Abstract
Adoptive T cell therapy (ACT) for the treatment of established cancers is actively being pursued in clinical trials. However, poor in vivo persistence and maintenance of anti-tumor activity of transferred T cells remain major problems. Transforming growth factor beta (TGFβ) is a potent immunosuppressive cytokine that is often expressed at high levels within the tumor microenvironment, potentially limiting T cell mediated anti-tumor activity. Here, we used a model of autochthonous murine prostate cancer to evaluate the effect of cell intrinsic abrogation of TGFβ signaling in self/tumor specific CD8 T cells used in ACT to target the tumor in situ. We found that persistence and anti-tumor activity of adoptively transferred effector T cells deficient in TGFβ signaling was significantly improved in the cancerous prostate. However, over time, despite persistence in peripheral lymphoid organs, the numbers of transferred cells in the prostate decreased and the residual prostate infiltrating T cells were no longer functional. These findings reveal that TGFβ negatively regulates the accumulation and effector function of transferred self/tumor specific CD8 T cells and highlight that, when targeting a tumor antigen that is also expressed as a self-protein, additional substantive obstacles are operative within the tumor microenvironment, potentially hampering the success of ACT for solid tumors.
Introduction
The recent FDA approval of two cancer immunotherapies, a vaccine (Sipuleucel-T) for treatment of prostate cancer (1), and an anti-CTLA-4 blocking antibody (ipilimumab) for treatment of metastatic melanoma (2), has highlighted the ability to modulate the immune system to attack tumors. An alternative therapeutic strategy, which is being actively pursued in multiple clinical settings, is adoptive T cell therapy (ACT), in which tumor-reactive T cells are generated and/or expanded ex vivo from T cells isolated from the blood or tumor of cancer patients and then infused back into the patient (3). Although efficacy has clearly been demonstrated (4–6), difficulty sustaining adequate numbers and function of tumor-reactive T cells following transfer into patients has hindered success (7). This in part reflects immunosuppressive tumor microenvironments, which can inhibit rather than stimulate potentially effective anti-tumor T cell responses (8). Tumor cells can express inhibitory ligands for T cells and recruit inhibitory cells, and both can secrete immunosuppressive cytokines that render tumor-infiltrating lymphocytes (TILs) unresponsive or dysfunctional (8). Furthermore, T cells isolated directly from the patient for use in ACT are often of only low avidity, since most of the identified tumor antigens are self-proteins and endogenous self/tumor specific T cells that bear high affinity TCRs are deleted in the thymus (9, 10). However, one potential advantage of ACT over in vivo augmentation of endogenous responses is the ability to genetically engineer T cells to improve function prior to infusion, such as by expressing high affinity tumor-specific TCRs, abrogating T cell intrinsic negative regulators, or disrupting inhibitory signaling pathways that may be engaged in the tumor microenvironment (9, 11).
Transforming growth factor beta (TGFβ) is a pleiotropic cytokine that plays important roles in maintaining normal tissue homeostasis and inhibiting autoimmune responses, and depending on the context can promote or suppress tumor growth (12–17). The bioactive form of TGFβ binds to the TGFβ-type I and TGFβ-type II serine/threonine kinase receptor complexes, resulting in receptor mediated phosphorylation of downstream transcription factors Smad 2 and Smad 3 (17). TGFβ signaling is anti-proliferative, causing G1 cell cycle arrest in a variety of cell types, including epithelial and T cells (18, 19). Many tumors evade the cytostatic and anti-proliferative effects of TGFβ by acquiring mutations in the TGFβ receptor and/or downstream Smad signaling proteins (17). Activated T cells however, express higher levels of the TGFβ receptor and can produce TGFβ (20, 21). Molecular analysis of naïve CD8 T cells in vitro has revealed that TGFβ suppresses key molecules involved in the effector and cytolytic activities of T cells, including expression of IFNγ (22). Inhibition of TGFβ signaling by mechanisms such as neutralizing antibodies or kinase inhibitors is being pursued in clinical trials (23), but significant therapeutic benefits have not yet been reported. This may partly reflect failure to achieve full blockade of TGFβ, particularly in tumor tissues. Moreover, administering these agents at doses high enough to sustain full blockade may be too toxic. In the context of ACT, it would be possible to selectively abrogate the potentially profound immunosuppressive activity of TGFβ only in the T cells being used to target the tumor.
Prostate cancer is currently being pursued as a target for expanding applicability of T cell mediated immunotherapy. In large part this reflects identification of immunogenic prostate-restricted antigens that are expressed in malignant and normal prostate tissues but not other tissues that might be potential targets of toxicity, and that can elicit cytolytic T cell responses (24). However, TGFβ is present and necessary for normal prostate homeostasis, and is found in increased levels in the malignant prostate (25, 26), which can pose a substantive obstacle to T cell therapy of this tumor. Expression of a dominant negative form of TGFβRII (DNR-TGFβRII) or abrogation of TGFβ production exclusively in T cells of mice that develop autochthonous prostate cancer can delay tumor growth (21, 27), suggesting TGFβ interferes with the development and/or expression of an endogenous response. Studies in transplantable tumor models also demonstrated that TGFβ signaling blockade improves the therapeutic efficacy of tumor-reactive T cells (28–30).
Many tumor therapy studies have been performed using transplantable tumor cell lines, and such models, while advancing the discovery and testing of tumor therapies, have limitations. Injection of a large number of tumor cells is often necessary for successful implantation, with many cells dying rapidly after injection, which can induce an immune response prior to establishment of the tumor (31). More importantly, these tumors do not develop in the same organ-specific environment of tumors that develop and grow in situ. Autochthonous tumor models, in which the tumor develops “spontaneously” usually from enforced expression of a driver oncogene, also have some limitations, but do allow study of tumors derived from the organ of origin that develop over months in the context of a normal host immune system. Therefore, we used the Transgenic Adenocarcinoma of the Mouse Prostate (TRAMP) mouse, which expresses the SV40 T antigen under the prostate-specific probasin promoter, resulting in spontaneously arising prostate adenocarcinoma (32). The pathogenesis of prostate cancer in these mice has been well studied and models many aspects of human prostate cancer, including development of prostate intraepithelial neoplasia by 12 weeks of age and progression through distinct histological stages of adenocarcinoma (33, 34). We crossed these mice with the Probasin Ovalbumin Expressing Transgenic (POET1) mice, which express a membrane bound form of the model antigen ovalbumin (OVA) driven by the prostate-specific ARR2PB rat probasin promoter (35). TRAMPxPOET1 mice, denoted TRAMPOVA, express a targetable self/tumor antigen (OVA) in the context of a spontaneously arising prostate cancer. The use of OVA as a model self/tumor antigen allowed analysis of the efficacy in ACT of high affinity OVA-specific CD8 T cells, derived from OTI TCR transgenic mice (36), and of targeting a prostatic self-antigen with T cells in which TGFβ signaling has been abrogated to overcome a potentially substantive obstacle to antitumor activity in the environment of a cancerous prostate gland.
Materials and Methods
Mice
TRAMP mice (32) and were obtained from N. Greenberg (Fred Hutchinson Cancer Research Center (FHCRC), Seattle, WA). POET mice (35) and were obtained from T. Ratliff (Purdue University, West Lafayette, IN). TGFβRIIFlx/Flx mice were provided by D. Dichek (University of Washington (UW), Seattle, WA) with permission from S. Karlsson (Lund University, Lund, Sweden) (12). Distal Lck-Cre (d-lckCre) mice (37), which express Cre recombinase under control of the distal Lck promoter, were provided by P. Fink (UW, Seattle, WA) with permission from N. Killeen (University of California, San Francisco, CA). OTI TCR transgenic mice (36) containing CD8 T cells specific for the immunodominant epitope (SIINFEKL) of ovalbumin (OVA), were a gift from M. Bevan (UW, Seattle, WA). Ly5.1 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). To generate prostate cancer mice expressing a targetable self/tumor antigen, TRAMP+/+ male mice were crossed to female POET1+/− to generate to generate F1 mice hemizygous for the SV40 transgene and OVA expression (TRAMPOVA), and littermates expressing SV40 transgene only (TRAMP). All TRAMPOVA and TRAMP mice used were between 25–27 weeks of age, at which time all mice have high grade neoplasia (34). To generate OVA specific TGFβRII deficient mice (TGFβRII KO), mice expressing floxed TGFβRII genes (TGFβRIIFlx/Flx) were first bred to d-lckCRE mice or OTILy5.1 mice. The F1 offspring were bred together to produce mice harboring OTILy5.1 CD8 T cells with a conditional deletion of TGFβRII in mature CD8 T cells (OTILy5.1×TGFβRIIFlx/Flx×d-lckCRE). OTILy5.1×TGFβRIIFlx/Flx littermates were used as WT donors. All mice were maintained under specific pathogen-free conditions at the UW under the guidelines of the Institutional Animal Use and Care Committee.
Peptide
SIINFEKL peptide was synthesized by the Immune Monitoring Lab at FHCRC (Seattle, WA). Peptide was reconstituted in 100% DMSO at 10mg/ml and stored at −20°C.
Cell isolation
Mice were euthanized by cervical dislocation. Spleens were mechanically disrupted with the back of a 3mL syringe, filtered through a 70 µm strainer and red blood cells lysed with ammonium chloride potassium buffer. Cells were washed twice with complete RPMI media (RPMI 1640 supplemented with 2 µM glutamine, 100U/ml penicillin/streptomycin and 10% fetal calf serum). Prostate draining lymph nodes (peri-aortic; PDN) were dissociated with microscope slides. Prostate lobes were microdissected and weighed. Individual lobes were divided in half, with half used for histology and half digested with collagenase D (Roche) and DNAse I (Fermenta) for 1 hour at 37°C. Digested tissue was mechanically disrupted through a 40 µm strainer.
In vitro activation and adoptive transfer
Single cell suspensions were generated from spleens of OTILy5.1 WT and OTILy5.1 TGFβRII KO mice. CD4 and B cells were depleted using αCD4 and αB220 DynaBeads (Invitrogen). Remaining cells were co-cultured with irradiated (3000 rads) congenic splenocytes pulsed with SIINFEKL (10−1 µg/ml) at a 1:5 ratio and 25 U/mL human recombinant interleukin 2 (IL2, National Institute of Allergy and Infectious Diseases) in complete RPMI media. On day 5, OTI cells, which express the TCR chains Vα2 and Vβ5, were quantitated based on cell count and percent of 7AAD−CD8+Vα2+Vβ5+ cells by flow cytometry. Cells were washed twice with HBSS prior to injection of 5–7×106 OTI cells into the lateral tail vein of mice at a volume of 0.2mL.
Flow cytometry
All single cell suspensions were washed with staining buffer (PBS + 1% fetal calf serum) prior to phenotypic and functional characterization. The following antibodies were purchased from eBiosciences: CD8α, Ly5.1, IFNγ, TNFα, and PD-1. Surface staining was done at 4°C in staining buffer. Ki-67 (BD Biosciences) and Bim (Cell Signaling) staining was performed using the eBiocience fixation/permeabilization buffer kit per manufacturer’s instructions. Briefly, following surface staining with CD8 and Ly5.1 antibodies, cells were fixed, permeabilized and stained with antibody to Ki-67 and Bim. A secondary PE-anti-rabbit Fab2 fragment (Invitrogen) was used to detect Bim. Intracellular cytokine staining was performed using the Cyofix/Cytoperm Plus kit (BD Biosciences) per manufacturer’s instructions. Briefly, single cell suspensions from spleen, lymph node, and prostate were stimulated directly ex vivo for 5 hours with 10−1 µg/ml SIINFEKL peptide and congenic (Ly5.2+) splenocytes in the presence of Brefeldin A. Following surface staining with CD8 and Ly5.1, cells were fixed, permeabilized and stained with antibodies to IFNγ and TNFα. Flow cytometric analysis was performed using FACSCanto and LSRII at the Cell Analysis Facility, Department of Immunology, UW. Flow data was analyzed with FlowJo8.8.7 (Tree Star, Inc, Ashland, OR).
Prostate histology and immunohistochemistry
For hematoxylin and eosin (H&E) staining, microdissected prostate lobes were fixed in 4% paraformaldehyde then stored in 70% ethanol until processed by the Experimental Histopathology core at the FHCRC, Seattle, WA. Histologic sections were evaluated by a comparative medicine pathologist blinded to group assignments. Images were captured using a Nikon Eclipse 80i microscope with DS-Fi1 digital camera and NIS Elements software.
For immunofluorescence staining, microdissected prostate lobes were frozen in optimal cutting temperature (Sakura). Seven µM frozen prostate sections were cut on a cryostat. Sections were fixed with ice-cold acetone and blocked with PBS + 1% goat serum prior to staining. Primary antibodies included: Ly5.1-PE (eBioscience), rat anti-mouse PD-L1 (eBioscience), MHC Class I-PE (eBioscience) and rat IgG2a isotype control (eBioscience). When required, secondary goat anti-rat-alexa fluor 488 (Invitrogen) was used. All slides were counterstained with DAPI (Invitrogen). Slides were analyzed on a Leica fluorescence microscope, and photographic images captured with an Orca-ER digital camera and assembled into RGB images with Image J and Adobe Photoshop.
Antibody blockade treatment
Monoclonal αPD-1 (29F.1A12) (38), αPD-L1 (10F.9G2) (39) and αPD-L2, (3.2) (40) antibodies were provided by G. Freeman (Harvard Medical School, Boston, MA). To assure adequate blockade, the timing and dose of administration of these antibodies established for each individual antibody (41) was used. 200 µg of each blocking antibody was injected i.p. into recipient mice starting on the day of T cell transfer and continued every 3 days until mice were euthanized.
Statistical Analysis
Bar graphs are displayed as mean ± SEM. Statistical analyses were performed with Prism version 5.0, GraphPad Software, using unpaired two-tailed Student t tests. A p value of <0.05 was considered statistically significant.
Results
Abrogation of TGFβ signaling increases the accumulation of transferred prostate self/tumor antigen-specific CD8 T cells
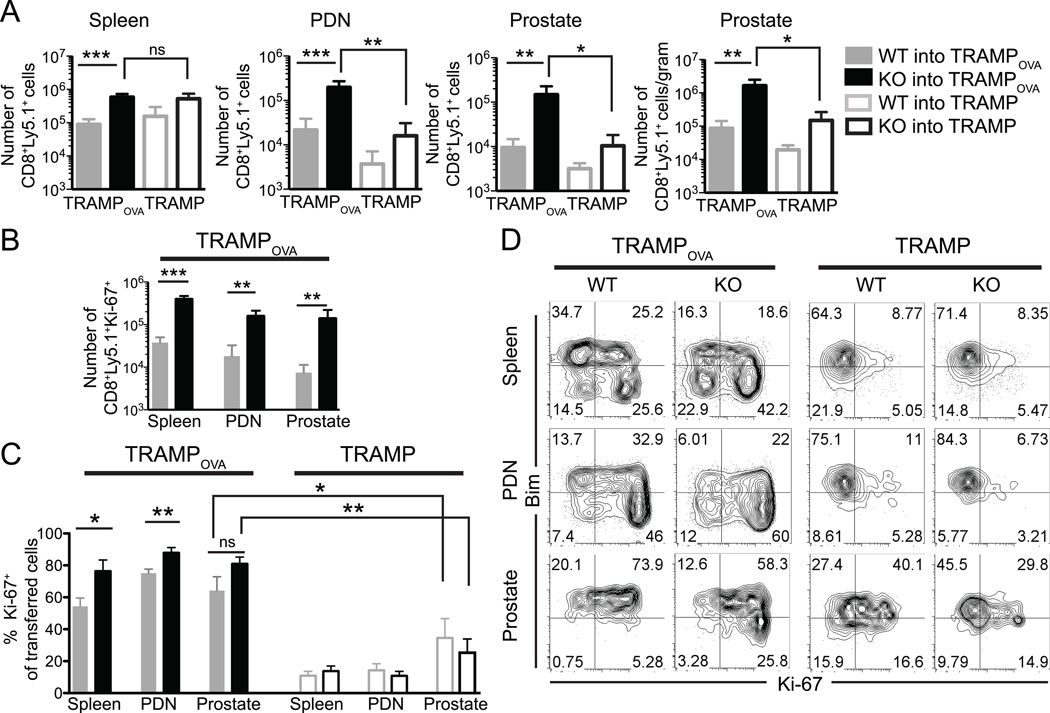
To investigate the T cell intrinsic role of TGFβ in the setting of ACT of prostate cancer, we transferred 5–7×106 in vitro activated OTI WT and TGFβRII KO CD8 T cells into tumor bearing 25–27 week old TRAMPOVA and TRAMP males. We first assessed if abrogating TGFβ signaling affected expansion of the transferred cells and found a significantly increased accumulation of TGFβRII KO cells compared to WT cells in the spleen, PDN and prostate of TRAMPOVA mice 1 week post transfer (Fig. 1A). To account for potential differences in prostate size, cells/gram of prostate tissue was also calculated, and a similar increase of TGFβRII KO cells was observed. To determine if the preferential accumulation of TGFβRII KO cells was antigen-specific, WT and TGFβRII KO cells were also transferred into TRAMP hosts (which do not express OVA in the prostate). Significantly less TGFβRII KO cells were detected in the PDN and prostate of TRAMP mice compared to TRAMPOVA mice (Fig. 1A), and there was no significant difference between the numbers of WT cells in TRAMPOVA compared to TRAMP mice or between the numbers of WT and TGFβRII KO cells in any of the tissues examined in TRAMP mice. This data suggests cell intrinsic TGFβ signaling negatively impacts the accumulation of prostate self/tumor antigen-specific CD8 T cells in the context of responding to self-antigen.
Figure 1. Increased accumulation of TGFβRII deficient prostate self/tumor antigen specific CD8 effector T cells in TRAMPOVA mice.
(A) 5–7×106 effector WT or TGFβRII KO cells were transferred i.v. into 25–27 week old TRAMPOVA and TRAMP hosts. Mice were euthanized 1 week post transfer and spleen, PDN and prostates were analyzed. (A) Cell numbers were quantitated based on total cell counts and percent of CD8+Ly5.1+ cells from flow cytometric analysis. For the prostate, numbers of transferred cells/gram of tissue is also shown. No significant differences were detected between WT and TGFβRII KO cells from each organ in TRAMP mice. (B) Numbers of CD8+Ly5.1+Ki-67+ WT and TGFβRII KO cells isolated from various organs in TRAMPOVA mice (C). Percent of transferred T cells expressing Ki-67 in the spleen, PDN and prostate of TRAMPOVA and TRAMP mice. No significant differences were detected between WT and TGFβRII KO cells from each organ in TRAMP mice. (A–C) Data represents pooled results from at least 3 independent experiments (n=2–3 mice/group/experiment for TRAMPOVA hosts and n=1–2 mice/group/experiment for TRAMP hosts). Bar graphs include mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 (unpaired Student’s t test). (D) Representative flow plots of Ki-67 and Bim expression by transferred cells isolated from TRAMPOVA and TRAMP mice. Plots are gated on CD8+Ly5.1+ cells. Results from 2 independent experiments.
The increased accumulation of TGFβRII KO cells could be a result of increased proliferation, as TGFβ signaling can inhibit cellular proliferation (17). Intracellular staining of WT and TGFβRII KO cells directly ex vivo for the proliferation marker, Ki-67 revealed that significantly increased numbers of TGFβRII KO cells expressing Ki-67+ in the spleen, PDN and prostate of TRAMPOVA mice (Fig. 1B). The enhanced proliferation was largely antigen-specific, as Ki-67 expression was greatly reduced in all transferred cells isolated from TRAMP mice, indicating that antigen exposure induced transferred cells to remain cycling for at least 1 week (Fig. 1C). The increased percentage of Ki-67+ WT cells in TRAMPOVA mice compared to TRAMP mice despite the failure to accumulate suggested that WT cells in TRAMPOVA mice may have a higher rate of apoptosis. TGFβ signaling upregulates the BH-3 only pro-apoptotic protein Bcl-2-interacting mediator of cell death (Bim) (42, 43), and a higher percentage of TGFβRII KO cells were Bimlow compared to WT cells in all organs examined in TRAMPOVA mice, especially in the proliferating (Ki-67+) population (Fig. 1D), whereas no differences between WT and TGFβRII KO cells were observed in TRAMP mice. These results suggest abrogation of TGFβ signaling increases the accumulation of prostate self/tumor antigen-specific CD8 T cells in part through increased proliferation and in part through reduced apoptosis by decreasing expression of pro-apoptotic proteins.
Abrogation of TGFβ signaling increases the effector function of transferred prostate tumor/self antigen-specific CD8 T cells
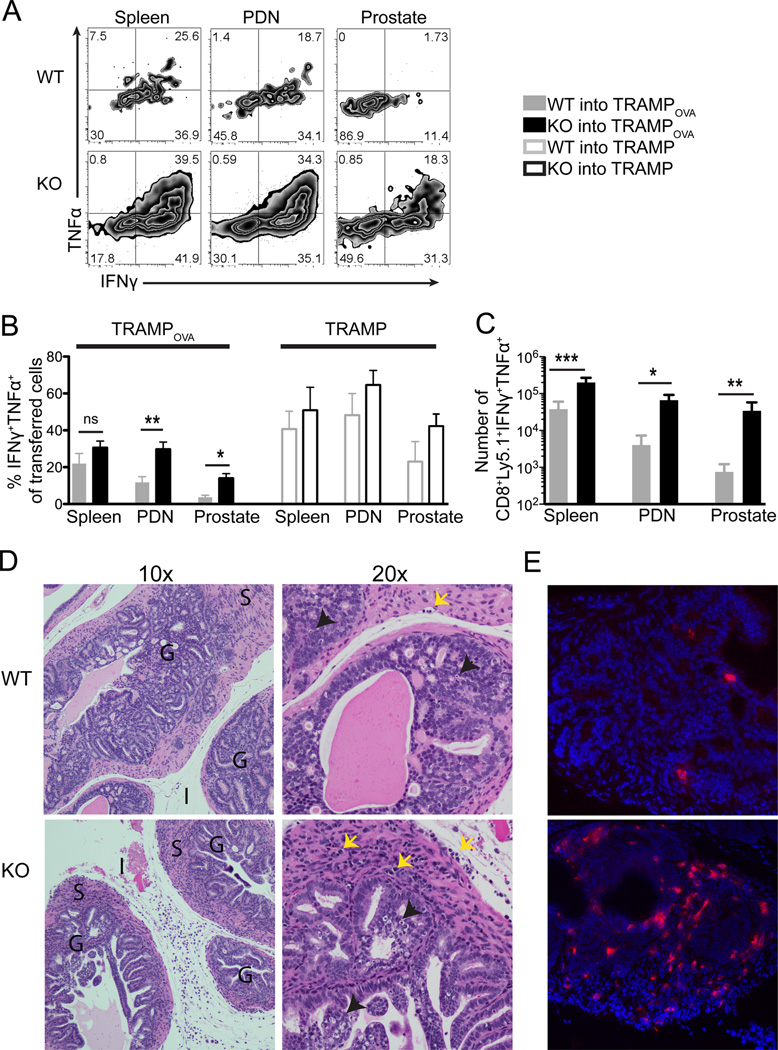
The ability of tumor-specific CD8 T cells to produce effector cytokines is critical for tumor regression (44, 45). Therefore, transferred T cells were harvested at 1 week post transfer, stimulated for 5 hours ex vivo with SIINFEKL peptide, and analyzed for cytokine production by intracellular staining. Abrogation of TGFβ signaling significantly increased the percentage and number of transferred cells capable of co-producing IFNγ and TNFα in the prostate and PDN (Fig. 2A–C). However, TGFβRII KO cells in the prostate of TRAMPOVA mice exhibited attenuated cytokine production compared to TGFβRII KO cells in the spleen, suggesting an additional TGFβ independent, organ-specific suppression of cellular function in the prostate (p=0.0018).
Figure 2. Transferred TGFβRII KO CD8 effector T cells exhibit enhanced effector function, show increased cellular infiltration and mediate epithelial damage in the prostate.
Mice were euthanized 1 week post adoptive T cell transfer (same experimental protocol as Figure 1). (A–C) Intracellular IFNγ and TNFα expression by transferred WT and TGFβRII KO cells from spleen, PDN and prostate of TRAMPOVA mice following 5 hour ex vivo stimulation with SIINFEKL peptide. Plots are gated on CD8+Ly5.1+ cells. (A) Representative flow plots of cytokine production by transferred WT and TGFβRII KO cells. Numbers represent percent of gated cells in each quadrant. (B) Percentage of transferred WT and TGFβRII KO cells exhibiting the ability to co-produce both TNFα and IFNγ. No significant differences between WT and TGFβRII KO cells from each organ in TRAMP mice. (C) Numbers of cytokine producing WT and TGFβRII KO cells in TRAMPOVA mice. (A–C) Data represents pooled results from at least 3 independent experiments (n=2–3 mice/group for TRAMPOVA hosts and n=1–2 mice/group for TRAMP hosts). Bar graphs include mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 (unpaired Student’s t test). (D–E) Prostate lobes from TRAMPOVA mice receiving either WT or TGFβRII KO cells were micro-dissected and processed for histological analysis. (D) TRAMPOVA prostate lobes were processed and stained with hematoxylin & eosin. Two magnifications are shown, 10× and 20× objectives. The presence of neoplasia in the glands (G), cellular infiltrates in the surrounding fibromuscular stroma (S) and interstitium (I) of TRAMPOVA mice receiving TGFβRII KO cells is evident at 10×. Black arrowheads point to apoptotic cells and yellow arrows point to lymphoid cells at 20×. (E) Frozen sections of TRAMPOVA prostate lobes were stained with DAPI (blue) and Ly5.1 (red); 20×. (D–E) Histology slides show one representative mouse from each experimental group (n=3–5 mice/group) from at least 2 independent experiments.
This functional impairment in the prostate was antigen-specific, as there was no significant difference in cytokine production between transferred WT and TGFβRII KO cells in any of the organs examined in TRAMP mice. However, decreased percentages of WT and TGFβRII KO cells from TRAMPOVA PDN compared to TRAMP produced cytokines (for WT p=0.0018, for KO p=0.0020) and significantly decreased percentage of TGFβRII KO cells from the prostate of TRAMPOVA compared to TRAMP mice co-produced IFNγ and TNFα (p=0.006). These results indicate that at least a component of the functional defect in cytokine production is antigen-specific, that abrogation of TGFβ signaling partially rescues the defect, and that the observed dysfunction of prostate self/tumor antigen T cells is organ-specific and rapidly induced.
T cells deficient in TGFβ signaling mediate increased cellular infiltration and focal epithelial disruption in the prostates of TRAMPOVA mice
We examined tissue sections of the prostate to determine if the increased numbers and effector function of TGFβRII KO cells compared to WT cells led to increased destruction/damage to the prostate tumors. Mice were euthanized at 1 week post-transfer, and the prostate lobes micro-dissected and either processed for H&E staining or frozen for immunofluorescence staining. The prostates of TRAMPOVA mice that received WT cells showed intact glandular and tumor epithelium with few apoptotic bodies and little evidence of cellular infiltrates in the epithelium or the fibromuscular stroma (Fig. 2D). In contrast, prostates from TRAMPOVA mice receiving TGFβRII KO cells had increased cellular infiltrates in the fibromuscular stroma, including both the interstitium and smooth muscle layer surrounding the glands, and evidence of epithelial disruption with areas of focal necrosis within the gland (Fig. 2D). The infiltrates contained adoptively transferred T cells, as immuno-histochemical staining of frozen prostate sections revealed increased Ly5.1+ cells in prostate glands of mice receiving TGFβRII KO cells compared to WT cells (Fig. 2E).
Despite evidence of increased anti-tumor activity in TRAMPOVA mice treated with TGFβRII KO cells, prostatic inflammation was not sustained
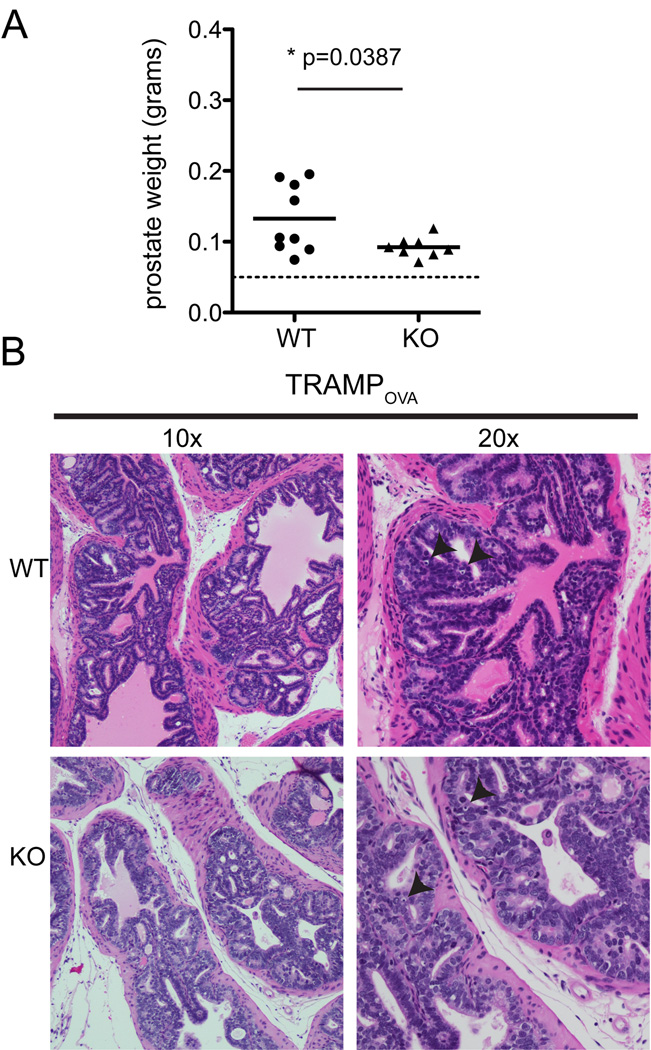
To determine if transfer of WT or TGFβRII KO cells affected tumor burden, prostates of treated mice were harvested 3 weeks post T cell transfer and weighed, with prostate weight used as a surrogate for tumor burden, as described (33). There was a small, but statistically significant, decrease in the prostate weight of TRAMPOVA mice receiving TGFβRII KO cells compared to mice receiving WT cells (Fig. 3A). However, histology specimens obtained 3 weeks post transfer showed few cellular infiltrates in the interstitium, no significant infiltration of mononuclear cells in the smooth muscle or gland, and no epithelial destruction in TRAMPOVA mice receiving WT or TGFβRII KO cells (Fig. 3B). Despite the decrease in prostate weight, neoplasia was still present in mice treated with TGFβRII KO cells. Thus, the increased infiltration of TGFβRII KO cells and anti-tumor activity observed at 1 week post-transfer in TRAMPOVA prostates was transient, and not sufficient for persistent therapeutic efficacy.
Figure 3. Cellular infiltration in the prostates of TRAMPOVA mice receiving TGFβRII KO cells was not sustained.
Prostates were microdissected and analyzed 3 weeks post transfer of WT and TGFβRII KO T cells. (A) Prostate weights at 3 weeks post T cell transfer. Dashed line marks prostate weight of age-matched healthy C57BL/6 prostate. Symbols represent individual mice and bar shows mean weight. (unpaired Student’s t test). (B) H&E staining of TRAMPOVA prostates at 3 weeks post T cell transfer show absence of cellular infiltrates and epithelial damage. Black arrowheads point to single, rare apoptotic cells.
Increased accumulation of TGFβRII KO prostate-specific T cells is sustained in the peripheral lymphoid organs but not in the prostate
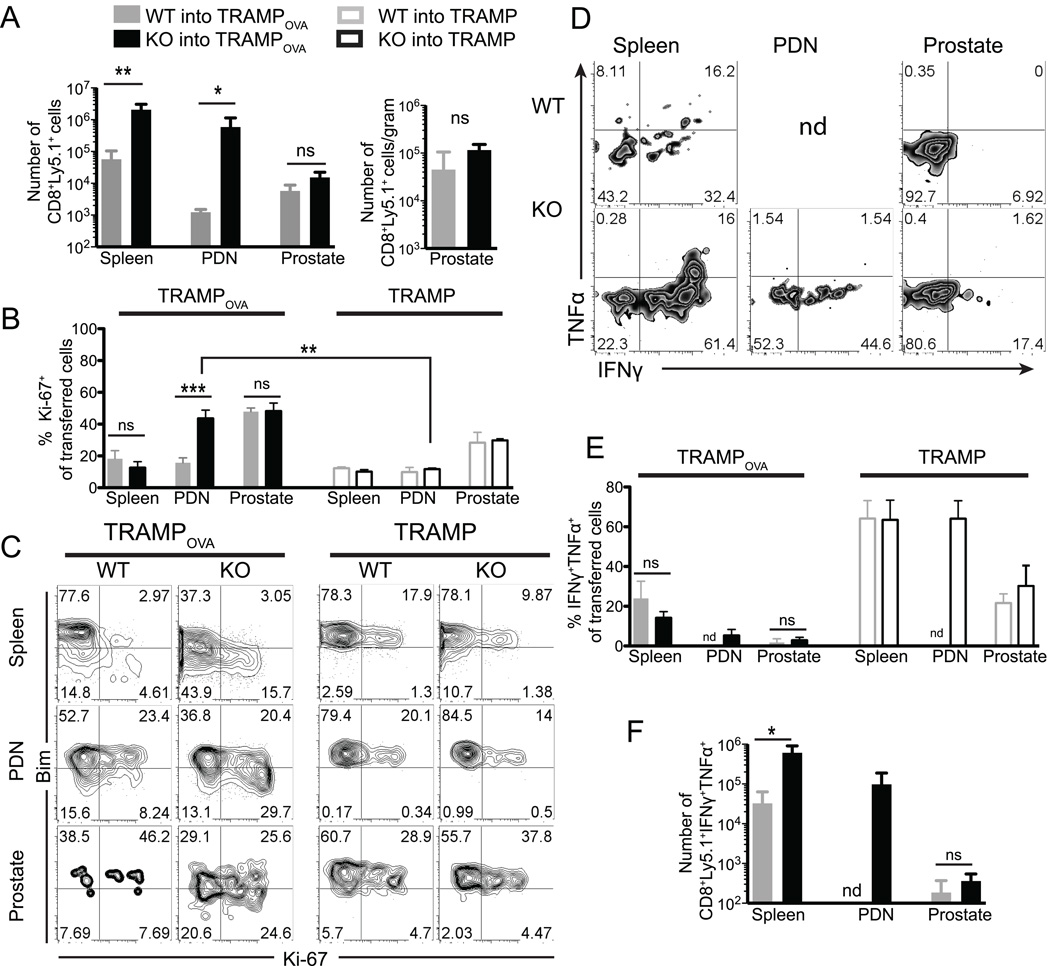
The limited efficacy suggested transferred T cells did not persist and/or became dysfunctional, obstacles also encountered in human ACT (7). To determine if the enhanced accumulation and function of TGFβRII KO cells evident at week 1 was maintained, mice were examined at week 3 post-T cell transfer. No significant differences in accumulation, proliferation or effector functions were observed between WT and TGFβRII KO cells in the prostate (Fig. 4). In contrast, increased numbers of TGFβRII KO cells compared to WT cells were still demonstrable in the spleen and PDN of TRAMPOVA mice (Fig. 4A), and there was no significant change in the number of TGFβRII KO cells in the spleen and PDN of TRAMPOVA mice at week 3 compared to week 1 (spleenwk 1: 5.4×105 cells, spleenwk 3: 2.8×106 cells, p = 0.1682; and PDNwk 1: 2×105 cells, PDNwk 3: 8.1×105 cells, p = 0.1765). Analysis of proliferation by staining for Ki-67 revealed that only in the PDN did a higher percentage of TGFβRII KO cells express Ki-67 compared to WT cells or to TGFβRII KO cells in TRAMP hosts (Fig. 4B). Similar to week 1, a higher percentage of TGFβRII KO cells were Ki-67+ Bimlow compared to WT cells in TRAMPOVA mice, but a higher fraction of TGFβRII KO cells were now Bimhigh compared to week 1 (Fig. 4C). Thus, TGFβ signaling prevents accumulation of prostate-specific cells in peripheral lymphoid organs, but additional factors beyond TGFβ signaling appear to contribute to the lack of persistence of prostate infiltrating cells.
Figure 4. TGFβRII KO cells persist up to 3 weeks in the peripheral lymphoid organs but lose function and no longer accumulate in the prostate of TRAMPOVA mice.
Mice were euthanized and analyzed 3 weeks post adoptive transfer (same experimental protocol as Figure 1) (A) Numbers of adoptively transferred WT and TGFβRII KO cells were quantitated in the spleen and PDN of TRAMPOVA mice. Total WT and TGFβRII KO cells in the prostate are also expressed as cells per gram of prostate. (B) Ki-67 expression in transferred cells at week 3 post transfer. No significant differences were detected between WT and TGFβRII KO cells from each organ in TRAMP mice. (C) Representative flow plots of Ki-67 and Bim expression by transferred cells isolated from TRAMPOVA and TRAMP mice. Flow plots are gated on CD8+Ly5.1+ cells. Results from 2 independent experiments. (D) Representative flow plots of cytokine production by WT and TGFβRII KO cells (gated on CD8+Ly5.1+ cells). Numbers represent percent of gated cells in each quadrant. (E) Percentage of transferred WT and TGFβRII KO cells that co-produce TNFα and IFNγ 5 hour ex vivo peptide stimulation. No significant differences were detected between WT and TGFβRII KO cells from each organ in TRAMP mice. (F) Number of transferred TGFβRII KO cells in each tissue that produce TNFα and IFNγ. (A–B, D–F) Results represent pooled data from at least 3 independent experiments (n=1–3 mice/group/experiment). Bar graphs show mean ± SEM. *P<0.05, **P<0.01, ***P<0.001 (unpaired Student’s t test).
By week 3 post transfer, prostate-infiltrating TGFβRII KO cells were also severely attenuated in effector cytokine production and increased dual-cytokine producing TGFβRII KO cells compared to WT cells were no longer detected in the prostate (Fig. 4D–F). Increased numbers of IFNγ+TNFα+ TGFβRII KO cells were still present in the spleen and PDN compared to WT cells (we were unable to recover sufficient numbers of WT cells from the PDN of TRAMPOVA mice at 3 weeks to analyze cytokine production) (Fig. 4F). WT and TGFβRII KO cells were also transferred into TRAMP mice and analyzed for cytokine production, revealing 2 important findings. First, similar to week 1 post-transfer, the majority of transferred cells recovered from TRAMP mice at week 3 post-transfer produced both cytokines (Fig. 2B, Fig. 4E), suggesting the decreased cytokine production by transferred cells in TRAMPOVA mice was due to persistent cognate antigen recognition. Second, both TGFβRII KO cells and WT cells isolated from the prostate showed a decreased ability to produce cytokines compared to transferred cells isolated from the spleen (TGFβRII KO cells p = 0.1446; WT cells p = 0.0370), suggesting factors within the prostate tumor microenvironment impacts the activity of these cells in an antigen-independent manner. Thus, despite persistence of TGFβRII KO cells in the periphery of TRAMPOVA mice, by week 3 TGFβRII KO cells no longer accumulate in the prostate and are severely attenuated in effector function.
TRAMPOVA prostate tumors express MHC Class I and maintain expression following adoptive transfer
MHC Class I expression is necessary for target cell destruction, sustained infiltration, and retention of CD8 lymphocytes in tissues (46), and tumor cells can down-regulate MHC Class I expression as a form of immune evasion (47). Although MHC Class I expression is not readily detectable on normal B6 prostate cells, it has been shown to be up-regulated in TRAMP prostate tumors (48). To determine if TRAMP prostate tumors maintained MHC Class I expression following cell transfer, we stained frozen prostate sections before and after transfer of WT or TGFβRII KO cells with anti-MHC Class I antibody, and found sustained Class I expression with no detectable change in TRAMPOVA prostates following therapy (Supplementary Figure 1).
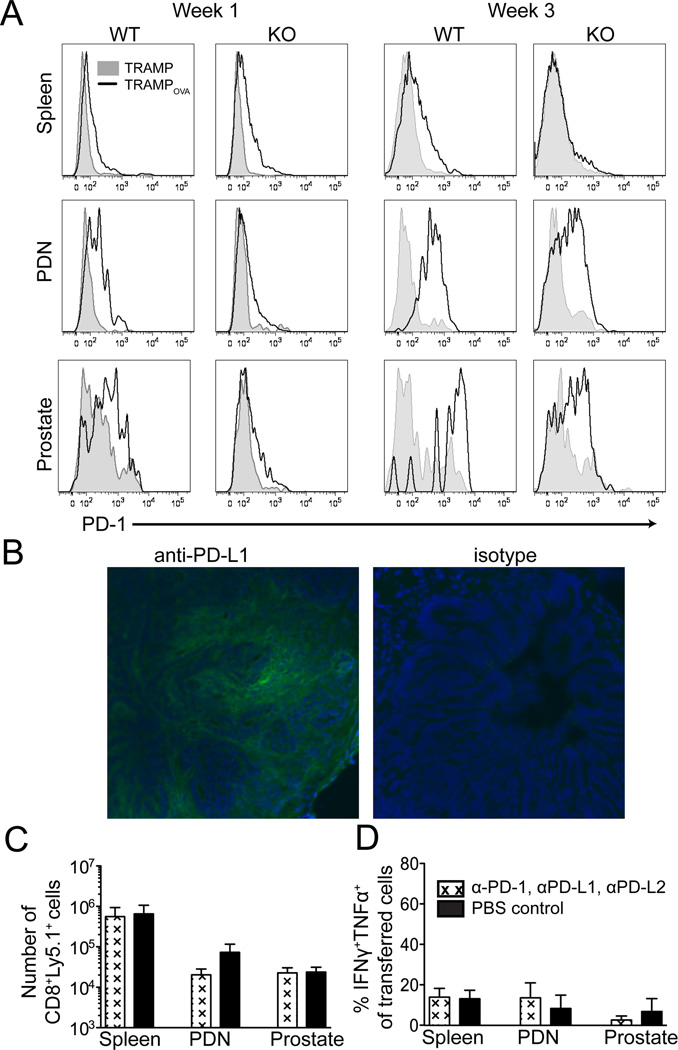
Persisting transferred TGFβRII KO T cells express PD-1 and TRAMPOVA prostates express the ligand, PD-L1
The failure of prostate-infiltrating TGFβRII KO cells to mediate continued significant prostate tumor damage, in addition to the decrease in proliferation and attenuation of effector cytokine production observed by week 3, suggested the transferred T cells might become functionally exhausted. Chronic antigen exposure can lead to T cell exhaustion (49, 50), which is characterized by a progressive hierarchical loss of CD8 T cell functions. Generally, the ability to produce IL2, maintain a high proliferative capacity, and kill targets ex vivo are lost first, followed by loss of TNFα production and partial loss of IFNγ production, then complete loss of IFNγ production, and eventually cell death (51, 52). Programmed death 1 (PD-1), an inhibitory co-receptor up-regulated in many settings of T cell exhaustion, has been reported to be expressed on human prostate tumor infiltrating CD8 T cells (53). At 1 week post-transfer, WT cells expressed higher levels of PD-1 in the PDN and prostate of TRAMPOVA mice than TRAMP mice (Fig. 5A). Abrogation of TGFβ signaling resulted in lower PD-1 expression at 1 week on transferred cells in the prostate and PDN of TRAMPOVA mice. However, at week 3 post-transfer, both TGFβRII KO and WT cells expressed high levels of PD-1 in the PDN and prostate of TRAMPOVA mice. This pattern of PD-1 expression correlated with the severity of the observed functional defect, suggesting PD-1 signaling may be inhibiting anti-tumor activity in the prostate, and that the defects in the prostate and PDN may reflect in part consequences of continued antigen recognition. There are currently 2 known ligands for PD-1, PD-1 ligand 1 (PD-L1; B7-H1) and PD-1 ligand 2 (PD-L2; B7-DC) (54). PD-L1 is up-regulated on many human tumors, including prostate cancer (55), and high PD-L1 expression in some tumor tissues correlates with a decrease in CD8 T cell infiltrates (54). Analysis of frozen TRAMPOVA prostates 3 weeks post-transfer of TGFβRII KO cells revealed PD-L1 was expressed on prostate epithelium (Fig. 5B).
Figure 5. PD-1 and PD-L1 are expressed respectively by persisting transferred T cells and the prostate tumor in treated TRAMPOVA mice, but blockade of PD-1 signaling does not further increase accumulation or effector function of TGFβRII KO cells at 3 weeks post transfer.
(A) PD-1 expression on WT and TGFβRII KO cells at week 1 and week 3 post transfer. Histograms are gated on CD8+Ly5.1+ cells. The WT or TGFβRII KO cells transferred into TRAMPOVA hosts shown with a black line, and cells transferred into TRAMP hosts in shaded grey. (B) PD-L1 expression of TGFβRII KO cell treated TRAMPOVA prostates 3 weeks post transfer. For PD-1 blocking experiments, blocking antibodies or PBS were administered i.p. every 3 days starting on the day of T cell transfer until mice were euthanized at 3 weeks post transfer. (C) Numbers of persisting transferred cells in TRAMPOVA mice treated with antibody or PBS. (D) Percentage of transferred TGFβRII KO cells co-producing TNFα and IFNγ following 5 hour ex vivo peptide stimulation. All results represent pooled data from 3 independent experiments (n=2–3 mice/group/experiment for mice treated with blocking antibodies and n=1–2 mice/group/experiment for control PBS treated). No significant differences between treated and untreated mice were detected (unpaired Student’s t test).
Blockade of PD-1 signaling does not further improve anti-tumor activity of TGFβRII deficient cells
PD-1 blockade has enhanced anti-tumor activity in transplantable tumor models (56, 57) and recently phase I human clinical trials of PD-1 blockade in cancer patients have demonstrated anti-tumor activity for certain cancers (58–61). However, in the TRAMP model, despite increased PD-1 expression on prostate-specific CD8 T cells, breeding TRAMP mice onto a PD-L1−/− background was reported to not prevent tolerization of prostate-specific CD8 T cells (62). Since PD-1 may signal through interactions with other known ligands, such as PD-L2, or unidentified ligands, we examined if blockade of PD-1 signaling in TGFβRII KO cells with a combination of PD-1, PD-L1 and PD-L2 blocking antibodies could promote more persistent and effective anti-tumor activity. In vitro activated TGFβRII KO cells were transferred into TRAMPOVA hosts, and cohorts of mice received either 200µg of each blocking antibody or PBS i.p. every 3rd day, starting on the day of T cell transfer. Mice were euthanized 3 weeks following treatment and assayed for T cell function and tumor burden. No significant differences were found between the numbers or function, as reflected by cytokine production, of TGFβRII KO cells in the spleen, PDN or prostate in mice that received the blocking antibody cocktail or control PBS (Fig. 5C–D). Prostates were also weighed and examined histologically, and no significant differences were detected (data not shown). As this could reflect limitations to these Abs effectively penetrating in situ tumor sites, we stained recovered TGFβRII KO cells with a secondary antibody to the IgG isotype of the blocking PD-1 antibody, and detected Ab bound to transferred T cells in the PDN and prostates of TRAMPOVA but not TRAMP mice (data not shown). These results suggest that, despite expression of PD-1 on transferred TGFβRII KO cells, and expression of PD-L1 on prostate tumor cells, antibody blockade of PD-1 signaling is not adequate to significantly synergize with the enhancement initially achieved by blockade of TGFβ signaling, implying additional inhibitory pathways are operative in the environment of prostate cancers.
Discussion
ACT is being actively pursued in clinical trials to treat malignancies, with successes reported in some cancers (4–6), but, even for tumors with identifiable tumor target antigens, substantive obstacles to broad applicability and the achievement of predictable and reproducible benefits remain. In this study we investigated if cell intrinsic abrogation of TGFβRII signaling in self/tumor antigen specific CD8 T cells could enhance the efficacy of in vitro activated effector T cells in ACT of prostate cancer, using an autochthonous model of murine prostate cancer that replicates many characteristics of human disease. The small but significant decrease in the prostate weight of TRAMPOVA mice receiving TGFβRII KO cells compared to mice receiving WT cells at 3 weeks post transfer was consistent with enhanced anti-tumor activity. However, unlike some transplantable models in which TGFβR blockade in tumor-reactive T cells resulted in complete elimination of the tumor (29, 30), anti-tumor activity in the TRAMP model was not sustained, suggesting additional barriers are present for targeting a tumor in situ.
Lack of persistence and failure to maintain in vivo anti-tumor activity following T cell transfer are frequent problems in clinical ACT targeting established tumors (7). We demonstrated that abrogation of TGFβ signaling was adequate to numerically sustain transferred T cells in distal secondary lymphoid organs, but additional immunosuppressive factors operative within the prostate and possibly PDN eventually rendered cells remaining at these sites dysfunctional. Although transferred cells in the PDN and prostate upregulated the inhibitory receptor, PD-1, antibody blockade of PD-1 signaling failed to significantly synergize with abrogation of TGFβ signaling, with no evidence of maintenance or restoration of anti-tumor activity detectable at 3 weeks post-T cell transfer. Analysis of the successful PD-1 blockade studies performed in the setting of chronic lymphocytic choriomeningitis virus (LCMV) infection revealed that PD-L1 blockade selectively restored the function of PD-1int but not PD-1hi LCMV-specific CD8 T cells (63). It appears likely that the transferred cells in our model resemble the PD-1hi LCMV-specific CD8 T cell subset. The reason for lack of efficacy with this subset is not likely due to insufficient blockade but rather that additional inhibitory receptors, such as CTLA-4 (64), LAG3 (65), TIM-3 (56) and/or 2B4 (66), may be simultaneously expressed and limiting T cell function. In fact, we found LAG3 expressed at increased levels at 3 weeks post-transfer on TGFβRII KO cells in the prostate and PDN but not the spleen of TRAMPOVA mice compared to TRAMP mice (data not shown). Temporary restoration of cytotoxicity of endogenous prostate-specific CD8 T cells following αLAG3 treatment and vaccination has been reported (65). However, whether blockade can augment the benefits of TGFβR disruption and/or synergize with other blocking reagents for treatment of in situ tumors remains unknown.
The context in which a T cell encounters antigen influences function and differentiation state (67). Thus, many additional events may be contributing to the failure of transferred effector cells to maintain function while targeting a prostate tumor. First, since a self-antigen is being targeted, transferred cells are likely encountering antigen not only on tumor cells but also normal prostate cells and/or dendritic cells (DCs) presenting the peptide in a tolerogenic context. Chronic antigen stimulation alone can induce T cell exhaustion (49, 50), and in some settings this exhaustion is not rescued by PD-1 blockade (50), as may be occurring in the prostate. Studies in the chronic LCMV infection model have also demonstrated that cell-intrinsic TGFβ blockade can lead to increased numbers of LCMV-specific CD8 T cells and promote clearance of chronic LCMV, but, in experimental conditions in which the viral antigen is not cleared, the TGFβR-deficient T cells also become functionally exhausted (43). Second, tumor associated DCs (TADCs) have been identified in TRAMP prostate tumors and can directly suppress naïve prostate-specific CD8 T cells (68), therefore, it is possible continuous encounters by transferred self/tumor-specific effector T cells with TADCs in the prostate prevent sustained anti-tumor activity. DC vaccines may transiently augment and/or restore the activity of prostate infiltrating T cells (69–71).
Additional cell extrinsic factors may also contribute to the immunosuppressive tumor environment, including Foxp3+ regulatory T cells (Tregs). Similar to published studies (72), we found increased numbers of CD4+Foxp3+ cells in 25 week-old TRAMPOVA prostates compared to healthy age-matched male mice. To test if Foxp3+ Tregs play a dominant role in suppressing adoptively transferred effectors, we bred TRAMPOVA mice to Foxp3DTR mice (73). In preliminary studies utilizing the TRAMPOVA×Foxp3DTR mice, in which near complete ablation of Foxp3+ T cells (>97%) can be achieved, no enhanced infiltration or cytokine production by transferred TGFβRII KO cells in the prostates of TRAMPOVA mice was observed (data not shown). Moreover, these Treg-depleted mice developed systemic autoimmunity, as previously reported (73), affirming the inherent difficulties associated with pursuing effective global depletion of Tregs as a therapeutic strategy for treating tumors.
Our findings have implications for human adoptive therapy. We found increased function of both WT and TGFβRII KO cells in the spleen and PDN compared to the prostate. The greater dysfunction at the site where the activity is actually required highlights the importance of analyzing intra-tumoral T cells when assessing the function of T cells targeting an established tumor. Evidence supporting this conclusion has also been provided in studies of melanoma patients, in which tumor infiltrating lymphocytes in metastatic lesions can exhibit an exhausted profile whereas T cells of the same specificity in the blood are functional (74).
These studies are the first to assess the effect of cell-intrinsic abrogation of TGFβRII signaling in self/tumor specific CD8 T cells in the context of ACT for a spontaneous solid cancer. The initial increase in accumulation of TGFβRII KO prostate-specific T cells and delay in loss of anti-tumor activity in the prostate does offer a window of opportunity for additional interventional therapies that could potentially result in synergistic anti-tumor activity before T cells become functionally impaired. Adjunctive therapies, such as radiation or chemotherapy, can augment anti-tumor activity of prostate-specific T cells (71, 75, 76). We recently demonstrated that lymphopenia-induced proliferation could transiently restore the function of tolerant T cells (77). These data together suggest that lymphodepletion of TRAMP mice may synergize with abrogation of TGFβRII to increase therapeutic efficacy. Additionally, identifying and targeting tumor-specific antigens not expressed by normal cells may circumvent or delay functional exhaustion by reducing the extent of persistent antigen stimulation. However, while some unique tumor-specific epitopes have been discovered in select tumors, tumor-specific antigens are often unique to each patient and the majority of antigens being targeted in clinical trials, including all known targetable prostate cancer antigens, are self-antigens (24, 78–80).
In conclusion, our results highlight some of the obstacles to ACT for solid tumors, and emphasize the need for testing potential ACT strategies in preclinical models that emulate the development and environment of tumors to identify and address potential pitfalls. The nature and relative importance of particular immunosuppressive mechanisms may vary with different tumor types, and a more complete analysis of the individual obstacles will likely be invaluable for designing combinatorial strategies to target selected tumors with T cells.
Supplementary Material
Acknowledgements
We thank I. Roberts for excellent technical assistance, S. Hernandez for advice on working with the TRAMP model, S. Knoblaugh and J. Randolph-Habecker for advice on histology, A. Farr for use of his fluorescent microscope and I. Stromnes for helpful discussion.
This work was supported by the National Institute of Health R01 CA33084 (to P.D.G.) and P01 AI56299 (to G.J.F.). C.K.C. received support as provided by the Cancer Research Institute Institutional Pre-Doctoral Award and a Pre-doctoral Prostate Cancer Training Award from the U.S. Army Medical Research and Materiel Command under W81XWH-09-1-0139
Abbreviations used in this paper
- ACT
adoptive T cell therapy
- DNR-TGFβRII
dominant negative form of TGFβRII
- LCMV
lymphocytic choriomeningitis virus
- OVA
ovalbumin
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
- PD-L2
programmed death ligand 2
- TGFβ
transforming growth factor beta
- TGFβRII
transforming growth factor beta receptor II
- TRAMP
Transgenic Adenocarcinoma of the Mouse Prostate
- PDN
prostate draining lymph node
- POET
prostate ovalbumin expressing transgenic
References
- 1.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 2.Kwek SS, Cha E, Fong L. Unmasking the immune recognition of prostate cancer with CTLA4 blockade. Nat Rev Cancer. 2012;12:289–297. doi: 10.1038/nrc3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, June CH. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002842. 95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger C, Turtle CJ, Jensen MC, Riddell SR. Adoptive transfer of virus-specific and tumor-specific T cell immunity. Curr Opin Immunol. 2009;21:224–232. doi: 10.1016/j.coi.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho WY, Blattman JN, Dossett ML, Yee C, Greenberg PD. Adoptive immunotherapy: engineering T cell responses as biologic weapons for tumor mass destruction. Cancer Cell. 2003;3:431–437. doi: 10.1016/s1535-6108(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 10.Offringa R. Antigen choice in adoptive T-cell therapy of cancer. Curr Opin Immunol. 2009;21:190–199. doi: 10.1016/j.coi.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt TM, Ragnarsson GB, Greenberg PD. T cell receptor gene therapy for cancer. Hum Gene Ther. 2009;20:1240–1248. doi: 10.1089/hum.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leveen P, Larsson J, Ehinger M, Cilio CM, Sundler M, Sjostrand LJ, Holmdahl R, Karlsson S. Induced disruption of the transforming growth factor beta type II receptor gene in mice causes a lethal inflammatory disorder that is transplantable. Blood. 2002;100:560–568. doi: 10.1182/blood.v100.2.560. [DOI] [PubMed] [Google Scholar]
- 13.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and - independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 14.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 15.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pu H, Collazo J, Jones E, Gayheart D, Sakamoto S, Vogt A, Mitchell B, Kyprianou N. Dysfunctional transforming growth factor-beta receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. Cancer Res. 2009;69:7366–7374. doi: 10.1158/0008-5472.CAN-09-0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ewen ME, Sluss HK, Whitehouse LL, Livingston DM. TGF beta inhibition of Cdk4 synthesis is linked to cell cycle arrest. Cell. 1993;74:1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- 19.Wolfraim LA, Walz TM, James Z, Fernandez T, Letterio JJ. p21Cip1 and p27Kip1 act in synergy to alter the sensitivity of naive T cells to TGF-beta-mediated G1 arrest through modulation of IL-2 responsiveness. J Immunol. 2004;173:3093–3102. doi: 10.4049/jimmunol.173.5.3093. [DOI] [PubMed] [Google Scholar]
- 20.Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donkor MK, Sarkar A, Savage PA, Franklin RA, Johnson LK, Jungbluth AA, Allison JP, Li MO. T cell surveillance of oncogene-induced prostate cancer is impeded by T cell-derived TGF-beta1 cytokine. Immunity. 2011;35:123–134. doi: 10.1016/j.immuni.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Pennison M, Pasche B. Targeting transforming growth factor-beta signaling. Curr Opin Oncol. 2007;19:579–585. doi: 10.1097/CCO.0b013e3282f0ad0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elkord E. Immunology and immunotherapy approaches for prostate cancer. Prostate Cancer Prostatic Dis. 2007;10:224–236. doi: 10.1038/sj.pcan.4500964. [DOI] [PubMed] [Google Scholar]
- 25.Barrack ER. TGF beta in prostate cancer: a growth inhibitor that can enhance tumorigenicity. Prostate. 1997;31:61–70. doi: 10.1002/(sici)1097-0045(19970401)31:1<61::aid-pros10>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 26.Lee C, Sintich SM, Mathews EP, Shah AH, Kundu SD, Perry KT, Cho JS, Ilio KY, Cronauer MV, Janulis L, Sensibar JA. Transforming growth factor-beta in benign and malignant prostate. Prostate. 1999;39:285–290. doi: 10.1002/(sici)1097-0045(19990601)39:4<285::aid-pros9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Diener KR, Woods AE, Manavis J, Brown MP, Hayball JD. Transforming growth factor-beta-mediated signaling in T lymphocytes impacts on prostate-specific immunity and early prostate tumor progression. Lab Invest. 2009;89:142–151. doi: 10.1038/labinvest.2008.123. [DOI] [PubMed] [Google Scholar]
- 28.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Jang TL, Yang X, Park I, Meyer RE, Kundu S, Pins M, Javonovic B, Kuzel T, Kim SJ, Van Parijs L, Smith N, Wong L, Greenberg NM, Guo Y, Lee C. Infiltration of tumor-reactive transforming growth factor-beta insensitive CD8+ T cells into the tumor parenchyma is associated with apoptosis and rejection of tumor cells. Prostate. 2006;66:235–247. doi: 10.1002/pros.20340. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Q, Yang X, Pins M, Javonovic B, Kuzel T, Kim SJ, Parijs LV, Greenberg NM, Liu V, Guo Y, Lee C. Adoptive transfer of tumor-reactive transforming growth factor-beta-insensitive CD8+ T cells: eradication of autologous mouse prostate cancer. Cancer Res. 2005;65:1761–1769. doi: 10.1158/0008-5472.CAN-04-3169. [DOI] [PubMed] [Google Scholar]
- 31.Willimsky G, Blankenstein T. The adaptive immune response to sporadic cancer. Immunol Rev. 2007;220:102–112. doi: 10.1111/j.1600-065X.2007.00578.x. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, Cunha GR, Donjacour AA, Matusik RJ, Rosen JM. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci U S A. 1995;92:3439–3443. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan-Lefko PJ, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 34.Hsu CX, Ross BD, Chrisp CE, Derrow SZ, Charles LG, Pienta KJ, Greenberg NM, Zeng Z, Sanda MG. Longitudinal cohort analysis of lethal prostate cancer progression in transgenic mice. J Urol. 1998;160:1500–1505. [PubMed] [Google Scholar]
- 35.Lees JR, Charbonneau B, Hayball JD, Diener K, Brown M, Matusik R, Cohen MB, Ratliff TL. T-cell recognition of a prostate specific antigen is not sufficient to induce prostate tissue destruction. Prostate. 2006;66:578–590. doi: 10.1002/pros.20307. [DOI] [PubMed] [Google Scholar]
- 36.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 37.Zhang DJ, Wang Q, Wei J, Baimukanova G, Buchholz F, Stewart AF, Mao X, Killeen N. Selective expression of the Cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J Immunol. 2005;174:6725–6731. doi: 10.4049/jimmunol.174.11.6725. [DOI] [PubMed] [Google Scholar]
- 38.Keir ME, Latchman YE, Freeman GJ, Sharpe AH. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol. 2005;175:7372–7379. doi: 10.4049/jimmunol.175.11.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, Greenfield EA, Liang SC, Sharpe AH, Lichtman AH, Freeman GJ. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 40.Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, Sharpe AH, Umetsu DT, Dekruyff RH. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 2010;3:81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 42.Wildey GM, Howe PH. Runx1 is a co-activator with FOXO3 to mediate transforming growth factor beta (TGFbeta)-induced Bim transcription in hepatic cells. J Biol Chem. 2009;284:20227–20239. doi: 10.1074/jbc.M109.027201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tinoco R, Alcalde V, Yang Y, Sauer K, Zuniga EI. Cell-intrinsic transforming growth factor-beta signaling mediates virus-specific CD8+ T cell deletion and viral persistence in vivo. Immunity. 2009;31:145–157. doi: 10.1016/j.immuni.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barth RJ, Jr, Mule JJ, Spiess PJ, Rosenberg SA. Interferon gamma and tumor necrosis factor have a role in tumor regressions mediated by murine CD8+ tumor-infiltrating lymphocytes. J Exp Med. 1991;173:647–658. doi: 10.1084/jem.173.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 46.Dissanayake D, Gronski MA, Lin A, Elford AR, Ohashi PS. Immunological perspective of self versus tumor antigens: insights from the RIP-gp model. Immunol Rev. 2011;241:164–179. doi: 10.1111/j.1600-065X.2011.01014.x. [DOI] [PubMed] [Google Scholar]
- 47.Garrido F, Cabrera T, Aptsiauri N. "Hard" and "soft" lesions underlying the HLA class I alterations in cancer cells: implications for immunotherapy. Int J Cancer. 2010;127:249–256. doi: 10.1002/ijc.25270. [DOI] [PubMed] [Google Scholar]
- 48.Nanda NK, Birch L, Greenberg NM, Prins GS. MHC class I and class II molecules are expressed in both human and mouse prostate tumor microenvironment. Prostate. 2006;66:1275–1284. doi: 10.1002/pros.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mueller SN, Ahmed R. High antigen levels are the cause of T cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2009;106:8623–8628. doi: 10.1073/pnas.0809818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bucks CM, Norton JA, Boesteanu AC, Mueller YM, Katsikis PD. Chronic antigen stimulation alone is sufficient to drive CD8+ T cell exhaustion. J Immunol. 2009;182:6697–6708. doi: 10.4049/jimmunol.0800997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 53.Sfanos KS, Bruno TC, Meeker AK, De Marzo AM, Isaacs WB, Drake CG. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crane CA, Panner A, Murray JC, Wilson SP, Xu H, Chen L, Simko JP, Waldman FM, Pieper RO, Parsa AT. PI(3) kinase is associated with a mechanism of immunoresistance in breast and prostate cancer. Oncogene. 2009;28:306–312. doi: 10.1038/onc.2008.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, Tangsombatvisit S, Grosso JF, Netto G, Smeltzer MP, Chaux A, Utz PJ, Workman CJ, Pardoll DM, Korman AJ, Drake CG, Vignali DA. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, Koren-Michowitz M, Shimoni A, Nagler A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 60.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM. Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012 doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai A, Higham E, Eisen HN, Wittrup KD, Chen J. Rapid tolerization of virus-activated tumor-specific CD8+ T cells in prostate tumors of TRAMP mice. Proc Natl Acad Sci U S A. 2008;105:13003–13008. doi: 10.1073/pnas.0805599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hurwitz AA, Foster BA, Kwon ED, Truong T, Choi EM, Greenberg NM, Burg MB, Allison JP. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
- 65.Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, Drake CG. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.West EE, Youngblood B, Tan WG, Jin HT, Araki K, Alexe G, Konieczny BT, Calpe S, Freeman GJ, Terhorst C, Haining WN, Ahmed R. Tight regulation of memory CD8(+) T cells limits their effectiveness during sustained high viral load. Immunity. 2011;35:285–298. doi: 10.1016/j.immuni.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 68.Watkins SK, Zhu Z, Riboldi E, Shafer-Weaver KA, Stagliano KE, Sklavos MM, Ambs S, Yagita H, Hurwitz AA. FOXO3 programs tumor-associated DCs to become tolerogenic in human and murine prostate cancer. J Clin Invest. 2011;121:1361–1372. doi: 10.1172/JCI44325. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Anderson MJ, Shafer-Weaver K, Greenberg NM, Hurwitz AA. Tolerization of tumor-specific T cells despite efficient initial priming in a primary murine model of prostate cancer. J Immunol. 2007;178:1268–1276. doi: 10.4049/jimmunol.178.3.1268. [DOI] [PubMed] [Google Scholar]
- 70.Higham EM, Shen CH, Wittrup KD, Chen J. Cutting edge: delay and reversal of T cell tolerance by intratumoral injection of antigen-loaded dendritic cells in an autochthonous tumor model. J Immunol. 2010;184:5954–5958. doi: 10.4049/jimmunol.1000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hess Michelini R, Freschi M, Manzo T, Jachetti E, Degl'Innocenti E, Grioni M, Basso V, Bonini C, Simpson E, Mondino A, Bellone M. Concomitant tumor and minor histocompatibility antigen-specific immunity initiate rejection and maintain remission from established spontaneous solid tumors. Cancer Res. 2010;70:3505–3514. doi: 10.1158/0008-5472.CAN-09-4253. [DOI] [PubMed] [Google Scholar]
- 72.Degl'Innocenti E, Grioni M, Capuano G, Jachetti E, Freschi M, Bertilaccio MT, Hess-Michelini R, Doglioni C, Bellone M. Peripheral T-cell tolerance associated with prostate cancer is independent from CD4+CD25+ regulatory T cells. Cancer Res. 2008;68:292–300. doi: 10.1158/0008-5472.CAN-07-2429. [DOI] [PubMed] [Google Scholar]
- 73.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 74.Baitsch L, Baumgaertner P, Devevre E, Raghav SK, Legat A, Barba L, Wieckowski S, Bouzourene H, Deplancke B, Romero P, Rufer N, Speiser DE. Exhaustion of tumor-specific CD8 T cells in metastases from melanoma patients. J Clin Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wada S, Yoshimura K, Hipkiss EL, Harris TJ, Yen HR, Goldberg MV, Grosso JF, Getnet D, Demarzo AM, Netto GJ, Anders R, Pardoll DM, Drake CG. Cyclophosphamide augments antitumor immunity: studies in an autochthonous prostate cancer model. Cancer Res. 2009;69:4309–4318. doi: 10.1158/0008-5472.CAN-08-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harris TJ, Hipkiss EL, Borzillary S, Wada S, Grosso JF, Yen HR, Getnet D, Bruno TC, Goldberg MV, Pardoll DM, DeWeese TL, Drake CG. Radiotherapy augments the immune response to prostate cancer in a time-dependent manner. Prostate. 2008;68:1319–1329. doi: 10.1002/pros.20794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schietinger A, Philip M, Schreiber H. Specificity in cancer immunotherapy. Semin Immunol. 2008;20:276–285. doi: 10.1016/j.smim.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Savage PA, Vosseller K, Kang C, Larimore K, Riedel E, Wojnoonski K, Jungbluth AA, Allison JP. Recognition of a ubiquitous self antigen by prostate cancer-infiltrating CD8+ T lymphocytes. Science. 2008;319:215–220. doi: 10.1126/science.1148886. [DOI] [PubMed] [Google Scholar]
- 80.Fasso M, Waitz R, Hou Y, Rim T, Greenberg NM, Shastri N, Fong L, Allison JP. SPAS-1 (stimulator of prostatic adenocarcinoma-specific T cells)/SH3GLB2: A prostate tumor antigen identified by CTLA-4 blockade. Proc Natl Acad Sci U S A. 2008;105:3509–3514. doi: 10.1073/pnas.0712269105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.