, Hersperger AR, Dolfi D, Wherry EJ, Ferrari G, Betts MR. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8 T cells. PLoS Pathog. 2010;6:e1000798. doi: 10.1371/journal.ppat.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Migueles SA, Laborico AC, Shupert WL, Sabbaghian MS, Rabin R, Hallahan CW, Van Baarle D, Kostense S, Miedema F, McLaughlin M, Ehler L, Metcalf J, Liu S, Connors M. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nature immunology. 2002;3:1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 55.Lichterfeld M, Kaufmann DE, Yu XG, Mui SK, Addo MM, Johnston MN, Cohen D, Robbins GK, Pae E, Alter G, Wurcel A, Stone D, Rosenberg ES, Walker BD, Altfeld M. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. The Journal of experimental medicine. 2004;200:701–712. doi: 10.1084/jem.20041270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 57.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, Routy JP, Haddad EK, Sekaly RP. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature medicine. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 58.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature immunology. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.