Abstract
Computer technology from the management of individual patient medical records to the tracking of epidemiologic trends has become an essential part of all aspects of modern medicine. Consequently, computers, including bedside components, point-of-care testing equipment, and handheld computer devices, are increasingly present in patients’ rooms. Recent articles have indicated that computer hardware, just as other medical equipment, may act as a reservoir for microorganisms and contribute to the transfer of pathogens to patients. This article presents basic microbiological concepts relative to infection, reviews the present literature concerning possible links between computer contamination and nosocomial colonizations and infections, discusses basic principles for the control of contamination, and provides guidelines for reducing the risk of transfer of microorganisms to susceptible patient populations.
Over the past 50 years, various forms of computer-based, information management applications have been developed and deployed in the clinical setting.1 During this time, many system developers have recognized the benefits associated with having computer hardware in the examination room2 or at the patient’s bedside in the hospital.3,4 More specifically, both Collen5 and, more recently, the Institute of Medicine6 have recognized the importance of having clinicians directly involved in data entry activities at the point of care in order to ensure accuracy and timeliness of the data. Finally, over the past several years the use of portable computing devices by clinicians in the patient’s presence has expanded considerably.7–9 While the need for and benefits of having computers at the patient’s bedside for use by clinicians has been well studied, little attention has been paid to the potential risks of infection to the patient that these devices might pose. A previous history of transfer of microorganisms from other inanimate environmental objects to patients17–24 suggests that the presence of computer hardware in the patient setting needs to be examined for this microbial transfer potential.
Therefore, the purpose of this article is to review current literature to determine the potential for transmission of pathogens via computer hardware and to review basic microbiologic and infection control procedures that might be used to determine and diminish the risk of microbial transfer to patients.
Review of Pertinent Microbiologic Concepts and Findings
Steps Preceding an Infection: Basic Definitions and Concepts
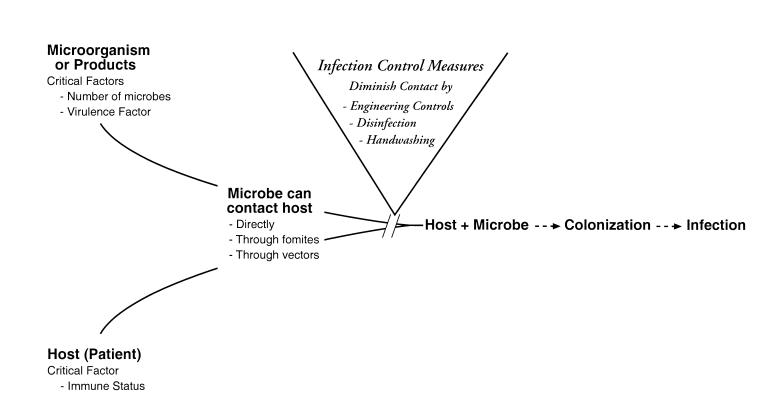
Humans are surrounded by a number of microorganisms, most of which are completely harmless and some of which are beneficial and even necessary for our existence. At times, however, our interaction with microbes can lead to an infection. An infection is the result of an interaction between a host (the patient) and a microorganism or some of its products (Figure 1▶). In general, at least four factors, some microbial-associated and some host-associated, determine whether an infection will occur. Microbial factors of importance include the number of microorganisms present. The more microorganisms present, the greater the chance of an infection. Secondly, the particular armamentarium of virulence factors that the microbe has will influence its ability to cause an infection. For example, a bacterium that produces a particularly potent toxin can be more apt to cause an infection than one that does not. Third, the most critical factor that the host brings to the interaction is immunologic status. A person who is immunosuppressed or immunocompromised due to any number of circumstances (Table 1▶) will be more susceptible to an infection. Finally, in order for an infection to occur, the microorganism or its products must come in contact with the host. Contact can happen in a number of different ways. The microbe might directly contact the host, or it might contact the host via an indirect route involving inanimate objects, called fomites, and/or living organisms, called vectors. The fomite, such as a piece of computer hardware, or the vector, such as a health care worker, becomes contaminated with a microbe and then serves as a reservoir for transmitting the microorganism to the host by some form of contact. Once the microbe reaches the host, a number of different associations are possible. The presence of a microbe in or on a host with growth and multiplication of that microorganism, but without tissue damage, is termed colonization.10 Once tissue damage begins, the colonization becomes an infection. Not all colonizations become infections, but all infections are generally preceded by colonizations.11
Figure 1 .
Steps potentially leading to infection and basic infection control interventions to decrease the risk of infection.
Table 1 .
Patients at Increased Risk for Infections
| Patients of extreme age, either very young or very old |
| Victims of severe trauma, such as burns or crush injuries |
| Malnourished patients |
| Patients with immunocompromising diseases (e.g., diabetes, AIDS) |
| Patients immunosuppressed for transplantation or chemotherapy |
Nosocomial Infections: What Are They and Why Be Concerned?
Nosocomial infections are infections that develop within a health care institution or are produced by organisms acquired during a stay at such a facility.12 These infections are not present or incubating at admission but are acquired by the patient during some interaction at the hospital or medical unit. Each year nosocomial infections cause a significant amount of morbidity, mortality, and increased medical costs. It is estimated that in the United States at least 2 million patients annually acquire nosocomial infections.13 These acquired infections directly cause approximately 19,000 deaths annually and contribute indirectly to an additional 80,000 deaths each year.14 Patients surviving nosocomial infections require longer hospital stays with increased medical support. It was estimated that in 1992 the annual cost to treat nosocomial infections was $4.5 billion.15 To put these figures in a more comprehensible context, a study published from the University of Michigan in 1999 compared the cost of treating patients who had acquired a nosocomial bloodstream infection with the cost of treating patients who had not. Of the patients who survived, the average cost for patients who had picked up a bloodstream infection while in the hospital compared to those who had not was $34,508 more per patient.16 Hence, these hospital-acquired infections are a concern because of both the human and economic tolls that they exact.
Computers as Microbial Reservoirs: Have Computers Been Linked to Nosocomial Infections?
It has long been recognized that inanimate objects in the patient’s environment can harbor microorganisms. These objects might be medical tools, such as stethoscopes,17 ear thermometers,18 or bronchoscopes,19 or common nonmedical objects, such as ball point pens,20 bedrails and bedside tables18,21 or plumbing components that introduce microbes into the bath water.22–24 Only recently have investigators begun to examine the microbial contamination on computer hardware and to ask if these microorganisms might play a role in patient acquired infections.
A search of the literature, using Medline, BIOSIS, and CINAHL databases, indicated that only a few studies investigating links between computers and patient colonizations and/or nosocomial infections presently exist in peer-reviewed scientific journals (Table 2▶). In 1995, in a letter to the editor, Masterton et al.25 linked refractory methicillin-resistant Staphylococcus aureus carriage in a nurse to contamination of home environmental objects, including a computer desk and joystick. Despite antibiotic treatment of the nurse, the bacterial carriage of this microbe was not eliminated until the home environment, including computer-related hardware, was decontaminated.
Table 2 .
Studies Investigating Computer Contamination and Patient Colonizations or Infections
| Infection Control Measures |
|||||
|---|---|---|---|---|---|
| Year | Author | Study Scope | Primary Findings | Before Study | Added After Study |
| 1995 | Masterton et al. | Case study, one home computer | MRSA on home computer contributed to MRSA carriage of nurse | None reported | Decontaminate home computer |
| 1998 | Isaacs et al. | 27 hospital computers tested 1 time | Antibiotic resistant microbes sought not found, but S. aureus and Pseudomonas isolated | Keyboard covers, 1 time/day disinfection | None reported |
| 1999 | Neely et al. | Epidemiologic study of A. baumannii colonization | A. baumannii colonization in patients linked to bedside computer keyboards | Keyboard covers, random cleaning | Daily keyboard disinfection; change in hand washing and gloving policy |
| 2000 | Bures et al. | Pulse field gel electrophoresis study of ICU infections | MRSA infections in patients directly linked to computers in ward | None reported | Keyboard covers, disinfected daily; hand washing enforced |
| 2001 | Devine et al. | 25 terminals cultured 1 time in 2 hospitals | 42% of computers positive for MRSA in hospital A, 8% positive in hospital B; hospital A had higher MRSA transfer rate | Hand washing in both hospitals, but monitored in hospital B | Enforce staff hand washing before and after patient contact |
| 2001 | Ivey et al. | Abstract of CPU fan contamination and fungi in patients rooms | No correlation between isolates on CPU fans and fungi in patient’s rooms | None reported | None reported |
In 1998, in a brief report by Isaacs et al.,26 27 keyboards in a burn unit were swabbed one time to determine if the keyboards could be contributing to an increase in antibiotic-resistant bacteria in their patients. Resistant isolates were not found, leading the authors to conclude that the computer keyboards were not a significant source of the spread of the resistant bacteria in their unit. It is interesting that while the two types of antibiotic-resistant bacteria that they sought were not found, other bacteria, Staphylococcus aureus and Pseudomonas, both of which are capable of causing serious infections in burn patients, were found on the computer terminals.
In 1999, Neely et al.27 reported a more extensive study in a burn unit in which there had been an increase in acquired Acinetobacter baumannii colonizations. An epidemiologic investigation showed this microorganism to be present more often on computer keyboard covers than on any other objects in the patients’ rooms. The increase in patient colonization coincided temporally with the introduction of bedside computers into the patients’ rooms. Once control measures were introduced to decrease the presence of microorganisms on the keyboards, the colonization rate for A. baumannii on the burn patients returned to its original low level. Such findings strongly suggest a link between contaminated computer keyboards and colonization in this group of patients.
In 2000, in an excellent study in an adult intensive care unit (ICU), Bures et al.28 cultured a number of microorganisms, including methicillin-resistant Staphylococcus aureus (MRSA), Enterococcus, and Enterobacter, from computer keyboards. Cultures from patients in the ICU showed similar microorganisms. Since MRSA can potentially be a particularly dangerous microbe, the MRSA on the keyboards was compared with the MRSA in the infected patients, using pulse-field gel electrophoresis, a particularly sensitive molecular genetics technique for distinguishing among isolates of the same genus and species. This technique showed that the MRSA causing clinical infection in two of the ICU patients was identical to the MRSA isolated from the keyboards, thereby establishing a direct connection between the infected patients and the computers.
In 2001, Devine et al.29 cultured for MRSA on ward computer terminals in two different hospitals. In hospital A, 12 terminals were cultured and 5 (42%) were positive for MRSA. In hospital B, 13 terminals were swabbed and 1 (8%) was positive for the bacteria. Not surprisingly, hospital A had a significantly higher rate of MRSA transmission for its patients than hospital B. These data are consistent with computer keyboards playing a role in the transmission of the bacteria.
To our knowledge, only one other study in the scientific literature addresses computers and microbial contamination in relation to patients. In this 2001 study, which has appeared only as a published abstract, Ivey et al.30 asked whether the fan in the computer processing unit (CPU) might be responsible for the dissemination of fungal flora in their ICU. Data showed little commonality between fungal cultures from the CPU dust and cultures from other room areas. The investigators concluded that computer fans in the ICU did not have a significant impact on the fungal infections in their unit.
The introduction of computers into critical care environments is a relatively new event. Consequently, the possible impact of the presence of these devices in patient care areas has not been well-studied. However, results from the first early studies presented above clearly demonstrate that the keyboards of computers, as of other bedside inanimate objects,18,19,22–24 can be reservoirs for microorganisms associated with colonized or infected patients. Whether other computer hardware, such as computer mice, rollerballs, touchscreens, joysticks or even portable handheld devices, might be a factor in the dissemination of microbes remains to be determined.
Microbial Survival and Transfer: What Factors Influence the Link between Computers and Patients?
Two factors that play a role in the link between any fomite, such as a piece of computer hardware, and the patient are the ability of a particular microbe to survive on a particular surface and the fact that various vectors, such as health care workers, can transfer microorganisms from one surface to another.
Microorganisms survive for different periods of time on different surfaces. Survival varies depending upon the particular microbe, the particular surface, and the concentration of the microorganism on the surface. In general, the greater the concentration of the microbe, the longer it survives.31–35 Survival can range from minutes to months. Obviously, if a microbe only survives for a few minutes on an inanimate object, such as the computer terminal, then the possibility of that microbe being acquired by a patient is quite small. However, conversely, if particular bacteria or fungi survive for weeks to months on a certain surface, then the odds of that organism being picked up by a patient or health care worker are considerably increased.
Most of the accessible components of computers are made of plastic. In a series of studies in which the survival of a variety of bacteria and fungi were determined on a number of different fabrics and plastics (including the plastic skins used to protect computer keyboards), microbial survival was often days to weeks on both types of surfaces.33–35 However, when there was a difference in survival between the fabrics and the plastics, the microbes tended to live longer on plastics. Hence, the long survival times of certain microorganisms, particularly on plastics such as those associated with computers, contributes to the possibility of computers acting as reservoirs for these microbes.
How do the microbes get from the surface to the patient? Controlled studies have shown that microorganisms can be readily transferred from inanimate objects to hands and visa versa. Rangel-Frausto et al.36 found that in 90% of their tests the yeast Candida albicans was transferred from a plastic surface to a person’s hands and that in 90% of their trials, the yeast was transferred from the hands to a plastic surface. In a more recent study, Noskin et al.37 showed that the bacterium Enterococcus faecium likewise was directly transferred from a vinyl surface to a person’s hands. Studies have also demonstrated that microbes can be transferred from person to person. In the Rangel-Frausto et al study,36 the yeast was transferred from hand to hand 69% of the time, and various outbreaks of both bacterial and fungal infections in patients have been traced to a specific individual health care worker.25,38,39 Hence, these studies indicate that it is quite possible for a long-lived microbe on a computer keyboard to be transferred to a staff member’s hands and then to a patient where it could potentially cause an infection.
Potential Solutions with Emphasis on Basic Infection Control Principles
All of the solutions presented below are quite simple, apply to most inanimate objects, and are based on one principle: before a microbe or its product can even potentially cause an infection in a patient, it must come in contact with that patient. Therefore all of the solutions discussed below have the single purpose of decreasing or eliminating the number of computer-associated microorganisms that come in contact with a patient (Figure 1▶).
Engineering or Process Controls Versus Behavioral Controls
In general, it is preferable to engineer the physical environment or configure a process so that it is difficult for an error, such as contamination, to occur rather than to depend on consistent, meticulous behavior alone to prevent errors (contamination). For example, in many patient rooms, space is at a premium, and it is possible that the computer terminal might be located close enough to the sink so that it could be splattered and thereby contaminated with microorganisms during the course of cleaning objects or hands. One control would be to advise staff to be careful not to splash the keyboard when using the sink; however, with multiple duties, it is unlikely that this care would always occur. A better control would be either to relocate the computer or to simply place a water impermeable barrier, such as a plastic panel, between the sink and the keyboard. With the barrier in place, the behavior of the people using the sink becomes a mute point as far as splashing the keyboard is concerned. Other examples of engineering and process controls are the use of a computer keyboard cover and of an infrared mouse to allow the process of computer cleaning/disinfection (see below) to be easier and more effective than relying on a person to meticulously clean the keyboard or the mechanical mouse without harming the hardware.
Such engineering or process controls may take a little forethought and may also involve a bit of expense. However, if they save staff time, decrease the need for continuous staff behavior surveillance and education, and/or prevent nosocomial infections, they are often worth the up-front time and expense.
Cleaning and Disinfecting
Cleaning is the removal of all foreign material, such as dirt and organic material from an object.40 Sterilization is the complete elimination or destruction of all forms of microbial life, while disinfection is a process that eliminates many or all pathogenic microorganisms, with the exception of bacterial spores, from inanimate objects.40 Since there are relatively few situations in which computer hardware would need to be sterilized, this discussion basically addresses disinfection, although many of the comments are also applicable to sterilization. Because dirt can harbor microbes from the normal disinfecting process, successful disinfection should be preceded by cleaning. However, certain disinfectant cleaners may accomplish both tasks in one process.
There is no perfect disinfecting agent; each chemical has its own advantages and disadvantages, depending on the situation in which it is used. Therefore, in any medical facility, the infection control personnel should be consulted about appropriate cleaning/disinfecting agents and procedures. Factors to be considered include the level of disinfection necessary for that particular computer, the potential types of organic and microbial contamination that might be present, and the cleaning/disinfecting agents available. When choosing these agents, besides efficacy in disinfection, issues such as patient and personnel safety (e.g., flammability, toxicities), ease of use (e.g., availability, need for pre-mixing), aesthetics (e.g., odors, color changes), and costs need be considered. Guidelines for selection and use of disinfectants are available in what is now a classic article in the infection control literature.41
In addition, one needs to assess the compatibility of the disinfecting chemical with the computer hardware to be cleaned/disinfected. Many chemical disinfectants require that the surface to be disinfected be exposed to the liquid disinfectant for 10 minutes. Such exposure could create an electrical or corrosive problem to certain pieces of computer hardware. In some circumstances, such as the computer keyboard, the problem of chemical damage to the keyboard components can be alleviated by the use of a thin plastic keyboard cover (aka skin), which can be liberally soaked with disinfectant without fear of compromising the computer.
Handwashing and Gloving
Microorganisms on the skin are generally divided into two categories. Resident flora are microbes that normally colonize or live on the skin of most individuals; they generally do not cause infections unless they are introduced into normally sterile body sites and/or unless the host is immunocompromised. In contrast, transient flora are microbes that are present on the skin for only a short time; they tend to be more pathogenic than the resident flora and are responsible for most nosocomial acquired infections.42 These transient or contaminant flora may be picked up by the hands of a health care worker; for example, when they touch a patient or any contaminated object, such as a computer component. Handwashing is a process which removes soil and transient microorganisms from the hands. Hence the simple process of handwashing has long been a mainstay of any control measure for reducing nosocomial infections.
Two basic types of soaps are available for handwashing: soaps that do contain an antimicrobial and soaps that do not. Because of concern about the emergence of resistance to antiseptics, antimicrobial soaps are generally not recommended for regular handwashing.43 However, there may be areas in a medical facility in which washing with antimicrobial soaps is preferred. Resident infection control personnel can advise about agents for hand hygiene, as well as on the specific handwashing procedure to use. Important factors include mechanical rubbing of the soap over all surfaces of the hands and an adequate period of rubbing to release the transient organisms.
In addition to soaps for handwashing, “waterless” agents are available. These alcohol rubs are presently being considered as a replacement for soap and water in the 2002 Guideline for Hand Hygiene of the CDC’s Healthcare Infection Control Practices Advisory Committee.44 It is important to realize that these agents are disinfectants and not cleaners. Therefore, any visible soil must first be removed before the alcohol will be completely effective. Also, it is recommended that after five or six consecutive uses, the hands be washed with soap and water to remove any build-up of agent.
A word of caution about gloves: gloves are not a substitute for handwashing. Generally, hands should be washed before gloves are donned; gloves should be picked up by the cuff to prevent contamination of the surface, which may touch a patient or clean object, and hands should be washed after gloves are removed.45,46 Gloves provide an extra amount of protection, and therefore may be used as an adjunct to handwashing, but not instead of handwashing. There can certainly be circumstances when gloves can be used to decrease the tranfer of microbes,47 but it is important to note that gloves alone, without an appropriate protocol for use, could potentially increase transfer, by giving the wearer a false sense of security. For example, washing one’s hands and putting on gloves prevent the wearer’s resident flora from touching the patient or computer and the patient or computer microbes from reaching the hands of the wearer. However, they do not prevent the wearer from tranferring microbes from the computer to the patient or visa versa, because the gloves can carry organisms from place to place or person to person as easily as the ungloved hands.
Practical Applications of Infection Control Principles in Medical Settings
Because each medical facility is somewhat different, the best infection control protocols needed for each piece of computer hardware or for any other inanimate object in the patient’s environment will vary with each situation. Hence, it would be impossible to provide specific protocols for all circumstances. However, applying the above basic infection control principles, one can address most computer-infection control situations, regardless of what computer component is involved or whether the computers are permanent room hardware or personal handheld devices. Furthermore, when introducing any piece of computer hardware into any medical situation, the following guidelines might be helpful.
Consult with the Infection Control Personnel at That Facility
The infection control personnel may be part of the performance improvement or risk management teams or have some other designation; however, all medical facilities should have some individual(s) dedicated to general infection control issues. There are several advantages to working with the local infection control staff. First, if a new piece of hardware is going into the patient’s area, staff will appreciate knowing this, because it constitutes a change in the patient’s environment. Hence should any changes in colonization rate or infection rate occur in the patients, the new elements of the environment could be immediately evaluated to see if they are a contributing factor. Secondly, the local infection control staff will know what the routine cleaning and disinfecting agents are as well as what the routine cleaning schedule is. From a practical point of view, if the hospital’s cleaning and disinfecting routines are appropriate for the new equipment, these accepted routines should be used rather than introducing a totally different protocol for one piece of equipment. Third, should special infection control-related protocols need to be established relative to the hardware, the infection control staff can advise the computer or technical services department as to how to monitor that the protocols are being correctly followed. Finally, the local staff are an excellent resource for the following two guidelines.
Determine the Risk Level of the Patients Served at Each Computer Hardware Location
Recognizing that microorganisms are ubiquitous and that most microbes are harmless to most people, it would be a waste of both time and money to impose more computer hardware infection control procedures than are needed to protect the patient population. On the other hand, being cognizant of the morbidity, mortality and costs of nosocomial infections, it is imperative that adequate infection control procedures are in place to protect high-risk patients (see Table 1▶). Hence, one needs to balance the infection control measures with the level of risk of the patients being served.
For example, in a clinic providing well-child check-ups and immunizations, infection control protocols could be minimal. A keyboard cover and perhaps a monitor cover, if the unit is in striking range of an examination table that might accommodate a male child without a diaper, would be a reasonable investment. The cleaning agents and cleaning frequency for these pieces of equipment could reasonably be the same as routinely used to clean something like the telephone in the same exam room. In contrast, in an intensive care unit or a facility serving immunocompromised patients, infection control measures would be much more rigorous. Keyboard covers should be disinfected more frequently than in the well-child clinic, and specific handwashing and perhaps gloving protocols, as recommended in past computer contamination studies (see Table 2▶),47 would be appropriate. Exactly how these issues are handled will depend in part on the following guideline.
Determine How that Piece of Computer Hardware is Being Used
The actual usage of the computer component also affects appropriate control measures. Do personnel go back and forth between the computer and the patient? Do staff enter a patient room simply to use the computer and then leave and go to another patient’s room? Does the piece of computer equipment move from room to room? In the case of the latter two questions, it is important to remember that anything in the patient’s environment, i.e. in the patient’s room, will probably be contaminated with microorganisms from that patient. In an intensive care unit situation, it is quite likely that the patient will be colonized by microorganisms that can cause nosocomial infections in other ICU patients. Therefore, anyone (such as a staff member) or anything (such as a portable computer) that contacts anything in the patient’s room should be considered to be contaminated and needs to be disinfected before leaving the room. For example, a staff person enters an ICU room, washes the hands, and dons gloves, according to hospital protocol; then the person enters data into the handheld computer and sets the device down in the patient’s room, retrieves the handheld computer, removes the gloves, and washes their hands before leaving the room. Because the handheld computer contacted a surface in the ICU patient’s room, it should be considered to be contaminated with the ICU patient’s flora, and it needs be recognized that the staff member, even though they followed all personnel protocols for handwashing, gloving etc upon entering and leaving the ICU, could still transfer microorganisms to the next ICU patient through his handheld computer device. Hence, if a piece of portable computer equipment comes in contact with any of the environment in a patient’s room, then that piece of equipment needs to be decontaminated before being brought into another ICU patient’s room. If the decontamination process for the piece of portable equipment is complicated, then consideration should be given to restricting the use of these portable devices in rooms of immunocompromised ICU patients.
Summary
Since the introduction of computers into the health care setting 50 years ago, there has been a growing recognition of the value of this technology in providing quality medical care. As the variety of computer devices has increased from PCs to various portable units and as the availability of software packages has grown from medical records programs to diagnostic aides, there has been an increased presence of computer hardware in all patient care venues from admissions and clinic areas to ICUs. Only recently have studies begun to investigate whether these computer devices can serve as fomites for the harboring and transfer of microorganisms involved in nosocomial colonizations or infections in hospitalized patients. Considering the long periods of time that some microorganisms can survive on plastic surfaces and the fact that microbes can be readily transferred from inanimate surfaces to hands and visa versa, it is not surprising that frequently-touched computer keyboards have been implicated in nosocomial colonizations and infections in various patient populations (see Table 2▶).
Control measures are quite simple and can include engineering modifications, such as the use of keyboard covers, cleaning and disinfection of appropriate computer hardware surfaces, and handwashing with or without gloving of pertinent personnel (see Figure 1▶). When these control practices are used and to what extent they are utilized will depend upon a balance between the amount of risk to the patient population being served (see Table 1▶) and the practical feasibility (time and cost) of the measures being considered. As expected, different medical facilities have instituted various levels of infection control relative to computer equipment (see Table 2▶). In general, the medical facility’s resident infection control staff can advise as to that facility’s routine control practices for medical devices. Working with this staff, specifics for the microbiologically safe use of individual computer hardware in a variety of medical settings can be determined. Observance of these simple control procedures can potentially decrease morbidity and mortality for patients and reduce medical care costs for hospitals and care giving organizations.
References
- 1.Collen MF. A history of medical informatics in the United States, 1950–1990. American Medical Informatics Association, Washington, DC, 1995.
- 2.Mitchell E, Sullivan F. A descriptive feast but an evaluative famine: Systematic review of published articles on primary care computing during 1980-97. Br Med J. 2001;322:279–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradshaw KE, Gardner RM, Clemmer TP, et al. Physician decision-making—evaluation of data used in a computerized ICU. Int J Clin Monit Comput. 1984;1(2):81–91. [DOI] [PubMed] [Google Scholar]
- 4.Wilson D, Nelson NC, Rosebrock BJ, et al. Using an integrated point of care system: A nursing perspective. Top Health Inf Manage. 1994;14(4):24–9. [PubMed] [Google Scholar]
- 5.Collen MF. General requirements for a medical information system (MIS). Comput Biomed Res. 1970;3(5):393–406. [DOI] [PubMed] [Google Scholar]
- 6.Dick RS, Steen EB (eds) The computer-based patient record: an essential technology for health care. Committee on Improving the Patient Record, Institute of Medicine, National Academy Press, Washington, DC, 1991.
- 7.Halpern NA, Burnett G, Morgan S, et al. Remote communication from a mobile terminal: an adjunct for a computerized intensive care unit order management system. Crit Care Med. 1995;23(12):2054–7. [DOI] [PubMed] [Google Scholar]
- 8.Tshopp M, Geissbuhler A. Use of handheld computers as bedside information providers. Medinfo. 2001;10(Pt 1):764–7. [PubMed] [Google Scholar]
- 9.Criswell DF, Parchman ML. Handheld computer use in U. S. family practice residency programs. J Am Med Inform Assoc. 2002;9(1):80–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson MM, Tweeten SM. General principles of epidemiology. In Pfeiffer JA (ed): APIC Text of Infection Control and Epidemiology, vol 1. Washington, DC, Association for Professionals in Infection Control and Epidemiology, 2000, pp 17–7.
- 11.McCormick R. Intensive Care Unit. In Pfeiffer JA (ed): APIC Text of Infection Control and Epidemiology, vol 1. Washington, DC: Association for Professionals in Infection Control and Epidemiology, 2000, pp 45–2.
- 12.McGowan JE Jr, Metchoch BG. Infection control epidemiology and clinical microbiology. In Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH (eds): Manual of Clinical Microbiology, 7th ed. Washington, DC, ASM Press; 1999, pp 107–8.
- 13.Centers for Disease Control and Prevention. Public health focus: surveillance, prevention, and control of nosocomial infections. MMWR. 1992;41:783–7. [PubMed] [Google Scholar]
- 14.Maki DG. Nosocomial infection in the intensive care unit. In Parrillo JE, Bone RC (eds): Critical Care Medicine: Principles of Diagnosis and Management. St. Louis, Mosby, 1995, pp 893–954.
- 15.Martone WJ, Jarvis WR, Culver DH, Haley RW. Incidence and nature of endemic and epidemic nosocomial infections. In Bennett JV, Brachman PS (eds): Hospital Infections. Boston, Little, Brown, 1992, pp 577–97.
- 16.DiGiovine B, Chenoweth C, Watts C, Higgins M. The attributable mortality and costs of primary nosocomial bloodstream infections in the intensive care unit. Am J Respir Crit Care Med. 1999;160:976–81. [DOI] [PubMed] [Google Scholar]
- 17.Boyce JM, Opal SM, Chow JW, et al. Outbreak of multidrug-resistant Enterococcus faecium with transferable vanB class vancomycin resistance. J Clin Microbiol. 1994;32:1148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porwancher R, Sheth A, Remphrey S, et al. Epidemiological study of hospital-acquired infection with vancomycin-resistant Enterococcus faecium: Possible transmission by an electronic ear-probe thermometer. Infect Control Hosp Epidemiol. 1997;18:771–4. [DOI] [PubMed] [Google Scholar]
- 19.Sorin M, Segal-Maurer S, Mariano N, et al. Nosocomial transmission of imipenem-resistant Pseudomonas aeruginosa following bronchoscopy associated with improper connection to the Steris System 1 processor. Infect Control Hosp Epidemiol. 2001;22:409–13. [DOI] [PubMed] [Google Scholar]
- 20.Datz C, Jungwirth A, Dusch H, et al. What’s on a doctors’ ball point pens? Lancet. 1997;350:1824. [DOI] [PubMed] [Google Scholar]
- 21.Slaughter S, Hayden MK, Nathan N, et al. A comparison of the effect of universal use of gloves and gowns with that of glove use alone on acquisition of vancomycin-resistant enterococci in a medical intensive care unit. Ann Intern Med. 1996; 125:448–56. [DOI] [PubMed] [Google Scholar]
- 22.Weber DJ, Rutala WA, Blanchet CN, et al. Faucet aerators: A source of patient colonization with Stenotrophomonas maltophilia. Am J Infect Control. 1999;27:59–63. [DOI] [PubMed] [Google Scholar]
- 23.Berrouane YF, McNutt L, Buschelman BJ, et al. Outbreak of severe Pseudomonas aeruginosa infections caused by a contaminated drain in a whirlpool bathtub. Clin Infect Dis. 2000; 31:1331–7. [DOI] [PubMed] [Google Scholar]
- 24.Vochem M, Vogt M, Doring G. Sepsis in a newborn due to Pseudomonas aeruginosa from a contaminated tub bath. N Engl J Med. 2001;345:378–9. [DOI] [PubMed] [Google Scholar]
- 25.Masterton RG, Coia JE, Notman AW, et al. Refractory methicillin-resistant Staphylococcus aureus carriage associated with contamination of the home environment. J Hosp Infect. 1995;29:318–9. [DOI] [PubMed] [Google Scholar]
- 26.Isaacs D, Daley A, Dalton D, et al. Swabbing computers in search of nosocomial bacteria. Ped Infect Dis J. 1998;17:533. [DOI] [PubMed] [Google Scholar]
- 27.Neely AN, Maley MP, Warden GD. Computer keyboards as reservoirs for Acinetobacter baumannii in a burn hospital. Clin Infect Dis. 1999;29:1358–60. [DOI] [PubMed] [Google Scholar]
- 28.Bures S, Fishbain JT, Uyehara CFT, et al. Computer keyboards and faucet handles as reservoirs of nosocomial pathogens in the intensive care unit. Am J Infect Control. 2000;28:465–70. [DOI] [PubMed] [Google Scholar]
- 29.Devine J, Cooke RPD, Wright EP. Is methicillin-resistant Staphylococcus aureus (MRSA) contamination of ward-based computer terminals a surrogate marker for nosocomial MRSA transmission and handwashing compliance? J Hosp Infect. 2001;48:72–5. [DOI] [PubMed] [Google Scholar]
- 30.Ivey JC, Oomen B, Forstall G. Fungal contamination related to personal computer devices installed in a hospital intensive care unit. Am Society for Microbiol Abstrs. 2001; L-1, p 469.
- 31.Dickgiesser N, Ludwig C. Examinations on the behaviour of gram positive and gram negative bacteria on aluminum foil. Zentbl Bakteriol Hyg Abt I Orig B. 1979;168:493–506. [PubMed] [Google Scholar]
- 32.Watson SP, Clements MO, Foster SJ. Characterization of the starvation-survival response of Staphylococcus aureus. J Bacteriol. 1998;180:1750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol. 2000;38:724–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neely AN. A survey of gram-negative bacteria survival on hospital fabrics and plastics. J Burn Care Rehabil. 2000; 21:523–7. [DOI] [PubMed] [Google Scholar]
- 35.Neely AN, Orloff MM. Survival of some medically important fungi on hospital fabrics and plastics. J Clin Microbiol. 2001; 39:3360–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rangel-Frausto MS, Houston AK, Bale MJ, et al. An experimental model for study of Candida suvival and transmission in human volunteers. Eur J Clin Microbiol Infect Dis. 1994; 13:590–5. [DOI] [PubMed] [Google Scholar]
- 37.Noskin GA, Bednarz P, Suriano T, et al. Persistent contamination of fabric-covered furniture by vancomycin-resistant enterococci: Implications for upholstery selection in hospitals. Am J Infect Control. 2000;28:311–3. [DOI] [PubMed] [Google Scholar]
- 38.Trick WE, Kioski CM, Howard KM, et al. Outbreak of Pseudomonas aeruginosa ventriculitis among patients in a neurosurgical intensive care unit. Infect Control Hosp Epidemiol. 2000;21:204–8. [DOI] [PubMed] [Google Scholar]
- 39.McNeil SA, Nordstrom-Lerner L, Malani PN, et al. Outbreak of sternal surgical site infections due to Pseudomonas aeruginosa traced to a scrub nurse with onychomycosis. Clin Infect Dis. 2001;33:317–23. [DOI] [PubMed] [Google Scholar]
- 40.Vyhlidal SA. Central services. In Pfeiffer JA (ed): APIC Text of Infection Control and Epidemiology, vol 1. Washington, DC, Association for Professionals in Infection Control and Epidemiology, 2000, pp 54A-3–54A-4.
- 41.Rutala WA. APIC guideline for selection and use of disinfectants. Am J Infect Control. 1996;24:313–342. [DOI] [PubMed] [Google Scholar]
- 42.Pittet D, Boyce JM. Hand hygiene and patient care: Pursuing the Semmelweis legacy. Lancet Infect Dis. 2001;9–20.
- 43.Larson E. Hygiene of the skin: When is clean too clean? Emerging Infect Dis. 2001;7:225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pugliese G, Favero MS (ed). Medical News Section. Alcohol rubs: CDC’s new hand-hygiene guidelines. Infect Control Hosp Epidemiol 2001;22:56–7. [Google Scholar]
- 45.Hannigan P, Shields JW. Handwashing and use of examination gloves. Lancet. 1998;351:571. [DOI] [PubMed] [Google Scholar]
- 46.Maley MP. Compliance with hand washing. Infect Control Hosp Epidemiol. 2000;21:4. [DOI] [PubMed] [Google Scholar]
- 47.Neely AN, Maley MP. Dealing with contaminated computer keyboards and microbial survival. Am J Infect Control 2001;29:131–2. [DOI] [PubMed] [Google Scholar]