Abstract
Prior studies have examined differences in brain volume between PTSD and control subjects. Convergent findings include smaller hippocampus and medial prefrontal cortex volumes in PTSD. However, post-traumatic stress symptoms (PTSS) exist on a spectrum, and neural changes may occur beyond the diagnostic threshold of PTSD. We examined the relationship between PTSS and gray matter among combat-exposed U.S. military veterans. Structural brain MRI was obtained on 28 combat veterans from Operations Enduring and Iraqi Freedom. PTSS were assessed using the Clinician-Administered PTSD Scale (CAPS). Thirteen subjects met criteria for PTSD. Subjects were unmedicated, and free of major comorbid psychiatric disorders. Images were analyzed using voxel-based morphometry, and regressed against the total CAPS score and trauma load. Images were subsequently analyzed by diagnosis of PTSD vs. non-PTSD. CAPS scores were inversely correlated with subgenual cingulate (sgACC), caudate, hypothalamus, insula, and left middle temporal gyrus (MTG). Group contrast revealed smaller sgACC, caudate, hypothalamus, left insula, left MTG, and right MFG in the PTSD group. PTSS are associated with abnormalities in limbic structures that may underlie the pathophysiology of PTSD. These abnormalities exist on a continuum with PTSS, beyond a diagnosis of PTSD.
Keywords: neuroimaging, anxiety, circuitry, trauma, PTSD
1. Introduction
Post-traumatic stress disorder (PTSD) and posttraumatic stress symptoms (PTSS) are highly prevalent in military service members returning from Operations Enduring/Iraqi Freedom (OEF/OIF) (Hoge et al., 2006). As many as 30% of returning veterans have been found to have PTSD or significant PTSS one year post-deployment (Thomas et al., 2010). There is a great need to better understand the pathophysiology of PTSD and discover biomarkers of the illness. This knowledge could eventually aid in the detection of vulnerability (or resilience) prior to, or shortly after, trauma exposure, or be used as an additional assessment for illness severity in PTSD.
One approach of elucidating the pathophysiology of PTSD has been the use of structural brain MRI, with comparison between PTSD and control groups. Convergent findings include smaller hippocampus and medial prefrontal cortex (mPFC) volumes, including the anterior cingulate cortex (ACC). Several studies also suggest reduced insula volumes in PTSD (Corbo et al., 2005; Chen et al., 2006; Kasai et al., 2008). The hippocampus is important for new memory consolidation, processing of contextual emotional memory, and inhibitory feedback on the HPA axis (Leuner and Gould, 2010). The mPFC plays important roles in emotion regulation and internal expression of emotion/fear conditioning (Phillips et al., 2008). The ventromedial (vm)PFC in particular supports fear extinction recall (Quirk et al., 2006).
Studies of subjects with PTSD suggest both overgeneralized fear responses and impaired fear extinction (e.g. (Peri et al., 2000; Orr et al., 2000; Jovanovic et al., 2010)). Functional brain studies have documented increased amygdala activation to a variety of emotional stimuli (Shin and Liberzon, 2010), and studies of fear extinction have revealed decreased activation of the vmPFC and hippocampus (e.g. (Milad et al., 2009; Bremner et al., 2005)). The above findings, along with structural brain studies, have led to a working model of PTSD in which the amygdala shows enhanced activation to emotional stimuli, with impaired recruitment of the vmPFC and hippocampus in modulating and extinguishing fear responses.
However, some studies have found no differences in these brain structures in PTSD subjects compared to trauma-exposed or healthy controls (for review of structural brain studies in PTSD, see (Shin and Liberzon, 2010)). These null findings may reflect variation in patient populations, trauma types, magnetic resonance imaging (MRI) acquisition parameters, morphometric analysis (e.g., manual tracing vs. automated algorithms), medication exposure, and relatively small effect sizes for some brain regions (Karl et al., 2006).
Another possibility for inconsistency in the literature is the variability in PTSD symptom severity across studies, e.g. (Fennema-Notestine et al., 2002; Gilbertson et al., 2002; Apfel et al., 2011). However, PTSS exist on a spectrum, and different thresholds of defining PTSD could lead to different findings. One potential solution to this is the use of a dimensional symptom approach, in particular by examining the neural correlates of PTSS across all trauma-exposed individuals (below and above the PTSD threshold). The study of PTSS is in line with the shift towards the dimensional assessment of symptoms and psychopathology being proposed in DSM-5 (Helzer and American Psychiatric Association, 2008). Dimensional analyses may also indicate greater specificity of neural changes to PTSS (and thus PTSD).
Several studies have identified correlations between regional brain volumes and PTSD symptom severity within the PTSD group only. For example, ACC volume (Woodward et al., 2006; Yamasue et al., 2003) and hippocampal volume (Gilbertson et al., 2002; Bremner et al., 2003; Villarreal et al., 2002) have been inversely correlated with PTSD severity in subjects with a diagnosis of PTSD. A meta-analysis found that reductions in hippocampal volume were more likely to manifest in subjects with severe PTSD when compared to subjects with moderate PTSD and controls (Karl et al., 2006). To our knowledge, no prior studies have examined the correlation of PTSS and gray matter volume in trauma-exposed individuals both with and without a diagnosis of PTSD.
To this end, this study aims to complement previous morphometric studies of PTSD by examining the relationship between PTSS and regional brain volumes in combat-exposed OEF/OIF military veterans, with subthreshold and threshold PTSD. We hypothesized that greater PTSS would be associated with smaller volumes in the ACC, hippocampus, and insula. However, smaller regional brain volumes could be a result of trauma exposure itself (and not simply PTSS). For example, smaller hippocampal volume has been associated with both trauma exposure and PTSD (Woon et al., 2010). Furthermore, childhood and adult trauma may have differential impacts on brain volume (Lupien et al., 2009). For example, the hippocampus appears particularly sensitive to childhood trauma (Andersen et al., 2008), whereas the ACC remains sensitive into adulthood (e.g. (Kasai et al., 2008)). We therefore included self-reported childhood and adult trauma exposure with PTSS in the same model. Finally, we conducted a group contrast to evaluate potential differences in brain volume in veterans with and without PTSD in order to facilitate comparison with the dimensional approach.
2. Methods
2. 1. Subjects
Twenty-eight combat-exposed OEF/OIF veterans (25 males, 3 females; mean age 29.6) were recruited from the Pittsburgh area through public media advertisements. DD Form 214 documentation of prior military service was obtained from all potential participants. Subjects were assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First et al., November 2002). Past month PTSS were assessed using the Clinician-Administered PTSD Scale (CAPS) (Blake et al., 1995). Thirteen subjects met full criteria for PTSD as identified by the CAPS, using the F1/I2 criteria (Weathers et al., 2001). Subjects were unmedicated, and free of major comorbid psychiatric disorders as determined by the SCID. Depressive symptom severity was measured using the Beck Depression Inventory (BDI) (Beck et al., 1961). Level of combat exposure was measured with the Combat Exposure Scale (CES) (Keane et al., 1989). Participants completed the Trauma History Questionnaire (THQ) which is a self-report measure for exposure to a wide variety of traumas. It includes the number of times an event occurred and age at the time of exposure (Green, 1996). The number of trauma types experienced (i.e. number of THQ questions positively endorsed), before and after age 18 were summed to indicate childhood and adult trauma load, respectively.
Exclusion criteria for the study were: history of traumatic brain injury or neurological disease, current (past month) alcohol or illicit substance abuse or dependence, use of greater than 14 alcoholic drinks per week over the past month and confirmed on prospective diaries, and failure to meet screening criteria for MRI, including the presence of metal clips, heart pacemakers, claustrophobia and possibility of pregnancy for women of child-bearing age. Written informed consent was obtained from all participants after procedures were explained according to University of Pittsburgh Institutional Review Board guidelines.
2.2. MRI acquisition
MRI structural brain images were acquired using a 3.0 Tesla Siemens Trio MRI scanner (Siemens, Malvern, PA) at the University of Pittsburgh Magnetic Resonance Research Center. Three-dimensional sagittal high-resolution MPRAGE scans were acquired with the following parameters: TE: 2.98ms, TR: 2300ms, slice thickness: 1.2mm, 160 slices, flip angle: 9°, FOV: 256×240mm, inversion time (IT): 900 ms and image matrix 256 by 240 that covered the entire brain.
2.3. Image preprocessing and VBM
Image processing and voxel based morphometry (VBM) were performed with Statistical Parametric Mapping, Version 8 (SPM8, Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk/spm), that was executed in Matlab 7.2 (Mathworks, Sherborn, MA). DICOM files were converted to NIFTI-1 format (http://nifti.nimh.nih.gov). As per our prior work (Almeida et al., 2009), converted files were segmented into gray and white matter, and normalized to standard international consortium for brain mapping template using a unified model integrated within SPM8. Standard SPM values were selected to bias regularization (0.0001) and FWHM cutoff (60 mm). Voxel values were modulated by the Jacobian determinants derived from the spatial normalization; brain structures that had volumes reduced after spatial normalization thereby had total counts decreased by an amount proportional to the degree of volume discounted. The final voxel resolution after normalization was 2×2×2 mm. Images were smoothed with a 12-mm Gaussian kernel. For analysis of the hippocampus only, images were smoothed with a 4-mm Gaussian kernel as suggested by prior studies (Kasai et al., 2008; Maguire et al., 2000).
2.4. Statistical analysis
Processed gray matter images were analyzed in a multiple regression model within SPM8 to test our primary hypothesis, that greater PTSS would be associated with smaller gray matter in the ACC, hippocampus, and insula. Regressors included the total (past month) CAPS score, childhood trauma load, and adult trauma load. All images were covaried for total gray matter volume (GMV) as determined by Easy Volume (Cyril Pernet, http://www.sbirc.ed.ac.uk/cyril/cp_download.html). Given the significant findings with regression against the total CAPS score (see below), secondary analyses were conducted within SPSS (version 18) on extracted cluster averages that were adjusted for total GMV. First, current depression scores from the BDI and age were added as additional regressors to examine whether these variables accounted for part of the correlation between CAPS-measured PTSS severity and GMV. Second, normalized cluster averages were entered into a multivariate regression with CAPS symptom subscores (B: re-experiencing, C: avoidance/numbing, and D: hyperarousal) to identify whether specific categories of PTSD symptoms were associated with smaller regional brain volumes observed with total CAPS scores. Finally, a voxel-wise group contrast was conducted within SPM8 by dividing subjects into diagnostic groups of PTSD (n=13) and non-PTSD (n=15), with total GMV as a covariate, to compare methods of contrast (i.e. dimensional vs. group contrast). Regional GMV findings were considered significant for uncorrected P<0.001 at the voxel level with a minimum spatial extent of 50 contiguous voxels. For Pearson/Spearman correlations and t-tests in SPSS, results were considered significant at P<0.05, two-tailed. For additional multivariate regressions of extracted cluster values associated with total CAPS score, Bonferroni correction was applied based on the number of clusters examined (P<0.05/4 or 0.013).
3. Results
3.1. Demographics and psychometrics
Means and standard deviations are shown in Table 1 for age, total CAPS score, combat exposure, BDI score, and number of childhood and adult trauma types. One subject also met criteria for generalized anxiety disorder in the past month. No other concurrent psychiatric illnesses were identified. Past DSM-IV diagnoses are listed in Table 1. Correlational analysis revealed that CAPS score was significantly associated with the BDI (r=0.84, P<0.001) and adult trauma load (r=0.46, P=0.015). Adult trauma load was correlated with the BDI (r=0.43, P=0.021), CES (r=0.38, P=0.046), and childhood trauma load (r=0.46, P=0.013). Comparison of clinical variables between the diagnostic groups revealed that the PTSD group had significantly greater total CAPS scores (t26=6.2, P<0.001), BDI (t26=3.7, P<0.01), and adult trauma load (t26=2.1, P<0.05) relative to the non-PTSD group (Table 1). The groups did not differ on other variables.
Table 1.
Demographic and clinical variables
| All Subjects | PTSD | non-PTSD | |
|---|---|---|---|
|
| |||
| n | 28 | 13 | 15 |
| Sex (F) | 3 (11%) | 2 (15%) | 1 (7%) |
| Age | 29.6 (5.3) | 28.9 (4.2) | 30.1 (6.3) |
| CAPS total | 29.5 (22.1) | 47.5 (17.5) | 13.9 (10.9) |
| Beck Depression score | 5.8 (7.5) | 10.5 (8.9) | 1.8 (2.0) |
| Combat Exposure Scale | 19.4 (10.9) | 22.1 (10.0) | 17 (11.4) |
| Childhood trauma load | 0.9 (1.2) | 0.9 (1.4) | 0.8 (1.1) |
| Adult trauma load | 5.9 (3.0) | 7.1 (2.4) | 4.9 (3.1) |
|
| |||
| Past DSM-IV diagnoses | |||
|
| |||
| Major Depressive Disorder | 3 (11%) | 3 (23%) | 0 (0%) |
| Dysthymic Disorder | 1 (4%) | 1 (8%) | 0 (0%) |
| Generalized Anxiety Disorder | 1 (4%) | 1 (8%) | 0 (0%) |
| Panic Disorder | 1 (4%) | 1 (8%) | 0 (0%) |
| Alcohol Dependence | 5 (18%) | 4 (31%) | 1 (7%) |
| Cocaine Abuse | 1 (4%) | 0 (0%) | 1 (7%) |
| Cannabis abuse | 3 (11%) | 2 (15%) | 1 (7%) |
Values represent either count (percent) or mean (standard deviation). Values in bold are statistically different between the PTSD and non-PTSD groups, two-tailed t-test, P<0.05. CAPS=Clinician-administered PTSD Scale (past month).
3.2. Gray matter volume
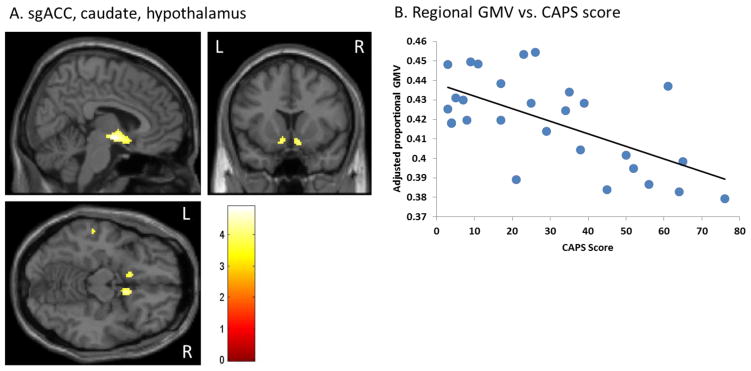
Whole brain regression in SPM8 revealed that CAPS score was inversely correlated with gray matter in a large cluster comprising the subgenual anterior cingulate cortex (sgACC, BA25), caudate nucleus, and hypothalamus (Table 2, Figure 1). CAPS score was also inversely correlated with left and right anterior insula (BA13) (Figure 2), and left middle temporal gyrus (MTG, BA21) (Table 2, Supplemental Figure 1). Childhood trauma load was inversely correlated with the left inferior parietal cortex (BA2), and cerebellum (Table 2, Supplemental Figure 2). No significant correlations were observed between adult trauma load and regional brain volume. There were no significant correlations with any of the above variables and hippocampal volume.
Table 2.
Regional gray matter findings
| Cluster Peak Coordinates | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Contrast | Region | Z-score | k | x | y | z |
| ↑CAPS, ↓GMV | sgACC (BA25), Caudate, Hypothalamus | 4.00 | 352 | 6 | −4 | −8 |
| Left Insula (BA13) | 3.87 | 180 | −32 | −12 | 16 | |
| Right Insula (BA13) | 3.89 | 111 | 36 | −10 | 20 | |
| Left Middle Temporal Gyrus (BA21) | 3.78 | 50 | −60 | −26 | −10 | |
|
| ||||||
| ↑Child Trauma, ↓GMV | Left Posterior Inferior Cerebellum | 3.98 | 429 | −44 | −70 | −54 |
| Left Posterior Superior Cerebellum | 3.35 | 108 | −28 | −68 | −26 | |
| Right Posterior Inferior Cerebellum | 3.39 | 124 | 16 | −76 | −52 | |
| Right Posterior Superior Cerebellum | 3.49 | 58 | 36 | −70 | −26 | |
| Left Inferior Parietal Lobe (BA2) | 3.95 | 67 | −42 | −30 | 38 | |
|
| ||||||
| non-PTSD>PTSD | sgACC (BA25), Caudate, Hypothalamus | 4.44 | 813 | 10 | 8 | −14 |
| Left Insula (BA13) | 3.68 | 109 | −32 | −12 | 18 | |
| Right Middle Frontal Gyrus (BA10) | 4.37 | 92 | 38 | 42 | 14 | |
| Left Middle Temporal Gyrus (BA21) | 3.79 | 86 | −64 | −26 | −10 | |
Summary of smaller gray matter volume associated with greater post-traumatic stress symptoms (↑CAPS, ↓GMV), greater child trauma load (↑Child Trauma, ↓GMV), or PTSD compared to non-PTSD. Regions shown were significant at uncorrected P<0.001, threshold k=50 voxels. k=cluster size. CAPS=Clinician-administered PTSD Scale. GMV=gray matter volume. Peak coordinates based on Montreal Neurological Institute atlas.
Figure 1.
CAPS score is inversely correlated with sgACC, caudate, and hypothalamus GMV. (A) sgACC, caudate, hypothalamus. (B) Regional GMV vs. CAPS score. Scatterplot depicts proportional GMV values averaged over all voxels in the cluster, and adjusted for total GMV. Extracted values shown here are not adjusted for depression scores or age.
Figure 2.
CAPS score is inversely correlated with insula GMV. (A) Left and right insula. (B) Left insula GMV vs. CAPS score. (C) Right insula GMV vs. CAPS score. Scatterplots depict proportional GMV values averaged over all voxels in the cluster, and adjusted for total GMV. Extracted values shown here are not adjusted for depression scores or age.
3.3. Secondary analyses
3.3.1. Covariation for combat exposure, depressive symptoms, and age
When CES scores, BDI scores, and age were added as additional regressors against extracted average cluster values within SPSS, findings remained unchanged. CAPS score remained inversely correlated with gray matter in the sgACC cluster (β=−0.75, P=0.004), left insula (β=−0.68, P=0.004), right insula (β=−0.83, P=0.002), and left MTG (β=−0.68, P=0.007). CES and BDI scores were not significantly correlated with GMV in the above areas. Age was moderately inversely correlated with the left insula cluster (β=−0.35, P=0.027), but showed no correlation with GMV in the other extracted regions.
3.3.2. Correlations with CAPS subscores
Multivariate regression of CAPS subscores and extracted average cluster values revealed that avoidance/numbing scores (C criteria) primarily accounted for reduced gray matter in the sgACC cluster (β=−0.64, P=0.008), left insula (β=−0.70, P=0.003), and right insula (β=−0.68, P=0.003). Avoidance/numbing scores showed a trend for smaller left MTG (β=−0.38, P=0.087). Re-experiencing (B criteria) and hyperarousal (D criteria) scores were not significantly associated with regional GMV.
3.3.3. Group contrast
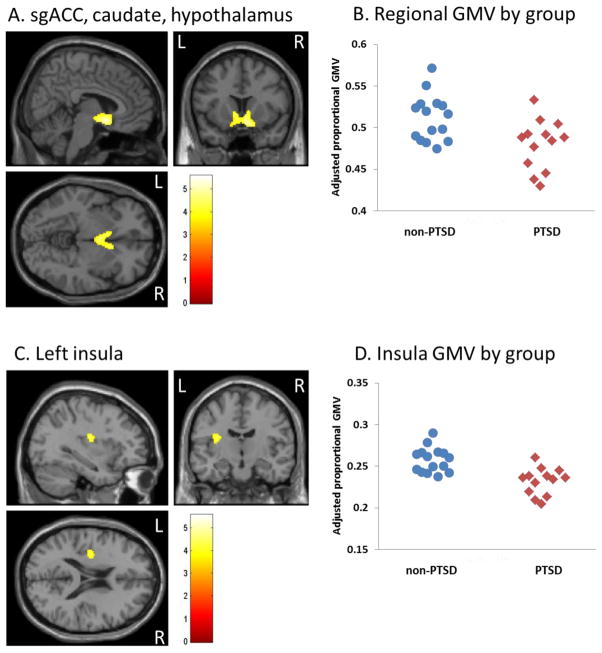
In order to compare methods of contrast (dimensional/regression compared to group contrasts), subjects were categorized by diagnosis of PTSD vs. non-PTSD. Contrast between these two groups was largely consistent with the regression analysis. Relative to the non-PTSD group, the PTSD group showed reduced gray matter in the same large cluster comprising the sgACC, caudate, and hypothalamus (Figure 3A, 3B). The PTSD group showed reduced gray matter in the left, but not right, insula (Figure 3C, 3D), left MTG (BA21), and right MFG (BA10) (Table 2). Relative reductions in GMV in the PTSD group were: sgACC cluster 6.3%, left insula 10.1%, left MTG 6.6%, and right MFG 11.8%. Similar to the regression analysis, there was no significant difference in hippocampal volumes between the PTSD and non-PTSD groups.
Figure 3.
Subjects with PTSD exhibit smaller sgACC, caudate, hypothalamus, and left insula GMV compared to non-PTSD. (A) sgACC, caudate, hypothalamus. (B) Scatterplot depicting individual sgACC/caudate/hypothalamus regional GMVs by diagnostic group. (C) Left insula. (D) Scatterplot depicting individual left insula regional GMVs by diagnostic group. Scatterplots depict proportional GMV values averaged over all voxels in the cluster, and adjusted for total GMV. Extracted values shown here are not adjusted for depression scores or age.
4. Discussion
To our knowledge, this is the first reported study to examine the correlation of PTSS and regional gray matter in trauma-exposed individuals both with and without PTSD. PTSS were inversely correlated with gray matter in the sgACC, caudate, hypothalamus, anterior insula, and left MTG. The caudate, sgACC, and anterior insula in particular may underlie the pathophysiology of PTSD as discussed in more detail below, and are consistent with working theoretical models of brain pathophysiology in PTSD (Shin and Liberzon, 2010). Furthermore, there was no significant correlation between these brain regions and childhood or adult trauma load. Together, this suggests specificity of these brain changes with PTSS, and that they are not simply due to depressive symptoms or trauma exposure itself.
When contrasted by diagnosis (PTSD and non-PTSD), smaller volumes were observed in the sgACC, caudate, left (but not right) insula, left MTG, and right MFG in the PTSD group, largely similar to the regression analysis. The size of these gray matter reductions (6–12%) is similar to differences observed in studies of individuals with major depression (Koolschijn et al., 2009). The consistency of findings using these two methods suggests the utility of whole brain correlation with symptom dimensions beyond diagnostic groups. The dimensional analysis, however, suggests that these neural changes are specific to PTSS and likely occur even below the diagnostic threshold of PTSD. This approach could be useful to complement standard group contrasts and is in line with the dimensional approaches proposed for DSM-5 and the proposed NIMH Research Domain Criteria (RDoC, with particular relevance for the fear/extinction domain) (Helzer and American Psychiatric Association, 2008; Insel et al., 2010).
Our study revealed that PTSS correlated with smaller caudate volume. The caudate nucleus, part of the striatum, is heavily involved in reward anticipation and response. Abnormal functioning within this structure has been documented in depression (Eshel and Roiser, 2010), substance abuse (Volkow et al., 2011), and PTSD, e.g. (Elman et al., 2009; Vythilingam et al., 2009; Sailer et al., 2008). Impaired recruitment of the striatum may in part mediate the anhedonia that is characteristic of depression, addictive disorders, and PTSD. Indeed, deep brain stimulation of the striatum has been found to alleviate symptoms of depression, including anhedonia (Malone et al., 2009; Schlaepfer et al., 2008). In depression, lower caudate volume has been associated with anhedonia and greater depression severity (Pizzagalli et al., 2009). Together, these findings suggest disruption of the reward processing system may represent a common underlying impairment in PTSD, depression, and substance abuse, and may in part explain the high comorbidity of these illnesses.
PTSS also correlated with smaller ACC volume. The ACC is a heterogeneous structure supporting different functions. While the dorsal ACC supports executive/cognitive processing and voluntary control of emotion regulation, the rostral ACC supports automatic emotion regulation, conflict monitoring, and attentional control of emotional stimuli. Both of these areas appear to support the expression of fear, pain, and emotion in humans (Phillips et al., 2008). The sgACC in particular shows homology to the infralimbic cortex in rats and plays a prominent role in visceromotor regulation and extinction of fear memory (Phillips et al., 2008; Quirk et al., 2006). Abnormalities in the medial prefrontal cortex (mPFC), including the sgACC, have been documented in PTSD in both structural and functional neuroimaging studies. Structural findings include reduced volumes of the orbitofrontal cortex (Thomaes et al., 2010), ACC (rostral and dorsal) (Woodward et al., 2006), rostral ACC (Rauch et al., 2003), dorsal ACC (Chen et al., 2006; Yamasue et al., 2003; Thomaes et al., 2010; Kitayama et al., 2006), and sgACC (Rauch et al., 2003). Furthermore, prior research suggests that lower ACC volume may be an acquired change following exposure to stressful life events (Papagni et al., 2011) and the development of PTSD (Kasai et al., 2008). A number of studies (but not all) have also demonstrated reduced activation of the mPFC in PTSD (for review see (Shin and Liberzon, 2010)). These findings have led to a working model of PTSD in which amygdala activation to emotional stimuli is thought to proceed relatively unchecked by mPFC, particularly the vmPFC, which has more direct connections to the amygdala, e.g. (Ghashghaei et al., 2007). In an fMRI study, re-experiencing and avoidance symptoms were inversely correlated with ACC activation (Hopper et al., 2007), and fear extinction paradigms show impaired recruitment of the vmPFC (Milad et al., 2009; Bremner et al., 2005; Rougemont-Bucking et al., 2011). These studies suggest impairments in fear extinction and inhibitory control of the amygdala that are mediated, at least in part, by the vmPFC. The correlation of PTSS with lower sgACC volume in the current study would be consistent with this model. The specific correlation of avoidance/numbing symptoms is somewhat more difficult to reconcile with this model, though could reflect engagement of compensatory mechanisms (and therefore circuitry) given the impairment in fear extinction that would normally have occurred via the sgACC.
Finally, PTSS correlated with lower bilateral anterior insula volume, though only the left insula was significantly different in the group contrast. The anterior insula is an important part of the limbic circuitry which is involved in the representation of interoceptive, emotional states (Critchley, 2005). Three prior studies, to our knowledge, have also documented reduced insula volumes in PTSD (Corbo et al., 2005; Chen et al., 2006; Kasai et al., 2008). Functional neuroimaging studies have, however, shown greater insula activation in response to emotional stimuli, which appears to be a commonality in multiple anxiety disorders and normal fear conditioning (Etkin and Wager, 2007). A recent study also demonstrated increased insula activity as a result of combat exposure itself, even in the absence of PTSD (van Wingen et al., 2011). In the context of these studies, the current findings suggest that trauma exposure induces, and PTSS are correlated with, reduced insula volume but increased insula activation to emotional stimuli. One possibility may be that reductions in insula volume represent a compensatory response to prolonged, heightened insula activation. Alternatively, increased activity may reflect a compensatory response for reduced volume. The reason for the dissociation between insula volume and activity remains unclear at this time, and merits further study.
Contrary to our main hypothesis, there was no relationship between PTSS and hippocampal volume, or differences in hippocampal volume between the PTSD and non-PTSD groups. Some previous studies also failed to find hippocampal volume differences in PTSD, e.g. (Fennema-Notestine et al., 2002; Bonne et al., 2001), suggesting variability of this finding in the literature. Furthermore, PTSD duration has been associated with smaller hippocampal volumes (Apfel et al., 2011; Felmingham et al., 2009). Subjects in the current study, being more recent war veterans, will not yet have had chronicity of PTSD to the extent of veterans in prior studies, which could explain the absence of hippocampal findings in this study. As mentioned earlier, hippocampal volume reductions also appear to be more evident with severe PTSD (Karl et al., 2006). Subjects with a full diagnosis of PTSD in this study had an average CAPS score of 47.5 (a moderate level of PTSD on average) and could therefore account for the lack of hippocampal grey matter differences.
There are several limitations to the current study. First, these findings are correlational only and do not prove causation. Nevertheless, the combination of correlational and group contrast approaches, and accounting for depression, age, and trauma load, does suggest specificity of these brain changes with PTSS. Second, this is a cross sectional study which makes it difficult to assess whether smaller regional brain volumes are a preexisting vulnerability factor (trait), alterations with PTSS itself (state), or a compensatory change. More longitudinal studies will be needed to address these limitations. Third, these findings may not generalize to other populations or trauma types, given the heavily male, combat veteran sample. Fourth, findings may not generalize to individuals with more severe PTSD (e.g. CAPS>80). The relatively restricted range in CAPS scores could also account for some of the similarity between the regression and group contrasts. It is nonetheless notable that some of the findings (smaller ACC and insula) have also been documented with more severe PTSD. This suggests that such changes are present even in individuals with milder PTSS. Finally, this is a relatively small number of subjects and would merit replication with a larger sample.
While subjects were free of any current alcohol or substance abuse, this study did not have measures of cumulative, lifelong alcohol use which could affect the current results. Chronic alcoholism has been associated with reduced grey matter in the superior frontal and motor cortices, cerebellum, and hippocampus (Spampinato et al., 2005; Hedges and Woon, 2010). However, PTSS were not associated with grey matter reductions in these areas in our study, making it less likely that lifetime alcohol use would account for the observed changes. In addition, removal of the 5 subjects with a history of alcohol dependence from the VBM analysis did not change the pattern of results.
There are a number of strengths of the present study. This is one of few studies to examine brain changes in OEF/OIF combat veterans. Subjects were healthy and free of major concurrent psychiatric comorbidities including alcohol and substance abuse or dependence. Subjects were medication-free, and medication is a common potential confound in clinical studies. This study was also able to account for childhood and adult trauma load which represent important potential confounds in studies of PTSD.
In summary, the current study revealed that PTSS are correlated with reduced gray matter in the sgACC, caudate, hypothalamus, and anterior insula. The similarity of these findings with the group contrast suggests that they exist on a spectrum in trauma-exposed individuals, and are present even in subthreshold PTSD. With further study, this could eventually help to identify individuals at risk for (or resilient to) developing full PTSD following traumatic events. These regions play an important role in mediating extinction of fear memory, reward, and anxious states. As such, abnormalities in these areas may represent pathophysiological changes in PTSD, a disorder characterized by heightened and generalized fear memory, anhedonia, and anxiety, all of which may be subserved by different neural circuits.
Supplementary Material
Acknowledgments
This work was supported by the Department of Defense Congressionally Directed Medical Research Program PT073961 (AG), and the National Institute of Mental Health MH083035 (AG), MH076971 (MP), MH088913 (MP). These data have been published in abstract form for the Society of Biological Psychiatry 2011 Annual Meeting.
Footnotes
Financial disclosures
Dr. Germain has served as a consultant for Concurrent Technologies Corporation. Drs. Herringa, Phillips, Almeida, and Insana report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida JR, Akkal D, Hassel S, Travis MJ, Banihashemi L, Kerr N, Kupfer DJ, Phillips ML. Reduced gray matter volume in ventral prefrontal cortex but not amygdala in bipolar disorder: significant effects of gender and trait anxiety. Psychiatry Research. 2009;171:54–68. doi: 10.1016/j.pscychresns.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. The Journal of Neuropsychiatry and Clinical Neurosciences. 2008;20:292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel BA, Ross J, Hlavin J, Meyerhoff DJ, Metzler TJ, Marmar CR, Weiner MW, Schuff N, Neylan TC. Hippocampal volume differences in gulf war veterans with current versus lifetime posttraumatic stress disorder symptoms. Biological Psychiatry. 2011;69:541–548. doi: 10.1016/j.biopsych.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. Journal of Traumatic Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. The American Journal of Psychiatry. 2001;158:1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychological Medicine. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. The American Journal of Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Chen S, Xia W, Li L, Liu J, He Z, Zhang Z, Yan L, Zhang J, Hu D. Gray matter density reduction in the insula in fire survivors with posttraumatic stress disorder: a voxel-based morphometric study. Psychiatry Research. 2006;146:65–72. doi: 10.1016/j.pscychresns.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Corbo V, Clement MH, Armony JL, Pruessner JC, Brunet A. Size versus shape differences: contrasting voxel-based and volumetric analyses of the anterior cingulate cortex in individuals with acute posttraumatic stress disorder. Biological Psychiatry. 2005;58:119–124. doi: 10.1016/j.biopsych.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. The Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Roiser JP. Reward and punishment processing in depression. Biological Psychiatry. 2010;68:118–124. doi: 10.1016/j.biopsych.2010.01.027. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Whitford TJ, Falconer E, Kemp AH, Peduto A, Bryant RA. Duration of posttraumatic stress disorder predicts hippocampal grey matter loss. Neuroreport. 2009;20:1402–1406. doi: 10.1097/WNR.0b013e3283300fbc. [DOI] [PubMed] [Google Scholar]
- Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biological Psychiatry. 2002;52:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: Nov, 2002. [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BL. Trauma History Questionnaire. In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Sidran Press; Lutherville, Md: 1996. pp. 366–369. [Google Scholar]
- Hedges DW, Woon FL. Alcohol use and hippocampal volume deficits in adults with posttraumatic stress disorder: A meta-analysis. Biological Psychology. 2010;84:163–168. doi: 10.1016/j.biopsycho.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Helzer JE American Psychiatric Association. Dimensional Approaches in Diagnostic Classification: Refining the Research Agenda for DSM-V. 1. American Psychiatric Association; Arlington, Va: 2008. [Google Scholar]
- Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq or Afghanistan. JAMA: The Journal of the American Medical Association. 2006;295:1023–1032. doi: 10.1001/jama.295.9.1023. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Frewen PA, van der Kolk BA, Lanius RA. Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. Journal of Traumatic Stress. 2007;20:713–725. doi: 10.1002/jts.20284. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neuroscience and Biobehavioral Reviews. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Kasai K, Yamasue H, Gilbertson MW, Shenton ME, Rauch SL, Pitman RK. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biological Psychiatry. 2008;63:550–556. doi: 10.1016/j.biopsych.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane T, Fairbank J, Caddell J, Zimering R, Taylor K, Mora C. Clinical evaluation of a measure to assess combat exposure. Psychological Assessment. 1989;1:53–55. [Google Scholar]
- Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. Journal of Affective Disorders. 2006;90:171–174. doi: 10.1016/j.jad.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annual Review of Psychology. 2010;61:111–40. C1–3. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, Frith CD. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone DA, Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biological Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. Effects of stressful life events on human brain structure: a longitudinal voxel-based morphometry study. Stress (Amsterdam, Netherlands) 2011;14:227–232. doi: 10.3109/10253890.2010.522279. [DOI] [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008;13:829, 833–57. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. The American Journal of Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, Whalen PJ, Makris N. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR. Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neuroscience & Therapeutics. 2011;17:227–236. doi: 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer U, Robinson S, Fischmeister FP, Konig D, Oppenauer C, Lueger-Schuster B, Moser E, Kryspin-Exner I, Bauer H. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia. 2008;46:2836–2844. doi: 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35:169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato MV, Castillo M, Rojas R, Palacios E, Frascheri L, Descartes F. Magnetic resonance imaging findings in substance abuse: alcohol and alcoholism and syndromes associated with alcohol abuse. Topics in Magnetic Resonance Imaging: TMRI. 2005;16:223–230. doi: 10.1097/01.rmr.0000192175.26243.a7. [DOI] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer N, de Ruiter MB, van Balkom AJ, Smit JH, Veltman DJ. Reduced anterior cingulate and orbitofrontal volumes in child abuse-related complex PTSD. The Journal of Clinical Psychiatry. 2010;71:1636–1644. doi: 10.4088/JCP.08m04754blu. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, Hoge CW. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Archives of General Psychiatry. 2010;67:614–623. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Molecular Psychiatry. 2011;16:664–671. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal G, Hamilton DA, Petropoulos H, Driscoll I, Rowland LM, Griego JA, Kodituwakku PW, Hart BL, Escalona R, Brooks WM. Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biological Psychiatry. 2002;52:119–125. doi: 10.1016/s0006-3223(02)01359-8. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Baler RD, Goldstein RZ. Addiction: pulling at the neural threads of social behaviors. Neuron. 2011;69:599–602. doi: 10.1016/j.neuron.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vythilingam M, Nelson EE, Scaramozza M, Waldeck T, Hazlett G, Southwick SM, Pine DS, Drevets W, Charney DS, Ernst M. Reward circuitry in resilience to severe trauma: an fMRI investigation of resilient special forces soldiers. Psychiatry Research. 2009;172:75–77. doi: 10.1016/j.pscychresns.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depression and Anxiety. 2001;13:132–156. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Martinez C, Schaer M, Eliez S. Decreased anterior cingulate volume in combat-related PTSD. Biological Psychiatry. 2006;59:582–587. doi: 10.1016/j.biopsych.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Woon FL, Sood S, Hedges DW. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2010;34:1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Kasai K, Iwanami A, Ohtani T, Yamada H, Abe O, Kuroki N, Fukuda R, Tochigi M, Furukawa S, Sadamatsu M, Sasaki T, Aoki S, Ohtomo K, Asukai N, Kato N. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.