Abstract
Macrophage migration inhibitory factor (MIF) is involved in tumorigenesis by facilitating tumor proliferation and evasion of apoptosis; however, its role in tumor immunity is unclear. In this study, we investigated the effect of MIF on the progression of the syngenic, CT26 colon carcinoma and the generation of tumor regulatory T cells (Tregs). The results showed that the tumor growth rate was significantly lower in MIF knockout (MIF−/−) mice than in wild type (MIF+/+) mice. Flow cytometric analysis of both spleen and tumor cells revealed that MIF−/− mice had significantly lower levels of tumor-associated CD4+Tregs than MIF+/+ mice. The splenic cells of MIF−/− mice also showed a decrease in CD8+Tregs, which was accompanied by an increase in CD8-induced tumor cytotoxicity. Interestingly, the inducible Treg response in spleen cells to anti-CD3/CD28+IL-2+TGF-β was greater in MIF−/− mice than in MIF+/+ mice. Spleen cells of MIF−/− mice, stimulated with anti-CD3/CD28, produced lower levels of IL-2, but not TGF-β, than those of MIF+/+ mice, which was recovered by the addition of recombinant MIF. Conversely, a neutralizing anti-MIF Ab blocked anti-CD3-induced IL-2 production by splenocytes of MIF+/+ mice and suppressed the inducible Treg generation. Moreover, the administration of IL-2 into tumor-bearing MIF−/− mice restored the generation of Tregs and tumor growth. Taken together, our data suggest that MIF promotes tumor growth by increasing Tregs generation through the modulation of IL-2 production. Thus, anti-MIF treatment might be useful in enhancing the adaptive immune response to colon cancers.
Keywords: Macrophage migration inhibitory factor, tumor, regulatory T cells, IL-2
Introduction
Within the immune system, macrophage migration inhibitory factor (MIF) is considered a broad-spectrum proinflammatory cytokine. MIF was originally identified as a T cell-derived lymphokine (1). T cells activated by specific antigen, mitogens, or anti-CD3 antibody (Ab) show increased expression of MIF mRNA and protein (2). Anti-MIF antibodies (Abs) inhibit T-cell proliferation and Ab production from B cells (2). MIF also is known to be involved in angiogenesis, tumor growth, and metastasis (3). For example, administration of anti-MIF mAb into mice bearing the human melanoma tumor, G361, significantly decreases tumor growth and neovascularization (4). In EG7 tumor-bearing mice, treatment with anti-MIF Abs result in an accumulation of CD4+ and CD8+ T cells at the tumor sites (5). However, in tumor conditions, it is still unclear whether and how host-derived MIF affects T cell immunity, independent of the angiogenic and proliferative effect of MIF on tumor cells.
All solid tumors are embedded in a stromal microenvironment consisting of immune cells, such as macrophage and lymphocytes, as well as non-immune cells. In particular, tumor-derived CD4+ regulatory T cells (CD4+Tregs) have been extensively studied in many different types of cancer (6, 7). Antigen-specific CD4+Tregs at tumor sites significantly suppress immune responses, leading to immune tolerance to tumor cells (8). Inducible CD4+Tregs suppress immune responses through a cell contact or soluble factor-dependent mechanism once they are activated by exposure to a specific antigen (9). Although the origin of CD4+Tregs remains largely unknown, they may be converted from CD4+CD25− naïve T cells and antigen-experienced effector cells in the suppressive cytokine milieu of tumor sites or they may arise by proliferation and expansion of naturally occurring CD4+CD25+T cells after antigen stimulation (10). CD4+Tregs in turn may promote local tumor growth and also contribute to the systemic progression of tumors into the peripheral blood or lymphoid organs (11). Thus, an increased Treg response may be a major obstacle for immunotherapy of cancer. It has become clear that a better understanding of the mechanisms and actions of Tregs in tumor immunity is critical in developing an effective tumor vaccine or immunotherapy (12). However, the function of MIF in the biology of Tregs is unknown.
In this study, we have investigated the role of MIF in tumor progression and the generation of tumor-associated Tregs. We demonstrated first that tumor growth rate was significantly lower in MIF−/− mice than in MIF+/+ mice when syngeneic colon cancer (CT26) cells were injected. Flow cytometric analysis of both spleen and tumor cells revealed that MIF−/− mice had significant lower levels of tumor-associated Tregs than MIF+/+ mice. The splenic cells of MIF−/− mice also showed a decrease in CD8+Tregs, which was accompanied by an increase in CD8-induced tumor cytotoxicity. In contrast, when splenic T cells were induced by anti-CD3/CD28+IL-2+TGF-β, the number of Tregs was significantly higher in MIF−/− than in MIF+/+ mice, suggesting that these stimuli are associated with a reduction in Treg development in MIF−/− mice. As expected, MIF−/− mice showed significantly lower levels of IL-2 production by spleen cells stimulated with anti-CD3/CD28 than MIF+/+ mice. Moreover, the administration of IL-2 into MIF−/− mice restored both the generation of Tregs and colocn carcinoma growth. We conclude that MIF promotes tumor progression in mice by increasing intra-tumor Tregs through the regulation of IL-2 production.
Materials and Methods
Mice
Mice genetically deficient in mif (MIF−/− mice) were backcrossed onto the BALB/c background (generation N10) (13). Age and sex-matched wild-type BALB/c mice (MIF+/+) were used as a control. All mice were 8–12 weeks of age unless specified otherwise. The mice were maintained in specific pathogen-free conditions and were used according to guidelines of the Institutional Animal Care Committee.
Induction and determination of tumor growth in mice
To determine the effect of MIF on tumor growth, CT26 tumor cells (an undifferentiated colon cancer cell line), were injected into syngeneic MIF−/− and MIF+/+ mice as described previously (14). Briefly, CT26 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Welgene, Daegu, South Korea) supplemented with 10% fetal bovine serum (FBS; Wisent Bioproducts, St. Bruno, QC, Canada). The cultured cells were resuspended in PBS, and 1×106 cells (suspended in 0.1 ml of PBS) then were injected subcutaneously into the upper flank of MIF−/− and MIF+/+ mice. Tumor size was estimated every day by orthogonal linear measurements made with Vernier calipers according to the formula: volume (mm3) = [(width, mm)2 × (length, mm)]/2 (15).
Flow cytometry analysis
Single cell suspensions were prepared from the tumor tissues and spleens of MIF−/− and MIF+/+ mice after tumor inoculation. The cells of tumor tissues and spleen, obtained from MIF−/− and MIF+/+ mice, were resuspended in staining buffer (Hanks’ Balanced Salt Solution (HBSS; Welgene) containing 2% FBS and stained for 1 hour with the following antibodies conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE) or allophycocyanin (APC); anti-CD4 Ab (eBioscience, San Diego, CA), anti-CD8 Ab (eBioscience), anti-CD122 Ab (eBioscience), anti-CD132 Ab (BD Pharmingen, San Diego, CA), anti-CTLA4 Ab (eBioscience), anti-GITR Ab (eBioscience), anti-Foxp3 Ab (eBioscience), anti-CD25 Ab (eBioscience), and ant-CD44 Ab (eBioscience). Isotype Ab (eBioscience) was used as a control. For the staining of Foxp3 and CTLA, the cells were fixed and treated with permeabilization buffer (eBioscience). The three-color samples were acquired using a FACS Canto (BD Biosciences, San Jose, CA) equipped with Diva software. Data were analyzed with Flowjo (Tree Star, Ashland, OR) software. Representative dot plots for CD4+CD25+FoxP+ T cells are shown in Supplementary Fig.1A.
In vitro culture of splenic cells
Spleens were isolated from MIF−/− and MIF+/+ mice and prepared as single-cell suspensions. The splenic cells then were resuspended in RPMI 1640 (Welgene) supplemented with FBS (Wisent Bioproducts). To induce Tregs, splenic cells were plated at a concentration in 96-well plates, and stimulated with pre-coated anti-CD3 Ab and anti-CD28 Ab (BD Pharmingen) in the absence or presence of murine IL-2 (1 ng/ml; R&D system, Minneapolis, MN) plus TGF-β (3 ng/ml; Peprotech, Rockey Hill, NJ). Cells were cultured for 72 hours, harvested, and used for flow cytometry analysis. In some experiments, recombinant MIF or anti-MIF Ab was added to spleen cells stimulated with anti-CD3 Ab plus anti-CD28 Ab in order to determine the effect of MIF on IL-2 production by splenic T cells. Mouse recombinant MIF (rMIF) was prepared as the native protein from an Escherichia coli expression system and purified free of endotoxin by C8 chromatography as described previously (16). Anti-MIF Ab (NIHIII.D.9) and non-immune IgG were isolated from mouse ascites by protein-A IgG purification kit (Thermo Scientific, Rockford, IL) according to the manufacturer’s instructions. The MIF receptor antagonist, 3-(3-hydroxybenzyl)-5-methylbenzooxazol-2-one, (a.k.a. Debio1036), was synthesized as described previously (17).
Enzyme-linked immunosorbent assay (ELISA) for cytokines
Cytokine concentration was determined in the culture supernatants of splenic cells and in the sera by ELISA. Spleen cells of tumor-bearing MIF−/− and MIF+/+ mice were plated in flat bottom 96-well plates, and then stimulated with plate-bound anti-CD3 (BD Pharmingen) and anti-CD28 Ab (BD Pharmingen) for 48 or 72 hours. Levels of IL-2, IFN-γ, IL-10 and TGF-β in the culture supernatants were measured by ELISA, according to manufacturers’ instructions (R&D Systems). Serum MIF levels were also determined by ELISA kit (USCN Life Science and Technology, Wuhan, China).
Immuno-histochemical staining of tumor tissues
Immuno-histochemical staining for CD3 was performed in tumor tissues to assess the infiltration of CD3+ T cells. Tumor tissue was dissected, fixed in a formalin solution (Sigma-Aldrich, St. Louis, MO), and paraffin-embedded. Nonspecific binding was blocked by treating the sections (5 µm) with 10% normal goat serum at 37°C for 1 hour. The sections were incubated overnight at 4°C with rabbit anti-CD3 Ab (Abcam, Cambridge, MA) and then for 60 minutes at room temperature with biotinylated secondary Ab (Santa Cruz Biotechnology, Santa Cruz, CA). Sections then were treated for 30 minutes at room temperature with 3,3’-diaminobenzidine to reveal the antigen. Counterstaining was performed using hematoxylin.
Detection of MIF mRNA
Total RNA was isolated from CT26 tumor cells, and spleen cells of MIF+/+ and MIF−/− mouse using the RNeasy Mini Kit (Quagen, Valencia, CA), according to the manufacturer’s instructions. Reverse transcription was performed using oligo(dT) as the random primer for reverse transcription and specific MIF primers and GAPDH primers for PCR. Reverse transcription was carried out using MyCycler (Bio-Rad Laboratories Ltd, Hemel Hempstead, United Kingdom). The following primers were used to amplify a 174-bp fragment of MIF: 5’-ACGACATGAACGCTGCCAAC-3’ and 5’-ACCGTGGTCTCTTATAAACC-3’; GAPDH: 5’-GCAGTGGCAAAGTGGAGATT-3’ and 5’-TAGTAGAGGCGGGGAAGACG.. The amplification profile for MIF was 28 cycles of denaturation at 94°C (30 seconds), annealing at 60°C (45 seconds), and extension at 60°C (1 minute), followed by extension for 10 minutes at 72°C. Afterwards, the PCR products were resolved by electrophoresis on a 1% agarose gel.
Cell-mediated cytotoxicity assay
The CT-26 target cells (5×104/well) were added to effector CD8+ T cells, which were obtained from the spleens of MIF+/+ or MIF−/− mice 30 days after the tumor inoculation, in 96-well flat-bottom plates at effector(E): target(T) cell at a ratio of 40:1. After co-incubation of both cell types for 4 or 8 hours at 37°C, cytotoxicity was assessed by measuring the concentrations of lactate dehydrogenase (LDH) released in the culture supernatants using the CytoTox 96 Assay kit (Promega, Madison, WI). The specific lysis is calculated by the equation of [(experimental release) - (spontaneous release) / (target maximum) - (target spontaneous release)]. Spontaneous LDH release in the absence of effector cells was below 10% of the maximal LDH release by a detergent. All experimental procedures and assays were performed in triplicate more than two times, which showed similar results.
Recombinant IL-2 treatment in vivo
To determine the role of IL-2 in tumor progression and Treg generation in MIF−/− mice, the mice were injected intraperitoneally with IL-2 (2,500 or 12,500 IU/day/mouse) 14 times every other day after the tumor cell inoculation; carrier-free, recombinant anti-mouse IL-2 Ab was purchased from R&D systems. For comparison, the tumor-bearing MIF−/− and MIF+/+ mice without IL-2 injection were maintained under the same conditions. After 30 days, tumor tissue cells and spleen cells were isolated from the three groups of mice. The cells were cultured, harvested, and used for flow cytometry analysis for Tregs.
Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Comparisons of the numerical data between groups were performed by paired or unpaired Mann-Whitney U-test or ANOVA. P values of less than 0.05 were considered statistically significant.
Results
Tumor growth is suppressed in MIF−/− mice
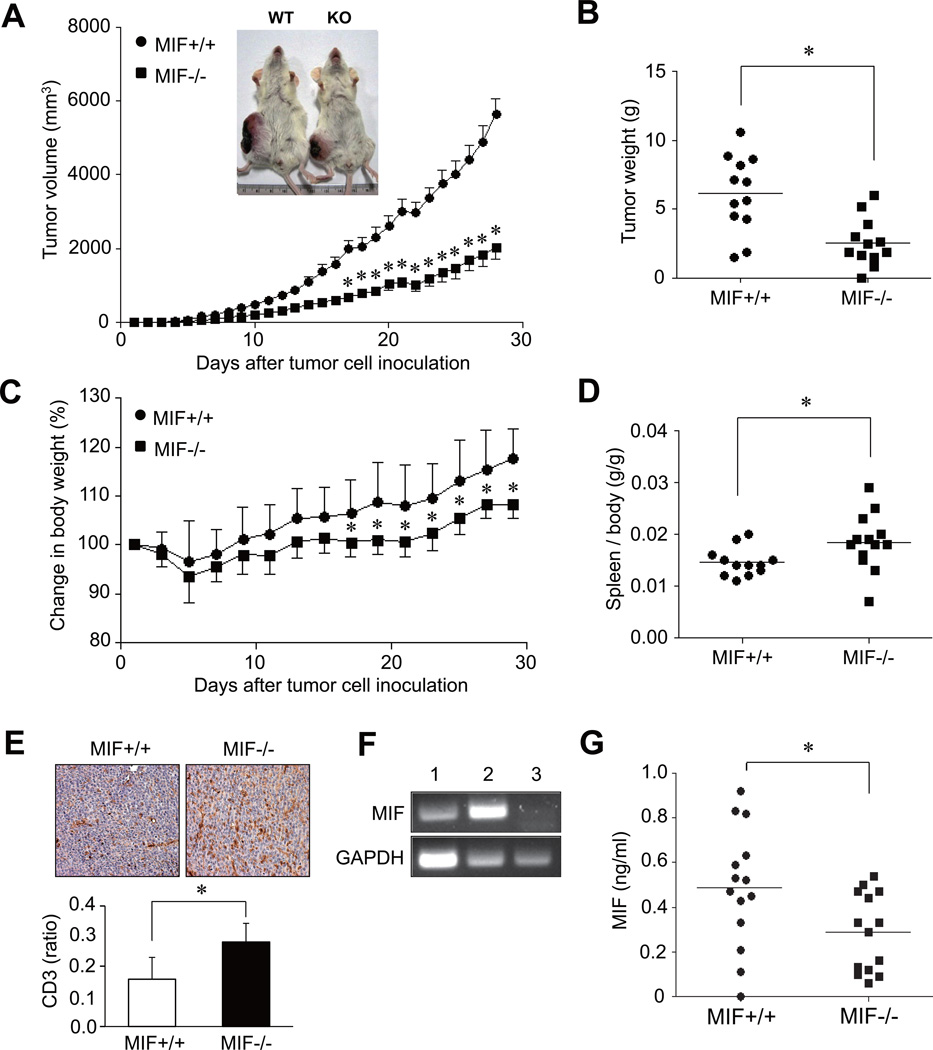
Recent studies have demonstrated that MIF is critically involved in the processes of cell proliferation and tumor angiogenesis (3–5, 14). However, it remains unclear if MIF of host origin or produced by tumors, independently affects tumor progression. To address this question, we established an animal model of colon cancer by inoculating CT-26 mouse colon carcinoma cells (1×106) into syngeneic MIF−/− and MIF+/+ mice. We first observed a sustained reduction in tumor growth in MIF−/− mice when compared to MIF+/+ mice (Fig. 1A). In parallel, MIF−/− mice showed a smaller change in body weight than MIF+/+ mice, as observed for 30 days after tumor inoculation (Fig. 1C). Moreover, the weight of tumors, which were excised 30 days after tumor injection, was significantly lower in MIF−/− than in MIF+/+ mice (Fig. 1B), whereas spleen weight were higher in MIF−/− mice (Fig. 1D), indicating that host MIF is crucial to CT-26 tumor cell growth. Similarly, when 4T1 mouse mammary carcinoma cells (1×106) were injected into the mice by the same protocol, their growth also was significantly reduced in MIF−/− mice (n=9 for each group, Supplementary Fig. 1B), suggesting that the effect of host MIF on tumor growth is not limited to the CT-26 colon cancer.
Figure 1. Reduced growth of the CT26 colon carcinoma in syngeneic MIF−/− mice.
(A and B) Decrease in tumor size and weight in MIF−/− mice. CT26 colon cancer cells (1×106/mouse) were administered subcutaneously into the right flank of MIF−/− mice and wild type (MIF +/+) mice. The tumor volume was calculated using the formula of width2×length×0.5 (length > width). Four weeks after the tumor inoculation, tumor mass was excised and its weight was measured. *, P < 0.001. Representative photographs are shown inside the figure; WT: wild type, KO: knock-out. (C) Changes in body weight of MIF−/− mice versus MIF +/+ mice after the tumor injection. *, P < 0.05. (D) Comparison of spleen weight between MIF−/− and MIF +/+ mice, which was assessed at 4 weeks after the tumor injection. *, P < 0.05. (E) Immuno-histochemical staining of tumor mass of MIF−/− mice (n=6) and MIF+/+ mice (n=6) using anti-CD3 antibody. The number of T cells was manually counted and presented as a ratio of T cells over non-T cells, including tumor cells. Data are the mean ± SD of CD3 ratios. (F) MIF mRNA expression in CT-26 tumor cells. Total RNA was isolated from CT26 colon cancer cells (lane 1), spleen cells of MIF+/+ mouse (lane 2), and spleen cells of MIF−/− mouse (lane 3). The mRNA expressions in these cells were determined by reverse transcription PCR analysis. Expression of GAPDH mRNA is presented as a control. (G) MIF concentrations in the sera of tumor-bearing MIF+/+ mice (n=14) and MIF−/− mice (n=14). Four weeks after the tumor inoculation, the sera were collected from the two groups of mice, and circulating MIF levels were measured by ELISA. *, P < 0.05.
Tumor growth can be affected by several factors, one of which is regulation of growth promoting cytokines (18, 19). For example, anti-MIF Ab treatment of EG.7 tumor-bearing mice increases T lymphocyte infiltration of tumors (5). To investigate whether the inhibition of tumor growth in MIF−/− mice is associated with T cell-mediated antitumor immunity, we analyzed the composition of T cells infiltrated in the tumor tissues of MIF−/− and MIF+/+ mice. By immuno-histochemical staining, the percentage of CD3+ T cells was significantly higher in tumor tissues of MIF−/− mice than those of MIF+/+ mice (Fig. 1E), indicating that MIF regulates T cell trafficking into tumor tissues (5).
It has been reported that CT-26 tumor cells intrinsically produce significant amounts of MIF (3), and that tumor-derived MIF may replenish the deficiency of host MIF in MIF-deficient mice. To address this issue, we determined MIF expression in the CT-26 tumor cells and the sera of MIF−/− mice. As reported previously (3), we found that CT26 cell line expressed MIF mRNA (Fig. 1F). MIF was also detectable in the sera of MIF−/− mice, but its levels were significantly lower in MIF−/− mice (n=14) than in MIF+/+ mice (n=14) (mean [±SD] serum concentration: 0.28±0.17 ng/ml for MIF−/− mice versus 0.49±0.26 ng/ml for MIF+/+ mice, P < 0.05, Fig. 1G). Given that host tissues of MIF−/− mice did not express MIF (Fig. 1F), the source of MIF in the sera of MIF−/− mice is the CT26 tumor cells.
Reduction of Tregs in tumor-bearing MIF−/− mice
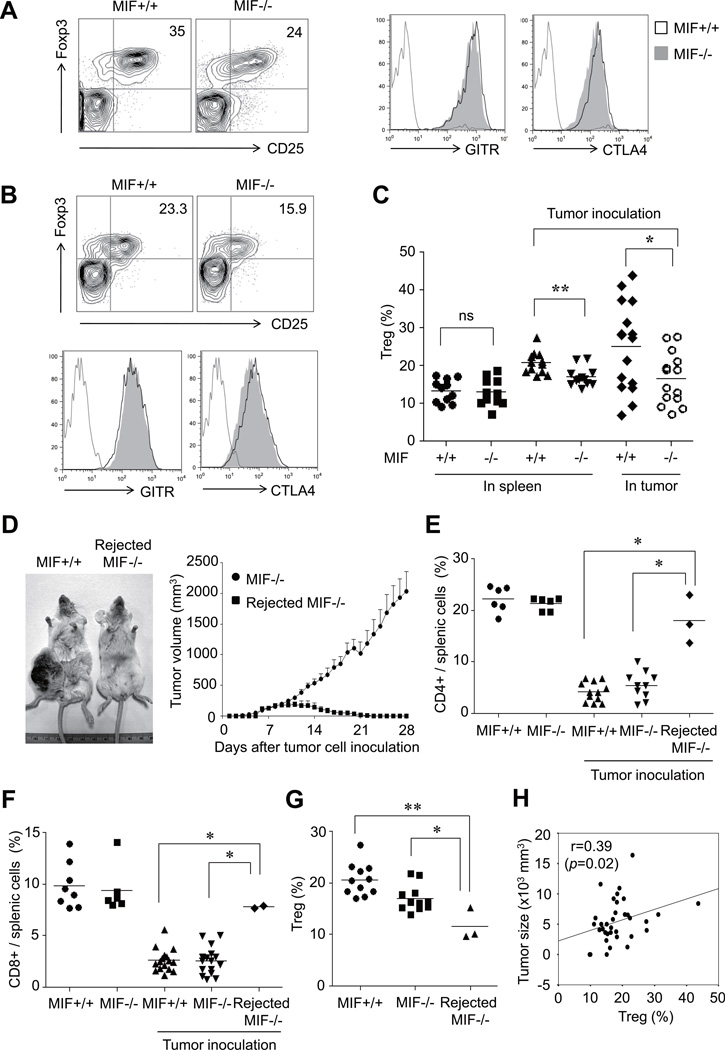
Our next experiment investigated the potential role of Tregs in the differential growth of CT26 colon cancer cells in MIF−/− and MIF+/+ mice. Under tumor microenvironments, Tregs can be differentiated, expanded, recruited and activated via multiple mechanisms (12, 20). As a result, Tregs are commonly found in the tumor itself, the peripheral blood or the lymphoid organs of the tumor-bearing host, and they in turn critically contribute to tumor growth and metastasis. To determine the role of MIF in the generation of Tregs, we first examined the levels of CD4+CD25+Foxp3+ T cell (CD4+Tregs) in MIF−/− and MIF+/+ mice. As seen in Fig. 2A to 2C, the percentage of CD4+Tregs over total CD4+ T cells was significantly lower in tumor tissue and the spleens of MIF−/− mice than in those of MIF+/+ mice. Morover, the percentage of CD4+Tregs over total CD4+ T cells also was reduced in tumor tissue from MIF−/− mice implanted with 4T1 mouse mammary carcinoma cells (Supplementary Fig. 1C), which is in parallel with the data on the decrease in 4T1 tumor growth in MIF−/− mice (Supplementary Fig. 1B). We next measured the expression of functional markers for Tregs, including glucocorticoid-induced TNF-related protein (GITR) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) by CD4+Foxp3+ T cells in MIF−/− mice and MIF+/+ mice injected with CT-26 tumor cells. The result showed that GITR and CTLA-4 expression levels by CD4+Foxp3+ T cells in both spleen and tumor tissue were not different between the two groups (Fig. 2A and Fig. 2B), suggesting that their regulatory function may not be altered in MIF−/− mice.
Figure 2. Decrease in CD4+Tregs in MIF−/− mice.
(A) Decrease in CD4+Tregs in MIF−/− mouse as compared to MIF+/+ mouse. Representative data of similar results in more than five mice are shown. Using flow cytometry analysis, the frequency of CD4+CD25+Foxp3+ T cells, CD4+GITR+ T cells, and CD4+CTLA4+ T cells was determined in single cell suspensions of tumor mass of MIF−/− versus MIF+/+ mouse. (B) Representative flow cytometry analysis of CD4+Tregs in the spleen of MIF−/− mouse versus MIF+/+ mouse. (C) Comparison of the percentage of CD4+Tregs in the spleen and tumor tissue between tumor-bearing MIF−/− and MIF+/+ mice. The frequency of Tregs was calculated as the percentage of CD4+CD25+Foxp3+ cells in the CD4+ T-cell population. Mice without tumor inoculation were used as a control. **, P < 0.01. (D) Tumor rejection in MIF−/− mice. Left panel represents a photograph of tumor-bearing and tumor-rejected MIF−/− mouse taken 4 weeks after tumor inoculation. Right panel shows changes in tumor volume in tumor-rejected versus tumor-bearing MIF−/− mice. Representative data are shown and the arrow indicates the injection site. (E and F) Recovery of CD4+ and CD8+ T cells in the spleen of tumor-rejected MIF−/− mice. The frequency of CD4+ or CD8+ T cells was determined by flow cytometry and calculated as the percentage of CD4+ or CD8+ cells in the splenocytes from mice. Tumor-naïve MIF−/− and MIF +/+ mice were used as a control. *, P < 0.05. (G) Decrease in CD4+ Tregs in the spleen of tumor-rejected MIF−/− mice, as compared to that of tumor-bearing MIF−/− and MIF +/+ mice. The frequency of Treg was calculated as the percentage of CD4+CD25+Foxp3+ cells in the CD4+ T-cell population. *, P < 0.05, **, P < 0.01. (H) Correlation of the frequency of CD4+Tregs with tumor size determined at 4 weeks after tumor injection, in all of MIF−/− and MIF+/+ mice tested.
Although infiltration of lymphocytes is almost always observed in both human and experimental animal cancers (21, 22), complete rejection of tumors is extremely rare. Interestingly, we identified three cases (15%) of complete rejection out of 20 MIF−/− mice within 30 days after tumor inoculation, but never in wild type mice (n>30) (P < 0.05). When we compared the composition of T cells in the spleen of three groups of mice: 1) tumor-bearing, 2) tumor-rejected, and 3) tumor-naive mice; the percentages of splenic CD4+ and CD8+ T cells were increased to normal levels in tumor-rejected, MIF−/− mice and was comparable to that observed in the spleen of tumor naïve mice (Fig. 2D to 2F). Tumor-rejected MIF−/− mice also had lower levels (%) of CD4+Tregs within the splenic composition of CD4+T cells than tumor-bearing MIF−/− mice or MIF+/+ mice (Fig. 2G). Moreover, tumor size, as determined 30 days after the tumor inoculation, correlated well with the percentages of CD4+Tregs when assessed in MIF−/− and MIF+/+ mice (r=0.39, Fig. 2H).
Taken together, MIF−/− mice harbored more CD4+ and CD8+ T cells but fewer CD4+Tregs in the spleen and/or tumor tissues than MIF+/+ mice, which may account for diminished tumor growth in MIF−/− mice.
Increase in inducible CD4+Tregs in tumor-bearing MIF−/− mice
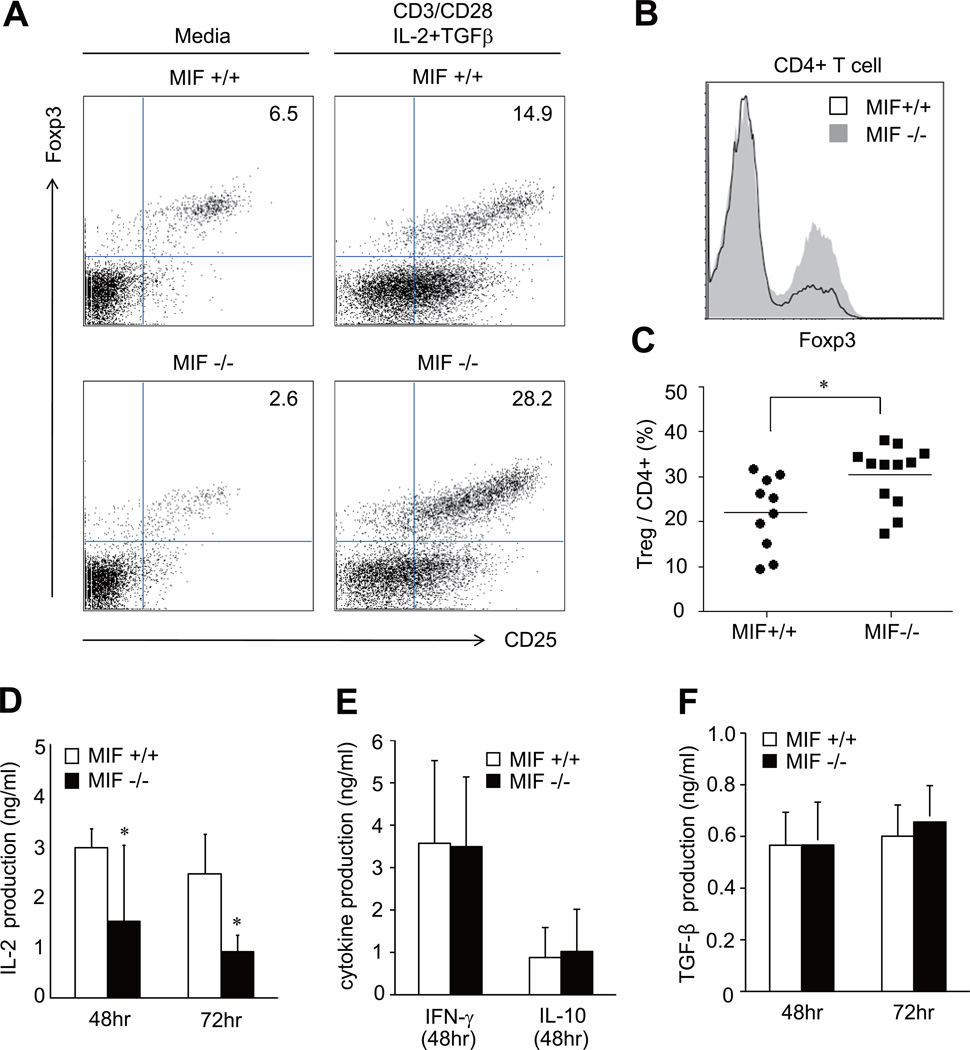
T cells cultured with IL-2, TGF-β and TCR-triggering stimuli have more inducible Tregs than T cells cultured with media or TCR triggering stimuli alone (23, 24). We next compared the number of inducible Tregs in spleen cells activated by the aforementioned stimuli in MIF−/− mice and wild type mice. As expected, when sorted CD4+ T cells were stimulated by anti-CD3/CD28 Ab+IL-2+TGF-β, the frequency of CD4+Tregs was markedly increased. Interestingly, in contrast with basal Tregs, the number of inducible Tregs was significantly greater within the CD4+ T cell population of MIF−/− than MIF+/+ mice (Fig. 3A to 3C). The percentage of inducible CD4+Tregs (%) among total splenic cells stimulated with anti-CD3/CD28 Ab+IL-2+TGF-β showed similar results (data not shown). We postulated that insufficient generation of these stimuli under tumor conditions may be associated with decreased Tregs in MIF−/− mice. To address this question, we measured the levels of IL-2, IFNγ, IL-10, and TGF-β production by spleen cells stimulated with anit-CD3 plus anti-CD28 Ab in order to simulate TCR triggering by tumor antigens (25). Spleen cells from tumor bearing MIF−/− mice showed significantly lower levels of IL-2 production than spleen cells from tumor bearing MIF+/+ mice (Fig. 3D). A decrease in IL-2 production also was observed in the splenic cells of MIF-deficient mice without tumor inoculation (data not shown). Nevertheless, the production of IFNγ, IL-10, and TGF-β by spleen cells was not different between the MIF−/− and MIF+/+ groups (Fig. 3E and 3F). These data indicate that MIF may act as upstream of IL-2 production and IL-2-mediated Treg genentation, but it may not affect IL-10 and TGF-β production by splenocytes.
Figure 3. Inducible CD4+Treg response and cytokine production in spleen cells of MIF−/− versus MIF+/+ mice.
(A and B) Representative data and quantification of inducible CD4+Tregs. Sorted CD4+ T cells of the spleen of tumor-naïve mice were stimulated with or without anti-CD3/CD28 Ab+IL-2 (1 ng/ml) +TGF-β (3 ng/ml). After 72 hours of stimulation, the frequency of CD4+CD25+Foxp3+ in CD4+ cells was determined by flow cytometry. Overlay histogram for Foxp3 is also shown in Fig. 3B. (C) Comparison of the percentage of inducible CD4+Tregs between tumor-naive MIF−/− and MIF +/+ mice. *, P < 0.05. (D to F) Cytokine production by splenic T cells of tumor-bearing MIF−/− versus MIF+/+ mice. The spleen cells (1×106) of the two groups of mice were stimulated with anti-CD3/CD28 Ab for 48 or 72 hours. Levels of IL-2, IFN-γ, IL-10 and TGF-β in the culture supernatants were measured by ELISA. *, P < 0.05.
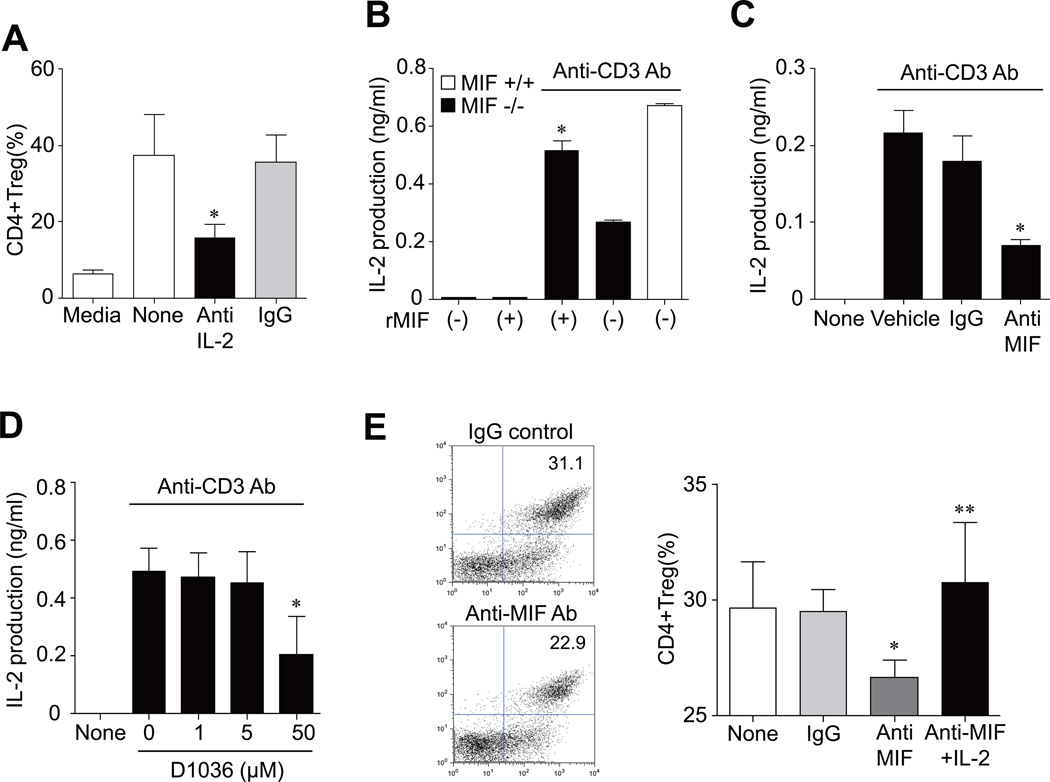
To address this isssue, we added anti-IL-2 Ab to spleinc CD4+ T cells stimulated with anti-CD3/CD28 Ab plus TGF-β (3 ng/ml). As reported previously (24), anti-IL-2 Ab strongly inhibited the inducible CD4+Treg response (Fig. 4A). We next treated recombinant MIF to MIF-deficient splenocytes. The result showed that addition of MIF (500 ng/ml) restored IL-2 release by MIF−/− splenocytes stimulated with anti-CD3 Ab+anti-CD28 Ab (Fig. 4B). Conversely, neutralizing anti-MIF Ab, but not isotype control Ab, blocked anti-CD3-induced IL-2 production by splenocytes of MIF+/+ mice (Fig. 4C). Moreover, Debio1036, a benzoxazolone compound that specifically inhibits MIF binding and activation of its transmembrane receptor CD74 (17), also suppressed anti-CD3-induced IL-2 production by splenocytes of MIF+/+ mice (Fig. 4D). In parallel, anti-MIF Ab treatment suppressed the CD4+Treg generation in the splenic CD4+ T cells of MIF+/+ mice stimulated with anti-CD3/CD28 Ab plus TGF-β, which was completely recovered by exogenous IL-2 (5 ng/ml) (Fig. 4E). Taken together, these results suggest that defective IL-2 production is an important factor explaining reduced Treg generation in MIF−/− versus MIF+/+ mice.
Figure 4. MIF controls IL-2-mediated Treg generation.
(A) Suppression of inducible CD4+ Tregs by anti-IL-2 Ab. Splenic CD4+ cells of MIF+/+ mice were stimulated with anti-CD3/CD28 Ab (CD3/CD28) plus TGF-β (3 ng/ml) in the presence of anti-IL-2 Ab (0.1 µg/ml) or isotype control IgG (IgG). After 72 hours of incubation, the frequency of CD4+CD25+Foxp3+ T cells over CD4+ cells was determined by flow cytometry analysis. Data shows mean±SD. *, P < 0.05 versus isotype control Ab. (B) Restoration of IL-2 production by recombinant MIF. Splenic T cells (2×105) of MIF−/− mice were stimulated with anti-CD3/CD28 Ab in the presence of recombinant MIF (500 ng/ml) for 48 hours. IL-2 levels the culture supernatants were measured by ELISA. *, P < 0.05 versus IL-2 production by splenic cells of MIF+/+ mice treated with anti-CD3/CD28 Ab alone. (C and D) Inhibition of IL-2 production by neutralizing anti-MIF Ab (C) or MIF receptor antagonist (D). Splenic cells (2×105) of MIF+/+ mice were stimulated with anti-CD3/CD28 Ab in the absence or presence of anti-MIF Ab (100 µg/ml) for 48 hours or D1036 (a MIF receptor antagonist: 1, 5, and 50 µM) for 24 hours. Isotype control IgG (100 µg/ml) was used as a control. IL-2 levels in the culture supernatants were measured by ELISA. *, P < 0.05 versus isotype control Ab or anti-CD3/CD28 Ab alone. (E) Effect of anti-MIF Ab on the inducible CD4+Treg production. Splenic CD4+ cells of MIF+/+ mice were cultured in RPMI1640 supplemented with 10% FBS, and stimulated with anti-CD3/CD28 Ab (CD3/CD28) plus TGF-β (3 ng/ml) in the presence of anti-MIF Ab (50 µg/ml) or isotype control IgG (50 µg/ml). After 72 hours of incubation, the frequency of CD4+Tregs was determined by flow cytometry analysis. A representative is shown on the left. Bar graph on the right shows the mean±SD of six independent experiments. *, P < 0.05 versus isotype control Ab. **, P < 0.01 versus anti-MIF Ab alone.
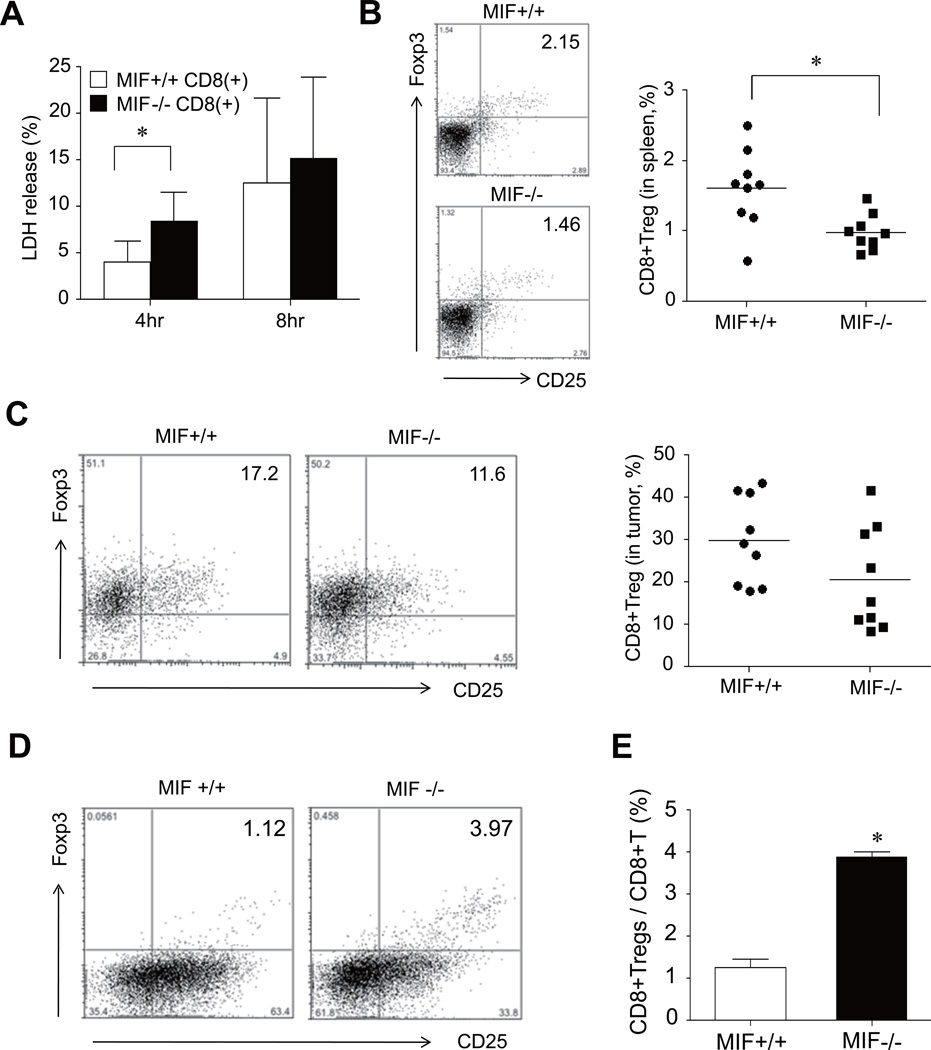
Increased cytotoxicity and decreased CD8+Tregs in tumor-bearing MIF−/− mice
To be effective, tumor-specific cytotoxicity must not only be initiated, but also be vigorous and sustained so as to achieve successful tumor regression (26, 27). Although a decrease in CD4+ Tregs may be linked to the regression of tumor growth in MIF−/− mice, it also is possible that CD8+ cells exhibit an enhanced, intrinsic cytotoxicity against tumor cells in the setting of MIF deficiency. To test the potential role of MIF in CD8+ cell-mediated cytotoxicity, CT-26 target cells were co-cultured with effector CD8+ T cells obtained from the spleens of MIF+/+ and MIF−/− mice, and cytotoxicity assessed by an LDH release assay. As shown in Fig. 5A, CD8+ effector T cells sorted from the spleens of MIF−/− mice showed a significant increase in the cytotoxicity response when compared with corresponding cells sorted from MIF+/+ mice. We next investigated the presence of immuno-suppressive CD8+ regulatory cells, CD8+Tregs, which have been also identified as a T cell population with immune suppressive properties in vitro (28). We tested whether tumor-bearing MIF−/− mice also have a decreased number of CD8+Tregs within the total CD8+ T cell effector population that is used in the cytotoxicity assay, as shown above for CD4+ T cells. As illustrated in Fig. 5B, tumor-bearing MIF−/− mice indeed had a lower number of CD8+Tregs in the spleen than MIF+/+ mice. The number of CD8+Tregs in the tumor mass also was lower in MIF−/− mice (Fig. 5C). Similar to CD4+Tregs, the frequency of CD8+Tregs of total splenic T cells stimulated with anti-CD3/CD28 Ab+IL-2+TGF-β was significantly higher in MIF−/− than in MIF+/+ mice (Fig. 5D and 5E). Taken together, these results suggest that MIF suppresses tumor-specific cytotoxicity by CD8+ T cells to promote the generation of CD8+Tregs.
Figure 5. Changes in tumor-specific cytotoxicity and CD8+Tregs in MIF−/− mice.
(A) Increase in tumor cell death induced by CD8+ T cells of MIF−/− mice. The CT26 carcinoma cells were subcutaneously injected in MIF−/− mice and MIF+/+ mice, and CD8+ T cells in the spleens of the two groups of mice then were isolated using anti-CD8 micro-beads. The CD8+ effector cells were co-cultured with CT26 target cells with E:T ratios of 40:1 for 4 hours and 8 hours. Cytotoxicity was measured by lactate dehydrogenase (LDH) release. *, P < 0.05. (B and C) Decrease in CD8+Tregs in tumor-bearing MIF−/− mice. Four weeks after the tumor injection, spleen cells and tumor tissues were harvested from MIF−/− mice and MIF+/+ mice. The frequency of CD8+CD25+Foxp3+ T cells was assessed by flow cytometry and calculated as the percentage of CD8+CD25+Foxp3+ cells in the CD8+ T-cell population. The representative data are shown in the left panel. *, P < 0.05. (D and E) Inducible CD8+ Tregs in tumor-naïve MIF−/− mice (n=3) and MIF+/+ mice (n=3). The splenic cells were stimulated by anti-CD3/CD28 Ab+IL-2+TGF-β as described in Materials and Methods. The number of CD8+CD25+Foxp3+ T cells was determined by flow cytometry. *, P < 0.05 versus MIF+/+ mice. A representative is shown in the left panel.
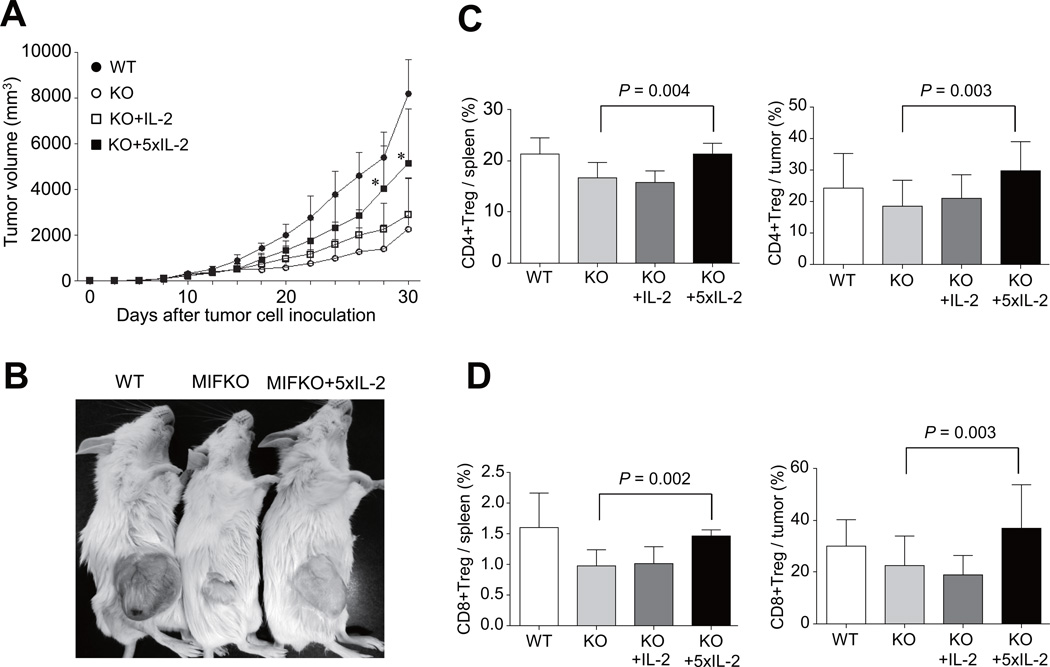
IL-2 treatment restores tumor growth and Tregs generation in MIF−/− mice
The dominant function of IL-2 appears to be in the maintenance of immune homeostasis and tolerance to self. IL-2 has been critically linked to the biology of regulatory T cells (29, 30). Most Tregs constitutively express the high-affinity IL-2R, which is comprised of the IL-2Rα, IL-2Rβ and IL-2Rγ protein (31). Based on the data on IL-2 production levels in MIF−/− mice, we investigated if exogenous IL-2 restores the generation of Tregs and tumor growth. To this end, we injected recombinant IL-2 intra-peritoneally into MIF−/− mice 14 times every other day for 30 days after the tumor cell inoculation. As shown in Figs. 6A to 6C, the rate of tumor growth was partially restored in MIF−/− mice by the administration of IL-2, and this effect was associated with an increase in the frequency of CD4+Tregs in the spleen (Fig. 6C). In parallel, IL-2-injected MIF−/− mice had an increased level of GITR and CTLA-4 in tumor infiltrated CD4+T cells when compared to vehicle-treated MIF−/− mice (data not shown). The frequency of CD8+Tregs also was significantly higher in the spleens of MIF−/− mice treated with IL-2 than in those of control mice (Fig. 6D). Together, these results indicate that IL-2 promotes the generation of CD4+ and CD8+Tregs, which in turn may contribute to the restoration of tumor growth in MIF−/− mice.
Figure 6. Recombinant IL-2 reverses tumor growth and Treg generation in MIF−/− mice.
(A) Tumor-bearing MIF−/− mice were injected with recombinant 2,500 IU IL-2 (IL-2, n=3), or 12,500IU IL-2 (5×IL-2, n=9) 14 times every other day for 30 days every day after the tumor cell inoculation. MIF+/+ (n=5) and MIF−/− mice (n=9) injected with vehicle alone were used as a control. Tumor growth rate was monitored every day. *, P < 0.05. (B) Appearance of vehicle-treated MIF+/+ mice, vehicle-treated MIF−/− mice, and IL-2-injected MIF−/− mice at 4 weeks after tumor cell inoculation. (C and D) The percentage of CD4+ and CD8+Tregs in the spleens and tumor tissues of IL-2-treated MIF−/− mice (n=3 for IL-2, n=9 for 5×IL-2), vehicle-treated MIF−/− (n=9), and vehicle-treated MIF+/+ mice (n=5), determined by flow cytometry analysis.
Discussion
Tumor-infiltrating immune cells are crucial to combating cancer and their activity correlates with disease prognosis and survival (32, 33). The identification of antigens from various tumors that are recognized by T cells further supports the importance of anti-tumor immunity and sets the stage for the development of more effective and antigen-specific cancer immunotherapies (34). Various clinical trials indicate that immunotherapy with cancer antigen can induce antigen-specific immune responses in the majority of patients (34, 35), but such immune responses are too weak or transient to produce therapeutic benefit to cancer patients. One of the factors contributing to this therapeutic failure is the presence of Tregs (12). Tregs induce immune tolerance by suppressing host immune responses against self- or non-self antigens; thus, limiting anti-tumor immunity and promoting tumor growth (6–12).
MIF was discovered as a cytokine secreted by activated T cells (1). Bacher et al. have reported that MIF plays an important regulatory role in the activation of T cells (2). Mitogen- or antigen-activated T cells express significant quantities of MIF mRNA and protein, and neutralization of MIF inhibits IL-2 production and T cell proliferation in vitro, decreasing the TH cell response to soluble Ag in vivo (2). It also has been shown that neutralizing anti-MIF Ab inhibits T cell proliferation and IL-2 production in vitro, and suppresses antigen-driven T cell activation and Ab production in vivo (36). However, the effect of MIF on tumor-associated Tregs remains undefined. In this study, we demonstrated first that the growth rate of the CT26 colon carcinoma was significantly lower in MIF−/− mice than in MIF+/+ mice. MIF−/− mice showed a higher infiltration of T cells in tumor tissues, whereas MIF deficient hosts had lower levels of tumor-associated Tregs in the spleen and tumor tissue than MIF+/+ mice. The decrease in tumor growth and Treg infiltration into the tumor tissue was similarly noted in MIF−/− mice implanted with 4T1 breast cancer cells, suggesting that the effect of host MIF on tumor growth and Treg production is not limited to CT-26 colon cancer. Together, these results suggest that MIF plays a role in the generation of Tregs in tumor-bearing mice.
Given that MIF promotes angiogenesis (4,14), it is noteworthy that T cell infiltration into tumor tissues was enhanced in MIF−/− mice, where angiogenesis is markedly repressed (37). Since Tregs inhibit the proliferation and activation of T cells by tumor antigens (38), a decrease in Tregs in MIF−/− mice may increase T cell activation and proliferation triggered by tumor antigens in the tumor tissues, leading to a reduction in tumor mass by a cytotoxic T lymphocyte (CTL) response. It is also a possibility that MIF has a role in the regulation of anti-tumor T cell trafficking. The finding that anti-MIF Ab promotes the migration of T lymphocytes into EG.7 tumors and augments CD8+ T cell specific anti-tumor activity supports this notion (5).
A direct inhibitory function for MIF in TH1-dependent CTL responses also has been described by Abe et al.(5). These authors found that administration of a neutralizing anti-MIF mAb to tumor-bearing mice significantly increases the CTL response by splenic cells, but the exact mechanism of this effect remains unclear. By using a co-culture system of CT26 cells and sorted CD8+ T cells, we demonstrated that CD8+ effector cells from MIF−/− mice show a significant increase in an anti-CT26 CTL response that was accompanied by a decrease in CD8+Tregs. In these tumor-bearing mice, CD8+Tregs expressed both CD25 and Foxp3 molecules, which are shared by CD4+Tregs. CD8+Tregs have been identified to mediate immuno-suppression in cancer and other diseases (39, 40), although some groups have questioned the immunosuppressive properties of these cells (41). For example, CD8+Tregs suppress antigen-activated CD4+ T cells in a TCR-specific manner restricted by the MHC class Ib molecule, Oa-1 (40, 42). In colorectal cancer, CD8+CD25+Foxp3+Tregs are present in tumor-infiltrating lymphocytes and suppress anti-tumor T cell immunity (43). Our results, together with previous reports (40, 42, 43), suggest that MIF promotes the generation of CD8+Tregs, which may in turn play a role in promoting tumor growth by decreasing CTL response.
Another possibility for the increased CD8 cytotoxicity in MIF−/− mice could be alteration in antigenicity. It has been reported that MIF mediates many of its inflammatory properties by interacting with the CD74-CD44 MIF receptor complex (44). We and others have demonstrated that the CT26 cell line expresses MIF (ref. 3 and Fig. 1F) as well as the MIF binding receptor CD74 (45). We also found that these cells express the MIF signaling receptor component, CD44, as determined by flow cytometry analysis (data not shown). Therefore, tumor-derived MIF in MIF−/− mice may increase the antigenicity of the tumor in an autocrine manner to enhance an CD8+ T cell, anti-tumor immune response. If this were the case, then CT26 cells may be rejected by tumor infiltrating CD8 T cells in MIF −/− mice (as seen in Fig. 2D) via recognition of MHC class I associated MIF derived peptides. In a similar way, it is also possible that the MIF−/− mice might not be completely syngeneic with the tumors.
What is the possible mechanism by which the generation of CD4+ and CD8+Tregs is suppressed in the CT26 colon carcinoma-bearing MIF−/− mice? Recent studies have linked chronic inflammation to cancer progression (46–49). Cancer is concomitantly associated with inflammation that may create a specific cytokine environment favoring the expansion of Tregs (50, 51). Suppressive cytokines such as IL-10, TGF-β, and IL-2 that are secreted by tumor cells, tumor-infiltrating T cells, and macrophages, not only recruit Tregs to tumor sites but also favor the conversion of non-suppressive T cells into Tregs with suppressive function (50, 51). Our work underscores the importance of MIF, as an upstream of IL-2, in the generation of Tregs and in the suppression of tumor immunity. We demonstrated that IL-2 secretion by splenic cells stimulated with anti-CD3/CD28 Ab was significantly lower in MIF−/− mice, and that addition of recombinant MIF restored IL-2 release by MIF−/− splenocytes stimulated with anti-CD3 Ab. Conversely, neutralizing anti-MIF Ab or the small molecule, Debio1036, which inhibits the interaction between MIF and its receptor CD74, blocked anti-CD3-induced IL-2 production by splenocytes of MIF+/+ mice and suppressed the inducible CD4+Treg generation. Moreover, administration of recombinant IL-2 into tumor-bearing MIF−/− mice restored the generation of Tregs and tumor growth. These observations indicate that MIF controls the generation of CD4+ and CD8+ Tregs through the induction of IL-2, suggesting that IL-2 play a critical role in the activation and maintenance of tumor-associated Tregs in vivo, particularly in MIF-deficient conditions.
It is unexpected that inducible CD4+ and CD8+Treg responses by stimulation with anti-CD3/CD28 Ab+IL-2+TGF-β were greater in MIF−/− mice than in MIF+/+ mice. It has been reported that in EG.7 tumor-bearing mice, anti-MIF Ab treatment significantly enhances expression of the common γc chain (CD122) of IL-receptor complex (5), which is required for intracellular signaling of IL-2 (29, 30) and mature CD8+ T cell survival. However, we did not find any difference in the expression of γc chain in the CD4+ and CD8+ T cells stimulated without or with anti-CD3/CD28 or with anti-CD3/CD28 Ab+IL-2+TGF-β between the two groups of mice. Therefore, it seems likely that an increase in inducible Tregs response in MIF−/− mice is not due to γc chain over-expression in the CT26 colon carcinoma model, and other mechanisms, such as a compensatory increase in IL-2 signaling efficiency, may be involved in this process.
With regard to cancer biology, a better understanding how Tregs are generated in the tumor microenvironment and how their suppressive function can be blocked is fundamentally important to improve the therapeutic potential of cancer vaccines. Our data suggest that MIF promotes tumor growth by increasing Treg generation and upregulating IL-2 production. These findings raise the possibility that MIF may be employed therapeutically to augment the suppressive function of Tregs in human disease, and that anti-MIF Ab might be useful to enhance the adaptive immune response against certain cancers.
Supplementary Material
Acknowledgements
We thank all members of the Institute of Bone and Joint Diseases at the Catholic University of Korea.
This work was supported by grants from the Korea Healthcare technology R&D Project, Ministry for Health, Welfare and Family Affairs (No. A092258), and National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (R33-2008-000-10064-0 and 2009-0080087), and NIH (R01 AR050498)
References
- 1.David JR. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966;56:72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacher M, Metz CN, Calandra T, Mayer K, Chesney J, Lohoff M, Gemsa D, Donnelly T, Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996;93:7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun B, Nishihira J, Yoshiki T, Kondo M, Sato Y, Sasaki F, Todo S. Macrophage migration inhibitory factor promotes tumor invasion and metastasis via the Rho-dependent pathway. Clin Cancer Res. 2005;11:1050–1058. [PubMed] [Google Scholar]
- 4.Shimizu T, Abe R, Nakamura H, Ohkawara A, Suzuki M, Nishihira J. High expression of macrophage migration inhibitory factor in human melanoma cells and its role in tumor cell growth and angiogenesis. Biochem Biophys Res Commun. 1999;264:751–758. doi: 10.1006/bbrc.1999.1584. [DOI] [PubMed] [Google Scholar]
- 5.Abe R, Peng T, Sailors J, Bucala R, Metz CN. Regulation of the CTL response by macrophage migration inhibitory factor. J Immunol. 2001;166:747–753. doi: 10.4049/jimmunol.166.2.747. [DOI] [PubMed] [Google Scholar]
- 6.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 7.Piersma SJ, Welters MJ, van der Burg SH. Tumor-specific regulatory T cells in cancer patients. Hum Immunol. 2008;69:241–249. doi: 10.1016/j.humimm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19:217–223. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 10.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6:577–583. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 11.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 12.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 13.Mizue Y, Ghani S, Leng L, McDonald C, Kong P, Baugh J, Lane SJ, Craft J, Nishihira J, Donnelly SC, Zhu Z, Bucala R. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci U S A. 2005;102:14410–14415. doi: 10.1073/pnas.0507189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesney J, Metz C, Bacher M, Peng T, Meinhardt A, Bucala R. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol Med. 1999;5:181–191. [PMC free article] [PubMed] [Google Scholar]
- 15.Taetle R, Rosen F, Abramson I, Venditti J, Howell S. Use of nude mouse xenografts as preclinical drug screens: in vivo activity of established chemotherapeutic agents against melanoma and ovarian carcinoma xenografts. Cancer Treat Rep. 1987;71:297–304. [PubMed] [Google Scholar]
- 16.Bernhagen J, Mitchell RA, Calandra T, Voelter W, Cerami A, Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF) Biochemistry. 1994;33:14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- 17.Hare AA, Leng L, Gandavadi S, Du X, Cournia Z, Bucala R, Jorgensen WL. Optimization of N-benzyl-benzoxazol-2-ones as receptor antagonists of macrophage migration inhibitory factor (MIF) Bioorganic & Medicinal Chemistry Letters. 2010;20:5811–5814. doi: 10.1016/j.bmcl.2010.07.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 19.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 20.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 21.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Pautu JL, Kumar L. Intratumoral T cells and survival in epithelial ovarian cancer. Natl Med J India. 2003;16:150–151. [PubMed] [Google Scholar]
- 23.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 24.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 25.Li Q, Carr A, Ito F, Teitz-Tennenbaum S, Chang AE. Polarization effects of 4-1BB during CD28 costimulation in generating tumor-reactive T cells for cancer immunotherapy. Cancer Res. 2003;63:2546–2552. [PubMed] [Google Scholar]
- 26.Akazawa T, Masuda H, Saeki Y, Matsumoto M, Takeda K, Tsujimura K, Kuzushima K, Takahashi T, Azuma I, Akira S, Toyoshima K, Seya T. Adjuvant-mediated tumor regression and tumor-specific cytotoxic response are impaired in MyD88-deficient mice. Cancer Res. 2004;64:757–764. doi: 10.1158/0008-5472.can-03-1518. [DOI] [PubMed] [Google Scholar]
- 27.Caldwell SA, Ryan MH, McDuffie E, Abrams SI. The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases. J Immunol. 2003;171:2402–2412. doi: 10.4049/jimmunol.171.5.2402. [DOI] [PubMed] [Google Scholar]
- 28.Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant JC, Menegaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut. 2009;58:520–529. doi: 10.1136/gut.2008.158824. [DOI] [PubMed] [Google Scholar]
- 29.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 30.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 31.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 33.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang RF. Enhancing antitumor immune responses: intracellular peptide delivery and identification of MHC class II-restricted tumor antigens. Immunol Rev. 2002;188:65–80. doi: 10.1034/j.1600-065x.2002.18807.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos L, Hall P, Metz CN, Bucala R, Morand EF. Role of macrophage migration inhibitory factor (MIF) in murine antigen-induced arthritis: interaction with glucocorticoids. Clin. Exp. Immunol. 2001;123:309–314. doi: 10.1046/j.1365-2249.2001.01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson JM, Coletta PL, Cuthbert RJ, Scott N, MacLennan K, Hawcroft G, Leng L, Lubetsky JB, Jin KK, Lolis E, Medina F, Brieva JA, Poulsom R, Markham AF, Bucala R, Hull MA. Macrophage migration inhibitory factor promotes intestinal tumorigenesis. Gastroenterology. 2005;129:1485–1503. doi: 10.1053/j.gastro.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 38.Shevach EM. Fatal attraction: tumors beckon regulatory T cells. Nat Med. 2004;10:900–901. doi: 10.1038/nm0904-900. [DOI] [PubMed] [Google Scholar]
- 39.Jiang H, Chess L. An integrated view of suppressor T cell subsets in immunoregulation. J Clin Invest. 2004;114:1198–1208. doi: 10.1172/JCI23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarantopoulos S, Lu L, Cantor H. Qa-1 restriction of CD8+ suppressor T cells. J Clin Invest. 2004;114:1218–1221. doi: 10.1172/JCI23152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer CT, Floess S, Baru AM, Lahl K, Huehn J, Sparwasser T. CD8+ Foxp3+ T cells share developmental and phenotypic features with classical CD4+ Foxp3+ regulatory T cells but lack potent suppressive activity. Eur J Immunol. 2011;41:716–725. doi: 10.1002/eji.201040913. [DOI] [PubMed] [Google Scholar]
- 42.Jiang H, Chess L. The specific regulation of immune responses by CD8+T cells restricted by the MHC class Ib molecule, Qa-1. Annu Rev Immunol. 2000;18:185–216. doi: 10.1146/annurev.immunol.18.1.185. [DOI] [PubMed] [Google Scholar]
- 43.Rifa'i M, Kawamoto Y, Nakashima I, Suzuki H. Essential roles of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity. 2006;25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maharshak N, Cohen S, Lantner F, Hart G, Leng L, Bucala R, Shachar I. CD74 is a survival receptor on colon epithelial cells. World J. Gastroenterol. 2010;16:3258–3266. doi: 10.3748/wjg.v16.i26.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 48.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 49.Bucala R, Donnelly SC. Macrophage migration inhibitory factor: a probable link between inflammation and cancer. Immunity. 2007;26:281–285. doi: 10.1016/j.immuni.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 50.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 51.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.