Abstract
Silibinin is a natural compound isolated from milk thistle seed extracts, and has traditionally been used as a hepatoprotectant. A number of studies have also established the cancer therapeutic and chemopreventive role of silibinin in both in vitro and in vivo models. The low density lipoprotein receptor-related protein-6 (LRP6) is an essential Wnt co-receptor for the Wnt/β-catenin pathway and represents a promising target for cancer prevention and therapy. In the present study, we found that silibinin was able to repress endogenous LRP6 expression and block Wnt3A-induced LRP6 phosphorylation and Wnt/β-catenin signaling activation in HEK293 cells. Importantly, silibinin was also able to suppress endogenous LRP6 expression and phosphorylation and block Wnt/β-catenin signaling in prostate cancer PC-3 and DU-145 cells and breast cancer MDA-MB-231 and T-47D cells. Mechanistically, silibinin inhibited LRP6 promoter activity and decreased LRP6 mRNA levels in prostate and breast cancer cells. Finally, we demonstrated that silibinin displayed anticancer activity with IC50 values comparable to those shown to suppress LRP6 expression and Wnt/β-catenin signaling activities in prostate and breast cancer cells. Our data indicate that silibinin is a novel small molecule Wnt/β-catenin signaling inhibitor by suppressing Wnt co-receptor LRP6 expression at the transcription level, and that the anti-cancer activity of silibinin is associated with its inhibitory effect on Wnt/LRP6 signaling.
Keywords: Wnt signaling, LRP6, β-catenin, silibinin, cancer, drug discovery
1. Introduction
The canonical Wnt/β-catenin signaling pathway is a critical pathway that regulates cell proliferation, migration, and differentiation, thus making it a powerful regulator of embryonic development and tumorigenesis [1–3]. Wnt proteins, which are secreted glycoproteins, bind to the low-density lipoprotein receptor-related protein5/6 (LRP5/6) and Frizzled (Fzd) to activate Wnt/β-catenin signaling [1–3]. In the absence of Wnts, β-catenin is sequestered in a complex that consists of the adenomatous polyposis coli (APC) tumor suppressor, Axin, glycogen synthase kinase-3β (GSK3β), and casein kinase 1 (CK1). This complex formation induces the phosphorylation of β-catenin by CK1 and GSK3β, which results in the ubiquitination and the subsequent degradation of β-catenin by the 26S proteasome. The action of this complex is inhibited upon binding of Wnt to its cell-surface receptors Fzd and LRP. The LRP-Wnt-Fzd binding results in stabilization of cytosolic β-catenin, which then translocates into the nucleus where it interacts with T-cell factor/lymphoid enhancing factor (TCF/LEF) to induce the expression of downstream target genes that regulate cell cycle, growth, and progression [1–3].
Wnt proteins can activate the canonical Wnt/β-catenin signaling pathway only in the presence of the Wnt co-receptor LRP5 or LRP6 [3]. LRP5/6 appears to transduce Wnt/β-catenin signal by binding and recruiting Axin to the cell membrane. Recruitment of Axin by Wnt binding to LRP5 was shown to activate the canonical Wnt signaling pathway by inducing the degradation of Axin, which is critical for β-catenin phosphorylation and degradation. It has been demonstrated that a PPSPXS motif, which is reiterated five times in the LRP6 intracellular domain and is conserved among LRP5, LRP6, and their Drosophila homolog, Arrow, is sequentially phosphorylated by GSK3β and CK1 upon Wnt stimulation. Phosphorylation of the PPSPXS motif provides a docking site for Axin binding [3].
Silibinin is a major component of silymarin, which is the most popular botanical medicine consumed by patients with hepatitis C [4]. Silibinin has also been shown to exert significant anti-neoplastic effects in a variety of in vitro and in vivo cancer models, including skin, breast, lung, colon, bladder, prostate and kidney carcinomas, and is currently being evaluated clinically for these pathological conditions [4–7]. Importantly, recent studies have demonstrated that the chemopreventive and chemotherapeutic effects of silibinin are associated with its activity against Wnt/β-catenin signaling [8–16]. It has been reported that silibinin is able to suppress Wnt/β-catenin signaling in hepatic cancer cells [8], melanoma cells [16], prostate cancer cells [15], and colorectal cancer cells [10] in vitro. Furthermore, silibinin also displayed inhibitory effects on Wnt/β-catenin signaling in small intestinal polyps of APCmin/+ mice [9, 11], azoxymethane-induced colonic tumors in A/J mice [12] and 1,2-dimethylhydrazine-induced colon cancer in Wistar rats [13]. Together, these studies indicate that silibinin is a potential Wnt/β-catenin signaling inhibitor. However, the exact mechanism underlying silibinin-induced inhibition of Wnt/β-catenin signaling was unclear. In the current study, we demonstrated for the first time that silibinin is a novel Wnt/β-catenin signaling inhibitor by suppressing Wnt co-receptor LRP6 expression at the transcription level.
2. Materials and Methods
2.1. Materials
Silibinin was purchased from Sigma. Plasmid pCS-Myc-hLRP6 containing the full-length human LRP6 cDNA was provided by Dr. Christof Niehrs (Deutsches Krebsforschungszentrum, Heidelberg, Germany), and plasmid pGST-E-cadherin was provided by Dr. Gail Johnson (University of Rochester). LRP6 and actin promoter reporter constructs were purchased from SwitchGear Genomics. The TOPFlash luciferase construct was from Upstate Biotechnology. A β-galactosidase-expressing vector was from Promega. Polyclonal anti-LRP6 was from Santa Cruz Biotechnology. Monoclonal anti-phosphorylated-LRP6 and anti-Axin2 were purchased from Cell Signaling Technology. Monoclonal anti-β-catenin was from BD Biosciences. Monoclonal anti-actin was from Sigma. Peroxidase labeled anti-mouse antibody and ECL system were purchased from Amersham Life Science. The luciferase and β-galactosidase assay systems were from Promega. Tissue culture media, fetal bovine serum (FBS), and plastic-ware were obtained from Life Technologies, Inc. Proteinase inhibitor cocktail Complete™ was obtained from Boehringer Mannheim.
2.2. Cell culture and conditioned media
All cell lines were obtained from ATCC and grown under standard cell culture conditions at 37°C in a humidified atmosphere with 5% CO2. Human fibrosarcoma cancer HT1080 cells stably transfected with HA-tagged LRP6 or empty pLNCX2 vector have been described before [17]. The prostate cancer PC-3 and DU145 cells were cultured in RPMI-1640 medium containing 10% FBS, 2 mM of L-glutamine, 100 units/ml of penicillin, and 100 µg/ml of streptomycin. HEK293 cells, HT1080 cells and breast cancer MDA-MB-231 and T-47D cells were cultured in DMEM medium containing 10% of FBS, 2 mM of L-glutamine, 100 units/ml of penicillin, and 100 µg/ml of streptomycin. Wnt3A-conditioned medium (CM) and L cell control CM were prepared according to manufacturer’s specifications.
2.3. Luciferase reporter assay for Wnt/β-catenin signaling
Cells were plated into 24-well plates. After overnight culture, the cells were transiently transfected with the TOPFlash luciferase construct and β-galactosidase-expressing vector. After 24 h incubation, cells were treated with Wnt3A CM and silibinin. Cells were then lysed 24 h later and both luciferase and β-galactosidase activities were determined. The luciferase activity was normalized to the β-galactosidase activity.
2.4. Luciferase reporter assay for LRP6 promoter activity
Cells were plated into 24-well plates. After overnight culture, the cells were transiently transfected with the LRP6 promoter reporter construct or the actin promoter reporter plasmid. After 24 h incubation, cells were treated with silibinin. Cells were then lysed 24 h later and the luciferase activities were determined. The LRP6 promoter luciferase activity was normalized to the actin promoter luciferase activity.
2.5. Western blotting
Cells in 6-well plates were lysed in 0.5 ml of lysis buffer (phosphate-buffered saline containing 1% Triton X-100 and 1 mM PMSF) at 4°C for 10 min. Equal quantities of protein were subjected to SDS-PAGE under reducing conditions. Following transfer to immobilon-P transfer membrane, successive incubations with a primary antibody, and a horseradish peroxidase-conjugated secondary antibody were carried out for 60–120 min at room temperature. The immunoreactive proteins were then detected using the ECL system. Films showing immunoreactive bands were scanned by Hp Scanjet 5590.
2.6. Cytosolic free β-catenin analysis with GST-E-cadherin binding assay
The GST-E-cadherin binding assay was carried out as previously described [18]. Uncomplexed cytosolic free β-catenin present in 100 µg of total cell lysate was subjected to SDS-PAGE and detected using the monoclonal antibody to β-catenin.
2.7. RT-PCR
Total RNA was isolated from cell cultures using RNA-Bee (Tel-Test), and first-strand cDNA synthesis was performed using ProSTARTM Ultro HF RT-PCR Kit (Strategene) primed with oligo(dT) primer in a 20 µl reaction mixture containing 1 µg total RNA. For analysis of LRP6 mRNA levels, RT-PCR was performed with the specific LRP6 and GAPDH primers as previously described [17]. The PCR product was loaded onto a 1.2% Agarose gel and stained with ethidium bromide.
2.8. Cell proliferation assay
Cells were seeded into 96-well tissue culture treated microtiter plates at a density of 5000 cells/well. RPMI-1640 containing 1.5% FBS was used as assay media for PC-3 and DU-145 cells, while DMEM containing 1.5% FBS was used as assay media for MDA-MB-231 and T-47D cells. After 24h incubation, the cells were treated with silibinin at indicated concentrations for 72 h. Cell viability was measured by the Cell Titer Glo Assay, which is a luminescent assay that is an indicator of live cells as a function of metabolic activity and ATP content.
2.9. Statistics
Statistical analyses were performed using Student's unpaired t-test. Data were presented as mean ± SD
3. Results
3.1. Silibinin blocks Wnt/β-catenin signaling induced by Wnt3A in HEK293 cells
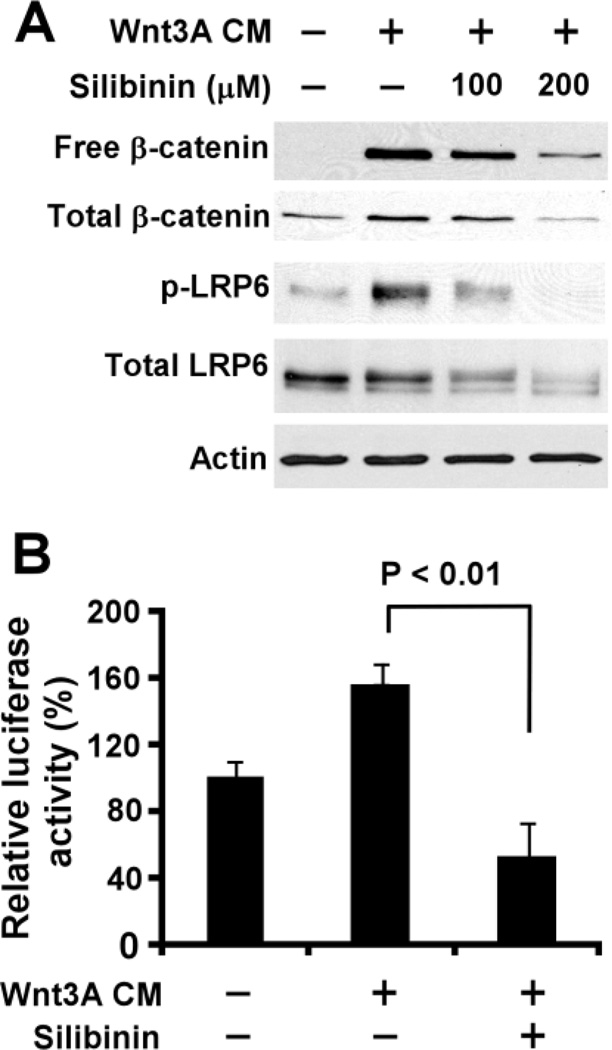
Uncomplexed cytosolic β-catenin (free β-catenin) is the active form of β-catenin that translocates to the cell nucleus to activate the transcription factors of the TCF/LEF family, leading to the transcription of Wnt target genes. To investigate the inhibitory effect of silibinin on Wnt/β-catenin signaling, we examined the level of cytosolic free β-catenin after silibinin treatment. As expected, Wnt3A CM treatment resulted in an increase of the cytosolic free β-catenin level in HEK293 cells. Notably, the increased level of cytosolic free β-catenin induced by Wnt3A was significantly reduced after silibinin treatment (Fig. 1A).
Fig.1.
Silibinin suppresses LRP6 expression and phosphorylation and inhibits Wnt3A-induced Wnt/β-catenin signaling in HEK293 cells. (A) HEK293 cells were treated with Wnt3A CM (20%) and silibinin at the indicated concentrations for 24 h. The levels of cytosolic free β-catenin and total cellular β-catenin, LRP6 and phospho-LRP6 (p-LRP6) were examined. All the samples were also probed with anti-actin antibody to verify equal loading. (B) HEK293 cells in 24-well plates were transiently transfected with TOPFlash construct and β-galactosidase-expressing vector in each well. After being incubated for 24 h, cells were treated with Wnt3A CM (5%) or silibinin (200 µM) plus Wnt3A CM (5%) for 24 h. The luciferase activity was then measured and normalized to the activity of the β-galactosidase.
To confirm the inhibitory effect of silibinin on Wnt/β-catenin signaling, we performed a Wnt/β-catenin signaling reporter assay to test whether silibinin is able to inhibit Wnt/β-catenin signaling in HEK293 cells. As expected, the increased TOPFlash activity induced by Wnt3A in HEK293 cells was blocked by silibinin (Fig. 1B).
3.2. Silibinin attenuates Wnt/β-catenin signaling in prostate cancer cells
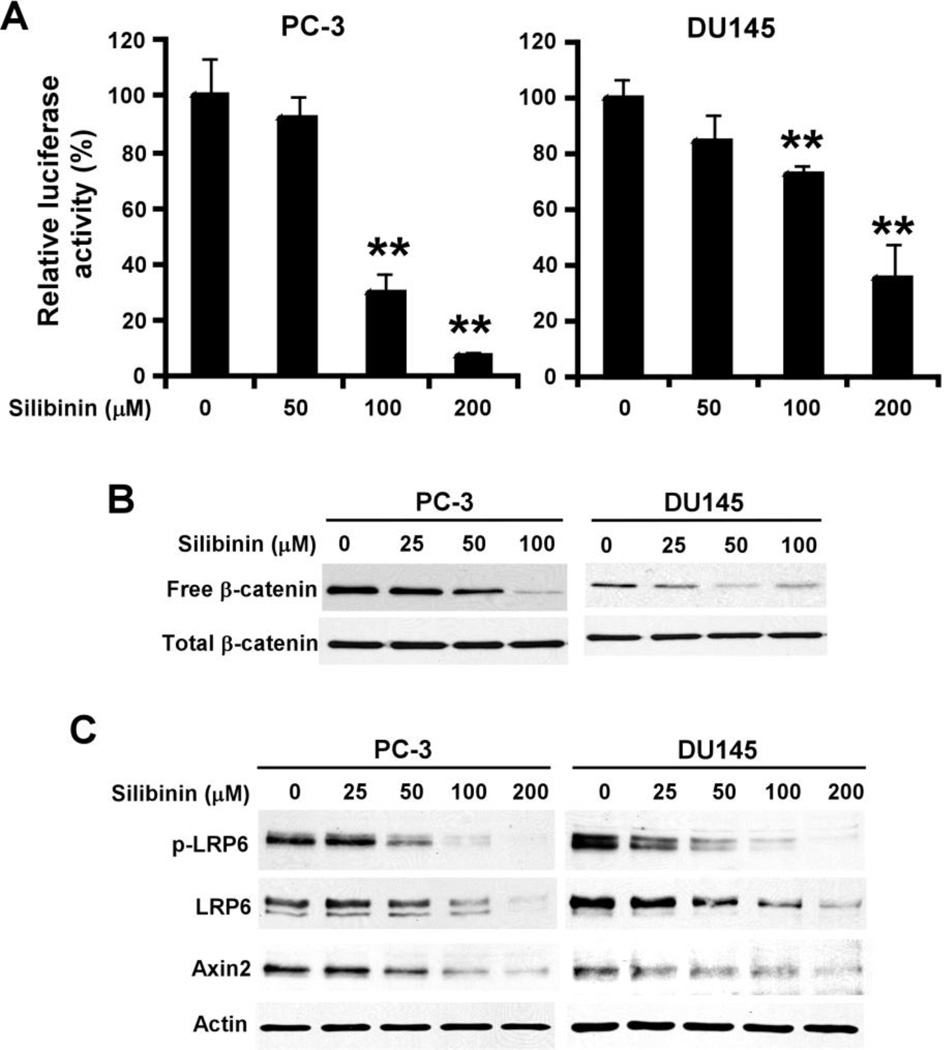
In our previous study, we demonstrated that the androgen-independent PC-3 and DU145 cells exhibit high levels of Wnt/β-catenin signaling and are sensitive to the treatment of the Wnt/β-catenin signaling inhibitors [19]. Thus, we selected PC-3 and DU145 cells to test whether silibinin attenuates Wnt/β-catenin signaling in Wnt-dependent cancer cells. When PC-3 and DU145 cells were transiently transfected with the Wnt/β-catenin signaling reporter TOPFlash, the TOPFLash luciferase activity was significantly decreased after silibinin treatment in prostate cancer cells (Fig. 2A). As expected, silibinin treatment also resulted in significant decreases of the cytosolic free β-catenin levels in PC-3 and DU145 cells (Fig. 2B).
Fig. 2.
Silibinin inhibits Wnt/β-catenin signaling in prostate cancer cells. (A) Prostate cancer PC-3 and DU145 cells were transiently transfected with TOPFlash construct and β-galactosidase-expressing vector. After being incubated for 24 h, cells were treated with silibinin for 24 h. The luciferase activity was then measured and normalized to the activity of the β-galactosidase. All the values are the average of triplicate determinations. **P < 0.01 versus corresponding control value. (B) Prostate cancer PC-3 and DU145 cells were treated with silibinin for 24 h. The levels of cytosolic free β-catenin and total cellular β-catenin were examined. (C) Prostate cancer PC-3 and DU145 cells were treated with silibinin at the indicated concentrations for 24 h. The levels of LRP6, phospho-LRP6 (p-LRP6) and Axin 2 were examined. All the samples were also probed with anti-actin antibody to verify equal loading.
Axin2 is a key transcriptional target of Wnt/β-catenin signaling. The expression level of Axin2 is the signature of the activation of Wnt/β-catenin signaling. As expected, silibinin treatment greatly reduced the expression of Axin2 in both PC-3 and DU145 cells (Fig. 2C).
3.3. Silibinin suppresses endogenous LRP6 expression and phosphorylation in HEK293 cells and prostate cancer cells
LRP6 is an essential Wnt co-receptor for the Wnt/β-catenin signaling pathway, and LRP6 phosphorylation is critical for Wnt/β-catenin signaling activation induced by Wnt proteins [3]. To explore the molecular mechanism underlying Wnt/β-catenin signaling inhibition by silibinin, we examined LRP6 expression and phosphorylation after silibinin treatment. As seen in Fig. 1A, treatment of Wnt3A CM markedly induced endogenous LRP6 phosphorylation in HEK293 cells, which was abolished by silibinin treatment. Moreover, the total cellular level of endogenous LRP6 was also greatly decreased (Fig. 1A).
Accumulating evidence indicates that activation of Wnt/β-catenin signaling in several types of cancers including prostate cancer is due to up-regulation of Wnt proteins and their receptors and/or down-regulation of secreted antagonists of the Wnt/β-catenin signaling pathway [20–26]. To that effect, we then tested the effects of silibinin on LRP6 in Wnt-dependent prostate cancer cells. As seen in Fig. 2C, silibinin treatment reduced the endogenous LRP6 phosphorylation and expression in a concentration dependent manner in both PC-3 and DU145 cells. These results suggest that silibinin targets LRP6 to inhibit Wnt/β-catenin signaling in prostate cancer cells.
3.4. Silibinin suppresses endogenous LRP6 expression and phosphorylation and blocks Wnt/β-catenin signaling in breast cancer cells
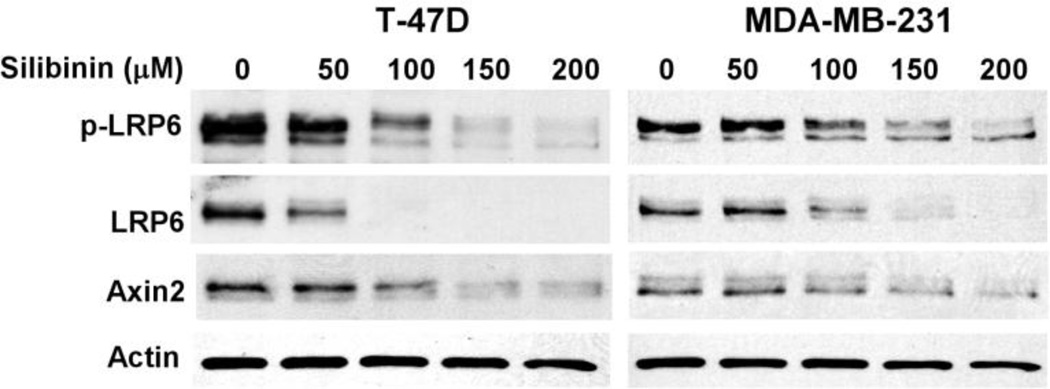
LRP6 plays an important role in breast cancer development and progression, and is a promising therapeutic target for triple negative breast cancer (TNBC) [23, 25, 26]. Hence, we tested the effects of silibinin on LRP6 in breast cancer cells. As seen in Fig.3, silibinin treatment reduced the levels of endogenous LRP6 phosphorylation and expression and specific Wnt/β-catenin signaling target Axin2 expression in a concentration dependent manner in both T-47D and MDA-MB-231. These results indicate that silibinin also targets LRP6 to inhibit Wnt/β-catenin signaling in breast cancer cells.
Fig. 3.
Silibinin suppresses LRP6 expression and Wnt/β-catenin signaling in breast cancer cells. MDA-MB-231and T-47D cells were treated with silibinin at the indicated concentrations for 24 h. The levels of LRP6, phospho-LRP6 (p-LRP6) and Axin 2 were examined. All the samples were also probed with anti-actin antibody to verify equal loading.
3.5. Silibinin suppresses LRP6 expression at the transcription level
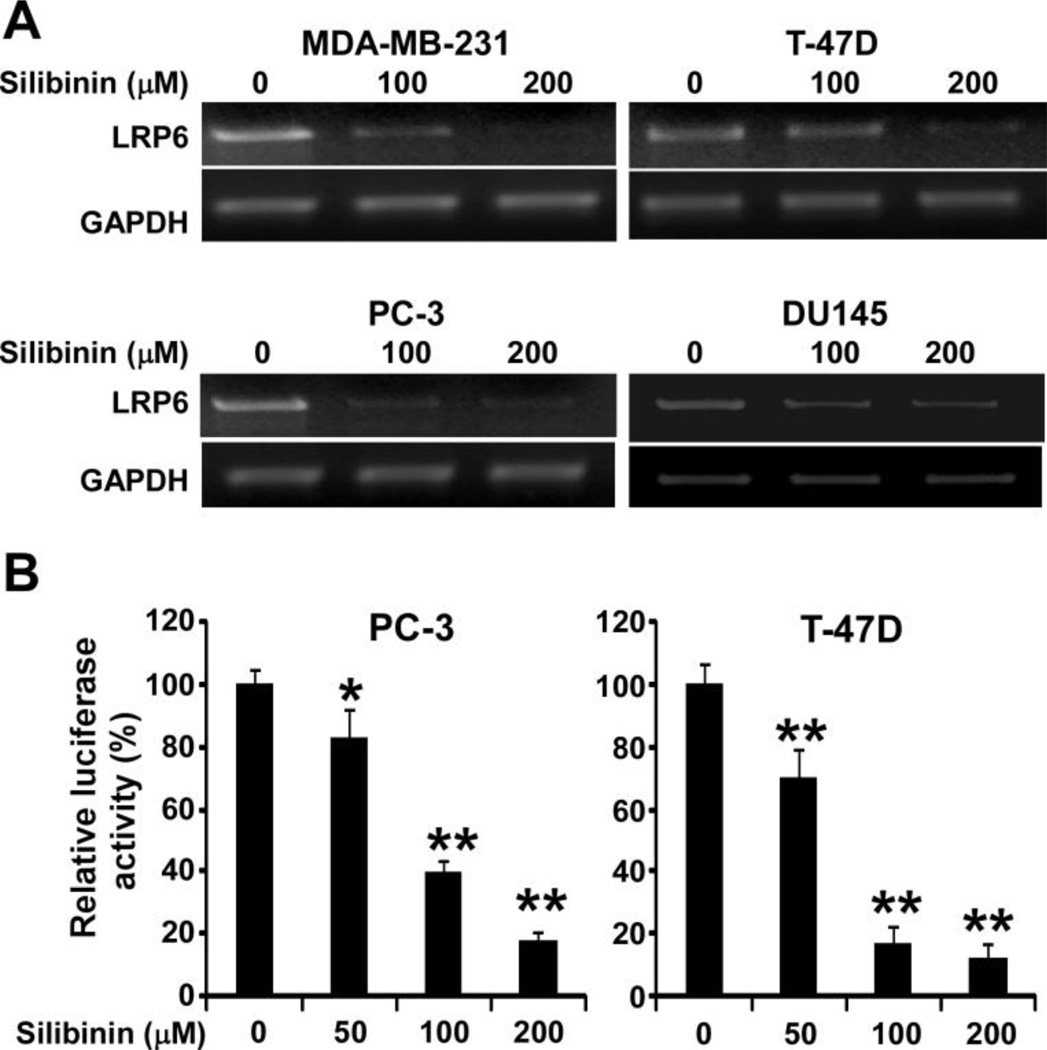
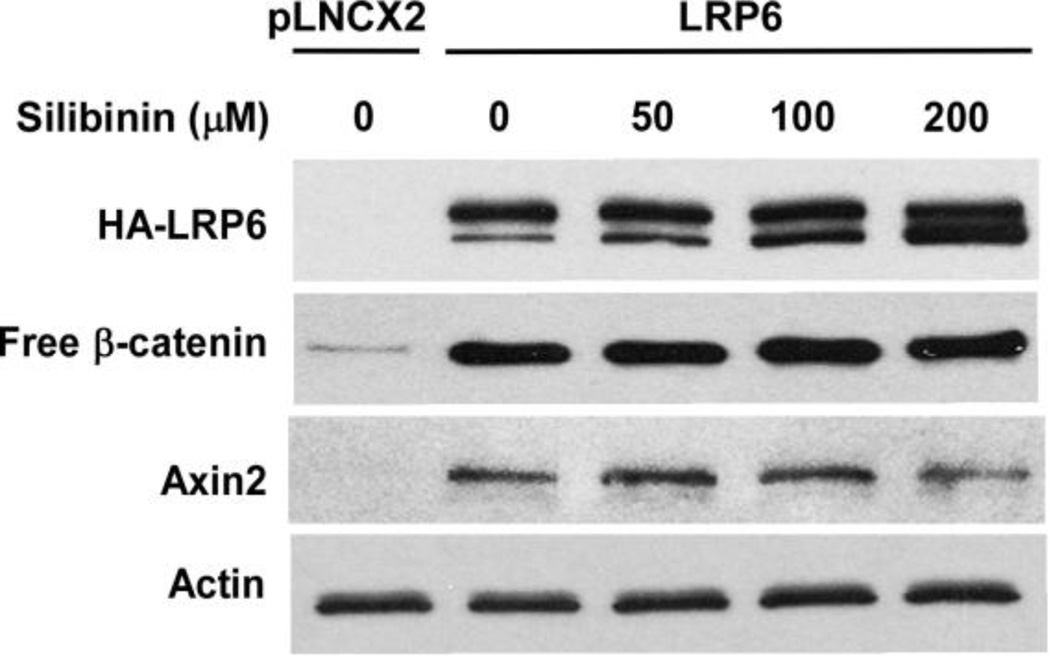
To explore the mechanism underlying silibinin-induced LRP6 down-regulation, we used semiquantitative RT-PCR techniques to determine the LRP6 transcript level in human cancer cells. As seen in Fig. 4A, silibinin negatively regulated LRP6 at the mRNA level in all four cancer cell lines tested. In addition, silibinin treatment inhibited the activity of the LRP6 promoter in a concentration dependent manner in both PC-3 and T-47D cells (Fig. 4B). Moreover, silibinin had no effect on enforced HA-LRP6 expression driven by CMV promoter in human fibrosarcoma cancer HT1080 cells (Fig. 5). In addition, silibinin was unable to block HA-LRP6-induced cytosolic free β-catenin elevation and Axin2 expression in HT1080 cells (Fig. 5). Together, these results indicate that silibinin regulates endogenous LRP6 expression at the transcription level and has no significant effect on LRP6 protein translation and degradation.
Fig. 4.
Silibinin suppresses LRP6 expression at the transcription level. (A) Cancer cells were treated with silibinin at the indicated concentrations for 24. Total RNA was extracted from the indicated cell lines, and RT-PCR was performed with primers for LRP6 and GAPDH. (B) Prostate cancer PC-3 cells and breast cancer T-47D cells were transiently transfected with the LRP6 promoter plasmid, or the actin promoter reporter plasmid. After being incubated for 24 h, cells were treated with silibinin at the indicated concentrations. The luciferase activities were then measured 24 h later, and the LRP6 promoter luciferase activity was normalized to the actin promoter luciferase activity. All the values are the average of triplicate determinations. *P < 0.05, **P < 0.01 versus corresponding control value.
Fig. 5.
Silibinin has no effect on enforced LRP6 expression. HT1080 cells stably transfected with HA-tagged LRP6 or empty pLNCX2 vector in 6-well plates were treated with silibinin at the indicated concentrations. Twenty-four hours later, the levels of HA-LRP6, Axin2 and cytosolic free β-catenin were examined by Western blotting. Samples were also probed with the anti-actin antibody to verify equal loading.
3.6. Inhibitory effect of silibinin on LRP/β-catenin signaling is involved in its anti-cancer activity
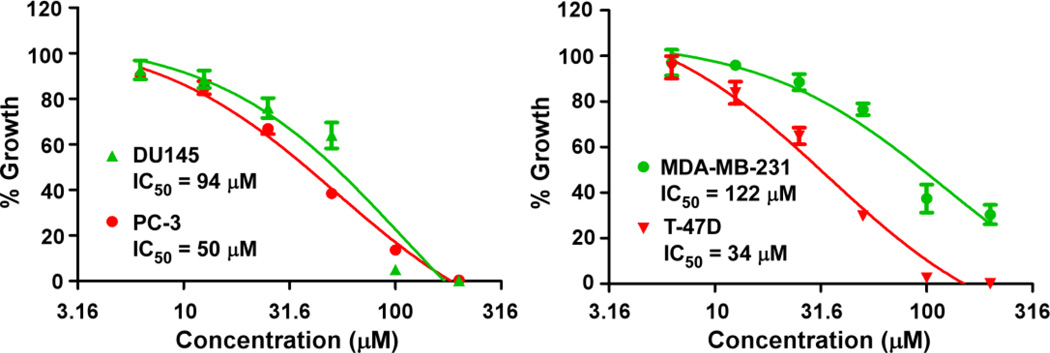
Given that silibinin can suppress LRP6 expression and block Wnt/β-catenin signaling in cancer cell, we then examined the effect of silibinin on cancer cell proliferation. As shown in Fig. 6, silibinin inhibited cancer cell proliferation with IC50 values 34–122 µM for the four tested cell lines. The IC50 values are comparable to those shown to suppress the activities of LRP6 expression and Wnt/β-catenin signaling in prostate PC-3 and DU145 and breast MDA-MB-231 and T-47D cancer cells. Furthermore, we found that HA-LRP6 transduced HT1080 cells were less sensitive to silibinin treatment than the corresponding pLNCX2 HT1080 cells. The IC50 value for HA-LRP6 HT1080 cells was 96 ± 2 µM (mean ± SD, n = 3), while IC50 value for the pLNCX2 HT1080 cells was 82 ± 1 µM (p < 0.01).
Fig. 6.
Silibinin inhibited prostate and breast cancer cell proliferation. Prostate cancer PC-3 and DU145 cells and breast cancer MDA-MB-231 and T-47D cells in 96-well plates were treated with silibinin for 72 h. Cell viability was measured by the Cell Titer Glo Assay system. All the values are the average of triplicate determinations.
Discussion
LRP6 is an essential co-receptor for the Wnt/β-catenin signaling pathway and is subjected to modulation by various secreted proteins. It appears that inappropriate expression of the Wnt proteins, Wnt receptors and Wnt signaling antagonists leads to aberrant activation of this pathway in many types of cancer including breast and prostate cancer [20–26]. The results present herein clearly demonstrate that silibinin is a novel small-molecule Wnt/β-catenin inhibitor that modulates Wnt/β-catenin signaling at the cell surface by suppressing Wnt co-receptor LRP6 expression at the transcription level. First, silibinin blocks Wnt3A-induced Wnt/β-catenin signaling activation in HEK293 cells; second, silibinin suppresses endogenous LRP6 expression and blocks Wnt3A-induced LRP6 phosphorylation in HEK293 cells; third, silibinin blocks Wnt/β-catenin signaling and reduces the levels of endogenous LRP6 expression and phosphorylation in prostate cancer PC-3 and DU-145 cells and breast cancer MDA-MB-231 and T-47D cells; and fourth, silibinin inhibits the LRP6 promoter activity and decreases the LRP6 mRNA level in prostate and breast cancer cells.
Wnt/β-catenin signaling has been implicated in different stages of mammary gland development and is important for mammary oncogenesis [23, 25, 26]. Initially, it was reported that Wnt/β-catenin signaling activation, as defined by β-catenin nuclear expression and overexpression of the Wnt/β-catenin target cyclin D1, was associated with a poorer prognosis in breast cancer patients [27]. Recent studies have also demonstrated that Wnt/β-catenin signaling activation is preferentially found in TNBC and is associated with a poor clinical outcome [28, 29]. Growing evidence indicates that LRP6 is a potential therapeutic target of breast cancer [26]. It has been demonstrated that LRP6 is up-regulated in human TNBC [30–32], and that transcriptional knockdown of LRP6 in TNBC MDA-MB-231 cells significantly decreased Wnt/β-catenin signaling, cell proliferation, and tumor growth in vivo [31]. Moreover, blocking Wnt/β-catenin signaling by N-myc downstream regulated gene-1 (NDRG1), a tumor metastasis suppressor which interacts with LRP6 and represses Wnt/β-catenin signaling, led to drastic suppression of metastatic phenotypes of mammary tumor cells in vitro and in vivo [33]. In addition, treatment of breast cancer cells with the LRP6 antagonist Nilcosmaide significantly inhibited cell proliferation [34]. Cytotoxic effects of silibinin on breast cancer cells were demonstrated by several studies [4–6]. In the present study, we found that silibinin was able to suppress LRP6 and inhibit Wnt/β-catenin signaling in breast cancer cells, and that its effects on Wnt/β-catenin signaling occurred at concentrations comparable to those required for inhibiting breast cancer cell proliferation. Our results indicate that the anti-breast cancer activity of silibinin is associated with its inhibitory effects on Wnt/LRP6 signaling.
Wnt/β-catenin signaling plays a significant role in prostatic development and tumorigenesis [22]. Over-expression of Wnt proteins and their receptors and epigenetic deregulation of Wnt/β-catenin signaling inhibitors contribute to aberrant activation of this pathway in prostate cancer [35–38]. LRP6 expression is significantly up-regulated in prostate patients with metastatic disease compared to those without metastasis, and is associated with a significantly increased risk of recurrent disease [33]. Furthermore, treatment of prostate cancer cells with Wnt3A CM or purified recombinant Wnt3A protein significantly enhanced cell growth and migration [39, 40], while treatment of prostate cancer cells with the LRP6 antagonist Dkk1 and Nilcosmaide significantly inhibited cell growth and migration [34, 40]. We have recently demonstrated that the recombinant Mesd protein, an universal inhibitor of LRP6 modulators, markedly inhibited Wnt/β-catenin signaling in prostate cancer PC-3 cells, and suppressed PC-3 cell proliferation in vitro and tumor growth in vivo [41, 42]. Studies have shown that silibinin exerts both preventive and therapeutic effects in different prostate tumor models and inhibits the proliferation of human prostate cancer cells in vitro and in vivo [4–6]. In the present study, we found that silibinin inhibited prostate cancer cell proliferation with IC50 values for prostate cancer PC-3 and DU145 cells of 50 µM and 94 µM, respectively. The IC50 values are comparable to those shown to suppress the activities of LRP6 and Wnt/β-catenin signaling in prostate cancer cells. Our results suggest that the inhibitory activities of silibinin on Wnt/β-catenin signaling contribute to its therapeutic and preventive effects against prostate cancer.
Aberrant activation of the Wnt/β-catenin signaling pathway is a necessary initiating event in the genesis of most colorectal cancers. Although genetic mutations of the Wnt/β-catenin signaling intracellular components APC, CTNNB1 (β-catenin encoding gene) and Axin2 are major contributing factors for colorectal cancers, it is now recognized that additional modulation of Wnt/β-catenin signaling is involved in colorectal tumor progression [24]. In particular, Wnt2, Fzd7, the secreted frizzled-related protein family and Wnt inhibitory factor-1 are dysregulated in colorectal cancer, and are able to modulate the Wnt/β-catenin pathway in colorectal cancer cells despite the presence of APC or CTNNB1 mutation [43–47]. It has been recently reported that the chemopreventive and chemotherapeutic effects of silibinin against colorectal cancer are associated with its activities against Wnt/β-catenin signaling [9–14]. In the present study, we have demonstrated that silibinin is a novel Wnt/β-catenin signaling inhibitor targeting LRP6. Future studies are required to address whether LRP6 is a potential therapeutic target for colorectal cancer.
Silibinin is a natural compound isolated from milk thistle seed extracts, and has traditionally been used as a hepatoprotectant [4]. Remarkably, silibinin is largely nontoxic and even very high doses are tolerated by animals (2 g/kg dose by oral gavage in mice) [48] and human (4.7 g/d orally given as silybin-phytosome) [49]. Many signaling pathways have been identified to be involved in the anticancer actions of silibinin [5, 6]. Disruption of Wnt/β-catenin signaling represents a great opportunity to determine if dietary supplements might have potential anticancer efficacy [1–3]. LRP6 is an essential coreceptor for the Wnt/β-catenin signaling pathway. Recently, salinomycin, a breast cancer stem cell killer [50], was demonstrated to be an inhibitor of Wnt/β-catenin signaling by inducing LRP6 degradation [51]. In the present study, we found that silibinin is a novel Wnt/β-catenin signaling inhibitor by suppressing LRP6 expression at the transcription level. Therefore, as a popular dietary supplement, silibinin has the potential to be developed as a new cancer prevention and therapy agent.
Highlights.
Silibinin blocks Wnt3A-induced Wnt/β-catenin signaling activation in HEK293 cells.
Silibinin suppresses LRP6 expression and phosphorylation in cancer cells.
Silibinin blocks LRP6 expression at the transcription level.
The anti-cancer activity of silibinin is linked to its effects on LRP6 signaling.
Acknowledgments
We are grateful to Dr. Christof Niehrs (Deutsches Krebsforschungszentrum, Heidelberg, Germany) for providing LRP6 cDNA, and Dr. Gail Johnson (University of Rochester) for providing GST-E Cadherin cDNA. This work was completed with the support of a grant from the National Institute of Health (R01CA124531).
Abbreviations
- APC
adenomatous polyposis coli
- CK1
casein kinase 1
- CM
conditioned medium
- GSK3β
glycogen synthase kinase-3β
- FBS
fetal bovine serum
- Fzd
Frizzled
- LDLR
low density lipoprotein receptor
- LRP6
low-density lipoprotein receptor-related protein-6
- TCF/LEF
T-cell factor/lymphoid enhancing factor
- TNBC
triple negative breast cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barker N, Clevers H. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 2.Polakis P P. Curr. Opin. Genet. Dev. 2007;17:45–51. doi: 10.1016/j.gde.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald BT, Tamai K, He H. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abenavoli L, Capasso R, Milic N, Capasso F. Phytother. Res. 2010;24:1423–1432. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- 5.Deep G, Agarwal R. Cancer Metastasis Rev. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Zeng J, Gao Y Y, He D. Expert Opin. Investig. Drugs. 2010;19:243–255. doi: 10.1517/13543780903533631. [DOI] [PubMed] [Google Scholar]
- 7.Cheung CW, Gibbons N, Johnson DW, Nicol DL. Anticancer Agents Med. Chem. 2010;10:186–195. doi: 10.2174/1871520611009030186. (2010) [DOI] [PubMed] [Google Scholar]
- 8.Ramakrishnan G, Lo Muzio L, Elinos-Báez CM, Jagan S, Augustine TA, Kamaraj S, Anandakumar P, Devaki T. Cell Prolif. 2009;42:229–240. doi: 10.1111/j.1365-2184.2008.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajamanickam S, Kaur M, Velmurugan B, Singh RP, Agarwal R. Pharm. Res. 2009;26:2558–2567. doi: 10.1007/s11095-009-9968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur M, Velmurugan B, Tyagi A, Agarwal C, Singh RP, Agarwal R. Neoplasia. 2010;12:415–424. doi: 10.1593/neo.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajamanickam S, Velmurugan B, Kaur M, Singh RP, Agarwal R. Cancer Res. 2010;70:2368–2378. doi: 10.1158/0008-5472.CAN-09-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ravichandran K, Velmurugan B, Gu M, Singh RP, Agarwal R. Clin. Cancer Res. 2010;16:4595–4606. doi: 10.1158/1078-0432.CCR-10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sangeetha N, Aranganathan S, Panneerselvam J, Shanthi P, Rama G, Nalini N. Eur. J. Pharmacol. 2010;643:93–100. doi: 10.1016/j.ejphar.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 14.Velmurugan B, Gangar SC, Kaur M, Tyagi A, Deep G, Agarwal R. Pharm. Res. 2010;27:2085–2097. doi: 10.1007/s11095-010-0207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deep G, Gangar SC, Agarwal C, Agarwal R. Cancer Prev. Res. 2011;4:1222–1232. doi: 10.1158/1940-6207.CAPR-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaid M, Prasad R, Sun Q, Katiyar SK. PLoS One. 2011;6:e23000. doi: 10.1371/journal.pone.0023000. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Li Y, Lu W, He X, Schwartz AL, Bu G. Oncogene. 2004;23:9129–9135. doi: 10.1038/sj.onc.1208123. [DOI] [PubMed] [Google Scholar]
- 18.Lu W, Kim KA, Liu J, Abo A, Feng X, Cao X, Li Y. FEBS Lett. 2008;582:643–650. doi: 10.1016/j.febslet.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 19.Lu W, Tinsley HN, Keeton A, Qu Z, Piazza GA, Li Y. Eur. J. Pharmacol. 2009;602:8–14. doi: 10.1016/j.ejphar.2008.10.053. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazieres J, He B, You L, Xu Z, Jablons DM. Cancer Lett. 2005;222:1–10. doi: 10.1016/j.canlet.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 21.Rubin JS, Barshishat-Kupper M, Feroze-Merzoug F, Xi ZF. Front. Biosci. 2006;11:2093–2105. doi: 10.2741/1952. [DOI] [PubMed] [Google Scholar]
- 22.Verras M, Sun Z. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Klarmann GJ, Decker A, Farrar WL. Epigenetics. 2008;3:59–63. doi: 10.4161/epi.3.2.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincan E, Barke N. Clin. Exp. Metastasis. 2008;25:657–663. doi: 10.1007/s10585-008-9156-4. [DOI] [PubMed] [Google Scholar]
- 25.Prosperi JR, Goss KH. Curr. Drug Targets. 2010;11:1074–1088. doi: 10.2174/138945010792006780. (2010) [DOI] [PubMed] [Google Scholar]
- 26.King TD, Suto MJ, Li Y. J. Cell. Biochem. 2012;113:13–18. doi: 10.1002/jcb.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin SY, Xia W, Wang JC, Kwong KY, Spohn B, Wen Y, Pestell RG, Hung MC. Proc. Natl. Acad. Sci. USA. 2000;97:4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khramtsov AI, Khramtsova GF, Tretiakova M, Huo D, Olopade OL, Goss KH. Am. J. Pathol. 2010;176:2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geyer FC, Lacroix-Triki M, Savage K, Arnedos M, Lambros MB, MacKay A, Natrajan R, Reis-Filho JS. Mod. Pathol. 2011;24:209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 30.Lindvall C, Zylstra CR, Evans N, West RA, Dykema K, Furge KA, Williams BO. PLoS One. 2009;4:e5813. doi: 10.1371/journal.pone.0005813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CC, Prior J, Piwnica-Worms D, Bu G. Proc. Natl. Acad. Sci. USA. 2010;107:5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, Somlo G, Yen Y. Oncogene. 2011;30:4437–4446. doi: 10.1038/onc.2011.145. [DOI] [PubMed] [Google Scholar]
- 33.Liu W, Xing F, Iiizumi-Gairani M, Okuda H, Watabe M, Pai SK, Pandey PR, Hirota S, Kobayashi A, Mo YY, Fukuda K, Li Y, Watabe K. EMBO Mol. Med. 2012;4:93–108. doi: 10.1002/emmm.201100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu W, Lin C, Roberts MJ, Waud WR, Piazza GA, Li Y. PLoS One. 2011;6:e29290. doi: 10.1371/journal.pone.0029290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, Rabbani SA. Cancer. 2004;101:1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- 36.Costa VL, Henrique R, Ribeiro FR, Carvalho JR, Oliveira J, Lobo F, Teixeira MR, Jerónimo C. Epigenetics. 2010;5:343–351. doi: 10.4161/epi.5.4.11749. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, Rantala J, Alanen K, Nees M, Kallioniemi O. Cancer Res. 2010;70:6735–6745. doi: 10.1158/0008-5472.CAN-10-0244. [DOI] [PubMed] [Google Scholar]
- 38.Uysal-Onganer P, Kawano Y, Caro M, Walker MM, Diez S, Darrington RS, Waxman J, Kypta RM. Mol. Cancer. 2010:55. doi: 10.1186/1476-4598-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verras M, Brown J, Li X, Nusse R, Sun Z. Cancer Res. 2004;64:8860–8866. doi: 10.1158/0008-5472.CAN-04-2370. [DOI] [PubMed] [Google Scholar]
- 40.Wang BE, Wang XD, Ernst JA, Polakis P, Gao WQ. PLoS One. 2008;3:e2186. doi: 10.1371/journal.pone.0002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu W, Liu CC, Thottassery JV, Bu G, Li Y. Biochemistry. 2010;49:4635–4643. doi: 10.1021/bi1001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin C, Lu W, Zhai L, Bethea T, Berry K, Qu Z, Waud WR, Li Y. FEBS Lett. 2011;585:3120–3125. doi: 10.1016/j.febslet.2011.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Caldwell GM, Jones C, Gensberg K, Jan S, Hardy RG, Byrd P, Chughtai S, Wallis Y, Matthews GM, Morton DG. Cancer Res. 2004;64:883–888. doi: 10.1158/0008-5472.can-03-1346. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB. Nat. Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 45.He B, Reguart N, You L, Mazieres J, Xu Z, Lee AY, Mikami I, McCormick F, Jablons DM. Oncogene. 2005;24:3054–3058. doi: 10.1038/sj.onc.1208511. [DOI] [PubMed] [Google Scholar]
- 46.Shi Y, He B, Kuchenbecker KM, You L, Xu Z, Mikami I, Yagui-Beltran A, Clement G, Lin YC, Okamoto J, Bravo DT, Jablons DM. Int. J. Cancer. 2007;121:1175–1181. doi: 10.1002/ijc.22848. [DOI] [PubMed] [Google Scholar]
- 47.Ueno K, Hiura M, Suehiro Y, Hazama S, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Neoplasia. 2008;10:697–705. doi: 10.1593/neo.08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal C, Singh RP, Dhanalakshmi S, Tyagi AK, Tecklenburg M, Sclafani RA, Agarwal R. Oncogene. 2003;22:8271–8282. doi: 10.1038/sj.onc.1207158. [DOI] [PubMed] [Google Scholar]
- 49.Flaig TW, Gustafson DL, Su LJ, Zirroli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. Invest. New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 50.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lauder ES. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu D, Choi MY, Yu J, Castro JE, Kipps TJ, Carson DA. Proc. Natl. Acad. Sci. USA. 2011;108:13253–13257. doi: 10.1073/pnas.1110431108. [DOI] [PMC free article] [PubMed] [Google Scholar]