Abstract
Sphingolipids, ubiquitous membrane lipids in eukaryotes, carry out a myriad of critical cellular functions. The past two decades have seen significant advances in sphingolipid research and in 2010, a first sphingolipid receptor modulator was employed as a human therapeutic. Further, cellular signaling mechanisms regulated by sphingolipids are being recognized as critical players in metabolic diseases. This review focuses on recent advances in cellular and physiological mechanisms of sphingolipid regulation and how sphingolipid signaling influences metabolic diseases. Progress in this area may contribute to new understanding and therapeutic options in complex diseases such as atherosclerosis, diabetes, metabolic syndromes and cancer.
1. Sphingolipid metabolism and signaling
Sphingolipids are eukaryotic specific lipids that carry out essential structural and functional roles (van Meer and Hoetzl, 2010). Within membranes sphingolipids are found associated with cholesterol and together they help form lipid domains. Thus sphingolipids are necessary for the formation of specialized membrane domains such as rafts and caveolae.
De novo sphingolipid biosynthetic pathway
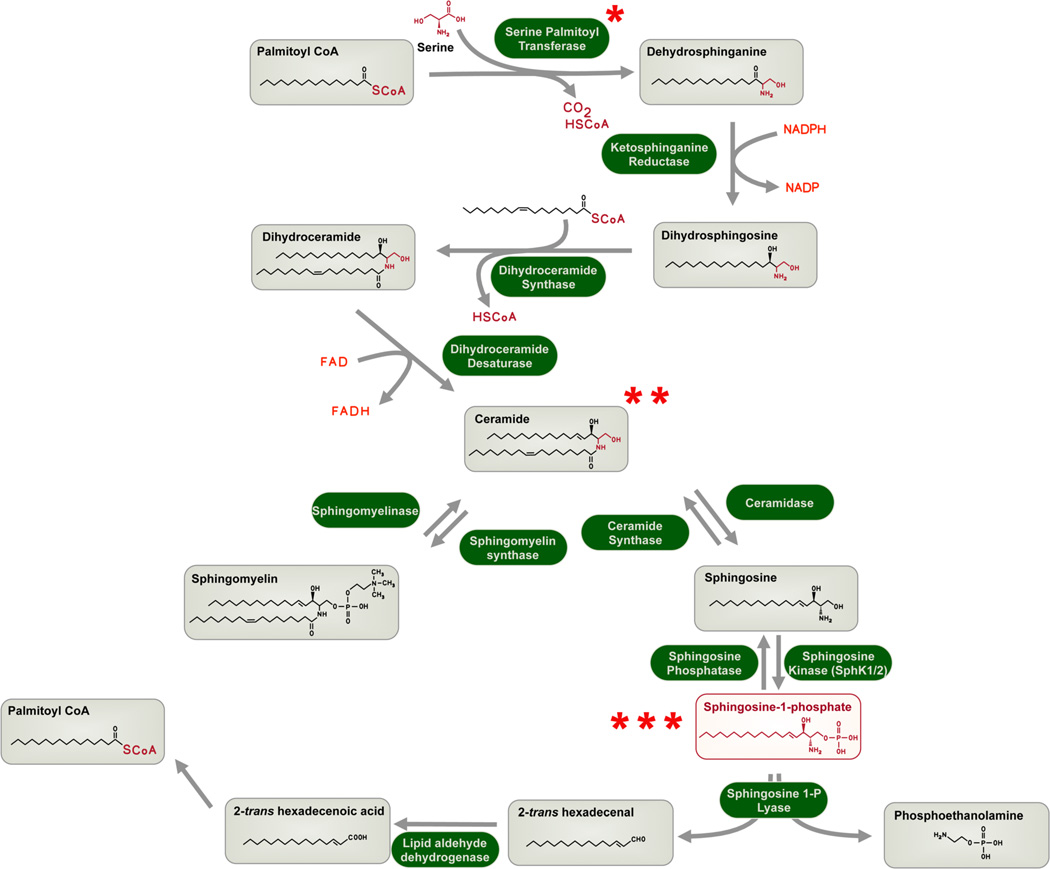
All eukaryotic cells have the capacity to produce sphingolipids via a de novo pathway in the endoplasmic reticulum (Bartke and Hannun, 2009). In this pathway, the amino acid serine is conjugated with the fatty acid, palmitoyl CoA by the rate limiting enzyme serine palmitoyl transferase (SPT). The product of the reaction - dihydrosphingosine (a.k.a sphinganine) is further converted to dihydro ceramide which is dehydrogenated into ceramide, a key intermediate. Specialized carrier proteins such as ceramide transfer protein (CERT) transports ceramide to the Golgi. Ceramide can follow several metabolic fates. For example it can be glycosylated to form glycosphingolipids, phosphorylated into ceramide 1-phosphate, acquire a polar head group to form sphingomyelin. SM and glycosphingolipids are stable, accumulate to high levels in cells and transported via vesicle-mediated mechanisms to specialized membrane domains. For example sphingomyelin accumulates in the outer leaflet of the plasma membrane as a major constituents of rafts (Figure 1).
Figure 1.
Sphingolipid metabolism. Key intermediates are boxed and enzymes are shown in green highlights. Key steps of regulation are indicated with asterisks. *- regulation at the step at the rate-limiting enzyme SPT. Please refer to figure 2 for details. **- ceramide transport from the ER to the Golgi needs the CERT protein which is subject to regulation by cellular signaling pathways. ***- intracellular S1P is secreted by the Spns2 transporter to mediate its extracellular actions on S1P receptors. Intracellular S1P is a key intermediate in the formation of phospholipids (via phosphoethanolamine) and triglycerols (via palmitoyl CoA).
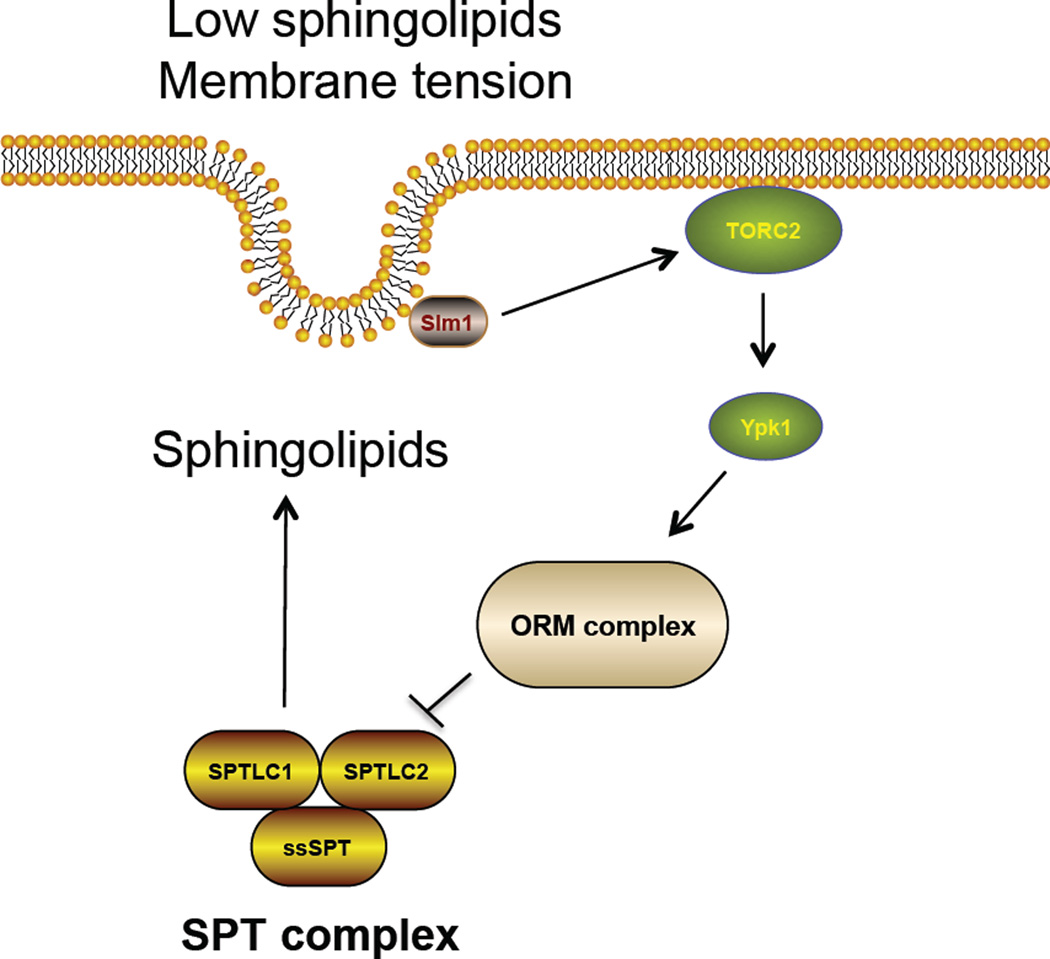
The control of sphingolipid synthesis by cells is regulated by substrate availability as well as by other mechanisms related to lipid composition and membrane homeostasis (Bartke and Hannun, 2009) (Breslow and Weissman, 2010). The availability of the substrate palmitoyl CoA enhances the de novo synthesis of sphingolipids. Since free palmitate levels rise in obesity and metabolic excess, sphingolipid flux through the de novo pathway is enhanced (Bikman and Summers, 2011; Holland and Summers, 2008). Recently, the Orm family proteins which inhibit the SPT enzymes were found to control de novo sphingolipid synthesis (Breslow et al., 2010) in the yeast S. cerevesiae. When sphingolipid levels are low, Orm proteins are highly phosphorylated by the protein kinase Ypk1, a yeast homologue of the mammalian serum and glucocorticoid-induced kinase. This modification blocks the ability of the Orm proteins to inhibit SPT enzyme complex in the ER and therefore enhances de novo sphingolipid synthesis (Roelants et al., 2011). Interestingly, Ypk1 kinase itself is activated by the protein kinase called target of rapamycin complex (TORC)-2 that can sense the membrane sphingolipid levels, especially ceramide (Aronova et al., 2008; Dickson, 2008). Recently, it was shown that sphingolipid depletion or plasma membrane stress in yeast (induced by mechanical stretching) activated translocation of the Slm1 protein to the Torc2 complex, activation of Torc2-dependent Ypk1 kinase, phosphorylation of Orm proteins and stimulation of sphingolipid synthesis via SPT. It is thought that increased sphingolipids reduce membrane stress and thus this system represents a homeostatic regulatory system for cellular membranes(Berchtold et al., 2012) (Figure 2). Whether this signaling system regulates sphingolipid synthesis in vertebrates and metazoans is not known but interestingly, regulation of the mTorc2-dependent Akt kinase (which is in the same AGC kinase family as the yeast Ypk1) is regulated by biomechanical forces in a caveolae-dependent manner(Yu et al., 2006).
Figure 2.
Regulation of SPT enzyme in S. cerevesie. Membrane tension changes or sphingolipid depletion leads to translocation of Slm1 protein and activation of TORC2 complex. This leads to increased activity of the Ypk1 kinase which phosphorylates the ORM complex and thus relieves the inhibitory activity of the SPT enzyme. This results in increased de novo synthesis of sphingolipids.
In mammalian cells, ceramide transport via the CERT protein also is sensitive to intracellular sphingolipid levels, which appear to regulate CERT function by PKD-dependent phosphorylation(Kumagai et al., 2007). Therefore, cells appear to possess the machinery to couple membrane sphingolipid levels to the biosynthetic pathway at the level of the rate-limiting enzyme SPT as well as at other key regulatory steps. However, it is important to stress that our knowledge of regulation of sphingolipid metabolism is significantly limited compared to other lipid regulatory pathways, i.e. sterol metabolism.
Sphingomyelinase pathway-derived mediators
An extensive network of enzymes metabolize sphingolipids into bioactive lipid mediators (Milhas et al., 2010) (Figure 1). For example, sphingomyelinase, which respond to extracellular signals, forms ceramide via a hydrolytic reaction. Ceramide is further converted to sphingosine by ceramidases. Sphingosine levels in cellular membranefs are kept low in part due to the action of ceramide synthase and sphingosine kinases (Sphk1 and Sphk2), which phosphorylate it into sphingosine 1-phosphate (S1P). In vertebrates, S1P is secreted into the extracellular space by specific transporters, one of which, spinster 2 homolog-2 (Spns2) was recently characterized (Kawahara et al., 2009).
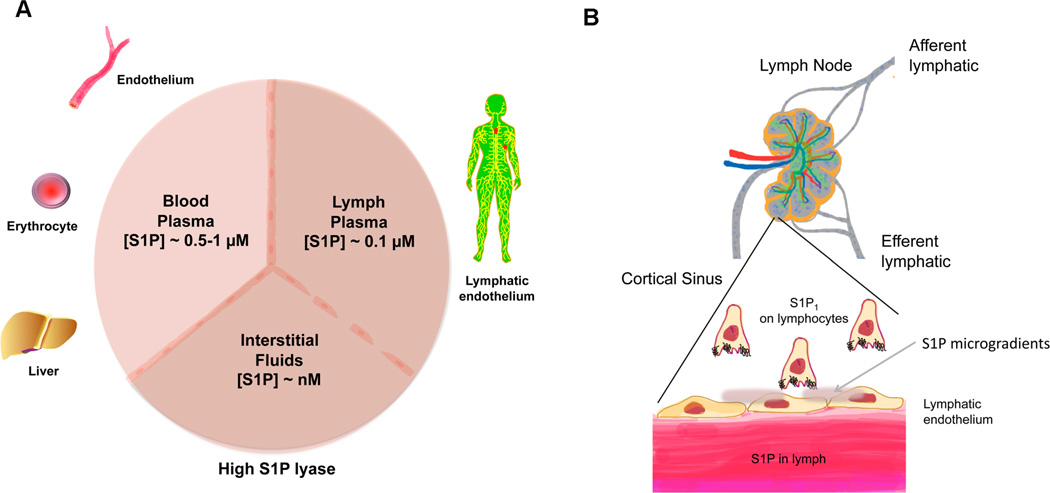
In mammalian plasma, high levels of S1P are found whereas interstitial fluids contain very low levels thus forming a gradient of S1P in different compartments (Pappu et al., 2007) (Figure 3A). Both hematopoietic cells and vascular endothelial cells contribute to high plasma S1P (Pappu et al., 2007; Venkataraman et al., 2008) and lymphatic endothelial cells (Pham et al., 2010) are thought to secrete S1P for the lymphatic circulation. Acute liver failure strongly suppresses plasma S1P levels (Yong-Moon Lee and Timothy Hla, unpublished observations). Further, production of HDL-associated ApoM which chaperones S1P (see below) by the liver may be important for high plasma S1P (Christoffersen et al., 2011). The action of Spns2 in vascular endothelium is important for maintaining high plasma S1P as global knockout as well as endothelial-specific knockout of Spns2 resulted in significant decreases in plasma S1P(Fukuhara et al., 2012). In contrast, it is not clear how S1P is exported out of hematopoietic cells even though ABCC1 was proposed to be the S1P transporter in mast cell lines (Mitra et al., 2006), Abcc1 knockout mice did not show alterations in plasma S1P levels (Lee et al., 2007). Since ABC transport inhibitors are nonspecific, much of the conclusions on S1P transport by this class of membrane proteins should be reassessed using more specific tools and/or genetic models.
Figure 3.
(A) S1P gradients. Plasma is enriched in S1P due to synthesis by red blood cells, endothelial cells and the liver, which provides ApoM, the physiological S1P carrier on HDL. 65% of plasma S1P is carried by ApoM in HDL whereas the remainder is largely associated with albumin. Lymph contains ~ 20% of plasma S1P which is contributed by lymphatic endothelial cells. In contrast, S1P is tissue interstitial fluids is kept low in part due to the high activity of degradative enzymes such as the S1P lyase. The formation of S1P gradients in critical in its physiological actions in immune and vascular systems. (B) Immune cell egress and S1P gradients. In lymph nodes and other secondary lymphoid organs, low S1P environment allows surface presentation of S1P1 receptor which is necessary for lymphocyte egress. High S1P in lymph and low S1P in the lymph node is needed for efficient egress. However, the LPP3 lipid phosphatase(Breart et al., 2011) and S1P transporter Spns2(Fukuhara et al., 2012) may allow microgradients of S1P at the nexus of lymphatic endothelium and lymphocytes.
HDL and other S1P chaperones
Plasma S1P is bound to HDL (~65%) and albumin (~30%). Interestingly, the ability of HDL to induce vasodilation, endothelial cell barrier function, cardioprotection and endothelial cell migration are dependent on S1P(Nofer et al., 2004; Sattler and Levkau, 2009). These studies suggest that the beneficial property of HDL to reduce the risk of cardiovascular disease may in part depend on its S1P chaperoning function. The molecular details of how HDL carries S1P was elusive until recently. Apolipoprotein M (ApoM) binds to S1P (Kd ~ 1 µM), co-crystallizes with S1P, delivers S1P to its receptors and is essential for S1P association with HDL in both mice and humans(Christoffersen et al., 2011). In Apom KO mice, endothelial barrier function was compromised even though significant plasma S1P was still present associated with other plasma proteins, such as albumin. It is currently unclear how HDL/ApoM complex interacts with cell surface S1P and HDL receptors. It is likely that S1P and HDL receptors cooperate upon HDL interaction with cell surfaces. In contrast to S1P, ceramide in plasma is associated mostly with VLDL and LDL(Khovidhunkit et al., 2004). In addition, glycosphingolipids are also associated with LDL and VLDL(Nilsson and Duan, 2006). The role of lipoprotein-associated sphingolipids in normal cardiovascular function and pathology is not well understood. It is likely that enrichment of sphingolipids in lipoproteins and cardiovascular cells contribute to the high abundance of S1P in the circulatory system.
S1P catabolic routes
S1P is degraded by several enzymes. S1P phosphatases-1 and -2 as well as lyso phospholipid phosphatase 3 (LPP3) and S1P lyase are involved in the degradation of this lipid mediator (Blaho and Hla, 2011; Breart et al., 2011) (Figure 1). The phosphatases convert S1P into sphingosine and thus feedback into sphingolipid metabolic pathway. However S1P lyase irreversibly degrades it into hexadecenal and phosphoethanolamine(Saba et al., 1997) which are intermediates in the phospholipid biosynthetic pathway. Recent work shows that further conversion of the highly reactive fatty aldehyde hexadecenal into hexadecenoic acid by hexadecenal dehydrogenase occurs in many cells. Since this ubiquitous pathway ultimately leads to the formation of Palmitoyl-CoA, it is important for conversion of sphingolipids to glycerolipids (Nakahara et al., 2012). Further details of S1P metabolism can be obtained in the following recent authoritative reviews (Bartke and Hannun, 2009; Breart et al., 2011; Cyster and Schwab, 2011; Maceyka et al., 2012).
Receptor-dependent signaling of S1P
Although all eukaryotes from single celled yeast to complex multicellular organisms possess the entire sphingolipid metabolic pathway, the extracellular appearance of S1P and its receptor dependent signaling modes seem to be vertebrate specific (Blaho and Hla, 2011). Indeed S1P transporter and its G protein coupled receptors are only present in the vertebrate genomes. There are 5 receptors for S1P named as S1P1–5. These receptors are closely related to lysophosphatidic acid and cannabinoid receptors and are encoded by 5 distinct genes (Hla and Brinkmann, 2011; Hla et al., 2001). They are expressed widely and couple to overlapping as well as distinct G proteins. Receptor signaling, cellular effects and biological effects have been extensively discussed in the several recent review articles (Blaho and Hla, 2011; Chun et al., 2010; Hla and Brinkmann, 2011; Maceyka et al., 2012). Most cells express one or more subtypes of S1P receptors. Therefore S1P has multiple biological effects in various organ systems.
The most well characterized effects of S1P are on vascular and immune systems (Blaho and Hla, 2011; Cyster and Schwab, 2011; Obinata and Hla, 2012). In the vascular system S1P activation of its receptors regulate vascular tone, vascular permeability, angiogenesis, vascular hyperplasia after injury, atherosclerosis and heart function. However in the immune system, the S1P gradient is critical for egress of immune cells from secondary lymphoid organs into the circulatory system. Therefore S1P receptor dependent trafficking paradigms are critical for normal immune homeostasis as well as inflammatory situations that occur in autoimmune diseases. A unique aspect of S1P signaling is that the ligand is present in abundance for the cardiovascular and blood-borne cells (Hla et al., 2008; Schwab et al., 2005). Given the dissociation constants of ligand receptor interactions are in the low nanomolar range (Hla et al., 2001; Lee et al., 1998), it is likely that cell surface receptors would be stimulated when exposed to plasma (total concentration of S1P in plasma is approximately 0.5–1 µM). Therefore receptor expression and subcellular localization are key to bioactivity of S1P signaling in cells(Oo et al., 2011). Thus, a given cell with S1P receptors on the plasma membrane will receive a more robust intracellular signal than the cell in which the receptors are sequestered in intracellular organelles.
Recently, an S1P receptor modulator called Fingolimod/ Gilenya was approved for the treatment of multiple sclerosis in over 40 countries (Brinkmann et al., 2010). It is a functional antagonist of the S1P1 receptor, by inducing irreversible internalization into endosomes which makes the lymphocytes refractory to the S1P-dependent egress step from lymphoid organs. Thus, Fingolimod treatment leads to disrupted trafficking of autoreactive T cells and suppression of autoimmune CNS inflammation. Thus S1P receptor-based therapeutics have entered the clinical era and numerous efforts are undertaken to further understand the biology of this lipid signaling pathway and to develop novel therapeutics in the control of various autoimmune and related diseases.
2. How do intracellular sphingolipids influence cell behavior?
Sphingolipid metabolism under normal cellular homeostasis is thought to be critical for fundamental cellular events such as membrane homeostasis, endocytosis, cell movement, nutrient transport and protein synthesis (Hannun and Obeid, 2008) (Breslow and Weissman, 2010). In addition, extracellular stimuli such as cytokines, hormones and cell stress (X-ray, UV irradiation) perturb sphingolipid metabolism via effects on both de novo syntheis and degradation (Gault et al., 2010; Morales et al., 2007; Stancevic and Kolesnick, 2010; Zeidan and Hannun, 2010). This results in localized increases in ceramide levels. This hydrophobic sphingolipid induces aggregation of lipid domains and forms ceramide-rich macrodomains. Such domains are large (> 200 nm to several µm in diameter) and are thought to influence membrane signaling events as described below (Cremesti et al., 2001; Grassme et al., 2003). Since lipid rafts localize signaling proteins, it is likely that ceramide-induced formation of macrodomains would alter composition, clustering and interaction of signaling proteins and/or lipids. Thus, alterations in ceramide levels likely influence lipid/ lipid, protein/lipid and protein/protein interactions within the membranes translating to transmission of intracellular signals.
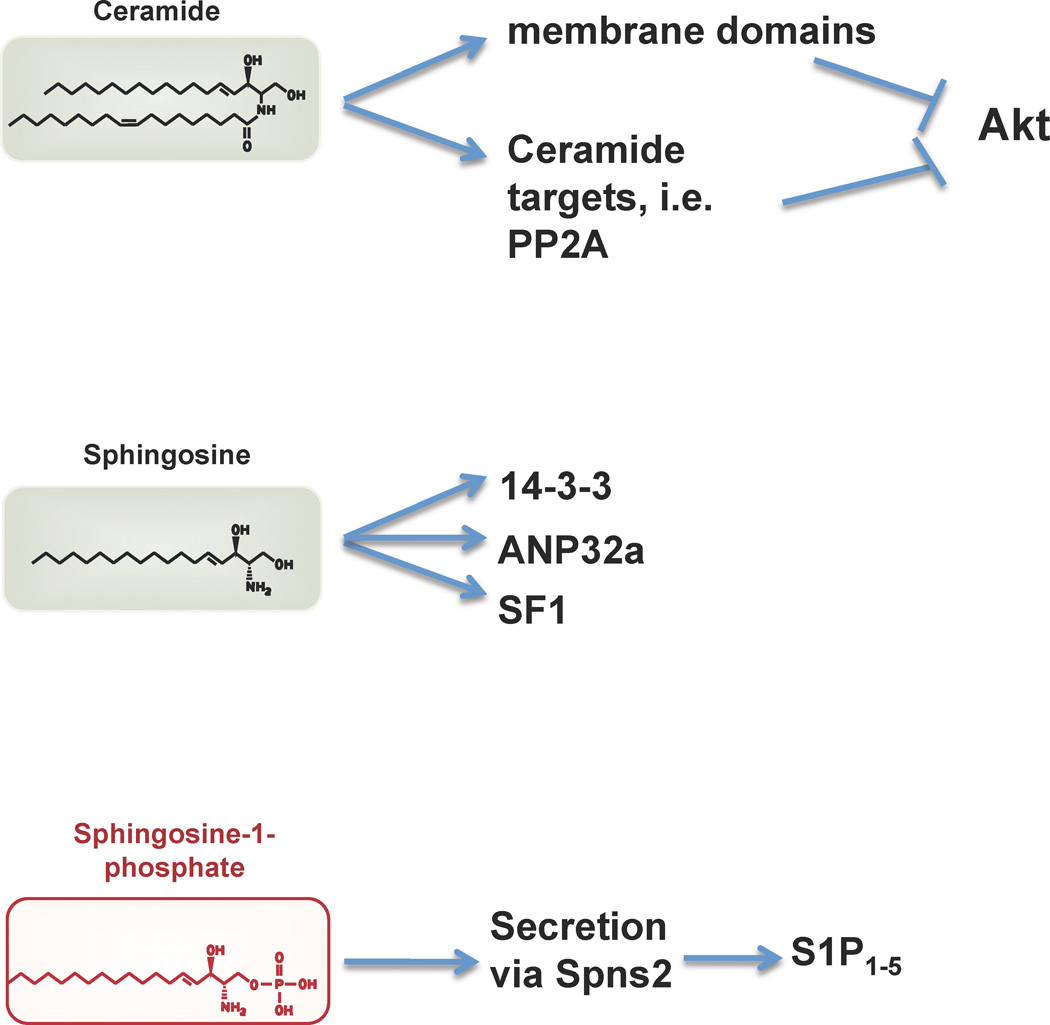
Further, it has been shown that ceramide can interact with a number of signaling proteins such as kinase suppressor of ras (KSR), atypical protein kinase-C (Yin et al., 2009; Zhang et al., 1997), c-Raf-1 (Basu et al., 1998), cathepsin D (Pettus et al., 2002) and ceramide-activated protein phosphatase (Chalfant et al., 2004; Dobrowsky et al., 1993). The physiological significance of such interactions remain to be further explored. In other words, it is not clear if such interactions occur in a classical second messenger mode whereby lipid/protein interaction changes the activity and/or localization of a particular signaling protein. However, it is likely that membrane domain perturbations induced by changes in ceramide levels profoundly alter the recruitment and/or composition of signaling proteins at a membrane locale. In some cases, alteration of ceramide content of specific organelle membranes may lead to biologically significant events. For example, the increase of in ceramide levels in the mitochondria is associated with apoptosis (Deng et al., 2008; Lee et al., 2011; Siskind et al., 2002). Thus, recent data support the model by which ceramides influence cellular signaling by membrane-intrinsic events (figure 4). This viewpoint has replaced the classical second messenger mode of action of ceramide, in which ceramide interacts with soluble proteins outside of membrane compartments.
Figure 4.
Mechanism of action of sphingolipid mediators. Ceramide is thought to act via alteration of membrane domains and signaling intermediates such as PP2A resulting in the inhibition of the protein kinase Akt. The ability of sphingosine to directly bind to 14-3-3-, ANP32a and the nuclear transcription factor SF1 may also be involved in cellular regulation. S1P is secreted by specific transporters such as Spns2 into the extracellular environment where it accumulates to high levels in plasma. S1P binds to and activates five G protein-coupled receptors (S1P1–5).
In addition to ceramide, sphingosine, which is produced by ceramidase, is also capable of altering cellular signaling reactions. It binds to the 14-3-3 protein isoforms, regulates their phosphorylation at the dimer interface and inhibits the anti-apoptotic functions of 14-3-3 (Woodcock et al., 2010; Woodcock et al., 2003). In addition, sphingosine binds directly to the ANP32a and ANP32b proteins which inhibit the protein phosphatase PP2A (Habrukowich et al., 2010). Increases in the intracellular levels of sphingosine or N,N’-dimethylsphingosine block the ability of ANP32 proteins to inhibit PP2A and thereby increase the activity of this key protein phosphatase. In addition, sphingosine binds to the nuclear receptor steroidogenic factor (SF1) and inhibits the transcription of genes such as CYP17 (Urs et al., 2007; Urs et al., 2006). Indeed in structural studies, sphingosine found in the ligand binding pocket of SF1(Li et al., 2005; Sablin et al., 2009). These studies support the concept that intracellular sphingosine levels which are determined by the activities of ceramidase and sphingosine kinases may fluctuate under various physiological conditions and regulate a myriad of cellular functions. However, it is not clear at present whether sphingosine levels play a critical role in the regulation of metabolic reactions as it was shown for ceramide (figure 4).
Some studies have suggested that S1P has intracellular actions (Hla et al., 1999; Spiegel et al., 1996; Spiegel and Milstien, 1995). Early studies suggested that intracellular S1P regulated calcium transients (Ghosh et al., 1990, 1994); however, no molecular entity was identified that could mediate intracellular S1P action to modulate calcium transients. Several studies have also suggested the role of intracellular S1P in the regulation of cell proliferation, actin dynamics, migration and cell survival using a variety of indirect approaches(Edsall et al., 2001; Olivera et al., 2003; Van Brocklyn et al., 1998). However, two recent studies by the Spiegel laboratory have identified the TNFα signaling intermediate, TRAF2 (Alvarez et al., 2010) and the nuclear factor histone deacetylase HDAC1 and 2 (Hait et al., 2009) as S1P binding molecules whose activities are modulated by binding to this sphingolipid. In the case of TRAF2, sphingosine kinase-1-derived S1P was reported to interact with and modulated its ubiquitin ligase activity. For HDAC1 and 2, sphingosine kinase-2-derived S1P inhibited their catalytic activity. Since S1P signals through the cell-surface G protein-coupled receptors which can influence both cytosolic and nuclear changes, the significance of these intracellular interactions are unclear. However, it is established that S1P (and dihydroS1P) play a critical metabolic function by serving as an intermediate in the biosynthesis of important building blocks of phospholipid synthesis, namely, fatty aldehyes which are subsequently converted into fatty acyl CoAs and phosphoethanolamine(Nakahara et al., 2012). This metabolic function is thought to be important in membrane homeostasis(Dobrosotskaya et al., 2002).
3. Extracellular signaling of sphingosine 1-phosphate
It is now established that extracellular S1P signals via its five GPCRs to regulate numerous organ systems (Blaho and Hla, 2011). Signaling properties of the S1P receptors are well characterized and some physiological roles of S1P have been elucidated by the analysis of mutant mice for receptors as well as the use of pharmacological tools (Chun et al., 2010). Some of the known biological processes regulated by S1P appear to be important in normal cellular handling of nutrients and thus dysregulated S1P signaling could contribute to metabolic disease. As a corollary, S1P-regulated cellular and physiological events could play critical roles in metabolic syndromes.
S1P and immune cell trafficking
The ability to regulate immune cell trafficking could be important in obesity-associated inflammation. It is known that S1P is essential for the egress of lymphocytes from thymus and secondary lymphoid organs as well as the re-entry of lymphocytes into peripheral lymphatics from tissue interstitium (Cyster and Schwab, 2011). In that scenario, high concentrations of S1P found in lymph, as well as local gradients established by reticuloendothelial system of egress portals activate the S1P1 receptor on lymphocytes and regulate successful extravasation (figure 3B). At the cellular level, lymphocytes appear to dynamically sample the environment with cellular protrusions and interact with resident reticuloendothelial cells. Given that the S1P1 receptor avidly induces Gi-dependent Rac activation, Akt phosphorylation, cortactin translocation, microtubule dynamics and F-actin assembly (Hla et al., 2001), it is likely that such GPCR-induced membrane protrusion events are necessary for efficient egress to occur. Analysis of T cell egress from lymph nodes also suggested that the balance of two competing chemokine signaling events determines egress rates. One signaling event, e.g., the balance between CXCL21/ CCR7 promotes T cell retention, whereas the other, e.g., S1P/ S1P1 promotes egress (Grigorova et al., 2009; Pham et al., 2010). Since hematopoietic cells in various environments, such as the bone marrow, spleen, lymph node, tissue interstitial spaces and the inflammatory milieu are exposed to numerous chemokine and lipid mediator gradients, competition between different GPCR signaling systems likely influence the cellular behavior and the likelihood of retention vs. egress. In addition, S1P-mediated signaling may play a role in the cellular arrangements into specific tissue architectures. For example, it is known that S1P2 receptor activates the G12/13 pathway to stimulate Rho GTPase resulting in the activation of PTEN, increased cAMP levels and inhibition of Akt and Rac GTPase (Michaud et al., 2010; Sanchez et al., 2007; Sanchez et al., 2005; Windh et al., 1999). These events result in reduced cell motility. Since the S1P2 receptor is critical for increased macrophage content in atherosclerotic plaques (Skoura et al., 2011) and the ability to commit B-cells to germinal centers (Green et al., 2011), cellular aggregation/ dispersion events which ultimately determine lymphoid architecture may also be regulated.
Such mechanisms could play important roles in obesity-associated inflammation in adipose tissue. For example, increased infiltration of CD8 T cells and inflammatory macrophages could result from S1P receptor-driven processes (Nishimura et al., 2009). Further, tissue retention of circulating hematopoietic progenitor cells and their differentiation into myeloid lineage (Massberg et al., 2007) could further enhance monocyte/ macrophage content of adipose tissues. The appearance of clusters of macrophages, commonly referred to as “crown-like structures” in adipose tissue of obese animals and humans (Morris et al., 2011), could also be regulated by S1PR-regulated events. The role of such migratory and collective cellular architectures may be important in dysregulated inflammatory reactions seen in metabolic syndromes and obesity-related disorders.
S1P and the vasculature
S1P signaling is also critical for vascular development and homeostasis. S1P1–3 receptors play significant roles in endothelial and vascular smooth muscle cells (Hla et al., 2001). In vascular smooth muscle, S1P2 and S1P3 regulate vasoconstriction, presumably due to the coupling to Gq/ IP3/ Ca2+ and G12/13/ Rho/ROCK signaling pathways (Kono et al., 2007; Murakami et al., 2010; Salomone et al., 2003; Szczepaniak et al., 2010). In contrast, coupling of S1P1 in endothelial cells to the eNOS enzyme, presumably due to Gi/ PI3K/ Akt pathway results in endothelium-dependent vasorelaxation (Dantas et al., 2003; Igarashi and Michel, 2009; Nofer et al., 2004). Thus, modulation of S1PRs can contribute to local vascular tone and systemic changes in blood pressure. Whether this plays a role in obesity-associated hypertension is not known. In the endothelium, S1P1 signaling is critical for VE-cadherin-dependent adherens junction assembly and regulation of vascular permeability (Jacobson and Garcia, 2007; Lee et al., 1999; McVerry et al., 2004; Sanchez et al., 2003). Regulation of cell-matrix interactions through strengthening of integrin-mediated events also plays a role in this phenomenon (Paik et al., 2001). In contrast, S1P2 promotes vascular permeability, due to its ability to induce Rho-dependent cell rounding and disruption of cell-cell junctions (Sanchez et al., 2007; Skoura et al., 2007). Interestingly, S1P2 mRNA is induced under hypoxic and inflammatory conditions. The ability to control vascular permeability by S1P receptors suggests that dysregulation of this system could participate in obesity-associated inflammatory conditions. Indeed, increased vascular permeability is an essential feature of inflammation.
S1P receptors regulate vascular development and angiogenesis (Liu et al., 2000; Paik et al., 2004). In obesity, concomitant with adipocyte hypertrophy and hyperplasia, increased neovascular growth or angiogenesis occurs. This is in fact essential since tissue growth and concomitant increases in oxygen and metabolic demands must be met by increased vascular supply. The ability of S1P1 to cooperate with angiogenic growth factors and stabilize neovessels (Lee et al., 1999) is likely critical in this context. Other S1P receptors, namely, S1P2 and S1P3 cooperate with S1P1 in embryonic angiogenesis (Kono et al., 2004). It is not known what the consequences are of S1PR dysfunction in obesity-associated angiogenesis.
Does S1P influence thrombosis and hemostasis?
In addition to the S1P ligand released by activated platelets presumably during thrombotic episodes (Yatomi, 2006), S1P signaling in the endothelial cells also appears to regulate thrombosis and hemostasis (Obinata and Hla, 2012). Activated protein C binds to its receptor and transactivates the S1P1 receptor to maintain the barrier function of the endothelium (Finigan et al., 2005). In addition, thrombin activation of its receptor PAR-1 results in S1P secretion and autocrine activation of S1P1 receptor which allows the recovery of the barrier dysfunction (Komarova et al., 2007). The role of S1P and its receptors in thrombosis, hemostasis and fibrinolysis are not well understood. However, given the cross talk between inflammatory, angiogenic and clotting pathways (Obinata and Hla, 2012), it is highly likely that S1P signaling in endothelial cells and platelets regulate such processes. During obesity, thrombotic episodes increase and factors that promote thrombosis as well as inhibit fibrinolysis increase. Whether S1P signaling and sphingolipid metabolic alterations play a role in such pathophysiologic scenarios need further investigation.
S1P and the pancreas
Endodermal organs, such as pancreas develop in response to intrinsic transcriptional cues, as well as endothelial- and blood-derived signals. Blood-derived S1P appears to be one such factor (Cleaver and Melton, 2003). Pancreatic mesenchymal bud development in the mouse is regulated by blood-derived S1P which acts on its receptors to regulate organ development and differentiation (Edsbagge et al., 2005; Serafimidis et al., 2011). Further, concomitant vascular development and endothelial cell signaling also regulates pancreatic development by both delivery of oxygen and nutrients as well as angiocrine signaling. In the adult, pancreatic beta cell survival and response to cytokines appear to be modulated by S1P receptors (Laychock et al., 2006; Rutti et al., 2009). In murine models, S1P2 receptor appears to mediate beta cell cytotoxicity whereas HDL activation of S1P receptors appears to attenuate inflammatory cytokine-induced beta cell loss.
In addition to these events, signaling of S1P receptors in adipocytes, muscle, liver and heart likely also regulate metabolic pathways and thus influence the pathophysiology of obesity and metabolic syndromes. However, specific mechanisms and biological effects remain poorly understood at present. A brief summary of S1P receptor functions in metabolic conditions is given in table I.
Table I.
Multiple S1P receptors in different cell types regulate various biological processes that have an impact in metabolic diseases. Some relevant examples from preclinical models are summarized.
| Cell Types in which S1P receptors function |
S1P receptor subtype |
Biological Process regulated by S1P receptors |
Potential Pathophysiological consequence in metabolic diseases |
Reference |
|---|---|---|---|---|
| T cells | S1P1R | Lymphocyte egress, phenotype switching | Immune response, inflammation |
Liu, G (2010) Cyster/ Schwab (2011) |
| B cells | S1P2R | Germinal center architecture | Humoral Immune response | Green, JA (2011) |
| Macrophages | S1P1R | Anti-inflammatory M2-like response | Innate immune response | Hughes, JE (2008) |
| Monocyte/macrophages | S1P2R | Monocyte trafficking/retention in tissues | Enhanced atherosclerosis/inflammation | Skoura, A (2011) |
| Endothelial cells | S1P1R, S1P3R | Adherens junction assembly, NO production | Normal vasomotor control, enhanced barrier function | Lee, MJ (2009) Nofer, JR (2004) |
| Endothelial cells | S1P2R | Adherens Junction disassembly, | Vascular inflammation |
Sanchez, T (2007) Skoura, A (2007) |
| Vascular smooth muscle cells | S1P3R, S1P2R | vasoconstriction | hypertension |
Salomone, S (2003) Murakami, A. (2010) |
| Pancreatic β-cells | S1P1R | Insulin secretion, islet survival | Normal glucose homeostasis |
Laychock, SG (2006) Fukuhara, S (2012) |
4. Sphingolipids in the control of glucose homeostasis
Recent work shows that increased levels of saturated fatty acids such as palmitate (16:0) results in the increased metabolic flux into the de novo sphingolipid biosynthetic pathway (Bikman and Summers, 2011; Samad et al., 2011; Yang et al., 2009). Therefore, in obesity and metabolic syndromes, increased levels of sphingolipids, in particular, ceramide, is observed. Secondly, activation of TLR4 by saturated fatty acids results in the upregulation of ceramide synthetic enzymes in a IκBβ kinase-dependent manner (Holland et al., 2011a). The net result is the increased levels of ceramide in various cellular membranes, which is associated with profound insulin resistance. The importance of this pathway is established in studies which show that inhibition of de novo sphingolipid synthesis by myriocin, an inhibitor of serine palmitoyl transferase (SPT), reversed the insulin resistance observed in murine models of obesity (Holland et al., 2007). Additionally, studies in myocytes cultured in vitro have shown that ceramide rather than sphingosine is responsible for fatty acid-mediated attenuation of insulin-stimulated glucose uptake and Akt phosphorylation (Chavez et al., 2005). These data point to the critical role of ceramide in metabolic dysregulation of glucose uptake and metabolism.
This was further underscored in a recent study which showed that adiponectin, a key homeostatic hormone acts on its receptors in adipocytes, cardiac muscle and liver to convert increased ceramide levels to S1P(Holland et al., 2011b). Adiponectin receptors induce ceramidase enzyme activity by a poorly understood mechanism resulting the formation of S1P via a sphingosine kinase-dependent pathway. This is important for reducing insulin resistance, cardiac dysfunction and liver pathologies associated with obesity and metabolic dysregulation.
A key question is how ceramide induces insulin resistance? As discussed above in section 3, ceramide levels in membranes greatly influence lipid domain structure and dynamics. Indeed, large macrodomains of membranes are formed when ceramide levels rise (Cremesti et al., 2002; Gulbins and Kolesnick, 2003). Furthermore, using cell culture models in which exogenous ceramide was introduced, it was found that translocation of Akt to plasma membrane via its PH domain was inhibited (Stratford et al., 2001). Moreover, activation of protein phosphatase PP2A (Dobrowsky et al., 1993) which can attenuate Akt signaling was observed. Thus, increased ceramide levels likely block insulin signaling by antagonizing the key signaling intermediate Akt, which is needed for stimulation of glucose transport as well as downstream transcriptional responses. It is also likely that additional steps are involved in various organelle membranes of the cell, via biophysical mechanisms as well as by modulation of key signaling intermediates.
In addition to ceramide, increased sphingolipid biosynthesis could lead to the activation of Sphk enzymes and the production of S1P. Extracellular S1P can modify function and survival of pancreatic beta cells in the islets. Treatment of isolated islets with HDL or S1P resulted in increased survival. In contrast, LDL induced increased islet apoptosis. Thus, extracellular S1P can influence insulin levels (Rutti et al., 2009). Indeed S1P enhanced survival of pancreatic islet cells in vitro (Laychock et al., 2006). Furthermore, S1P treatment acutely increases insulin secretion from pancreatic beta cell lines and in isolated islets in vitro(Fukuhara et al., 2012). It is not known if systemic S1P or local increases in S1P production, which can be induced by glucose via the activation of Sphk1 (Mastrandrea et al., 2010), is involved in physiological control of insulin secretion.
Although considered more as structural components of cell membranes, glycosphingolipids also play important roles in insulin sensitivity. Increased levels of glycosphingolipids which are found in genetic disorders of glycosphingolipid breakdown such as Gaucher’s disease are associated with resistance to insulin signaling as manifested by reduced insulin-stimulated glucose uptake(Aerts et al., 2011; Inokuchi, 2010). Although the mechanisms involved are not entirely clear, insulin receptor is inhibited by high glycosphingolipid microenvironment of the plasma membrane. Indeed, the ganglioside GM3 seem to directly associte with the insulin receptor and inhibits downstream signaling. Reduction of glycosphingolipid levels by inhibitors of synthesis, treatment with degrading enzymes or knockout of biosynthetic enzymes in mouse models reversed insulin resistance suggesting an important role for this family of sphingolipids(Aerts et al., 2011; Inokuchi, 2010). However, the relationships between ceramide- and glycosphingolipid-induced insulin resistance are not well understood. Whether they represent parallel systems or are interconnected is not clear at present. In addition, tissue specificity (i.e. muscle vs. adipose tissue) of ceramide vs. glycosphingolipid functions could also determine the overall pathophysiological state of insulin resistance.
These findings indicate that sphingolipids influence glucose metabolism in a variety of tissues. In metabolic syndrome, where abnormal lipid accumulation is seen in non-adipogenic tissues such as liver, muscle, heart and pancreas (Holland and Summers, 2008), increased sphingolipid levels likely play profound pathophysiological roles.
5. Sphingolipids at the cross-roads of amino acid, fatty acid and carbohydrate metabolism
The rate limiting enzyme in the sphingolipid biosynthetic pathway, SPT is is an oligomeric enzyme composed of three SPTLC subunits. SPTLC2 and SPTLC form heterodimers which have low enzymatic activity but upon association with the third subunit called ssSPT, greatly enhanced activity was seen. It is thought that heterotrimeric SPT isozymes or higher order oligomeric structures are functional(Lowther et al., 2012). Mutations in two such subunits were shown to cause a severe form of neuropathy called hereditary sensory autonomic neuropathy type I (HSAN1) (Garofalo et al., 2011). Interestingly, such mutations changed the property of this rate-limiting enzyme – in addition to reducing the catalytic efficiency, substrate promiscuity was observed resulting in the formation of abnormal sphingolipids. The mutant enzymes used alanine and glycine as atypical substrates and produced abnormal sphingolipids, deoxysphinganine and deoxymethylsphinganine, respectively (Gable et al., 2010). These sphingolipids lack the hydroxyl group at C1 and cannot be converted into phosphorylated and glycosylated complex sphingolipids. In addition, degradation enzymes including S1P lyase and phosphatases cannot metabolize these deoxysphingolipids. However, they can be N-acylated into ceramides and oxidized into sphingosines by the introduction of the trans double bond at C4–C5.
Since free sphingoid bases or ceramides can induce cell stress responses, and similar deoxysphingolipids isolated from marine sponges induce cellular toxicity, it was hypothesized that the formation of deoxysphingolipids cause neuronal toxicity in HSAN1 (Garofalo et al., 2011; Scherer, 2011). Indeed, Merrill and colleagues found that deoxysphingolipids were formed by treatment of cells or mice with fumonisin B1, which inhibits ceramide synthase (Zitomer et al., 2009). In fact, utilization of alanine by wild-type SPT occurs to a degree that results in the formation of abnormal sphingolipids. Fumonisin B1 by inhibiting the conversion of such sphingoid bases into ceramide species enabled the formation of deoxysphingolipids to be readily detected (Zitomer et al., 2009). However, by altering substrate availability, i.e. supplementation of L-serine, the formation of deoxysphingolipids was suppressed in a transgenic model of HSAN1 (C133W SPTLC1 mutant mouse) and in human HSAN1 patients. In the mouse, L-serine supplementation suppressed symptoms of neuropathy and male reproductive abnormalities, suggesting the pathogenic role of abnormal deoxysphingolipids (Garofalo et al., 2011).
Since the precursors of deoxysphingolipids are naturally occurring amino acids, such as, alanine and glycine, which are coupled to carbohydrate metabolic pathways (e.g. glycolysis), it is likely that altered levels of amino acids which occurs in metabolic syndrome and diabetes would lead to alterations in deoxysphingolipid levels. This was shown by the laboratory of Hornemann who examined the levels of deoxy and 1-hydroxylated sphingolipids in normal subjects and in patients with metabolic syndromes and diabetes(Bertea et al., 2010; Othman et al., 2012). They found significant upregulation of glycine- and alanine-derived sphingolipids in plasma of diabetics and metabolic syndrome patients compared to controls. These data are exciting because deoxy-sphingoid bases were enriched in the LDL and VLDL fractions in the plasma and could serve as useful biomarkers in metabolic syndromes. Furthermore, they could play a role in the pathogenesis of diabetic neuropathy as well as in other changes including microvascular alterations.
Future studies need to focus on cellular and molecular mechanisms, which mediate the cytotoxic reactions of the deoxysphingolipids. They can be present as free sphingoid base or as ceramides and thus could alter intracellular signaling pathways by interacting with specific downstream signaling proteins or alteration of membrane structure. This would allow novel approaches to reverse the pathologic phenotypes associated with deoxysphingolipids.
6. Sphingolipid metabolic connections with cellular lipid hemeostasis
Sphingolipid metabolism is tightly coupled to other lipid metabolic pathways, for example, sterol metabolism(Degroote et al., 2004). Changes in plasma membrane sphingomyelin results in major alterations in cellular cholesterol levels and trafficking, presumably due to the affinity of these two lipids. Thus, it was observed that sphingomyelinase treatment of cells, which reduces plasma membrane sphingomyelin, resulted in the increased levels of free cholesterol in the ER (Scheek et al., 1997) (van Meer and Hoetzl, 2010). This was shown to inhibit the activation of SREBP-1, thus resulting in the inhibition of cholesterol synthesis and uptake, primarily by the downregulation of HMG CoA reductase and LDL-R expression. Similarly, enhanced uptake of sphingomyelin resulted in trapping of sphingolipids and cholesterol in late endosomes and lysosomes, thus reducing free cholesterol at the ER, and increased SREBP-mediated upregulation of cholesterol synthesis and uptake. Thus, sphingolipid and cholesterol metabolic pathways are tightly linked which raises further questions about the consequences of such interactions in metabolic diseases such as atherosclerosis and diabetes.
Sphingolipid synthesis and breakdown appear to be critical for feedback control of phospholipid synthesis in Drosophila in which SREBP pathway senses levels of phosphatidyl enthanolamine (PE) (Dobrosotskaya et al., 2002). The de novo sphingolipid biosynthetic pathway is coupled to PE synthesis by providing the fatty acid substrate. When phosphorylated sphingoid bases are degraded, fatty acid aldehydes and phosphoethanolamine are produced by the S1P lyase reaction. Such intermediates are necessary for PE synthesis. Indeed, PE levels are sensed by SREBP, which in turn controls the sphingolipid biosynthetic enzymes. Whether this mechanism occurs in vertebrates is not known but this example illustrates the interconnections that exist between cellular lipid metabolic systems.
Recent studies in S. cerevesiae suggest a novel feedback control of sphingolipid biosynthesis involving TORC2 and Orm proteins(Aronova et al., 2008; Roelants et al., 2011; Tabuchi et al., 2006). Analysis of TORC2 subunit Avo3 (yeast homologue of the mammalian RICTOR) showed that TORC2 kinase controls ceramide levels. Specifically, TORC2 activates the Ypk2 kinase which is necessary for the activity of ceramide synthase enzyme. Previous studies by the laboratories of Dickson (Dickson, 2008) and Kozutsumi (Sun et al., 2000) have shown that sphingosine and similar long chain bases activate the Ypk2 kinase in a Phk1 and Phk2 kinase-dependent manner. This constitutes a feed-forward regulatory loop whereby reduced ceramide synthase activity leads to increased sphingosine and activation of Ypk2 and ceramide synthesis in a TORC2-dependent manner. In another study, activation of Ypk2 and TORC2 phosphorylated the Orm proteins and inhibited their repression of SPT activity(Breslow and Weissman, 2010; Roelants et al., 2011). Thus, Ypk2/ TORC2 pathway increases sphingolipid synthesis by activating ceramide synthase and SPT activity. Since TORC2 is an evolutionarily-conserved pathway regulated by nutrient availability, cell growth and cellular energy status(Laplante and Sabatini, 2009), recent evidence suggesting that it is intimately connected with sphingolipid pathways highlight the potential importance of these lipids in cellular metabolic regulation. This universality of this paradigm needs to be further explored.
7. Inflammation impact on sphingolipids
During inflammatory processes, mobilization of innate and adaptive immune cells to the tissues as well as activation of cytokine networks ensues. Such changes are regulated by sphingolipids and sphingolipid metabolism is altered in tissues as a response of these alterations. The trafficking of both naïve and activated adaptive immune cells is regulated by S1P signaling via the S1P receptor-1(Cyster and Schwab, 2011). In addition, dendritic and NK cell trafficking is also regulated, even though other S1P receptors such as S1P5 may be involved. Thus, S1P signaling is required for proper trafficking patterns which ensure the optimal magnitude and duration of both humoral and cellular immune responses. Evidence also exists for the regulation of cellular phenotype by S1PR signaling. Thus, the regulatory T cells (Treg), which are very important to attenuate inflammatory and autoimmune responses, is regulated by the S1P1/ Akt/ mTOR signaling axis(Liu et al., 2010). In multiple sclerosis patients who were administered the S1PR modulator Fingolimod, levels of circulating TH17 cells (which promote inflammatory reactions) is reduced whereas Treg numbers increase, suggesting that such changes underlie the efficacy of this pharmacological agent(Mehling et al., 2010). Whether S1P/ S1PR signaling controls myeloid cell influx, survival and egress is not known. However, macrophages express significant levels of the mRNA for S1P1 and S1P2. S1P1 function is associated with anti-inflammatory or M2 phenotype (Hughes et al., 2008) whereas S1P2 inhibits the chemokine-induced migration of macrophages to tissue spaces(Michaud et al., 2010). In addition, S1P2 function in macrophages appear to regulate the number of macrophages that infiltrate the atherosclerotic plaques in mouse models, perhaps due to its ability to reduce chemokine-induced motility and thereby block egress(Skoura et al., 2011). Further studies are needed to define the role of S1P receptors in obesity-induced inflammatory responses.
S1PRs in endothelial cells could also play a role in driving the extent and intensity of the inflammatory response in tissues. In vitro studies have shown that S1P1 and S1P2 exert opposing roles in regulating vascular permeability(Sanchez et al., 2007; Skoura and Hla, 2009). In addition, S1P2 mRNA expression appears to be induced in pathological states. Thus, by regulating plasma exudation, tissue inflammatory responses could be altered. It is known that deposition of plasma proteins such as fibrin into the interstitial spaces would induce an acute inflammatory response, since fibrin degradation products have profound effects on innate immune cells(van Hinsbergh, 2012). Whether S1P-regulated vascular changes play a role in obesity-associated inflammatory responses warrant further investigation.
In contrast to the well established roles of extracellular S1P/ S1PR signaling in various biological responses, the intracellular role of sphingolipids in inflammatory reactions is equivocal and its physiological role unclear. Even though sphingolipid turnover is stimulated by innate immune stimulation, for example, TNFα signaling(Peraldi et al., 1996) (Dressler et al., 1992), whether this is essential for the ability of the cytokine to function is not clear. Ceramide formation is induced in many systems, but its duration varies and may be related to membrane changes that occur secondary to cytokine action. Importantly, co-immunoprecipitation of the TNFα effector TRAF2 with Sphk1 was observed in transfected cells(Xia et al., 2002). In addition, a recent report suggested that Sphk1-derived intracellular S1P is a co-factor for the E3 ubiquitin ligase activity of TRAF2 and that this interaction is necessary for TNFα-induced NFκB activation(Alvarez et al., 2010). This interesting report was based entirely on in vitro findings. However, in our recent study of Sphk1 and Sphk2 double KO macrophages, TNFα-induced NFκB responses are indistinguishable from WT macrophages, suggesting that intracellular S1P is not essential for the TNFα-induced inflammation in these cells (Yuuan Xiong and Timothy Hla, unpublished observations). Further, Sphk1 (Michaud et al., 2006) and Sphk2 mice (Zemann et al., 2007) exhibit normal inflammatory responses and a recent report that claimed the essential role of Sphk1 in mouse models of sepsis (Puneet et al., 2010) was highlighted as potentially of ethical concern by the journal Science(Alberts, 2011), which raises serious questions about the conclusions in that paper which implied the critical role of Sphk1 in sepsis.
S1P, abundantly expressed in the vascular compartment, is an important player in maintaining normal homeostasis. However, its proper function may be required in the regulation of extent, intensity and duration of inflammatory reactions. Its mode of action is clearly different from classical lipid mediators such as eicosanoids which are produced upon demand and act rapidly to regulate inflammatory reactions(Shimizu, 2009). Whether, compartment-specific function of S1P regulates inflammation requires further study. As such, it could be critical in obesity associated inflammatory responses.
8. Potential involvement of sphingolipids in obesity-associated syndromes
Atherosclerosis
Sphingolipids play complex roles in initiation and progression of atherosclerosis, a disease which causes highest morbidity and mortality in developed and newly developing countries. Plasma sphingomyelin levels were correlated with incidence of cardiovascular disease even though this finding has been not universally confirmed (Chen et al., 2011; Jiang et al., 2000; Schlitt et al., 2006; Yeboah et al., 2010). Importantly, inhibition of SPT with myriocin reduced plasma sphingomyelin levels and reduced atherosclerotic lesions in murine models of atherosclerosis (Hojjati et al., 2005). These data suggest that sphingolipid metabolic alterations could play important roles in atherosclerosis.
In related studies, SMS2 enzyme which synthesizes sphingomyelin was shown to play important roles in atherosclerosis (Liu et al., 2009a; Liu et al., 2009b). Deletion of the Sms2 gene resulted in attenuation of plasma sphingomyelin level and reduced plaque burden in mouse models of atherosclerosis. Studies in macrophages suggest that SMS2 is required for the elevation of sphingomyelin and diacylglycerol, two lipids that are important for raft formation and PKC activation, respectively. Indeed, TLR2-induced NFκB activation was impaired in Sms2 KO cells (Hailemariam et al., 2008), suggesting that attenuated inflammatory mechanisms may explain the anti-atherogenic phenotype.
An important pro-atherogenic mechanism that sphingolipids regulate is the aggregation and retention of LDL and VLDL in the subendothelial space. Tabas and co-workers showed that secreted acid sphingomyelinase acts in LDL and VLDL to induce their aggregation, which is presumably mediated by the production of ceramide (Schissel et al., 1998). This event may cause increased uptake of aggregated lipoproteins by resident macrophages and their differentiation into foam cells. Thus, acid sphingomyelinase KO mice exhibited a profound reduction in atherosclerotic lesion formation(Devlin et al., 2008).
Given that increased sphingolipid entry and breakdown occurs in inflamed vascular wall, presumably due to the influx of pro-atherogenic lipoproteins and increased expression of secreted sphingomyelinases during inflammation(Tabas et al., 2007), it is likely that downstream sphingolipid mediators are also enhanced. In that context, we hypothesized that increased S1P in the vascular lesions would signal via S1P receptors to regulate atherosclerotic plaque progression. S1p2r KO exhibited profound reduction in plaque area in both LDLR and ApoE models of atherosclerosis (Skoura et al., 2011). Indeed, the function of S1P2 in macrophages seem to be important. Possible mechanisms include retention of macrophages in the plaque by the anti-migratory S1P2 receptor and the modulation of inflammatory phenotype (secretion of proinflammatory cytokines such as IL-1β and IL-18). Another study on S1p3r KO mice showed its importance in macrophage influx without alterations in atherosclerotic plaque size(Keul et al., 2011). However S1P1 and S1P2 are expressed at higher levels in macrophages and likely play more prominent roles in atherosclerosis in mouse models(Michaud et al., 2010). The effect of S1P1 in atherosclerosis has not been addressed in animal models. However, in vitro, S1P1 is necessary for the induction of the anti-inflammatory M2 phenotype(Hughes et al., 2008). The interplay of S1P receptors in endothelial cells, macrophages and vascular smooth muscle cells likely play important roles in initiation and progression of atherosclerosis. Since sphingolipids are critical in this vascular disease, further knowledge in this area will likely provide novel therapeutic opportunities in this challenging area of translational research.
Cancer
Sphingolipids play myriad and profound roles in cancer. For example, dietary sphingolipids suppress colon carcinogenesis in animal models(Merrill et al., 1995). Sphingosine kinases are elevated in numerous cancer models and are being actively pursued as a therapeutic target given that knock out of Sphk1 enzyme suppresses intestinal polyposis in mouse genetic models(Kohno et al., 2006). The reader is referred to the following recent reviews which address this issue in a comprehensive manner (Cuvillier, 2007; Fyrst and Saba, 2010; Pitson, 2011; Pyne and Pyne, 2010). The focus of our discussion will be how metabolic alterations in the sphingolipid signaling axis that occur in obesity have the potential to impact cancer development and progression‥
Obesity is an established risk factor for numerous malignancies (Calle and Kaaks, 2004). In addition to being a risk factor for the development of postmenopausal breast cancer, obesity is associated with an increased risk of cancers of the colon, esophagus, endometrium, kidney and pancreas (van Kruijsdijk et al., 2009). Moreover, obesity is increasingly recognized as a poor prognostic factor for many malignancies (Yoon et al., 2011). Several mechanisms appear to account for the link between obesity and carcinogenesis. Increased production of estrogen in excess adipose tissue has been suggested to contribute to the increased risk of postmenopausal breast cancer (Cleary and Grossmann, 2009). Multiple lines of evidence support a role for obesity-related effects on insulin levels and the insulin-like growth factor-1 axis, in addition to altered production of adipokines in the pathogenesis of cancer (van Kruijsdijk et al., 2009). Obesity also causes increased levels of proinflammatory mediators which may impact on the development and progression of cancer (Olefsky and Glass, 2010; Sinicrope and Dannenberg, 2011).
Given the link between obesity and cancer, it is worth considering whether sphingolipid alterations could be involved. Cancer development and progression are accelerated by the proinflammatory microenvironment, as exemplified in Helicobacter pylori-induced gastric cancer, Hepatitis B-induced liver cancer, among others. Indeed obesity causes systemic as well as local inflammation including increased myeloid cell infiltration, presence of crown-like structures of macrophage aggregates and increased pro-inflammatory cytokine production(Morris et al., 2011; Subbaramaiah et al., 2011). Since increased levels of ceramide and S1P modulate immune and inflammatory reactions, signaling of sphingolipids could contribute to the pro-oncogenic microenvironment. For example, increased S1P signaling in endothelial cells could induce angiogenesis and thereby induce tumor growth and metastasis. Recruitment of various types of immune cells in the tumor microenvironment could also be regulated by S1P signaling. For example, myeloid-derived suppressor cells are known to suppress anti-tumor immunity (Lindenberg et al., 2011) and whether trafficking or differentiation of such cells is regulated by S1P receptor signaling warrants investigation. In addition, membrane sphingomyelin and ceramide levels are known to be critical for antigen presentation and raft-dependent T cell receptor function(Levental et al., 2010). Systemic alterations in ceramide levels which are seen in obesity could be involved in suppression of cell mediated immunity against tumor antigens.
The provision of the inflammatory milieu in general could involve dysregulated sphingolipid signaling. Indeed, increased S1P1 signaling in tumor cells via the Stat3 pathway was shown to be critical for tumor development and metastasis in mouse models(Lee et al., 2010). S1P2 receptor is involved in regulation of tumor cell migration and angiogenesis(Lepley et al., 2005; Li et al., 2011; Li et al., 2008). Thus, the role of sphingolipids in obesity-induced pro-tumorigenic microenvironment warrants further investigation.
Summmary
Sphingolipid alterations occur in obesity and metabolic diseases. Importantly, specific sphingolipid signaling pathways, which influence major biological processes such as insulin sensitivity, lipid metabolism, inflammation and immune reactions are dysregulated. New knowledge on the biology of sphingolipids is anticipated to enhance our understanding of metabolic diseases and obesity-associated conditions. Further, it is likely that this area will prove fertile in the search for new therapeutic targets to control obesity and associated maladies.
Acknowledgements
This work is supported by NIH grants (HL49094, HL67330, HL70694, HL89934) to TH and NIH grant CA154481, the Breast Cancer Research Foundation, and the Botwinick-Wolfensohn Foundation to AJD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aerts JM, Boot RG, van Eijk M, Groener J, Bijl N, Lombardo E, Bietrix FM, Dekker N, Groen AK, Ottenhoff R, et al. Glycosphingolipids and insulin resistance. Advances in experimental medicine and biology. 2011;721:99–119. doi: 10.1007/978-1-4614-0650-1_7. [DOI] [PubMed] [Google Scholar]
- Alberts B. Editorial Expression of Concern. Science. 2011 [Google Scholar]
- Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, et al. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronova S, Wedaman K, Aronov PA, Fontes K, Ramos K, Hammock BD, Powers T. Regulation of ceramide biosynthesis by TOR complex 2. Cell metabolism. 2008;7:148–158. doi: 10.1016/j.cmet.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartke N, Hannun YA. Bioactive sphingolipids: metabolism and function. Journal of lipid research. 2009;50(Suppl):S91–S96. doi: 10.1194/jlr.R800080-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Bayoumy S, Zhang Y, Lozano J, Kolesnick R. BAD enables ceramide to signal apoptosis via Ras and Raf-1. The Journal of biological chemistry. 1998;273:30419–30426. doi: 10.1074/jbc.273.46.30419. [DOI] [PubMed] [Google Scholar]
- Berchtold D, Piccolis M, Chiaruttini N, Riezman I, Riezman H, Roux A, Walther TC, Loewith R. Plasma membrane stress induces relocalization of Slm proteins and activation of TORC2 to promote sphingolipid synthesis. Nature cell biology. 2012;14:542–547. doi: 10.1038/ncb2480. [DOI] [PubMed] [Google Scholar]
- Bertea M, Rutti MF, Othman A, Marti-Jaun J, Hersberger M, von Eckardstein A, Hornemann T. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids in health and disease. 2010;9:84. doi: 10.1186/1476-511X-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikman BT, Summers SA. Ceramides as modulators of cellular and whole-body metabolism. The Journal of clinical investigation. 2011;121:4222–4230. doi: 10.1172/JCI57144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho VA, Hla T. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chemical reviews. 2011;111:6299–6320. doi: 10.1021/cr200273u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breart B, Ramos-Perez WD, Mendoza A, Salous AK, Gobert M, Huang Y, Adams RH, Lafaille JJ, Escalante-Alcalde D, Morris AJ, et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. The Journal of experimental medicine. 2011;208:1267–1278. doi: 10.1084/jem.20102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, Weissman JS. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow DK, Weissman JS. Membranes in balance: mechanisms of sphingolipid homeostasis. Molecular cell. 2010;40:267–279. doi: 10.1016/j.molcel.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, Aradhye S, Burtin P. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nature reviews Drug discovery. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature reviews Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- Chalfant CE, Szulc Z, Roddy P, Bielawska A, Hannun YA. The structural requirements for ceramide activation of serine-threonine protein phosphatases. Journal of lipid research. 2004;45:496–506. doi: 10.1194/jlr.M300347-JLR200. [DOI] [PubMed] [Google Scholar]
- Chavez JA, Holland WL, Bar J, Sandhoff K, Summers SA. Acid ceramidase overexpression prevents the inhibitory effects of saturated fatty acids on insulin signaling. The Journal of biological chemistry. 2005;280:20148–20153. doi: 10.1074/jbc.M412769200. [DOI] [PubMed] [Google Scholar]
- Chen X, Sun A, Zou Y, Ge J, Lazar JM, Jiang XC. Impact of sphingomyelin levels on coronary heart disease and left ventricular systolic function in humans. Nutrition & metabolism. 2011;8:25. doi: 10.1186/1743-7075-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffersen C, Obinata H, Kumaraswamy SB, Galvani S, Ahnstrom J, Sevvana M, Egerer-Sieber C, Muller YA, Hla T, Nielsen LB, et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9613–9618. doi: 10.1073/pnas.1103187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Hla T, Lynch KR, Spiegel S, Moolenaar WH. International Union of Basic and Clinical Pharmacology. LXXVIII. Lysophospholipid receptor nomenclature. Pharmacological reviews. 2010;62:579–587. doi: 10.1124/pr.110.003111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Melton DA. Endothelial signaling during development. Nature medicine. 2003;9:661–668. doi: 10.1038/nm0603-661. [DOI] [PubMed] [Google Scholar]
- Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. The Journal of biological chemistry. 2001;276:23954–23961. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- Cremesti AE, Goni FM, Kolesnick R. Role of sphingomyelinase and ceramide in modulating rafts: do biophysical properties determine biologic outcome? FEBS letters. 2002;531:47–53. doi: 10.1016/s0014-5793(02)03489-0. [DOI] [PubMed] [Google Scholar]
- Cuvillier O. Sphingosine kinase-1--a potential therapeutic target in cancer. Anticancer Drugs. 2007;18:105–110. doi: 10.1097/CAD.0b013e328011334d. [DOI] [PubMed] [Google Scholar]
- Cyster JG, Schwab SR. Sphingosine-1-Phosphate and Lymphocyte Egress from Lymphoid Organs. Annual review of immunology. 2011 doi: 10.1146/annurev-immunol-020711-075011. [DOI] [PubMed] [Google Scholar]
- Dantas AP, Igarashi J, Michel T. Sphingosine 1-phosphate and control of vascular tone. American journal of physiology Heart and circulatory physiology. 2003;284:H2045–H2052. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- Degroote S, Wolthoorn J, van Meer G. The cell biology of glycosphingolipids. Seminars in cell & developmental biology. 2004;15:375–387. doi: 10.1016/j.semcdb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Deng X, Yin X, Allan R, Lu DD, Maurer CW, Haimovitz-Friedman A, Fuks Z, Shaham S, Kolesnick R. Ceramide biogenesis is required for radiation-induced apoptosis in the germ line of C. elegans. Science. 2008;322:110–115. doi: 10.1126/science.1158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin CM, Leventhal AR, Kuriakose G, Schuchman EH, Williams KJ, Tabas I. Acid sphingomyelinase promotes lipoprotein retention within early atheromata and accelerates lesion progression. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1723–1730. doi: 10.1161/ATVBAHA.108.173344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RC. Thematic review series: sphingolipids. New insights into sphingolipid metabolism and function in budding yeast. Journal of lipid research. 2008;49:909–921. doi: 10.1194/jlr.R800003-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrosotskaya IY, Seegmiller AC, Brown MS, Goldstein JL, Rawson RB. Regulation of SREBP processing and membrane lipid production by phospholipids in Drosophila. Science. 2002;296:879–883. doi: 10.1126/science.1071124. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. The Journal of biological chemistry. 1993;268:15523–15530. [PubMed] [Google Scholar]
- Dressler KA, Mathias S, Kolesnick RN. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255:1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- Edsall LC, Cuvillier O, Twitty S, Spiegel S, Milstien S. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. Journal of neurochemistry. 2001;76:1573–1584. doi: 10.1046/j.1471-4159.2001.00164.x. [DOI] [PubMed] [Google Scholar]
- Edsbagge J, Johansson JK, Esni F, Luo Y, Radice GL, Semb H. Vascular function and sphingosine-1-phosphate regulate development of the dorsal pancreatic mesenchyme. Development. 2005;132:1085–1092. doi: 10.1242/dev.01643. [DOI] [PubMed] [Google Scholar]
- Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. The Journal of biological chemistry. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Simmons S, Kawamura S, Inoue A, Orba Y, Tokudome T, Sunden Y, Arai Y, Moriwaki K, Ishida J, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. The Journal of clinical investigation. 2012;122:1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrst H, Saba JD. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6:489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gable K, Gupta SD, Han G, Niranjanakumari S, Harmon JM, Dunn TM. A disease-causing mutation in the active site of serine palmitoyltransferase causes catalytic promiscuity. The Journal of biological chemistry. 2010;285:22846–22852. doi: 10.1074/jbc.M110.122259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo K, Penno A, Schmidt BP, Lee HJ, Frosch MP, von Eckardstein A, Brown RH, Hornemann T, Eichler FS. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. The Journal of clinical investigation. 2011;121:4735–4745. doi: 10.1172/JCI57549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Advances in experimental medicine and biology. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh TK, Bian J, Gill DL. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990;248:1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Ghosh TK, Bian J, Gill DL. Sphingosine 1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. The Journal of biological chemistry. 1994;269:22628–22635. [PubMed] [Google Scholar]
- Grassme H, Cremesti A, Kolesnick R, Gulbins E. Ceramide-mediated clustering is required for CD95-DISC formation. Oncogene. 2003;22:5457–5470. doi: 10.1038/sj.onc.1206540. [DOI] [PubMed] [Google Scholar]
- Green JA, Suzuki K, Cho B, Willison LD, Palmer D, Allen CD, Schmidt TH, Xu Y, Proia RL, Coughlin SR, et al. The sphingosine 1-phosphate receptor S1P maintains the homeostasis of germinal center B cells and promotes niche confinement. Nature immunology. 2011;12:672–680. doi: 10.1038/ni.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova IL, Schwab SR, Phan TG, Pham TH, Okada T, Cyster JG. Cortical sinus probing, S1P1-dependent entry and flow-based capture of egressing T cells. Nature immunology. 2009;10:58–65. doi: 10.1038/ni.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- Habrukowich C, Han DK, Le A, Rezaul K, Pan W, Ghosh M, Li Z, Dodge-Kafka K, Jiang X, Bittman R, et al. Sphingosine interaction with acidic leucine-rich nuclear phosphoprotein-32A (ANP32A) regulates PP2A activity and cyclooxygenase (COX)-2 expression in human endothelial cells. The Journal of biological chemistry. 2010;285:26825–26831. doi: 10.1074/jbc.M110.147058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailemariam TK, Huan C, Liu J, Li Z, Roman C, Kalbfeisch M, Bui HH, Peake DA, Kuo MS, Cao G, et al. Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1519–1526. doi: 10.1161/ATVBAHA.108.168682. [DOI] [PubMed] [Google Scholar]
- Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, et al. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nature reviews Molecular cell biology. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology. 2011;76:S3–S8. doi: 10.1212/WNL.0b013e31820d5ec1. [DOI] [PubMed] [Google Scholar]
- Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, Kluk M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochemical pharmacology. 1999;58:201–207. doi: 10.1016/s0006-2952(99)00086-6. [DOI] [PubMed] [Google Scholar]
- Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids--receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- Hla T, Venkataraman K, Michaud J. The vascular S1P gradient-cellular sources and biological significance. Biochimica et biophysica acta. 2008;1781:477–482. doi: 10.1016/j.bbalip.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. The Journal of biological chemistry. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. The Journal of clinical investigation. 2011a;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell metabolism. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nature medicine. 2011b;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocrine reviews. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC. Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circulation research. 2008;102:950–958. doi: 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovascular research. 2009;82:212–220. doi: 10.1093/cvr/cvp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi J. Membrane microdomains and insulin resistance. FEBS letters. 2010;584:1864–1871. doi: 10.1016/j.febslet.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Jacobson JR, Garcia JG. Novel therapies for microvascular permeability in sepsis. Current drug targets. 2007;8:509–514. doi: 10.2174/138945007780362719. [DOI] [PubMed] [Google Scholar]
- Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- Keul P, Lucke S, von Wnuck Lipinski K, Bode C, Graler M, Heusch G, Levkau B. Sphingosine-1-phosphate receptor 3 promotes recruitment of monocyte/macrophages in inflammation and atherosclerosis. Circulation research. 2011;108:314–323. doi: 10.1161/CIRCRESAHA.110.235028. [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. Journal of lipid research. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, Ai Y, Ristimaki AP, Fyrst H, Sano H, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Molecular and cellular biology. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Science's STKE : signal transduction knowledge environment. 2007;2007:re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T, et al. Deafness and stria vascularis defects in S1P2 receptor-null mice. The Journal of biological chemistry. 2007;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- Kono M, Mi Y, Liu Y, Sasaki T, Allende ML, Wu YP, Yamashita T, Proia RL. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. The Journal of biological chemistry. 2004;279:29367–29373. doi: 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- Kumagai K, Kawano M, Shinkai-Ouchi F, Nishijima M, Hanada K. Interorganelle trafficking of ceramide is regulated by phosphorylation-dependent cooperativity between the PH and START domains of CERT. The Journal of biological chemistry. 2007;282:17758–17766. doi: 10.1074/jbc.M702291200. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Current biology : CB. 2009;19:R1046–R1052. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laychock SG, Sessanna SM, Lin MH, Mastrandrea LD. Sphingosine 1-phosphate affects cytokine-induced apoptosis in rat pancreatic islet beta-cells. Endocrinology. 2006;147:4705–4712. doi: 10.1210/en.2006-0456. [DOI] [PubMed] [Google Scholar]
- Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nature medicine. 2010;16:1421–1428. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Rotolo JA, Mesicek J, Penate-Medina T, Rimner A, Liao WC, Yin X, Ragupathi G, Ehleiter D, Gulbins E, et al. Mitochondrial ceramide-rich macrodomains functionalize Bax upon irradiation. PloS one. 2011;6:e19783. doi: 10.1371/journal.pone.0019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- Lee YM, Venkataraman K, Hwang SI, Han DK, Hla T. A novel method to quantify sphingosine 1-phosphate by immobilized metal affinity chromatography (IMAC) Prostaglandins & other lipid mediators. 2007;84:154–162. doi: 10.1016/j.prostaglandins.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepley D, Paik JH, Hla T, Ferrer F. The G protein-coupled receptor S1P2 regulates Rho/Rho kinase pathway to inhibit tumor cell migration. Cancer research. 2005;65:3788–3795. doi: 10.1158/0008-5472.CAN-04-2311. [DOI] [PubMed] [Google Scholar]
- Levental I, Grzybek M, Simons K. Greasing their way: lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49:6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- Li MH, Hla T, Ferrer F. Sphingolipid modulation of angiogenic factor expression in neuroblastoma. Cancer Prev Res (Phila) 2011;4:1325–1332. doi: 10.1158/1940-6207.CAPR-11-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MH, Sanchez T, Pappalardo A, Lynch KR, Hla T, Ferrer F. Induction of antiproliferative connective tissue growth factor expression in Wilms' tumor cells by sphingosine-1-phosphate receptor 2. Molecular cancer research : MCR. 2008;6:1649–1656. doi: 10.1158/1541-7786.MCR-07-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Choi M, Cavey G, Daugherty J, Suino K, Kovach A, Bingham NC, Kliewer SA, Xu HE. Crystallographic identification and functional characterization of phospholipids as ligands for the orphan nuclear receptor steroidogenic factor-1. Molecular cell. 2005;17:491–502. doi: 10.1016/j.molcel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lindenberg JJ, Fehres CM, van Cruijsen H, Oosterhoff D, de Gruijl TD. Cross-talk between tumor and myeloid cells: how to tip the balance in favor of antitumor immunity. Immunotherapy. 2011;3:77–96. doi: 10.2217/imt.10.95. [DOI] [PubMed] [Google Scholar]
- Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nature immunology. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Huan C, Chakraborty M, Zhang H, Lu D, Kuo MS, Cao G, Jiang XC. Macrophage sphingomyelin synthase 2 deficiency decreases atherosclerosis in mice. Circulation research. 2009a;105:295–303. doi: 10.1161/CIRCRESAHA.109.194613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang H, Li Z, Hailemariam TK, Chakraborty M, Jiang K, Qiu D, Bui HH, Peake DA, Kuo MS, et al. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arteriosclerosis, thrombosis, and vascular biology. 2009b;29:850–856. doi: 10.1161/ATVBAHA.109.185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. The Journal of clinical investigation. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther J, Naismith JH, Dunn TM, Campopiano DJ. Structural, mechanistic and regulatory studies of serine palmitoyltransferase. Biochemical Society transactions. 2012;40:547–554. doi: 10.1042/BST20110769. [DOI] [PubMed] [Google Scholar]
- Maceyka M, Harikumar KB, Milstien S, Spiegel S. Sphingosine-1-phosphate signaling and its role in disease. Trends in cell biology. 2012;22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, Huff IV, Junt T, Wagers AJ, Mazo IB, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrandrea LD, Sessanna SM, Del Toro A, Laychock SG. ATP-independent glucose stimulation of sphingosine kinase in rat pancreatic islets. Journal of lipid research. 2010;51:2171–2180. doi: 10.1194/jlr.M000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVerry BJ, Peng X, Hassoun PM, Sammani S, Simon BA, Garcia JG. Sphingosine 1-phosphate reduces vascular leak in murine and canine models of acute lung injury. American journal of respiratory and critical care medicine. 2004;170:987–993. doi: 10.1164/rccm.200405-684OC. [DOI] [PubMed] [Google Scholar]
- Mehling M, Lindberg R, Raulf F, Kuhle J, Hess C, Kappos L, Brinkmann V. Th17 central memory T cells are reduced by FTY720 in patients with multiple sclerosis. Neurology. 2010;75:403–410. doi: 10.1212/WNL.0b013e3181ebdd64. [DOI] [PubMed] [Google Scholar]
- Merrill AH, Jr, Schmelz EM, Wang E, Schroeder JJ, Dillehay DL, Riley RT. Role of dietary sphingolipids and inhibitors of sphingolipid metabolism in cancer and other diseases. The Journal of nutrition. 1995;125:1677S–1682S. doi: 10.1093/jn/125.suppl_6.1677S. [DOI] [PubMed] [Google Scholar]
- Michaud J, Im DS, Hla T. Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J Immunol. 2010;184:1475–1483. doi: 10.4049/jimmunol.0901586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud J, Kohno M, Proia RL, Hla T. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS letters. 2006;580:4607–4612. doi: 10.1016/j.febslet.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS letters. 2010;584:1887–1894. doi: 10.1016/j.febslet.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis : an international journal on programmed cell death. 2007;12:923–939. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- Morris PG, Hudis CA, Giri D, Morrow M, Falcone DJ, Zhou XK, Du B, Brogi E, Crawford CB, Kopelovich L, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami A, Takasugi H, Ohnuma S, Koide Y, Sakurai A, Takeda S, Hasegawa T, Sasamori J, Konno T, Hayashi K, et al. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: investigation based on a new S1P3 receptor antagonist. Molecular pharmacology. 2010;77:704–713. doi: 10.1124/mol.109.061481. [DOI] [PubMed] [Google Scholar]
- Nakahara K, Ohkuni A, Kitamura T, Abe K, Naganuma T, Ohno Y, Zoeller RA, Kihara A. The sjogren-larsson syndrome gene encodes a hexadecenal dehydrogenase of the sphingosine 1-phosphate degradation pathway. Molecular cell. 2012;46:461–471. doi: 10.1016/j.molcel.2012.04.033. [DOI] [PubMed] [Google Scholar]
- Nilsson A, Duan RD. Absorption and lipoprotein transport of sphingomyelin. Journal of lipid research. 2006;47:154–171. doi: 10.1194/jlr.M500357-JLR200. [DOI] [PubMed] [Google Scholar]
- Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nature medicine. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- Nofer JR, van der Giet M, Tolle M, Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Godecke A, Ishii I, Kleuser B, et al. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. The Journal of clinical investigation. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obinata H, Hla T. Sphingosine 1-phosphate in coagulation and inflammation. Seminars in immunopathology. 2012;34:73–91. doi: 10.1007/s00281-011-0287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Olivera A, Rosenfeldt HM, Bektas M, Wang F, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 1 induces G12/13-mediated stress fiber formation, yet promotes growth and survival independent of G protein-coupled receptors. The Journal of biological chemistry. 2003;278:46452–46460. doi: 10.1074/jbc.M308749200. [DOI] [PubMed] [Google Scholar]
- Oo ML, Chang SH, Thangada S, Wu MT, Rezaul K, Blaho V, Hwang SI, Han DK, Hla T. Engagement of S1P-degradative mechanisms leads to vascular leak in mice. The Journal of clinical investigation. 2011;121:2290–2300. doi: 10.1172/JCI45403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman A, Rutti MF, Ernst D, Saely CH, Rein P, Drexel H, Porretta-Serapiglia C, Lauria G, Bianchi R, von Eckardstein A, et al. Plasma deoxysphingolipids: a novel class of biomarkers for the metabolic syndrome? Diabetologia. 2012;55:421–431. doi: 10.1007/s00125-011-2384-1. [DOI] [PubMed] [Google Scholar]
- Paik JH, Chae S, Lee MJ, Thangada S, Hla T. Sphingosine 1-phosphateinduced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. The Journal of biological chemistry. 2001;276:11830–11837. doi: 10.1074/jbc.M009422200. [DOI] [PubMed] [Google Scholar]
- Paik JH, Skoura A, Chae SS, Cowan AE, Han DK, Proia RL, Hla T. Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes & development. 2004;18:2392–2403. doi: 10.1101/gad.1227804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 2007;316:295–298. doi: 10.1126/science.1139221. [DOI] [PubMed] [Google Scholar]
- Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. The Journal of biological chemistry. 1996;271:13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochimica et biophysica acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- Pham TH, Baluk P, Xu Y, Grigorova I, Bankovich AJ, Pappu R, Coughlin SR, McDonald DM, Schwab SR, Cyster JG. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. The Journal of experimental medicine. 2010;207:17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitson SM. Regulation of sphingosine kinase and sphingolipid signaling. Trends in biochemical sciences. 2011;36:97–107. doi: 10.1016/j.tibs.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Puneet P, Yap CT, Wong L, Lam Y, Koh DR, Moochhala S, Pfeilschifter J, Huwiler A, Melendez AJ. SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science. 2010;328:1290–1294. doi: 10.1126/science.1188635. [DOI] [PubMed] [Google Scholar]
- Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature reviews Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- Roelants FM, Breslow DK, Muir A, Weissman JS, Thorner J. Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19222–19227. doi: 10.1073/pnas.1116948108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutti S, Ehses JA, Sibler RA, Prazak R, Rohrer L, Georgopoulos S, Meier DT, Niclauss N, Berney T, Donath MY, et al. Low- and high-density lipoproteins modulate function, apoptosis, and proliferation of primary human and murine pancreatic beta-cells. Endocrinology. 2009;150:4521–4530. doi: 10.1210/en.2009-0252. [DOI] [PubMed] [Google Scholar]
- Saba JD, Nara F, Bielawska A, Garrett S, Hannun YA. The BST1 gene of Saccharomyces cerevisiae is the sphingosine-1-phosphate lyase. The Journal of biological chemistry. 1997;272:26087–26090. doi: 10.1074/jbc.272.42.26087. [DOI] [PubMed] [Google Scholar]
- Sablin EP, Blind RD, Krylova IN, Ingraham JG, Cai F, Williams JD, Fletterick RJ, Ingraham HA. Structure of SF-1 bound by different phospholipids: evidence for regulatory ligands. Mol Endocrinol. 2009;23:25–34. doi: 10.1210/me.2007-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomone S, Yoshimura S, Reuter U, Foley M, Thomas SS, Moskowitz MA, Waeber C. S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. European journal of pharmacology. 2003;469:125–134. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- Samad F, Badeanlou L, Shah C, Yang G. Adipose tissue and ceramide biosynthesis in the pathogenesis of obesity. Advances in experimental medicine and biology. 2011;721:67–86. doi: 10.1007/978-1-4614-0650-1_5. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Estrada-Hernandez T, Paik JH, Wu MT, Venkataraman K, Brinkmann V, Claffey K, Hla T. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. The Journal of biological chemistry. 2003;278:47281–47290. doi: 10.1074/jbc.M306896200. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:1312–1318. doi: 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- Sanchez T, Thangada S, Wu MT, Kontos CD, Wu D, Wu H, Hla T. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4312–4317. doi: 10.1073/pnas.0409784102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler K, Levkau B. Sphingosine-1-phosphate as a mediator of high-density lipoprotein effects in cardiovascular protection. Cardiovascular research. 2009;82:201–211. doi: 10.1093/cvr/cvp070. [DOI] [PubMed] [Google Scholar]
- Scheek S, Brown MS, Goldstein JL. Sphingomyelin depletion in cultured cells blocks proteolysis of sterol regulatory element binding proteins at site 1. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:11179–11183. doi: 10.1073/pnas.94.21.11179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer SS. The debut of a rational treatment for an inherited neuropathy? The Journal of clinical investigation. 2011;121:4624–4627. doi: 10.1172/JCI60511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schissel SL, Jiang X, Tweedie-Hardman J, Jeong T, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH Implications for atherosclerotic lesion development. The Journal of biological chemistry. 1998;273:2738–2746. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- Schlitt A, Blankenberg S, Yan D, von Gizycki H, Buerke M, Werdan K, Bickel C, Lackner KJ, Meyer J, Rupprecht HJ, et al. Further evaluation of plasma sphingomyelin levels as a risk factor for coronary artery disease. Nutrition & metabolism. 2006;3:5. doi: 10.1186/1743-7075-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Serafimidis I, Heximer S, Beis D, Gavalas A. G protein-coupled receptor signaling and sphingosine-1-phosphate play a phylogenetically conserved role in endocrine pancreas morphogenesis. Molecular and cellular biology. 2011;31:4442–4453. doi: 10.1128/MCB.05702-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T. Lipid mediators in health and disease: enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu Rev Pharmacol Toxicol. 2009;49:123–150. doi: 10.1146/annurev.pharmtox.011008.145616. [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. J Clin Oncol. 2011;29:4–7. doi: 10.1200/JCO.2010.32.1752. [DOI] [PubMed] [Google Scholar]
- Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. The Journal of biological chemistry. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura A, Hla T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovascular research. 2009;82:221–228. doi: 10.1093/cvr/cvp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura A, Michaud J, Im DS, Thangada S, Xiong Y, Smith JD, Hla T. Sphingosine-1-phosphate receptor-2 function in myeloid cells regulates vascular inflammation and atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:81–85. doi: 10.1161/ATVBAHA.110.213496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. The Journal of clinical investigation. 2007;117:2506–2516. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel S, Foster D, Kolesnick R. Signal transduction through lipid second messengers. Current opinion in cell biology. 1996;8:159–167. doi: 10.1016/s0955-0674(96)80061-5. [DOI] [PubMed] [Google Scholar]
- Spiegel S, Milstien S. Sphingolipid metabolites: members of a new class of lipid second messengers. The Journal of membrane biology. 1995;146:225–237. doi: 10.1007/BF00233943. [DOI] [PubMed] [Google Scholar]
- Stancevic B, Kolesnick R. Ceramide-rich platforms in transmembrane signaling. FEBS letters. 2010;584:1728–1740. doi: 10.1016/j.febslet.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratford S, DeWald DB, Summers SA. Ceramide dissociates 3'-phosphoinositide production from pleckstrin homology domain translocation. The Biochemical journal. 2001;354:359–368. doi: 10.1042/0264-6021:3540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, Zhou XK, Blaho VA, Hla T, Yang P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sun Y, Taniguchi R, Tanoue D, Yamaji T, Takematsu H, Mori K, Fujita T, Kawasaki T, Kozutsumi Y. Sli2 (Ypk1), a homologue of mammalian protein kinase SGK is a downstream kinase in the sphingolipid-mediated signaling pathway of yeast. Molecular and cellular biology. 2000;20:4411–4419. doi: 10.1128/mcb.20.12.4411-4419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepaniak WS, Pitt BR, McVerry BJ. S1P2 receptor-dependent Rho-kinase activation mediates vasoconstriction in the murine pulmonary circulation induced by sphingosine 1-phosphate. American journal of physiology Lung cellular and molecular physiology. 2010;299:L137–L145. doi: 10.1152/ajplung.00233.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- Tabuchi M, Audhya A, Parsons AB, Boone C, Emr SD. The phosphatidylinositol 4,5-biphosphate and TORC2 binding proteins Slm1 and Slm2 function in sphingolipid regulation. Molecular and cellular biology. 2006;26:5861–5875. doi: 10.1128/MCB.02403-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs AN, Dammer E, Kelly S, Wang E, Merrill AH, Jr, Sewer MB. Steroidogenic factor-1 is a sphingolipid binding protein. Molecular and cellular endocrinology. 2007;265–266:174–178. doi: 10.1016/j.mce.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs AN, Dammer E, Sewer MB. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology. 2006;147:5249–5258. doi: 10.1210/en.2006-0355. [DOI] [PubMed] [Google Scholar]
- Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, et al. Dual actions of sphingosine-1- phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. The Journal of cell biology. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hinsbergh VW. Endothelium--role in regulation of coagulation and inflammation. Seminars in immunopathology. 2012;34:93–106. doi: 10.1007/s00281-011-0285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- van Meer G, Hoetzl S. Sphingolipid topology and the dynamic organization and function of membrane proteins. FEBS letters. 2010;584:1800–1805. doi: 10.1016/j.febslet.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circulation research. 2008;102:669–676. doi: 10.1161/CIRCRESAHA.107.165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. The Journal of biological chemistry. 1999;274:27351–27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- Woodcock JM, Ma Y, Coolen C, Pham D, Jones C, Lopez AF, Pitson SM. Sphingosine and FTY720 directly bind pro-survival 14-3-3 proteins to regulate their function. Cellular signalling. 2010;22:1291–1299. doi: 10.1016/j.cellsig.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Woodcock JM, Murphy J, Stomski FC, Berndt MC, Lopez AF. The dimeric versus monomeric status of 14-3-3zeta is controlled by phosphorylation of Ser58 at the dimer interface. The Journal of biological chemistry. 2003;278:36323–36327. doi: 10.1074/jbc.M304689200. [DOI] [PubMed] [Google Scholar]
- Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, D'Andrea RJ, Gamble JR, Vadas MA. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. The Journal of biological chemistry. 2002;277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. American journal of physiology Endocrinology and metabolism. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y. Sphingosine 1-phosphate in vascular biology: possible therapeutic strategies to control vascular diseases. Current pharmaceutical design. 2006;12:575–587. doi: 10.2174/138161206775474404. [DOI] [PubMed] [Google Scholar]
- Yeboah J, McNamara C, Jiang XC, Tabas I, Herrington DM, Burke GL, Shea S. Association of plasma sphingomyelin levels and incident coronary heart disease events in an adult population: Multi-Ethnic Study of Atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:628–633. doi: 10.1161/ATVBAHA.109.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X, Zafrullah M, Lee H, Haimovitz-Friedman A, Fuks Z, Kolesnick R. A ceramide-binding C1 domain mediates kinase suppressor of ras membrane translocation. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2009;24:219–230. doi: 10.1159/000233248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon HH, Lewis MA, Shi Q, Khan M, Cassivi SD, Diasio RB, Sinicrope FA. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. J Clin Oncol. 2011;29:4561–4567. doi: 10.1200/JCO.2011.37.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. The Journal of clinical investigation. 2006;116:1284–1291. doi: 10.1172/JCI27100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan YH, Hannun YA. The acid sphingomyelinase/ceramide pathway: biomedical significance and mechanisms of regulation. Current molecular medicine. 2010;10:454–466. doi: 10.2174/156652410791608225. [DOI] [PubMed] [Google Scholar]
- Zemann B, Urtz N, Reuschel R, Mechtcheriakova D, Bornancin F, Badegruber R, Baumruker T, Billich A. Normal neutrophil functions in sphingosine kinase type 1 and 2 knockout mice. Immunol Lett. 2007;109:56–63. doi: 10.1016/j.imlet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yao B, Delikat S, Bayoumy S, Lin XH, Basu S, McGinley M, Chan-Hui PY, Lichenstein H, Kolesnick R. Kinase suppressor of Ras is ceramide-activated protein kinase. Cell. 1997;89:63–72. doi: 10.1016/s0092-8674(00)80183-x. [DOI] [PubMed] [Google Scholar]
- Zitomer NC, Mitchell T, Voss KA, Bondy GS, Pruett ST, Garnier-Amblard EC, Liebeskind LS, Park H, Wang E, Sullards MC, et al. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. The Journal of biological chemistry. 2009;284:4786–4795. doi: 10.1074/jbc.M808798200. [DOI] [PMC free article] [PubMed] [Google Scholar]