Abstract
Dyslipidemia, the condition of elevated serum triglycerides, elevated low-density lipoprotein cholesterol, and/or low high-density lipoprotein cholesterol, is a public health problem of growing concern. Dyslipidemia clusters with other disorders of the metabolic syndrome that together influence, and may derive from, chronic inflammation. While best recognized as a risk factor for atherosclerotic cardiovascular disease, lipid dysregulation has recently been shown to influence a variety of disease processes in several organ systems. This review highlights our current understanding of the role of cholesterol and its homeostatic trafficking in pulmonary physiology and pathophysiology. Gene-targeted mice deficient in regulatory proteins that govern reverse cholesterol transport (e.g., ATP Binding Cassette transporter G1, apolipoprotein E) have recently been shown to have abnormal lung physiology, including dysregulated pulmonary innate and adaptive immune responses to the environment. It has also recently been shown that diet-induced dyslipidemia alters trafficking of immune cells to the lung in a manner that may have important implications for the pathogenesis of acute lung injury, asthma, pneumonia, and other lung disorders. Conversely, cholesterol-targeting pharmacologic agents, such as statins, apolipoprotein mimetic peptides, and Liver X Receptor agonists, have shown early promise in the treatment of several lung disorders. An improved understanding of the precise molecular mechanisms by which cholesterol and its trafficking modify pulmonary immunity will be required before the full implications of dyslipidemia as a lung disease modifier, and the full potential of lipid-targeting agents as pulmonary therapeutics, can be realized.
Keywords: Pulmonary disease, Dyslipidemia, Statins, Metabolic Syndrome, Inflammation, Cholesterol
1. Introduction
Dyslipidemia, a condition involving elevated serum triglyceride (TG), elevated serum low density lipoprotein cholesterol (LDL-C), and/or low serum high density lipoprotein cholesterol (HDL-C), has become a health problem of growing concern in many industrialized countries and developing nations (reviewed in [1]). Approximately 21% of adults in the United States have elevated serum LDL-C (reviewed in [2]). Dyslipidemia is often associated with metabolic syndrome, which increases the risk for atherosclerosis and type II diabetes mellitus. Hypercholesterolemia has been widely studied in the context of cardiovascular inflammation, but its role in pulmonary immunity has only recently been considered. The issue of cholesterol and its role in pulmonary immunity is potentially of tremendous public health impact given the high rates of dyslipidemia and respiratory tract infections in the United States, and recent reports that widely used cholesterol-active therapies impact pulmonary immunity [3]. This review addresses our current understanding of how cholesterol levels are regulated in the lung and how cholesterol itself regulates immune responses in the lung. Furthermore, we discuss emerging roles for cholesterol regulatory proteins as potential targets for therapeutic development in chronic pulmonary disease.
2. Cholesterol transport, uptake, and excretion
Cholesterol is essential for the integrity of cellular membranes, maintaining membrane fluidity and membrane functions, including signal transduction. Cholesterol and TG homeostasis have been extensively studied in cardiovascular disease progression and have been t he topic of recent scholarly reviews (reviewed in [4, 5]). While a comprehensive treatment of cholesterol trafficking is beyond the scope of the present review, a basic understanding of the mechanisms by which cell and serum cholesterol levels are maintained is necessary for examining how these events translate to lung physiology and pathophysiology.
Cholesterol is either absorbed from dietary sources or synthesized in vivo in both liver and peripheral cells by a pathway whose rate-limiting step is regulated by hydroxymethyl-glutaryl coenzyme A reductase (HMGCR). Hepatic cholesterol, deriving both from de novo synthesis and uptake of intestinal chylomicron remnants, is packaged with apolipoprotein B (ApoB) into very low-density lipoprotein (VLDL) particles for export into the systemic circulation. VLDL is then progressively metabolized into low density lipoprotein (LDL), which serves as the major vehicle supplying cholesterol to peripheral cells (e.g., tissue macrophages) via low density lipoprotein receptor (LDLR) and scavenger receptors (SRs). Cholesterol levels of peripheral cells are, in turn, controlled by LDLR and HMGCR downregulation as well as by the homeostatic pathway for disposal of cellular cholesterol, termed ‘reverse cholesterol transport’ (RCT) (reviewed in [6, 7]).
Following hydrolysis from its esterified form, free cholesterol (FC) is effluxed from peripheral cells to serum lipid-poor/free apolipoprotein A-I (apoA-I) via the ATP Binding Cassette (ABC) transporter A1 (ABCA1) (reviewed in [8]), thereby forming nascent high density lipoprotein (HDL) particles in the first step of RCT. HDL then serves as an acceptor for further cellular cholesterol efflux mobilized via scavenger receptor B type I (SR-BI) and ABCG1 [9], along with cholesterol mobilized by diffusion and by export with macrophage apoE. Serum HDL cholesterol is then cleared by the liver after binding to hepatic SR-BI; alternatively, cholesterol transferred from HDL to LDL in the circulation by cholesteryl ester transfer protein (CETP) is cleared by hepatic LDLR. The liver finally disposes of cholesterol, in the form of FC and bile acids, by export of these sterols into the biliary tract for excretion in the feces. Notably, ABCA1, ABCG1, apoE, and CETP (in humans) are all target genes of the nuclear receptor Liver X Receptor (LXR), a transcription factor that serves as a cellular sensor for elevated oxysterol levels. As RCT is thought to be anti-atherogenic, active efforts are underway to develop and validate apoA-I mimetic peptides as well as synthetic LXR agonists that might reduce human atherosclerotic cardiovascular disease by promoting RCT in vivo [10].
Although cholesterol is essential for cellular integrity and metabolism, overloading of macrophages with cholesterol produces cytotoxic and inflammatory responses that are now known to contribute to atherosclerosis and other diseases. Overloading macrophages in vitro with exogenous cholesterol induces cytokine production via endoplasmic reticulum (ER) stress [11, 12]. Free cholesterol loading of membranes also activates Toll like Receptors (TLRs), as well as sensitizes TLRs to microbial ligands [13], while intracellular cholesterol overload activates the inflammasome [14]. The inflammasome is a large, multiprotein complex that activates caspase 1 to cleave pro-IL-1β and pro-IL-18 into a mature form [15, 16]. The activation of the inflammasome by cholesterol crystals has been shown to work through NLRP3 leading to the production of the proinflammatory cytokine IL-1 that has been implicated in cardiovascular disease pathogenesis [17, 18]. Thus, cholesterol homeostasis and inflammation are coupled in macrophages, with dysregulated cellular cholesterol loading inducing inflammatory responses. This ‘spontaneous’ activation of the innate immune system by cholesterol overload has been widely studied in the progression of chronic inflammatory diseases such as atherosclerosis, but has only recently been examined in the context of the lung.
3. Cholesterol and lipoproteins in lung physiology
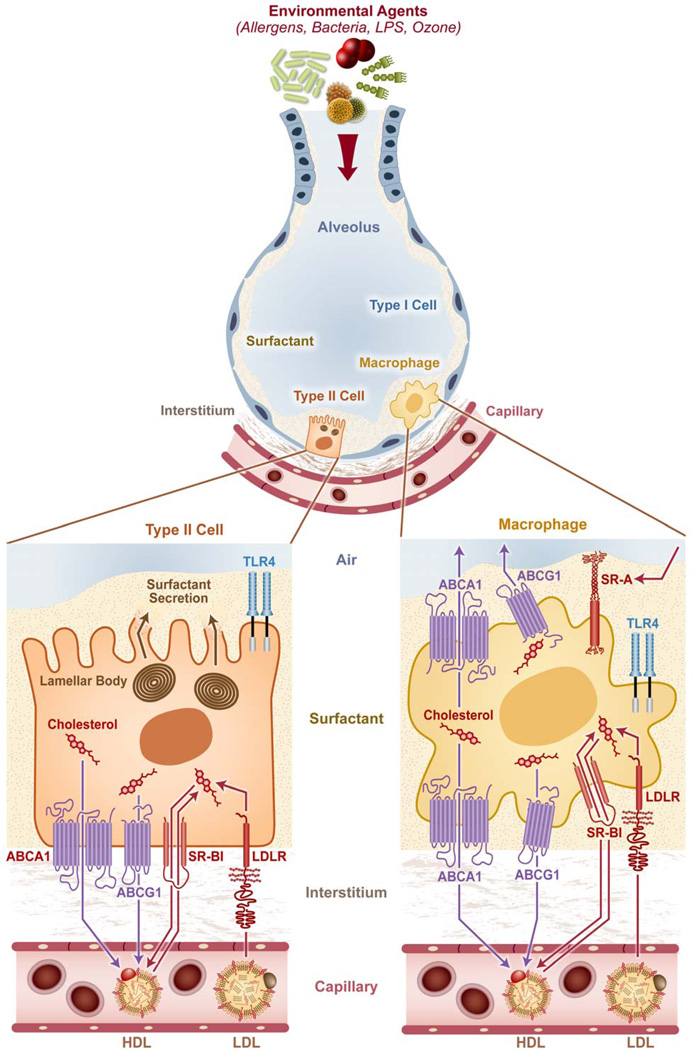
Although the lung has not traditionally been considered an organ sensitive to circulating lipoproteins and their cholesterol cargo, several reports over the years have suggested an important and perhaps even unique role for lipoproteins and cholesterol in pulmonary physiology (summarized in Figure 1). Circulating LDL and HDL are both taken up by the lung through specific receptors, and supply cholesterol to lung-resident cells, thereby inhibiting local pulmonary cholesterol biosynthesis [19, 20]. HDL also serves as the major source of the antioxidant vitamin E for alveolar epithelial type II cells [21], and promotes surfactant production by type II cells [22], and growth of lung fibroblasts [23]. Although cholesterol is essential for type II cell function, excessive amounts of cholesterol impair surfactant function, suggesting the critical importance of alveolar cholesterol homeostasis to normal lung physiology [24]. Indeed, increased dietary cholesterol has been shown to alter surfactant synthesis, composition, and function [25, 26]. Whether lipoprotein particles comparable to those described in the serum exist in the alveolar compartment remains unclear.
Figure 1. Cholesterol trafficking influences multiple cell types in the lung.
Alveolar epithelial type II cells and alveolar macrophages likely receive cholesterol from circulating low density lipoprotein and high density lipoprotein (LDL, HDL) through the LDL receptor (LDLR) and scavenger receptor B type I (SR-BI), respectively. HDL is also the major source of the antioxidant vitamin E for type II cells. Class A scavenger receptors (SR-A) on macrophages play a role in clearance of oxidized alveolar lipids that may otherwise mediate cytotoxic and pro-inflammatory effects. The disposal pathway for cholesterol from type II cells and macrophages involves the cholesterol efflux transporters ATP Binding Cassette (ABC) A1 and ABCG1, and perhaps also SR-BI. ABCA1 also mediates basolateral surfactant efflux from type II cells; deletion of either ABCA1 or ABCG1 leads to severe surfactant proteinosis and lipidosis. Disordered cholesterol/phospholipid trafficking through the lung, such as with ABCG1 deletion, alters immune cell trafficking to the lung as well as the lung’s immune responsiveness to a variety of environmental exposures, indicating that there is intimate crosstalk between lipid and immune homeostasis in the lung.
As surfactant lipids are directly exposed to environmental oxidants, oxidation of alveolar lipids into cytotoxic and pro-inflammatory species poses a unique and perpetual problem for the lung. Ozone forms bioactive oxysterols that, if not properly cleared by type A scavenger receptors (i.e., MARCO, SR-AI/II) on alveolar macrophages [27], induce apoptosis and cytotoxicity [28–30]. Moreover, a broad array of acute lung injury exposures has been reported to oxidize surfactant phospholipids into proinflammatory species that trigger inflammation through secondary activation of the TLR4 cascade [31]. Thus, homeostatic maintenance of alveolar lipids is directly coupled to alveolar inflammatory homeostasis and represents a unique challenge for the lung.
Several proteins that play a central role in RCT, including apoA-I, apoE, ABCA1, and ABCG1 are expressed in the lung. For many of these targets, pulmonary phenotypes observed upon gene-deletion suggest the importance of cholesterol homeostasis to lung physiology. Mice with a deletion of the RCT-promoting, HDL-associated apolipoprotein apoA-I have increased airway resistance, inflammatory cell recruitment, and airway collagen deposition in the steady state [32], whereas apoE-deficient mice, a dyslipidemic strain commonly utilized in atherosclerosis research, have reduced developmental alveologenesis and abnormal pulmonary function with increased airway resistance and static compliance [33]. Mice deficient in either of the cholesterol efflux transporters ABCA1 or ABCG1, both of which are expressed in alveolar macrophages and alveolar epithelium, have a pulmonary phenotype characterized by lipid overload [11, 34, 35]. ABCA1 null mice have an accumulation of excess cholesterol in alveolar macrophages and type II cells, alveolar proteinosis, and respiratory distress [34]. Naïve Abcg1−/− mice have a similar yet more pronounced pulmonary phenotype of lipid-overloaded alveolar macrophages and alveolar epithelial cells that is further complicated by increased steady state recruitment of a wide array of leukocyte subtypes to the lung [11, 35]. Abcg1−/− alveolar macrophages appear to play a central role in this phenotype, displaying increased constitutive cytokine synthesis and apoptosis [35]. Interestingly, the lung phenotype of Abcg1−/− mice is highly reminiscent of the rare human lung disease pulmonary alveolar proteinosis, in which alveolar macrophage ABCG1 deficiency has been implicated [36]. While there is good evidence that loading of cultured macrophages with exogenous cholesterol activates multiple inflammatory pathways, there is nevertheless little direct evidence at present that cholesterol overload in ABCG1-deleted mice is causally responsible for their constitutive lung inflammation.
4. Effects of cholesterol regulators on pulmonary immune responses to the environment
Recent studies have revealed important roles for several endogenous cholesterol trafficking regulators, including ABCG1, apoE, and LXR in pulmonary immune responses. We recently reported that Abcg1−/− mice have an exaggerated pulmonary response to both LPS inhalation and K. pneumoniae infection, characterized by enhanced PMN recruitment to the airspace as well as elevated airspace cytokines [37]. Increased alveolar neutrophilia in the infected Abcg1−/− lung was associated with enhanced bacterial clearance, suggesting that ABCG1 regulates pulmonary host defense. Interestingly, this phenotype appeared to be tissue-selective as Abcg1+/+ and Abcg1−/− mice had equivalent responses to intravenous bacteria and intraperitoneal LPS. Recently, our laboratory has also uncovered a novel role for ABCG1 in a murine model of allergic asthma involving ovalbumin (OVA) sensitization and challenge [38]. We find that ABCG1-deficient mice display reduced airway eosinophils, T helper (Th) 2 cytokines, and Th2 cells, and increased airway neutrophils and IL-17 after OVA sensitization and challenge, suggesting skewing of adaptive immune programs away from Th2 in the setting of ABCG1 deficiency. By contrast, the dyslipidemic murine strains Apoe−/− and Ldlr−/− were recently reported to display increased airway hyperresponsiveness and mucus production but normal airway inflammatory cell counts in a house dust mite model of murine asthma [39].
Several studies have recently indicated that LXR, a nuclear receptor for oxysterols that induces ABCG1 and apoE among several other RCT target genes, also regulates pulmonary immunity. We and others have reported that treatment of mice with synthetic LXR agonists decreases influx of PMNs into the airspace, upregulates antioxidant enzymes, and decreases proinflammatory cytokines in the airspace in multiple models of acute lung injury and infection [40–43]. Our laboratory also reported that pharmacologic activation of LXR compromises host defense against K. pneumoniae lung infection, likely due to reduced alveolar neutrophil recruitment [43]. This contrasts with a recent study by Korf et al. [44], wherein it was reported that LXR expression contributes to clearance of, and T cell specific immunity against Mycobacterium tuberculosis. Taken together, these studies indicate that regulation of pulmonary host defense by LXR may be pathogen-specific. As the lung is a major source of the endogenous LXR agonist cholestenoic acid, and serum cholestenoic acid is decreased in various chronic lung diseases [45], we speculate that reduced LXR tone in the injured lung may feed back to dysregulate inflammation and host defense during chronic lung disease.
5. Effects of diet-induced dyslipidemia on pulmonary immunity
Whereas metabolic syndrome and dyslipidemia have been clearly linked to several cardiovascular inflammatory phenotypes, little is known about the sensitivity of immune responses in the lung to systemic dyslipidemia. To address this knowledge gap, we recently reported the effects of diet-induced dyslipidemia on pulmonary innate immune responses in wild type (C57BL/6) mice [46]. We found that high cholesterol diet-fed mice displayed attenuated influx of PMNs into the airspace in response to LPS and K. pneumoniae, as well as compromised bacterial clearance from the lung. The reduced influx of PMNs in dyslipidemic mice stemmed from both deficient induction of airspace NF-κB-dependent cyto-/chemokines and impaired PMN chemotaxis, and could be recapitulated by intravenous injection of oxidized LDL into naïve normal diet-fed mice. Paradoxically, bacteria were cleared more effectively from the bloodstream during dyslipidemia. This was associated with basal circulating neutrophilia and serum cytokine induction in dyslipidemic mice, as well as hyperresponsiveness to systemically administered TLR ligands. Taken together, these data indicate that diet-induced dyslipidemia imparts a dysregulated compartmentalization of neutrophils, cytokines, and TLR responsiveness between serum and airspace, enhancing host defense in the former but compromising host defense in the latter.
In a report by Martens et al. [47], Apoe −/− mice were fed either a low cholesterol (LC) or high (HC) diet before infection with M. tuberculosis. Apoe −/− mice fed the LC diet were slightly more susceptible to M. tuberculosis, as evidenced by increased lung inflammation and bacterial lung burden. However, Apoe−/− mice fed the HC diet had a significant increase in M. tuberculosis susceptibility characterized by massive lung inflammation, heavy bacterial burden, and early mortality. The Apoe−/− HC diet-fed mice also had a defect in adaptive immunity with a failure to mount a protective Th1 immune response. The M. tuberculosis susceptibility of Apoe−/− mice increased with serum cholesterol, suggesting that their susceptibility was dependent upon cholesterol and not ApoE deficiency. Taken together, these studies indicate that dyslipidemia can alter pulmonary innate and adaptive immune responses, increasing susceptibility to pathogens in the lung.
The effect of diet-induced dyslipidemia has also been recently examined in adaptive immune responses in the lung. In a report by Yeh and colleagues [48], a HC diet in mice significantly increased airspace eosinophils, IL-5, and PGE2 but not allergen-specific serum IgE in an OVA sensitization and challenge model. By contrast, we recently reported an inverse relationship between serum total and non-HDL cholesterol and asthma in the U.S. population, suggesting that human subjects with the highest serum cholesterol levels are least likely to have asthma [49]. Clearly, further investigation of the relationship between serum lipoprotein cholesterol and asthma is warranted.
6. Cholesterol as a potential pharmacologic target in lung disease
Given the connection between cholesterol dysregulation and pulmonary immunity, it has become of interest to examine the efficacy against pulmonary disease of drugs targeting cholesterol transport (Table 1). In addition to reduction of serum cholesterol through inhibition of HMGCR in the mevalonate synthesis pathway of the liver and other organs, statins have been shown to have pleiotropic anti-inflammatory actions. While some effects of statins on pro-inflammatory signaling likely stem from reduction of lipid raft cholesterol, statins also attenuate pro-inflammatory signaling through depleting HMGCR-derived isoprenoids, and may also have HMGCR-independent effects upon inflammation (reviewed in [3]). Most in vivo studies of statins have not distinguished among these mechanisms.
Table I.
Reported efficacy of cholesterol-targeted agents in experimental models of lung disease.
| Lung disease model | Pharmacological agent | Outcomes | References |
|---|---|---|---|
| Asthma | Simvastatin | ↓ BAL eosinophils, Th1 and Th2 cytokines ↓ AHR ↓ TGF-β1-induced fibronectin |
[50, 83, 84] |
| Pravastatin | ↓ BAL eosinophils, IL-5, PGE2, MCP-1 | [51] | |
| LXR agonist | TO901317: ↓ HSMC migration/proliferation GW3965: ↔ BAL eosinophils or cytokines, ↑ AHR |
[41, 78] | |
| ApoA-I mimetics | 5A and D4F: ↓ BAL eosinophils, PMNs, lymphocytes, and Th2 and Th17 cytokines, ↓ AHR |
[56, 80] | |
| ApoE mimetics | ↓ BAL eosinophils, Th2 and Th17 cytokines, AHR, and IgE |
[39] | |
| Acute Lung Injury | Simvastatin | ↓ LPS-induced lung permeability, PMN influx, and NF-κB activation |
[85] |
| Lovastatin | ↓ BAL PMNs, proinflammatory cytokines, ↓ lung clearance of K. pneumoniae. |
[53, 85] | |
| LXR agonist | TO901317: ↓ BAL PMNs and clearance of K. pneumoniae. |
[43] | |
| ApoA-I mimetics | D-4F: ↓ influenza A-induced IL-6 and TNFα production by A549 cells, ↑ Type I interferon and viability |
[86] | |
| ApoA-I protein | ↓ IL-1B, IL-6, and TNF-a in BAL and lung injury after sepsis |
[87, 88] | |
| COPD/Emphysema | Simvastatin | ↓ lung injury, ↓ BAL MMP-9, VEGF, eNOS ↑ alveolar cell proliferation |
[52, 89] |
|
Idiopathic Pulmonary Fibrosis |
Simvastatin | ↓ CTGF, SMA, and collagen production from fibroblasts from healthy and IPF patients |
[90] |
| ApoA-I mimetics | ↓ BAL cellular influx, and lung collagende position |
[91] |
Abbreviations: AHR, Airway Hyperresponsiveness; BAL, Bronchoalveolar lavage; CTGF, connective tissue growth factor; eNOS, Endothelial NOS; HSMC, Human smooth muscle cells; IPF, Idiopathic Pulmonary Fibrosis; IL, Interluekin; LPS, Lipopolysaccharide; MCP-1, Monocyte chemotactic protein-1; MMP, Matrix metalloproteinase; PGE2, prostaglandin E2; PMN, polymorphonuclear leukocyte; SMA, Smooth muscle actin; Th, T helper; TGF-β1, Transforming growth factor β1; TNF, Tumor necrosis factor; VEGF, Vascular endothelial growth factor.
The benefits of statin therapy on inflammatory airway diseases have been demonstrated in multiple mouse models of lung disease, that together suggest wide-ranging potential for statins in therapy of human lung disorders. Both simvastatin and pravastatin reduced airspace inflammatory cells and Th2 cytokine production in mouse models of allergic asthma (OVA and house dust mite) [50, 51]. Lee et al. [52] reported that simvastatin inhibited lung parenchymal destruction and peribronchial and perivascular inflammatory cell infiltration in a murine model of smoking-induced emphysema through a reduction in levels of MMP-9, a major inflammatory mediator. Our group reported that lovastatin reduced airspace neutrophilia and protein leakage following LPS inhalation, a model of acute lung injury [53]. Beneficial effects of statins have also been observed in animal models of pulmonary hypertension and pulmonary fibrosis [54, 55].
Recently, the impact of statins on lung function has been examined in observational studies of human lung disease (Table 2). Several retrospective studies have reported that statins are independently associated with reduced risk of pneumonia and/or pneumonia-associated mortality [56–62]. However, a prospective study noted no significant relationship between statins and mortality or admission to an intensive care unit in a cohort of patients with community-acquired pneumonia [63]. In asthma, both simvastatin and atorvastatin treatment have been associated with reduced leukocytes and leukotrienes in sputum, as well an improvement in FEV1 [64–66], although not all studies have found a benefit in important clinical outcomes (reviewed in [67]). In COPD, statins were associated with reduced FEV1 decline, decreased intubations, and decreased mortality [68, 69]. Other observational studies have reported that statins may delay disease progression and improve survival in patients with pulmonary hypertension [70], and decrease the occurrence of acute rejection after lung transplant [71]. While varying results have been reported for statins in acute lung injury (ALI) [72, 73], a large NHLBI ARDS network study in progress, Statins for Acutely Injury Lungs from Sepsis (SAILS), will hopefully resolve the issue of statin efficacy in ALI and ARDS. Additional lipid-modifying agents that have been evaluated in cardiovascular disease include niacin and cholesterol ester transfer protein (CETP) inhibitors. Both niacin and CETP inhibitors have been successful in raising HDL levels and lowering LDL levels [74–76], however niacin failed to improve cardiovascular outcomes in a large trial (AIM-HIGH). While the CETP inhibitor torcetrapib has been associated with worsened clinical outcomes, more recently tested CETP inhibitors such as anacetrapib have shown more promise [75, 77]. Whether niacin and/or CETP inhibitors may have efficacy against lung disease remains an interesting, untested question.
Table 2.
Clinical outcomes of treatment of lung disease with statins.
| Pulmonary Disease |
Study Design | Outcome Associated with Statin Therapy |
Reference |
|---|---|---|---|
|
Age-related lung function decline |
Observational study | ↓ decline in FEV1 and FVC | [92] |
| Retrospective study of current/former smokers |
↓ decline in FEV1 and FVC | [93] | |
| COPD | Retrospective cohort study | ↓ exacerbations, ↓ intubations | [69] |
| Retrospective cohort, case control studies |
↓ COPD death | [94] | |
| Population-based analysis | ↓ COPD death | [95] | |
| Nested case control study | ↓ COPD hospitalization, ↓ death | [96] | |
| Retrospective cohort study | ↑survival after exacerbation | [68] | |
| Asthma | Prospective, randomized, placebo-controlled, crossover trial |
↑ FEV1, ↓ sputum eosinophils and symptoms in patients discontinuing ICS |
[64] |
| Prospective, randomized, placebo-controlled, double- blind crossover trial |
↔ PEF, asthma control ↓ sputum macrophage, LTB4 |
[66] | |
| Population-based study | ↓ hospitalization for asthma | [97] | |
| Prospective, randomized, placebo-controlled, double- blind trial |
↓ sputum eosinophil percentage in patients co-treated with ICS |
[65] | |
| Prospective, randomized, placebo-controlled, double- blind crossover trial |
↔ exhaled NO, lung function, sputum eosinophils |
[98] | |
| Retrospective cohort study | ↓ FEV1, ↑ medication requirement, ↑ symptoms compared with asthmatics not started on statins |
[99] | |
| Prospective, randomized, placebo-controlled trial |
↔ PEF ↑ quality of life score |
[76] | |
| ALI | Retrospective cohort study | ↔ VFD, organ failures, mortality | [72] |
| Prospective, randomized, placebo-controlled, double- blind trial |
↓ nonpulmonary organ dysfunction ↓ BALF interleukin-8 ↔ ICU mortality |
[73] | |
| IPF | Retrospective cohort study | ↔ survival | [100] |
|
Pulmonary Hypertension |
Prospective observational study |
↑ exercise capacity / improved HD ↓ disease progression |
[70] |
| Prospective, randomized, placebo-controlled trial of COPD patients with PH |
↑ exercise capacity ↓ dyspnea score, ↓ PAP |
[101] |
Abbreviations: ALI, Acute lung injury; BALF, Bronchoalveolar fluid; COPD, Chronic obstructive pulmonary disease; FEV1, Forced expiratory volume in 1 second; FVC, Forced vital capacity; HD, hemodynamics; ICS, inhaled corticosteroids; ICU, Intensive care unit; IPF, Idiopathic pulmonary fibrosis; LTB4, leukotriene B4; NO, nitric oxide; PAP, pulmonary artery pressure; PEF, peak expiratory flow; PH, pulmonary hypertension; VFD, ventilator-free days.
Additional cholesterol-targeting agents that have been studied in models of lung disease include LXR agonists and apolipoprotein mimetic peptides. In addition to the aforementioned efficacy against LPS- and bacteria-induced pulmonary neutrophilia [43], LXR agonists also have been shown to reduce pro-inflammatory responses in human airway smooth muscle cell cultures, suggesting that they may hold some promise in treatment of airway disease [78]. Peptides have been designed that mimic the secondary structure (i.e., amphipathic α-helical repeats) and anti-inflammatory/antioxidant function of the apoA-I holoprotein, and that have been validated, like apoA-I, to have atheroprotective and RCT-promoting activity in animal models. Much like apoA-I, apoA-I mimetics bind oxidized lipids, promote cellular cholesterol efflux, and exert potent anti-inflammatory effects in cell culture (reviewed in [79]). While well characterized in attenuation of atherosclerosis, these peptides have only recently been examined in pulmonary disorders.
Administration of the apoA-I mimetic peptide 5A prior to house dust mite (HDM) sensitization and challenge in a mouse model of asthma resulted in a significant reduction in airspace eosinophils, lymphocytes, and neutrophils, as well as attenuated pulmonary histopathology [80]. The reduction in airway inflammation was associated with a decrease in Th2 and Th17 cytokines. 5A also abrogated the development of airway hyperresponsiveness, and reduced several key features of airway remodeling, including goblet cell hyperplasia and the expression of collagen genes (Col1a1 and Col3a1). Similar findings were noted for the alternate apoA-I mimetic D-4F in an OVA sensitization and challenge model of asthma, wherein D-4F decreased airway hyperresponsiveness, cellular inflammation, and markers of oxidative stress [81]. D-4F has also interestingly been reported to attenuate lung inflammation induced by influenza A infection in mice [82]. Recently, an apoE mimetic peptide modeled after the LDLR-binding region of apoE holoprotein was also shown to reduce eosinophilic airway inflammation, airway cytokines, airway hyperresponsiveness, and goblet cell hyperplasia in a house dust mite model of allergic asthma in mice [39]. Taken together, these studies highlight the strong potential for apolipoprotein mimetics in treatment of pulmonary diseases, although the precise mechanisms underlying the efficacy of these agents remain unclear.
7. Conclusions
Dyslipidemia is highly prevalent in modern society, and clusters with obesity and other disorders of the metabolic syndrome that together center upon disordered inflammation. Emerging studies using gene-deleted mice and pharmacological tools indicate that cholesterol trafficking plays a surprisingly important and perhaps unique role in lung physiology and lung immune homeostasis. This unique relationship likely arises from the distinct biology and cholesterol requirements of the lung. To what extent obesity modifies lung disease through dysregulated lipid trafficking in the lung remains an unanswered question. Future studies will be required to differentiate the various roles of cholesterol regulators in trafficking of leukocytes to the lung, clearance of pathogens, and maintenance of pulmonary function. This intriguing area of research holds the promise of uncovering novel determinants of, and treatments for, chronic lung disease.
Acknowledgments
The authors thank Sue Edelstein for figure design and Drs. Don Cook and Bethany Hsia for critical review of the manuscript. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (Z01 ES102005).
ABBREVIATIONS
- ABCA1
ATP Binding Cassette transporter A1
- ABCG1
ATP Binding Cassette transporter G1
- AHR
Airway Hyperresponsiveness
- ALI
Acute lung injury
- Apo
apolipoprotein
- BAL
Broncheoalveolar lavage
- CETP
cholesteryl ester transfer protein
- CTGF
connective tissue growth factor
- COPD
Chronic obstructive pulmonary disease
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- FC
free cholesterol
- HD
hemodynamics
- HDL-C
high density lipoprotein cholesterol
- HMGCR
hydroxymethyl-glutaryl coenzyme A reductase
- HSMC
human smooth muscle cells
- ICS
inhaled corticosteroids
- ICU
Intensive care unit
- IL
interleukin
- IPF
Idiopathic pulmonary fibrosis
- LDL-C
low density lipoprotein cholesterol
- LDLR
low density lipoprotein receptor
- LPS
lipopolysaccharide
- LTB4
leukotriene B4
- LXR
Liver X Receptor
- MCP-1
monocyte chemotactic protein-1
- MMP
matrix metalloproteinase
- NO
nitric oxide
- OVA
ovalbumin
- PAP
pulmonary artery pressure
- PEF
peak expiratory flow
- PGE2
prostaglandin E2
- PH
pulmonary hypertension
- PMN
polymorphonuclear leukocyte
- RCT
reverse cholesterol transport
- SMA
smooth muscle actin
- SR
scavenger receptor
- TG
Triglyceride
- Th
T helper
- TGF-β1
transforming growth factor β1
- TLR
Toll-like Receptor
- TNF
tumor necrosis factor
- VEGF
vascular endothelial growth factor
- VFD
ventilator-free days
- VLDL
very low density lipoprotein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundy SM. Inflammation, metabolic syndrome, and diet responsiveness. Circulation. 2003;108:126–128. doi: 10.1161/01.CIR.0000082641.20034.6A. [DOI] [PubMed] [Google Scholar]
- 2.Kuklina EV, Yoon PW, Keenan NL. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999–2006. Jama. 2009;302:2104–2110. doi: 10.1001/jama.2009.1672. [DOI] [PubMed] [Google Scholar]
- 3.Hothersall E, McSharry C, Thomson NC. Potential therapeutic role for statins in respiratory disease. Thorax. 2006;61:729–734. doi: 10.1136/thx.2005.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hegele RA. Plasma lipoproteins: genetic influences and clinical implications. Nat Rev Genet. 2009;10:109–121. doi: 10.1038/nrg2481. [DOI] [PubMed] [Google Scholar]
- 5.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM. Promise of low-density lipoprotein-lowering therapy for primary and secondary prevention. Circulation. 2008;117:569–573. doi: 10.1161/CIRCULATIONAHA.107.720300. discussion 73. [DOI] [PubMed] [Google Scholar]
- 8.Lee JY, Parks JS. ATP-binding cassette transporter AI and its role in HDL formation. Curr Opin Lipidol. 2005;16:19–25. doi: 10.1097/00041433-200502000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Yvan-Charvet L, Pagler TA, Wang N, Senokuchi T, Brundert M, Li H, et al. SR-BI inhibits ABCG1-stimulated net cholesterol efflux from cells to plasma HDL. J Lipid Res. 2008;49:107–114. doi: 10.1194/jlr.M700200-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda T, Grillot D, Billheimer JT, Briand F, Delerive P, Huet S, et al. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2010;30:781–786. doi: 10.1161/ATVBAHA.109.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol. 2008;180:3560–3568. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–21772. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 13.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 16.Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signaling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 17.Rajamaki K, Lappalainen J, Oorni K, Valimaki E, Matikainen S, Kovanen PT, et al. Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One. 2010;5:e11765. doi: 10.1371/journal.pone.0011765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, et al. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 19.Nistor A, Simionescu M. Uptake of low density lipoproteins by the hamster lung. Interactions with capillary endothelium. Am Rev Respir Dis. 1986;134:1266–1272. doi: 10.1164/arrd.1986.134.6.1266. [DOI] [PubMed] [Google Scholar]
- 20.Hass MA, Longmore WJ. Regulation of lung surfactant cholesterol metabolism by serum lipopoteins. Lipids. 1980;15:401–406. doi: 10.1007/BF02534063. [DOI] [PubMed] [Google Scholar]
- 21.Kolleck I, Schlame M, Fechner H, Looman AC, Wissel H, Rustow B. HDL is the major source of vitamin E for type II pneumocytes. Free Radic Biol Med. 1999;27:882–890. doi: 10.1016/s0891-5849(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 22.Pian MS, Dobbs LG. Lipoprotein-stimulated surfactant secretion in alveolar type II cells: mediation by heterotrimeric G proteins. Am J Physiol. 1997;273:L634–L639. doi: 10.1152/ajplung.1997.273.3.L634. [DOI] [PubMed] [Google Scholar]
- 23.Bjorkerud S, Bjorkerud B. Lipoproteins are major and primary mitogens and growth promoters for human arterial smooth muscle cells and lung fibroblasts in vitro. Arterioscler Thromb. 1994;14:288–298. [PubMed] [Google Scholar]
- 24.Vockeroth D, Gunasekara L, Amrein M, Possmayer F, Lewis JF, Veldhuizen RA. Role of cholesterol in the biophysical dysfunction of surfactant in ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298:L117–L125. doi: 10.1152/ajplung.00218.2009. [DOI] [PubMed] [Google Scholar]
- 25.McCrae KC, Weltman B, Alyward S, Shaw RA, Sowa MG, Unruh HW, et al. The effect of elevated dietary cholesterol on pulmonary surfactant function in adolescent mice. Pediatr Pulmonol. 2008;43:426–434. doi: 10.1002/ppul.20772. [DOI] [PubMed] [Google Scholar]
- 26.Baritussio A, Enzi G, Inelmen EM, Schiavon M, de Biasi F, Allegra L, et al. Altered surfactant synthesis and function in rats with diet-induced hyperlipidemia. Metabolism. 1980;29:503–510. doi: 10.1016/0026-0495(80)90075-x. [DOI] [PubMed] [Google Scholar]
- 27.Dahl M, Bauer AK, Arredouani M, Soininen R, Tryggvason K, Kleeberger SR, et al. Protection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors MARCO and SR-AI/II. J Clin Invest. 2007;117:757–764. doi: 10.1172/JCI29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulfer MK, Murphy RC. Formation of biologically active oxysterols during ozonolysis of cholesterol present in lung surfactant. J Biol Chem. 2004;279:26331–26338. doi: 10.1074/jbc.M403581200. [DOI] [PubMed] [Google Scholar]
- 29.Sevanian A, Berliner J, Peterson H. Uptake, metabolism, and cytotoxicity of isomeric cholesterol-5,6-epoxides in rabbit aortic endothelial cells. J Lipid Res. 1991;32:147–155. [PubMed] [Google Scholar]
- 30.O'Callaghan YC, Woods JA, O'Brien NM. Comparative study of the cytotoxicity and apoptosis-inducing potential of commonly occurring oxysterols. Cell Biol Toxicol. 2001;17:127–137. doi: 10.1023/a:1010914306375. [DOI] [PubMed] [Google Scholar]
- 31.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang W, Xu H, Shi Y, Nandedkar S, Zhang H, Gao H, et al. Genetic deletion of apolipoprotein A-I increases airway hyperresponsiveness, inflammation, and collagen deposition in the lung. J Lipid Res. 2010;51:2560–2570. doi: 10.1194/jlr.M004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Massaro D, Massaro GD. Apoetm1Unc mice have impaired alveologenesis, low lung function, and rapid loss of lung function. Am J Physiol Lung Cell Mol Physiol. 2008;294:L991–L997. doi: 10.1152/ajplung.00013.2008. [DOI] [PubMed] [Google Scholar]
- 34.Bates SR, Tao JQ, Collins HL, Francone OL, Rothblat GH. Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L980–L989. doi: 10.1152/ajplung.00234.2005. [DOI] [PubMed] [Google Scholar]
- 35.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–4282. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 36.Thomassen MJ, Barna BP, Malur AG, Bonfield TL, Farver CF, Malur A, et al. ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J Lipid Res. 2007;48:2762–2678. doi: 10.1194/jlr.P700022-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Draper DW, Madenspacher JH, Dixon D, King DH, Remaley AT, Fessler MB. ATP-binding cassette transporter G1 deficiency dysregulates host defense in the lung. Am J Respir Crit Care Med. 2010;182:404–412. doi: 10.1164/rccm.200910-1580OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Draper DW, Gowdy KM, Madenspacher JH, Wilson RH, Whitehead GS, Nakano H, et al. ATP Binding Cassette Transporter G1 Deletion Induces IL-17-Dependent Dysregulation of Pulmonary Adaptive Immunity. J Immunol. 2012 doi: 10.4049/jimmunol.1101605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yao X, Fredriksson K, Yu ZX, Xu X, Raghavachari N, Keeran KJ, et al. Apolipoprotein E negatively regulates house dust mite-induced asthma via a low-density lipoprotein receptor-mediated pathway. Am J Respir Crit Care Med. 2010;182:1228–1238. doi: 10.1164/rccm.201002-0308OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong H, He J, Lee JH, Mallick E, Gao X, Li S, et al. Activation of the liver X receptor prevents lipopolysaccharide-induced lung injury. J Biol Chem. 2009;284:30113–30121. doi: 10.1074/jbc.M109.047753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birrell MA, Catley MC, Hardaker E, Wong S, Willson TM, McCluskie K, et al. Novel role for the liver X nuclear receptor in the suppression of lung inflammatory responses. J Biol Chem. 2007;282:31882–31890. doi: 10.1074/jbc.M703278200. [DOI] [PubMed] [Google Scholar]
- 42.Crisafulli C, Mazzon E, Paterniti I, Galuppo M, Bramanti P, Cuzzocrea S. Effects of Liver x receptor agonist treatment on signal transduction pathways in acute lung inflammation. Respir Res. 2010;11:19. doi: 10.1186/1465-9921-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smoak K, Madenspacher J, Jeyaseelan S, Williams B, Dixon D, Poch KR, et al. Effects of liver X receptor agonist treatment on pulmonary inflammation and host defense. J Immunol. 2008;180:3305–3312. doi: 10.4049/jimmunol.180.5.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korf H, Vander Beken S, Romano M, Steffensen KR, Stijlemans B, Gustafsson JA, et al. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J Clin Invest. 2009;119:1626–1637. doi: 10.1172/JCI35288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Babiker A, Andersson O, Lindblom D, van der Linden J, Wiklund B, Lutjohann D, et al. Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin. J Lipid Res. 1999;40:1417–1425. [PubMed] [Google Scholar]
- 46.Madenspacher JH, Draper DW, Smoak KA, Li H, Griffiths GL, Suratt BT, et al. Dyslipidemia induces opposing effects on intrapulmonary and extrapulmonary host defense through divergent TLR response phenotypes. J Immunol. 2010;185:1660–1669. doi: 10.4049/jimmunol.0903501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76:3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeh YF, Huang SL. Dietary cholesterol enhances pulmonary eosinophilic inflammation in a murine model of asthma. Int Arch Allergy Immunol. 2001;125:329–334. doi: 10.1159/000053834. [DOI] [PubMed] [Google Scholar]
- 49.Fessler MB, Massing MW, Spruell B, Jaramillo R, Draper DW, Madenspacher JH, et al. Novel relationship of serum cholesterol with asthma and wheeze in the United States. J Allergy Clin Immunol. 2009;124:967–974. e1–e15. doi: 10.1016/j.jaci.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKay A, Leung BP, McInnes IB, Thomson NC, Liew FY. A novel anti-inflammatory role of simvastatin in a murine model of allergic asthma. J Immunol. 2004;172:2903–2908. doi: 10.4049/jimmunol.172.5.2903. [DOI] [PubMed] [Google Scholar]
- 51.Yeh YF, Huang SL. Enhancing effect of dietary cholesterol and inhibitory effect of pravastatin on allergic pulmonary inflammation. J Biomed Sci. 2004;11:599–606. doi: 10.1007/BF02256124. [DOI] [PubMed] [Google Scholar]
- 52.Lee JH, Lee DS, Kim EK, Choe KH, Oh YM, Shim TS, et al. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med. 2005;172:987–993. doi: 10.1164/rccm.200501-041OC. [DOI] [PubMed] [Google Scholar]
- 53.Fessler MB, Young SK, Jeyaseelan S, Lieber JG, Arndt PG, Nick JA, et al. A role for hydroxyl-methylglutaryl coenzyme a reductase in pulmonary inflammation and host defense. Am J Respir Crit Care Med. 2005;171:606–615. doi: 10.1164/rccm.200406-729OC. [DOI] [PubMed] [Google Scholar]
- 54.Liu ZQ, Liu B, Yu L, Wang XQ, Wang J, Liu HM. Simvastatin has beneficial effect on pulmonary artery hypertension by inhibiting NF-kappaB expression. Mol Cell Biochem. 2011;354:77–82. doi: 10.1007/s11010-011-0807-4. [DOI] [PubMed] [Google Scholar]
- 55.Kim JW, Rhee CK, Kim TJ, Kim YH, Lee SH, Yoon HK, et al. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin Exp Pharmacol Physiol. 2010;37:1055–1063. doi: 10.1111/j.1440-1681.2010.05431.x. [DOI] [PubMed] [Google Scholar]
- 56.Myles PR, Hubbard RB, McKeever TM, Pogson Z, Smith CJ, Gibson JE. Risk of community-acquired pneumonia and the use of statins, ace inhibitors and gastric acid suppressants: a population-based case-control study. Pharmacoepidemiol Drug Saf. 2009;18:269–275. doi: 10.1002/pds.1715. [DOI] [PubMed] [Google Scholar]
- 57.van de Garde EM, Hak E, Souverein PC, Hoes AW, van den Bosch JM, Leufkens HG. Statin treatment and reduced risk of pneumonia in patients with diabetes. Thorax. 2006;61:957–961. doi: 10.1136/thx.2006.062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chalmers JD, Singanayagam A, Murray MP, Hill AT. Prior statin use is associated with improved outcomes in community-acquired pneumonia. Am J Med. 2008;121:1002–1007. e1. doi: 10.1016/j.amjmed.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 59.Mortensen EM, Pugh MJ, Copeland LA, Restrepo MI, Cornell JE, Anzueto A, et al. Impact of statins and angiotensin-converting enzyme inhibitors on mortality of subjects hospitalised with pneumonia. Eur Respir J. 2008;31:611–617. doi: 10.1183/09031936.00162006. [DOI] [PubMed] [Google Scholar]
- 60.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The effect of prior statin use on 30-day mortality for patients hospitalized with community-acquired pneumonia. Respir Res. 2005;6:82. doi: 10.1186/1465-9921-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schlienger RG, Fedson DS, Jick SS, Jick H, Meier CR. Statins and the risk of pneumonia: a population-based, nested case-control study. Pharmacotherapy. 2007;27:325–332. doi: 10.1592/phco.27.3.325. [DOI] [PubMed] [Google Scholar]
- 62.Thomsen RW, Riis A, Kornum JB, Christensen S, Johnsen SP, Sorensen HT. Preadmission use of statins and outcomes after hospitalization with pneumonia: population-based cohort study of 29,900 patients. Arch Intern Med. 2008;168:2081–2087. doi: 10.1001/archinte.168.19.2081. [DOI] [PubMed] [Google Scholar]
- 63.Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. Bmj. 2006;333:999. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Simvastatin in the treatment of asthma: lack of steroid-sparing effect. Thorax. 2010;65:891–896. doi: 10.1136/thx.2010.138990. [DOI] [PubMed] [Google Scholar]
- 65.Maneechotesuwan K, Ekjiratrakul W, Kasetsinsombat K, Wongkajornsilp A, Barnes PJ. Statins enhance the anti-inflammatory effects of inhaled corticosteroids in asthmatic patients through increased induction of indoleamine 2, 3-dioxygenase. J Allergy Clin Immunol. 2010;126:754–762. e1. doi: 10.1016/j.jaci.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. 2008;63:1070–1075. doi: 10.1136/thx.2008.100198. [DOI] [PubMed] [Google Scholar]
- 67.Zeki AA, Kenyon NJ, Goldkorn T. Statin drugs, metabolic pathways, and asthma: a therapeutic opportunity needing further research. Drug Metab Lett. 2011;5:40–44. doi: 10.2174/187231211794455217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soyseth V, Brekke PH, Smith P, Omland T. Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007;29:279–283. doi: 10.1183/09031936.00106406. [DOI] [PubMed] [Google Scholar]
- 69.Blamoun AI, Batty GN, DeBari VA, Rashid AO, Sheikh M, Khan MA. Statins may reduce episodes of exacerbation and the requirement for intubation in patients with COPD: evidence from a retrospective cohort study. Int J Clin Pract. 2008;62:1373–1378. doi: 10.1111/j.1742-1241.2008.01731.x. [DOI] [PubMed] [Google Scholar]
- 70.Kao PN. Simvastatin treatment of pulmonary hypertension: an observational case series. Chest. 2005;127:1446–1452. doi: 10.1378/chest.127.4.1446. [DOI] [PubMed] [Google Scholar]
- 71.Johnson BA, Iacono AT, Zeevi A, McCurry KR, Duncan SR. Statin use is associated with improved function and survival of lung allografts. Am J Respir Crit Care Med. 2003;167:1271–1278. doi: 10.1164/rccm.200205-410OC. [DOI] [PubMed] [Google Scholar]
- 72.Kor DJ, Iscimen R, Yilmaz M, Brown MJ, Brown DR, Gajic O. Statin administration did not influence the progression of lung injury or associated organ failures in a cohort of patients with acute lung injury. Intensive Care Med. 2009;35:1039–1046. doi: 10.1007/s00134-009-1421-8. [DOI] [PubMed] [Google Scholar]
- 73.Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O'Kane CM, Elborn JS, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study) Am J Respir Crit Care Med. 2011;183:620–626. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 74.Nicholls SJ, Brewer HB, Kastelein JJ, Krueger KA, Wang MD, Shao M, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. Jama. 2011;306:2099–2109. doi: 10.1001/jama.2011.1649. [DOI] [PubMed] [Google Scholar]
- 75.Gutstein DE, Krishna R, Johns D, Surks HK, Dansky HM, Shah S, et al. Anacetrapib, a novel CETP inhibitor: pursuing a new approach to cardiovascular risk reduction. Clin Pharmacol Ther. 2012;91:109–122. doi: 10.1038/clpt.2011.271. [DOI] [PubMed] [Google Scholar]
- 76.Braganza G, Chaudhuri R, McSharry C, Weir CJ, Donnelly I, Jolly L, et al. Effects of short-term treatment with atorvastatin in smokers with asthma--a randomized controlled trial. BMC Pulm Med. 2011;11:16. doi: 10.1186/1471-2466-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 78.Delvecchio CJ, Bilan P, Radford K, Stephen J, Trigatti BL, Cox G, et al. Liver X receptor stimulates cholesterol efflux and inhibits expression of proinflammatory mediators in human airway smooth muscle cells. Mol Endocrinol. 2007;21:1324–1334. doi: 10.1210/me.2007-0017. [DOI] [PubMed] [Google Scholar]
- 79.Getz GS, Reardon CA. Apolipoprotein A-I and A-I mimetic peptides: a role in atherosclerosis. J Inflamm Res. 2011;4:83–92. doi: 10.2147/JIR.S12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yao X, Dai C, Fredriksson K, Dagur PK, McCoy JP, Qu X, et al. 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite-induced asthma. J Immunol. 2011;186:576–583. doi: 10.4049/jimmunol.1001534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nandedkar SD, Weihrauch D, Xu H, Shi Y, Feroah T, Hutchins W, et al. D-4F, an apoA-1 mimetic, decreases airway hyperresponsiveness, inflammation, and oxidative stress in a murine model of asthma. J Lipid Res. 2011;52:499–508. doi: 10.1194/jlr.M012724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Lenten BJ, Wagner AC, Anantharamaiah GM, Garber DW, Fishbein MC, Adhikary L, et al. Influenza infection promotes macrophage traffic into arteries of mice that is prevented by D-4F, an apolipoprotein A-I mimetic peptide. Circulation. 2002;106:1127–1132. doi: 10.1161/01.cir.0000030182.35880.3e. [DOI] [PubMed] [Google Scholar]
- 83.Zeki AA, Franzi L, Last J, Kenyon NJ. Simvastatin inhibits airway hyperreactivity: implications for the mevalonate pathway and beyond. Am J Respir Crit Care Med. 2009;180:731–740. doi: 10.1164/rccm.200901-0018OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schaafsma D, McNeill KD, Mutawe MM, Ghavami S, Unruh H, Jacques E, et al. Simvastatin inhibits TGFbeta1-induced fibronectin in human airway fibroblasts. Respir Res. 2011;12:113. doi: 10.1186/1465-9921-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1026–L1032. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 86.Van Lenten BJ, Wagner AC, Navab M, Anantharamaiah GM, Hui EK, Nayak DP, et al. D-4F, an apolipoprotein A-I mimetic peptide, inhibits the inflammatory response induced by influenza A infection of human type II pneumocytes. Circulation. 2004;110:3252–3258. doi: 10.1161/01.CIR.0000147232.75456.B3. [DOI] [PubMed] [Google Scholar]
- 87.Jiao YL, Wu MP. Apolipoprotein A-I diminishes acute lung injury and sepsis in mice induced by lipoteichoic acid. Cytokine. 2008;43:83–87. doi: 10.1016/j.cyto.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 88.Yan YJ, Li Y, Lou B, Wu MP. Beneficial effects of ApoA-I on LPS-induced acute lung injury and endotoxemia in mice. Life Sci. 2006;79:210–215. doi: 10.1016/j.lfs.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi S, Nakamura H, Seki M, Shiraishi Y, Yamamoto M, Furuuchi M, et al. Reversal of elastase-induced pulmonary emphysema and promotion of alveolar epithelial cell proliferation by simvastatin in mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L882–L890. doi: 10.1152/ajplung.00238.2007. [DOI] [PubMed] [Google Scholar]
- 90.Watts KL, Sampson EM, Schultz GS, Spiteri MA. Simvastatin inhibits growth factor expression and modulates profibrogenic markers in lung fibroblasts. Am J Respir Cell Mol Biol. 2005;32:290–300. doi: 10.1165/rcmb.2004-0127OC. [DOI] [PubMed] [Google Scholar]
- 91.Kim TH, Lee YH, Kim KH, Lee SH, Cha JY, Shin EK, et al. Role of lung apolipoprotein A-I in idiopathic pulmonary fibrosis: antiinflammatory and antifibrotic effect on experimental lung injury and fibrosis. Am J Respir Crit Care Med. 2010;182:633–642. doi: 10.1164/rccm.200905-0659OC. [DOI] [PubMed] [Google Scholar]
- 92.Alexeeff SE, Litonjua AA, Sparrow D, Vokonas PS, Schwartz J. Statin use reduces decline in lung function: VA Normative Aging Study. Am J Respir Crit Care Med. 2007;176:742–747. doi: 10.1164/rccm.200705-656OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Keddissi JI, Younis WG, Chbeir EA, Daher NN, Dernaika TA, Kinasewitz GT. The use of statins and lung function in current and former smokers. Chest. 2007;132:1764–1771. doi: 10.1378/chest.07-0298. [DOI] [PubMed] [Google Scholar]
- 94.Frost FJ, Petersen H, Tollestrup K, Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 2007;131:1006–1012. doi: 10.1378/chest.06-1997. [DOI] [PubMed] [Google Scholar]
- 95.Ishida W, Kajiwara T, Ishii M, Fujiwara F, Taneichi H, Takebe N, et al. Decrease in mortality rate of chronic obstructive pulmonary disease (COPD) with statin use: a population-based analysis in Japan. Tohoku J Exp Med. 2007;212:265–273. doi: 10.1620/tjem.212.265. [DOI] [PubMed] [Google Scholar]
- 96.Mancini GB, Etminan M, Zhang B, Levesque LE, FitzGerald JM, Brophy JM. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006;47:2554–2560. doi: 10.1016/j.jacc.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 97.Huang CC, Chan WL, Chen YC, Chen TJ, Chou KT, Lin SJ, et al. Statin use in patients with asthma: a nationwide population-based study. Eur J Clin Invest. 2011;41:507–512. doi: 10.1111/j.1365-2362.2010.02434.x. [DOI] [PubMed] [Google Scholar]
- 98.Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol. 2007;119:328–335. doi: 10.1016/j.jaci.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 99.Ostroukhova M, Kouides RW, Friedman E. The effect of statin therapy on allergic patients with asthma. Ann Allergy Asthma Immunol. 2009;103:463–468. doi: 10.1016/S1081-1206(10)60261-X. [DOI] [PubMed] [Google Scholar]
- 100.Nadrous HF, Ryu JH, Douglas WW, Decker PA, Olson EJ. Impact of angiotensin-converting enzyme inhibitors and statins on survival in idiopathic pulmonary fibrosis. Chest. 2004;126:438–446. doi: 10.1378/chest.126.2.438. [DOI] [PubMed] [Google Scholar]
- 101.Lee TM, Chen CC, Shen HN, Chang NC. Effects of pravastatin on functional capacity in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Clin Sci (Lond) 2009;116:497–505. doi: 10.1042/CS20080241. [DOI] [PubMed] [Google Scholar]