Abstract
Huntington's Disease (HD) is a neurodegenerative disorder caused by a cytosine-adenine-guanine (CAG) triplet repeat-expansion in the Huntingtin (HTT) gene. Diagnosis of HD is classically defined by the presence of motor symptoms; however cognitive and depressive symptoms frequently precede motor manifestation, and may occur early in the prodromal phase. There are sparse data so far on functional brain correlates of depressive symptoms in prodromal-HD. A Stroop color-naming test was administered to 32 subjects in the prodromal phase of HD and 52 expansion-negative controls while performing functional magnetic resonance imaging at 3 Tesla. Networks of functional connectivity were identified using group independent component analysis, followed by an analysis of functional network interactions. A contrast of temporal regression-based beta-weights was calculated as a reflection of Stroop-interference related activity and correlated with Center for Epidemiologic Studies Depression (CES-D) scores. For secondary analysis, patients were stratified in two subgroups by median split of CAG repeat-length. Stroop-performance was independent of HTT mutation-carrier-status and CES-D score. Stroop-interference related activity of the ventromedial prefrontal cortex-node of the default-mode network, calculated by temporal-regression beta-weights, was more highly correlated with depressive symptoms in subjects in the prodromal phase of HD than in controls, differing significantly. The strength of this correlation and its difference from controls increased when a subgroup of patients with longer CAG repeat lengths was analyzed. These findings suggest that depressive symptoms in prodromal-HD subjects may reflect altered functional brain network activity in the context of early HD related brain alterations.
Keywords: Depression, Functional Connectivity, fMRI, BOLD, Neurodegeneration, DMN
1. Introduction
Huntington's Disease (HD) is a neurodegenerative disorder caused by a cytosine-adenine-guanine (CAG) triplet repeat expansion in the Huntingtin (HTT) gene (HD_Collaborative_Research_Group, 1993; Ross and Tabrizi, 2011). HD is inherited as an autosomal dominant condition, with nearly complete penetrance by age 65 (Langbehn et al., 2004). Prevalence of HD is 4–10 / 100000 in the western hemisphere (Tabrizi et al., 2009). Clinical manifestation of HD is classically defined by progressive motor dysfunction accompanied by cognitive decline and psychiatric symptoms (Walker, 2007). The length of the expanded CAG repeat is inversely correlated with onset age (Penney et al., 1997; Langbehn et al., 2004). Predictive genetic testing makes possible the identification of individuals with the expanded CAG repeat length who do not yet have sufficient motor signs to be diagnosed as affected (Gusella et al., 1983; Duyao et al., 1993; HD_Collaborative_Research_Group, 1993). Such individuals may have non-specific early cognitive or psychiatric symptoms (“prodromal-HD”). Depressive symptoms in prodromal individuals show an incidence more than twice the general population (Marshall et al., 2007) and have been repeatedly reported as early clinical findings in HD (Folstein et al., 1979; Paulsen et al., 2005; Duff et al., 2007; Julien et al., 2007; van Duijn et al., 2007). CAG-repeat length has been reported to be related to severity of psychiatric symptoms in the prodromal phase (Duff et al., 2007) and a recent study reports increased prevalence of incompletely penetrant Huntingtin alleles among individuals with major depressive disorder (Perlis et al., 2010). While the neurobiology of depression in the context of HD remains unclear, data from recent studies on depression in HD indicates the possible relevance of regional brain changes (Thu et al., 2010; Hobbs et al., 2011).
Several neuroimaging studies have reported significant abnormalities preceeding the clinical diagnosis of HD. These include striatal atrophy (Paulsen et al., 2006; Aylward, 2007), cortical-thinning (Rosas et al., 2005; Nopoulos et al., 2007; Klöppel et al., 2009), white-matter atrophy (Thieben et al., 2002; Reading et al., 2005; Paulsen et al., 2010; Rosas et al., 2010) and possibly smaller intracranial volume (Nopoulos et al., 2011). Alterations observable with blood oxygen level dependence (BOLD) functional magnetic resonance imaging (fMRI) suggest an association between cognitive changes and alterations in areas of executive function (Reading et al., 2004; Zimbelman et al., 2007; Wolf et al., 2008b) that appear to precede structural changes and possibly reflect complex processes involving neuronal dysfunction in the prodromal phase (Paulsen, 2009). Analysis of functional connectivity, refering to synchronous neuronal activity of spatially remote brain regions (Friston et al., 1993; van de Ven et al., 2004), appears to be a promising approach, capable of identifying patterns of impaired neuronal interaction. Independent component analysis (ICA) is a method of blind source signal separation, that can be applied to fMRI-signals to identify spatially distinct maps and corresponding time courses, representing functional brain networks (McKeown et al., 1998b; Calhoun et al., 2001; van de Ven et al., 2004; Beckmann et al., 2005; Calhoun et al., 2008b; Damoiseaux and Greicius, 2009). While loss of integrity has been reported for functional networks both in the context of depressive syndromes (Greicius et al., 2007; Grimm et al., 2009; Sheline et al., 2009; Sheline et al., 2010a) as well as in neurodegenerative diseases (Sorg et al., 2007; Sheline et al., 2010b), there has been relatively little work assessing functional connectivity in prodromal-HD. Thus far only one study reported alterations in lateral prefrontal network connectivity implicated in paradigms of cognitive challenge (Wolf et al., 2008a).
While there exists a broad literature on impairments of emotional processing in subjects in the prodromal phase of HD (Berrios et al., 2001; Kirkwood et al., 2002; Duff et al., 2007; Johnson et al., 2007; Julien et al., 2007; van Duijn et al., 2007; Vassos et al., 2007; Henley et al., 2008; Klöppel et al., 2010), there is an absence of fMRI studies on correlates of depressive symptomes in the prodrome of HD. The Stroop color-naming task challenges brain systems involved in executive function (Stroop, 1935; MacLeod, 1991; MacLeod and MacDonald, 2000). It has been demonstrated to reliably engage diverse spatially independent networks of functional connectivity, therefore making it a suitable paradigm for multivariate analysis of BOLD fMRI data using ICA (McKeown et al., 1998a; Harrison et al., 2008). Furthermore, Stroop task-performance is significantly impaired in patients with major depression (Benoit et al., 1992; Lemelin et al., 1997), and may serve as a trait marker for depression (Killian et al., 1984; Trichard et al., 1995; Paradiso et al., 1997; Videbech et al., 2004). This fits well with a concatenation of studies demonstrating interrelations of systems involved in cognitive and emotional processing and greater vulnerability of depressed patients to cognitive stress (Zihl et al., 1998; Ravnkilde et al., 2002; Majer et al., 2004; Reppermund et al., 2007).
We hypothesized, that depressive symptoms in individuals in the prodromal phase of HD may relate to altered functional brain network activity as a reflection of early HD related brain changes. The aim of this study was therefore to use ICA to first identify relevant brain networks of functional connectivity observable in fMRI data under a Stroop-Interference task. In a second step, functional activity of the identified networks was correlated with depressive symptoms, and correlation coefficients were then tested for significant differences between prodromal-HD and unaffected controls. Additionally an analysis using subgroups of the prodromal-HD sample based on Huntingtin CAG repeat length was performed to test significant effects for a possible relationship to the individual genetic load of the Huntingtin expansion mutation.
2. Methods
2.1. Study population
32 prodromal subjects were recruited through the Huntington’s Disease Center at Johns Hopkins University School of Medicine, and were subdivided for secondary analysis by median-split of CAG-repeat length in two groups (CAG ≤ 42 and > 42). The prodromal participants all had scores on the quantitative neurologic examination (QNE) (Folstein et al., 1983) < 10 (mean (SD) 3.35 (3.45)), and all scored below 5 on the chorea subscore (mean (SD) 0.48 (1.06)). Estimated years to onset of motor symptoms (YTO) was calculated based on CAG-repeat length of the mutated HTT allele and age (Langbehn et al., 2004) and disease burden score (DBS) was calculated as [(CAG-repeat length −35.5) * age] (Penney et al., 1997; Langbehn et al., 2004). Additionally 52 control subjects were recruited through Johns Hopkins University. None of the 84 participants had a history of severe mood-, obsessive compulsive-, psychotic disorder or substance abuse, however 5 controls and 7 subjects in the prodromal phase of HD reported antidepressant medication use within the last four weeks. Consent was obtained according to the Declaration of Helsinki (World_Medical_Association, 1991) and approved by the Johns Hopkins University Institutional Review Board.
Clinical personnel, trained in psychiatric patient evaluation, performed the following interviews and neuropsychological tests on the day of scanning: Center for Epidemiologic Studies-Depression scale (CES-D) for prevalent depressive symptoms (Radloff, 1977); Mini Mental State Exam (MMS) to screen for cognitive impairment (Folstein et al., 1975), and the National Adult Reading Test (NART) as an estimate of premorbid intelligence (Bright et al., 2002). CES-Depression scores in the prodromal-HD subgroup with CAG-repeat lengths > 42 were higher than controls (table 1) and correlated with CAG repeat length in the entire prodromal-HD sample (r=0.43, p=0.03). There were no significant correlations between CES-D and DBS (r=0.01), YTO (r=0.13), total QNE (r=−0.05) or age (r=−0.17).
Table 1.
Statistics of the study population, displayed are group-averages (SD) and p-values (t-tests) for expansion-negative controls, subjects in the prodromal phase of HD and the subgroups derived from the prodromal-HD sample.
| Controls (n=52) | Prodromal HD (n=32) |
CAG > 42 (n=16) |
CAG ≤ 42 (n=16) |
HD vs. Ctr. | CAG > 42 vs. Ctr. |
CAG ≤ 42 vs. Ctr. |
CAG > 42 vs. CAG ≤ 42 |
|
|---|---|---|---|---|---|---|---|---|
| Demographics and clinical measures | ||||||||
| Age [years] | 39.9 (9.8) | 40 (10.11) | 39.88 (9.53) | 40.13 (10.97) | 0.97 | 0.99 | 0.94 | 0.95 |
| sex (% females) | 44% | 63% | 50% | 75% | 0.11 | 0.69 | 0.03 | 0.15 |
| CAG - repeats [n] | - | 42.81 (3.43) | 44.75 (3.89) | 40.88 (1.09) | - | - | - | <0.001 |
| Disease Burden Score (DBS) | 281 (95) | 346 (66) | 215 (70) | - | - | - | <0.001 | |
| Estimated time to onset [years] | - | 14.84 (9.65) | 9.12 (3.56) | 20.56 (10.49) | - | - | - | <0.001 |
| CES-Depression score | 5.87 (6.55) | 6.97 (7.12) | 9.94 (7.71) | 4 (5.15) | 0.47 | 0.04 | 0.3 | 0.02 |
| Education [years] | 16.44 (2.78) | 14.88 (2.47) | 14.44 (2.75) | 15.31 (2.15) | 0.01 | 0.01 | 0.14 | 0.32 |
| MMS test score | 29.35 (0.83) | 29.16 (0.72) | 29 (0.81) | 29.31 (0.6) | 0.29 | 0.15 | 0.88 | 0.23 |
| HART test score | 111 (9.07) | 108 (7.39) | 107 (5.15) | 109 (8.97) | 0.18 | 0.1 | 0.64 | 0.29 |
| QNE score - total | - | 3.25 (3.44) | 4.88 (3.59) | 1.63 (2.45) | - | - | - | 0.01 |
| QNE score - chorea | - | 0.47 (1.04) | 0.69 (1.3) | 0.25 (0.68) | - | - | - | 0.24 |
| Volumes | ||||||||
| Intracranial Volume [ml] | 2117 (230) | 2078 (303) | 2041 (216) | 2116 (374) | 0.47 | 0.23 | 0.94 | 0.49 |
| Ventricles / Intracranial Volume [%] | 1.17 (0.40) | 1.44 (0.62) | 1.57 (0.55) | 1.38 (0.58) | 0.12 | 0.18 | 0.27 | 0.88 |
| Striatum / Intracranial Volume [%] | 1.25 (0.11) | 1.13 (0.16) | 1.04 (0.14) | 1.22 (0.15) | <0.001 | <0.001 | 0.29 | 0.0025 |
| Stroop performance | ||||||||
| accuracy, baseline [%] | 98.72 (1.73) | 98.8 (2.04) | 98.54 (2.64) | 99.06 (1.21) | 0.84 | 0.76 | 0.46 | 0.48 |
| accuracy, congruent task [%] | 98.91 (1.77) | 98.7 (1.78) | 98.54 (1.6) | 98.85 (1.83) | 0.6 | 0.46 | 0.91 | 0.63 |
| accuracy, incongruent task [%] | 95.93 (5.93) | 96.51 (5.89) | 96.04 (6.83) | 96.98 (4.95) | 0.43 | 0.95 | 0.23 | 0.48 |
| reaction time, baseline [ms] | 609 (102) | 600 (66.9) | 621 (70.3) | 577 (57.1) | 0.61 | 0.67 | 0.23 | 0.06 |
| reaction time, congruent task [ms] | 602 (112) | 603 (64.4) | 624 (70.7) | 581 (51.0) | 0.96 | 0.45 | 0.48 | 0.06 |
| reaction time, incongruent task [ms] | 701 (134) | 693 (90.4) | 692 (64.5) | 694 (113) | 0.77 | 0.8 | 0.85 | 0.96 |
2.2. Scan acquisition and structural analysis
Data were acquired at the FM Kirby Research Center for Functional Brain Imaging at Kennedy Krieger Institute on a Philips Intera 3T scanner (Philips Medical Systems, Best, the Netherlands) equipped with a multi-element receiver coil and SENSE head coil. Functional EPI acquisitions consisting of 34 slices were collected in the axial plane, aligned parallel to the line from the anterior commisure to the posterior commisure using the following parameters: TR = 2000 ms; TE = 35 ms; flip angle = 70°; 34 slices; FOV=240 mm; nominal resolution = 1.88×1.88×3 mm; no gap; SENSE factor = 2.0. The fMRI-paradigm was presented automatically triggered via ePrime19 software.
Whole-brain anatomy was assessed using T1-weighted, three-dimensional Magnetization Prepared Rapid Gradient Echo (MP-RAGE) using the following parameters: TR = 8.4 ms; TE = 3.8 ms; flip angle = 8°; 150 slices (no gap); FOV=230 mm; nominal resolution = 0.9×0.9×0.9 mm);
Large Deformation Diffeomorphic Metric Mapping (LDDMM) was used to quantify metric distances on anatomical structures in medical images (Miller et al., 2002; Huang et al., 2008; Oishi et al., 2009) for volume estimations (Giedd, 2004; Nopoulos et al., 2011) providing a measure of intracranial volume (ICV). ICV and ventricle volume per ICV, used as an indicator of general atrophy (Wolf et al., 2003; Nestor et al., 2008) did not differ significantly between subjects in the prodromal phase of HD and controls. Consistent with earlier neuroimaging studies, striatal-volume was significantly lower in the prodromal-HD sample (Paulsen et al., 2006; Aylward, 2007) (table 1).
2.3. fMRI paradigm
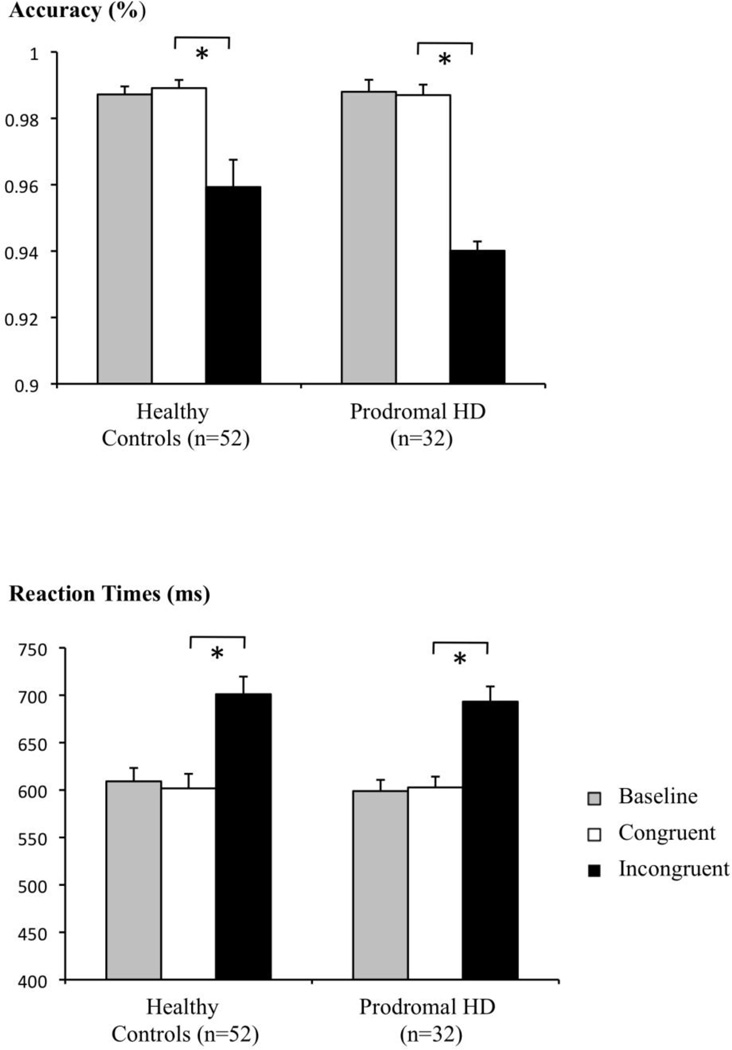
An MRI compatible version of the Stroop color-naming test was performed as described earlier (Stroop, 1935; MacLeod, 1991; Gruber et al., 2002) by projecting series of four X’s (baseline) or color word stimuli (congruent and incongruent tasks, respectively) onto a screen viewed using a mirror mounted to the head coil and using a control box with buttons representing each color to be named as an input device for the subject scanned. The interference effect was estimated for each participant as a measure of behavioral task-performance, by calculating the differences in reaction time and accuracy relating to the particular incongruent and congruent task, for each subject (figure 1) (Jensen, 1965; Jensen and Rohwer, 1966). All 84 subjects tested, achieved accuracies above 85%. Trials were separated by 10 s (5TR) of rest, resulting in a total of 466 s (233TR) for the entire paradigm (figure 2).
Figure 1.
Stroop-interference is reflected by decreased accuracy and increased reaction times in the incongruent versus the congruent tasks for both controls and prodromal-HD.
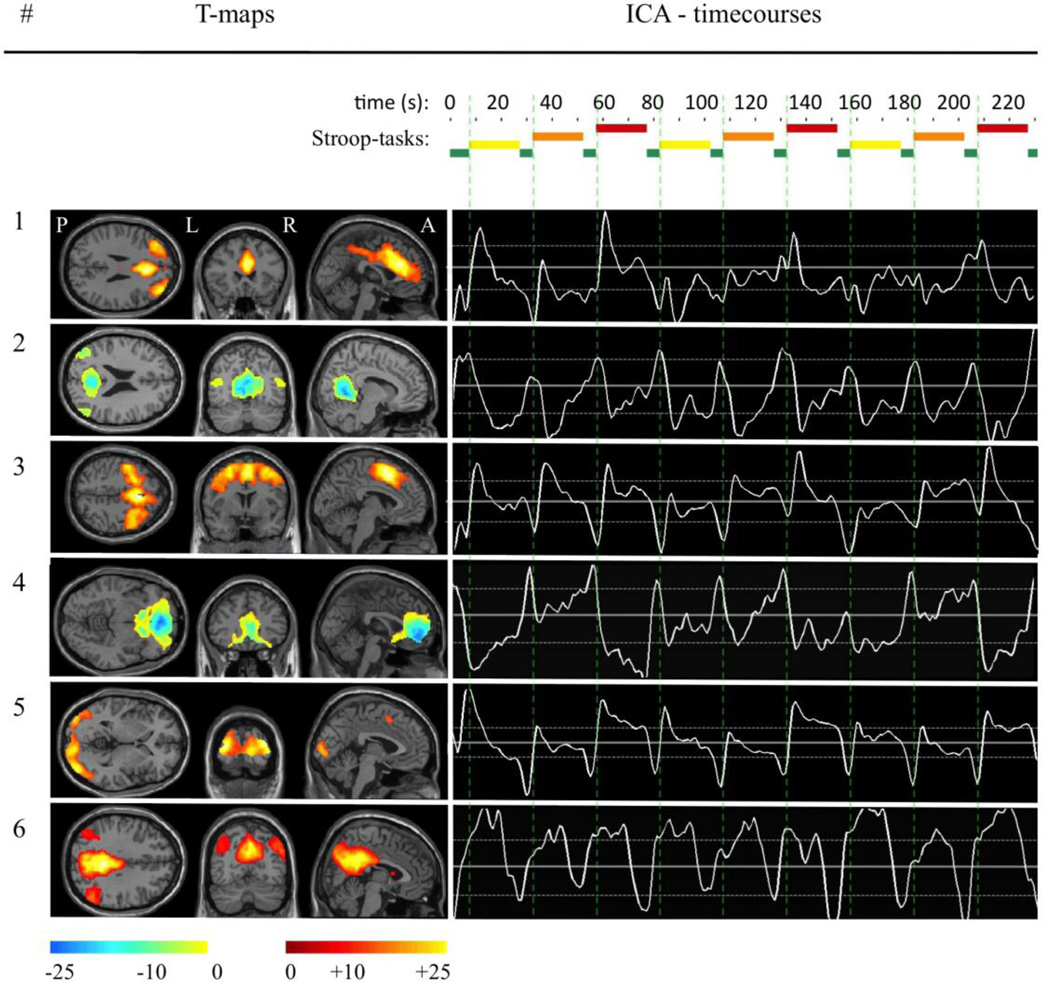
Figure 2.
Spatial extent of the identified components is indicated based on t-maps. ICA-timecourses indicate z-scores of component activation (solid white lines: means, dotted white lines: ± 0.2 SD) in relation to the Stroop-paradigm applied over time (TR). Green bars indicate rests between Stroop tasks, yellow: Stroop-baseline, orange: Stroop-congruent, red: Stroop-incongruent, vertical dotted green lines indicate the end of the rest-phases.
2.4. Analysis of functional MRI data
2.4.1. Data Preprocessing
Individual fMRI data were preprocessed by an initial correction for timing differences between slices, realignment, smoothing with a 6mm3 full width half-maximum Gaussian kernel, spatial normalization to MNI template space via the anatomical MPRAGE scan as suggested for Group spatial ICA (Van de Moortele et al., 1997; Calhoun et al., 2001; Jafri et al., 2008) using the Statistical Parametric Mapping (SPM5) software package (Friston, 1995), (http://www.fil.ion.ucl.ac.uk/spm/), high-pass frequency filter (128 s) and a correction for temporal autocorrelation.
2.4.2. Component identification
Group spatial ICA (Calhoun et al., 2001) was conducted for all 84 participants using the GIFT software (version 1.3h, http://icatb.sourceforge.net/) and Matlab 7.10 (MathWorks, Inc., Natick, Massachusetts, United States) as described earlier (Bell and Sejnowski, 1995; Beckmann et al., 2005; Calhoun et al., 2008a; Jafri et al., 2008). In brief, GIFT is an application developed in Matlab to perform group ICA for blind source signal separation of fMRI-data, resulting in spatially independent components of synchronous BOLD activity. Using GIFT, the following processes to perform spatial group ICA were applied to the entire sample of 84 subjects: After an initial step of data reduction using principal component analysis (PCA), ICA was used to identify independent components. In this study the Infomax algorithm (Bell and Sejnowski, 1995) was used in combination with the integrated ICASSO-function (http://www.cis.hut.fi/projects/ica/icasso) to maximize reliability of the ICA. Minimum description length (MDL) criteria were applied (Li et al., 2007) for component estimation, resulting in a total of 35 components. Then back reconstruction using components from ICA and results from the initial data reduction step was performed to compute the individual subject components and timecourses, which were scaled using z-scores. Spatial t-maps were generated indicating relative strength of component contribution and used for visualization. Using a design-matrix with information about the timing of the Stroop-interference tasks, the GIFT temporal sorting algorithm was performed to apply a multiple regression. This resulted in beta-weights representing component specific measures of task related BOLD-activity for each test condition (incongruent and congruent) and subject, which were used to calculate a contrast (incongruent – congruent) reflecting component specific Stroop-interference related activity. (Calhoun et al., 2008a; Jafri et al., 2008). Visualization was performed using GIFT v1.3h component viewer and xjView (http://www.alivelearn.net/xjview8). By applying a systematic approach to identify and sort out components related to physiological noise (Perlbarg et al., 2007; Stevens et al., 2007; Jafri et al., 2008), six components were selected as potentially relevant in a context of emotional processing and cognition.
2.5. Statistical analysis
Tests for differences in demographic, clinical or Stroop-performance related variables between subjects in the prodromal phase of HD and expansion negative controls were performed by using independent samples t-tests. A possible interaction between CES-Depression scores and Stroop-interference was tested using ANOVA.
Linear regression analysis was used to estimate the relationship of Stroop-interference related activity with CES-Depression scores of patients and controls separately. The following covariates were applied and found not to affect the correlations significantly: ratio of ventricular volume / intracranial volume as a reflection of relative volume differences (Wolf et al., 2003; Nestor et al., 2008), age, gender and antidepressive medication status. Correlation coefficients of patients and controls for each of the six selected components were tested for significant differences by performing a Fisher r to z transformation and estimation of standard error of the difference for two independent variables (Significance of the Difference Between Two Correlation Coefficients, Richard Lowry 2011, http://faculty.vassar.edu/lowry/rdiff.html) (Fisher, 1915; Edwards, 1984). A correction for multiple comparissons by a factor of 7 was performed, taking into account the total number of tests (Holm, 1979). Differences were considered significant at a level of 5% after correction (−log(p) > 2.15). In case of significant differences, an additional correlation and test for significant difference based on Fisher r to z transformation (Fisher, 1915; Edwards, 1984) was performed for the prodromal-HD subgroup with higher genetic load (CAG-repeat length > 42) versus controls. Additionally, possible relationships between Stroop-interference related component activity and CAG-repeat length were tested by performing linear regression analysis for all six components. Between network connectivity was calculated using correlation coefficients of BOLD-activity between the components identified with group-ICA (Joel et al., 2011).
Analyzed CES-Depression scores and beta-weights were tested for normal distribution and applicability of parametric testing by applying a one-sample Kolmogorov-Smirnov test, resulting in confirmation of the null hypothesis. Statistics were performed using the SPSS statistical software package for Windows, Version 17.0, between network connectivity was assessed using the Matlab 7.10 (R2010a) statistical toolbox.
T-maps of the six identified networks were tested in a two sample t-test using SPM5 for topographical differences in spatial distribution between subjects in the prodromal phase of HD and controls. A one sample t-test was performed on beta-weights derived from multiple regression (temporal sorting algorithm, GIFT software package, v1.3h) to test for task relatedness of the components (Calhoun et al., 2008a; Jafri et al., 2008). As a descriptive measure indicating general task-relatedness of all components for one specific task, means of the −log(p-values) were calculated.
3. Results
3.1. Stroop task-performance is similar in subjects in the prodromal phase of HD and controls, resulting in a significant effect of interference in both groups
A fMRI compatible version of the Stroop color-naming test (Stroop, 1935; MacLeod, 1991) was completed by 32 subjects in the prodromal phase of HD and 52 controls. A significant effect of Stroop-interference could be observed both for test-accuracy (p<0.05) and reaction time (p<0.01), as measured by a decrease in accuracy and increase in reaction time in the incongruent (prodromal-HD: mean (SD): 96.51 (5.89)%; 693 (90.4) ms; controls: mean (SD): 95.93 (5.93)%; 701 (134) ms) versus the congruent task (prodromal-HD: mean (SD): 98.7 (1.78)%; 603 (64.4) ms; controls: mean (SD): 98.91 (1.77)%; 602 (112) ms) (figure 1). Stroop-performance both in terms of test-accuracy and reaction times did not differ significantly between the populations tested (controls, all prodromal-HD, prodromal-HD with CAG > 42, prodromal-HD with CAG ≤ 42), when a two sample t-test was applied (table 1). Also the effect of Stroop-interference was not affected significantly by HTT mutation-carrier-status: Differences between incongruent and congruent task in acccuracy, prodromal-HD (mean(SD)): 4.69 (2.59), controls: 2.98 (0.82), F(1,83)=0.56, p=0.46; differences between incongruent and congruent task in reaction time, prodromal-HD: 90.49 (14.98), controls: 99.33 (11.69), F(1,83)=0.22, p=0.64. There was no significant interaction of interference with CES-Depression scores (accuracy: F(21,83)=0.45, p=0.98; reaction time: F(21,83)=1.14, p=0.33).
3.2. ICA decomposition of Stroop-interference related BOLD signal identifies six components reflecting functional brain network activity
By performing ICA-decomposition followed by a systematic approach of selection, a total of six components were identified (figure 2). Comparison of SPM t-maps of the six components did not reveal significant differences in spatial extent between subjects in the prodromal phase of HD and unaffected controls (no suprathreshold clusters at T=5.91, p=0.005). Based on measured between-network-connectivity (Van de Moortele et al., 1997; Calhoun et al., 2001; Jafri et al., 2008) (matrix correlation of component timecourses using the Matlab 7.10 (R2010a) statistical toolbox), components were categorized in two groups: a group of task activated elements (group A: #1, #3, #5, #6) and a group of task downregulated elements (group B: #2, #4). Using information of synchrony between networks, in addition to the anatomical information revealed by labelling using GIFT toolbox, all six components could be matched to previously reported functional networks (table 2) (Greicius et al., 2003; Beckmann et al., 2005; Fox et al., 2005; Damoiseaux et al., 2006; Seeley et al., 2007; Buckner et al., 2008; Calhoun et al., 2008a; Joel et al., 2011; Sämann et al., 2011). Between network connectivity of subjects in the prodromal phase of HD did not differ significantly from healthy controls (Correlation values were converted to z-scores using the Fisher r to z transformation, (Fisher, 1915)) (table 2).
Table 2.
Overview of BOLD-synchrony for the six components identified by ICA decomposition. Bold figures and colors indicate correlation-coefficients (pearson-r) as a reflection of between network connectivity of the entire sample of 84 subjects; italic figures indicate group specific pearson-r: on top controls, below prodromal-HD.
| Comp. # | Network | BA | Group | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Salience | 24 | A | 1 | −0.20 | 0.31 | −0.20 | 0.22 | 0.14 |
| 1 | −0.16 | 0.31 | −0.16 | 0.21 | 0.17 | ||||
| 1 | −0.28 | 0.30 | −0.27 | 0.22 | 0.08 | ||||
| 2 | Default Mode, Posterior Cingulate Cortex | 23 | B | −0.20 | 1 | −0.30 | 0.31 | −0.39 | −0.30 |
| −0.16 | 1 | −0.32 | 0.31 | −0.39 | −0.24 | ||||
| −0.28 | 1 | −0.26 | 0.32 | −0.38 | −0.38 | ||||
| 3 | Dorsal-frontoparietal Attention | 6 | A | 0.31 | −0.30 | 1 | −0.32 | 0.48 | 0.36 |
| 0.31 | −0.32 | 1 | −0.38 | 0.50 | 0.36 | ||||
| 0.30 | −0.26 | 1 | −0.23 | 0.44 | 0.35 | ||||
| 4 | Default Mode, Ventromedial prefrontal cortex | 10 | B | −0.20 | 0.31 | −0.32 | 1 | −0.35 | −0.13 |
| −0.16 | 0.31 | −0.38 | 1 | −0.40 | −0.22 | ||||
| −0.27 | 0.32 | −0.23 | 1 | −0.28 | 0.01 | ||||
| 5 | Extrastriate Visual Association | 19 | A | 0.22 | −0.39 | 0.48 | −0.35 | 1 | 0.43 |
| 0.21 | −0.39 | 0.50 | −0.40 | 1 | 0.42 | ||||
| 0.22 | −0.38 | 0.44 | −0.28 | 1 | 0.45 | ||||
| 6 | Attention | 31 | A | 0.14 | −0.30 | 0.36 | −0.13 | 0.43 | 1 |
| 0.17 | −0.24 | 0.36 | −0.22 | 0.42 | 1 | ||||
| 0.08 | −0.38 | 0.35 | 0.01 | 0.45 | 1 | ||||
| <= −0.3 | −0.3 – −0.09 | −0.09 – +0.09 | 0.09 – 0.3 | >= 0.3 | |||||
One sample t-tests for task relatedness of components (Calhoun et al., 2008a; Jafri et al., 2008) revealed increasing mean −log(p) values (SD) with increasing difficulty of the applied task (baseline: 1.24 (1.1); congruent: 2.51 (1.95); incongruent: 3.74 (2.87)) (table 3). Also differences of Stroop-interference related component activity between prodromal-HD and controls did not reach level of significance (5%, −log(p)=1.3) when an independent samples t-test was applied (Comp.#1: −log(p)=0.02, T=0.07; comp.#2: −log(p)=0.52, T=1.05; comp.#3: −log(p)=0.15, T=0.38; comp.#4: −log(p)=0.64, T=−1.2; comp.#5: −log(p)=0.04, T=−0.1; comp.#6: −log(p)=0.13, T=−0.33). Linear regression analysis of Stroop-interference related component activity with CAG-repeat length did not indicate significant relationships for either of the six identified components (Comp.#1: −log(p)=0.70, r=0.23; comp.#2: −log(p)=0.62, r=10.21; comp.#3: −log(p)=0.06, r=−0.03; comp.#4: −log(p)=0.32, r=0.13; comp.#5: −log(p)=0.03, r=−0.02; comp.#6: −log(p)=0.35, r=0.14).
Table 3.
Results from one sample t-tests to test for task-relatedness of component activity in the entire study population (n=84). Bold −log(p) values indicate significant relations, SEM% indicate variation as % of the group average.
| Comp. | Baseline | Congruent | Incongruent | |||
|---|---|---|---|---|---|---|
| # | −log (p) | SEM% | −log (p) | SEM% | −log (p) | SEM% |
| 1 | 1.77 | 3.85 | 1.6 | 5.56 | 2.58 | 3.03 |
| 2 | 0.42 | 8 | 0.56 | 8.7 | 0.68 | 8.7 |
| 3 | 3.19 | 2.67 | 5.07 | 2.13 | 5.84 | 2.11 |
| 4 | 0.54 | 5.17 | 1.02 | 3.32 | 2.62 | 2.86 |
| 5 | 2.04 | 2.25 | 4.46 | 2.41 | 10.21 | 1.47 |
| 6 | 0.1 | 3.92 | 1.68 | 4.08 | 2.74 | 3.08 |
| mean (SD) | 1.24 (1.1) | 2.51 (1.95) | 3.74 (2.87) | |||
3.3. Activity in a component comprising the ventro-medial prefrontal cortex correlates with CES-Depression scores in prodromal-HD subjects
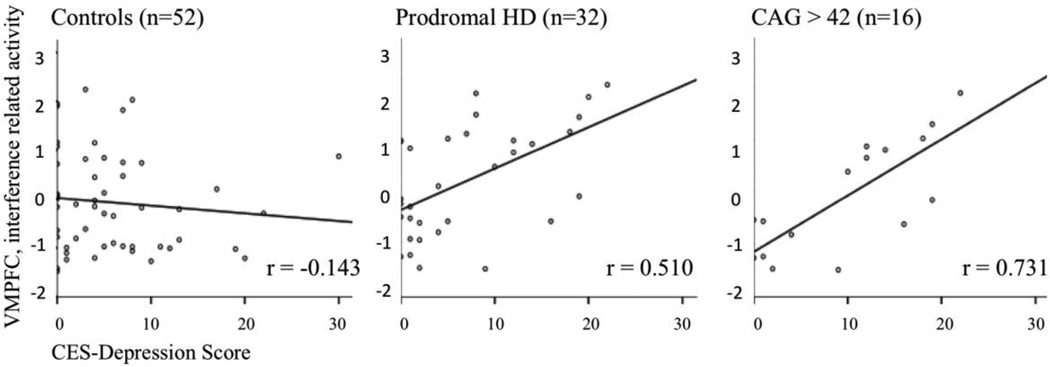
To identify components differing in the relation of component activity with depressive symptoms between patients and controls, a test for difference between correlation coefficients in two independent samples derived from Fisher r to z transformation was performed (Fisher, 1915; Edwards, 1984). Significant difference resulted only for the ventromedial prefrontal cortex component (z=3.02, −log(p)=2.6), r=−0.143 in controls versus r=0.510 in prodromal-HD (table 4). Strength of this correlation increased, when the analysis was limited to the prodromal-HD subgroup with CAG-repeat lengths > 42: r=0.731; in comparison to expansion negative controls z=3.45, −log(p)=3.22 (figure 3). Correlation between prodromal-HD with CAG-repeat lengths ≤ 42 (r=0.411) and > 42 (r=0.731) did not differ significantly (z=1.26, −log(p)=0.68).
Table 4.
Displayed are relationships of Stroop-interference related functional connectivity in all six components with CES-Depression scores for controls and prodromal-HD, respectively. Significant differences of correlation coefficients (Fisher, 1915; Edwards, 1984) are indicated in bold.
| Comp. | Controls (n=52) |
Prodromal HD (n=32) |
Difference of corr. coeff. |
|
|---|---|---|---|---|
| # | r | r | [z] | −log(p) |
| 1 | 0.11 | −0.01 | 0.51 | 0.21 |
| 2 | 0.15 | 0.27 | 0.56 | 0.25 |
| 3 | 0.20 | −0.11 | 1.31 | 0.72 |
| 4 | −0.14 | 0.51 | 3.02 | 2.6 |
| 5 | −0.11 | −0.01 | 0.44 | 0.18 |
| 6 | 0.08 | 0.23 | 0.65 | 0.28 |
Figure 3.
Linear regression analysis indicates a relationship between Stroop-interference related activity in the ventromedial prefrontal cortex (VMPFC) with CES-Depression scores in prodromal-HD but not in expansion negative controls. This effect appears stronger in the prodromal-HD subgroup with CAG repeat lengths > 42.
4. Discussion
Our main finding is a specific correlation in prodromal carriers of the HD mutation between depressive symptoms and Stroop-interference related BOLD activity in the ventromedial prefrontal cortex. This relationship was not observable in unaffected controls, indicating specificity for carriers of the HD mutation. In addition, this finding potentially relates to the extent of the Huntingtin expansion mutation, as the correlation with depressive symptoms increases in strength when a prodromal-HD subgroup with CAG repeat length greater than 42 is analyzed. Depressive symptoms during the prodromal phase of HD may therefore reflect alterations of brain network activity that involve particularly anterior subparts of the default-mode network and are associated with early HD related brain alterations. High levels of behavioral Stroop performance in prodromal-HD subjects may reflect conserved overall functional network connectivity at a moderate level of cognitive challenge and may also be consistent with the fact that no general differences in Stroop related network activity were observable between controls and HD subjects.
Affective symptoms are common in the prodrome of HD and may be a very early manifestation of the disease (Folstein et al., 1979; Cummings, 1995; Marder et al., 2000; Duff et al., 2007; Julien et al., 2007; van Duijn et al., 2007; Vassos et al., 2007; Tabrizi et al., 2011). However, they may be subtle, often not qualifying for a diagnosis according to DSM-IV (Duff et al., 2007; Tabrizi et al., 2009). In this study, the Center for Epidemiologic Studies Depression Scale (CES-D) was utilized (Radloff, 1977), as it has has been developed to measure depressed mood in population based non-clinical samples (Myers and Weissman, 1980) while other psychometric instruments such as the Hamilton Depression Rating Scale (HDRS), have been developed to assess severity of major depression in clinical settings (Hamilton, 1960). The CES-D scale therefore appears capable of quantifying subdiagnostic affective symptoms (Roberts and Vernon, 1983), thus reducing bias due to floor effects, which have been indicated as a particular challenge for psychometrics in the prodrome of HD (Tabrizi et al., 2009). While CES-Depression scores did not differ significantly between subjects in the prodromal phase of HD and controls, higher scores were measured in the subgroup with CAG-repeat length > 42. Additionally a relationship between CAG-repeat length and CES-Depression scores could be observed for the entire prodromal-HD sample, which is consistent with earlier reports on depressive symptoms in HD-mutation carriers and possibly reflects first signs of HD-related central nervous system alterations that precede manifestation of motor symptoms (Folstein et al., 1979; Paulsen et al., 2005; Duff et al., 2007; Julien et al., 2007; van Duijn et al., 2007; Tabrizi et al., 2009). Mild general atrophy and reduced striatal volume are consistent findings in prodromal-HD (Paulsen et al., 2006; Aylward, 2007; Aylward et al., 2010) and striatal atrophy in particular is clearly observable also in our data, reflecting distinct brain alterations that differ subjects with the HD mutation from controls (table 1). There were no further differences in demographic or clinical measures between subjects in the prodromal phase of HD and controls including Stroop task-performance and the effect of interference (Jensen, 1965; Jensen and Rohwer, 1966) (figure 1). The significant effect of interference observable on a behavioral level indicates proper implementation of the test in both samples and is consistent with earlier reports on high levels of neuropsychological test performance in prodromal-HD (Brandt et al., 2008).
By performing spatial ICA-decomposition of whole brain fMRI signals followed by a combined selection-process accounting for physiological nuisance contribution (Perlbarg et al., 2007; Stevens et al., 2007; Jafri et al., 2008), we identified six components that correspond to previously reported functional networks and may be relevant in a context of emotional and cognitive processing (figure 2) (Greicius et al., 2003; Beckmann et al., 2005; Fox et al., 2005; Damoiseaux et al., 2006; Buckner et al., 2008; Jafri et al., 2008; Sämann et al., 2011). These were categorized in two groups based on between network connectivity (Joel et al., 2011): Group A consists of components activated by the Stroop task (component #1, 3, 5 and 6) and is inverse-correlated to group B (component #2 and 4), which is consistent with an earlier report, suppressed by the Stroop task (Harrison et al., 2008) (table 2).
Our finding of correlations between activity in component #4 (which mainly represents the ventromedial prefrontal cortex) and depressive symptoms appears consistent with a concatenation of reports on altered emotional processing and impaired functional brain network activity: The default mode network is a set of brain regions with synchronous BOLD-activity at rest, which corresponds inversely to cognitive paradigms. It shows bilateral connectivity that converges on the posterior cingulate and includes the ventromedial prefrontal cortex as a frontal node (Biswal et al., 1995; Buckner et al., 2008). While the prefrontal cortex region has been reported to be involved in mood regulation (Keedwell et al., 2005; Fellows, 2007; Brassen et al., 2008; Koenigs et al., 2008), cognitive dysfunction is associated with altered default-mode network connectivity (Laxton et al., 2010; Petrella et al., 2011). Our ICA finding of a relationship between increased Stroop interference related activity in component #4 and high depression scores in prodromal-HD subjects might therefore reflect higher levels of effort necessary to successfully complete the task than controls, possibly indicating a compensatory mechanism for early HD related brain changes. This may be consistent with earlier reports on altered default-mode network activity in neurodegenerative diseases in general (Sorg et al., 2007; Buckner et al., 2008) and particularly with reports on increased activity of anterior brain networks in a context of depression (Greicius et al., 2007; Sheline et al., 2010a). The fact that there was no significant relationship between depressive symptoms and Stroop-interference related activity of component #2 might point towards disruptional disease effects between anterior and posterior parts of the default-mode network.
Temporal sorting of the identified components indicated a high task-relatedness in most of the identified functional networks, that increased with the level of challenge (table 3). In the incongruent condition, which was the most demanding block of the Stroop-interference paradigm, all components except #2 showed significant task-related modulation both in patients and controls. The lack of task relatedness observed may be due to an indistinctive suppression by attention and Stroop-interference, making it difficult to distinguish the different Stroop tasks and may be consistent with earlier reports (Harrison et al., 2008). However, this might also indicate a ceiling-effect and thus lack of sensitivity of the experimental approach used in our study.
The fact, that our finding of correlated ventromedial prefrontal cortex activity and depressive symptoms is enhanced in a subgroup of expansion positive individuals with particularly long CAG repeat lengths (figure 3), suggests a relation to individual genetic burden and may be consistent with a recent report on increased prevalence of incompletely penetrant HD alleles among individuals with major depressive disorder (Perlis et al., 2010). However, as the correlation coefficients between the subgroups with CAG repeat lengths ≤ 42 and > 42 did not differ significantly and also Stroop-interference related component activity did not correlate with CAG-repeat length, the effect of CAG-repeat length has to be interpreted with caution.
A limitation to performing ICA on BOLD fMRI signals is the fact that besides identifying components representing relevant networks of functional connectivity, artifacts related to movement and physiological noise will result (Beckmann et al., 2005; Damoiseaux and Greicius, 2009). For this reason we performed a selection procedure resulting in a set of components primarily within gray matter and of low temporal frequency (Perlbarg et al., 2007; Stevens et al., 2007; Jafri et al., 2008). While our methods allowed us to assess effects of interacting neurobiology of depressive symptoms and HD, no significant differences between controls and prodromal-HD subjects were detectable by ICA for spatial extent of the components, between-network connectivity and also Stroop interference related activity when using the described methods. The fact that we don’t see a general group difference in brain network activity in response to the Stroop paradigm may be consistent with the similar task performance in subjects in the prodromal phase of HD and controls. However, it can not be excluded that there might be subtle differences which were not detectable with the ICA methods applied in this study and may require a larger sample for sufficient power to be identified. Also, results may differ when other cognitive paradigms than Stroop-interference are applied.
As effects of serotonergic medication on BOLD fMRI signal have been reported (Anand et al., 2005; Windischberger et al., 2010), antidepressive medication status was used as a covariate in correlation analysis. While we did not find a significant relationship between antidepressive medication status and task-related component activity, we can not exclude an unspecific effect on our fMRI data, possibly affecting power of this analysis.
In this study, we have identified Stroop-interference associated activity in the ventromedial prefrontal cortex-node of the default-mode network as a correlate of depressive symptoms in prodromal-HD. The effect is possibly modulated by CAG repeat length of the mutated Huntingtin allele. Alterations of functional brain networks including systems involved in emotional processing and cognitive function may be a plausible pathomechanism and appear consistent with earlier considerations on converging neurobiology of both, with a possible integrating role of the ventromedial prefrontal cortex region (Zihl et al., 1998; Mayberg et al., 1999; Fellows, 2007; van Kesteren et al., 2010). Our data underline the potential of studying the prodromal phase of HD as a model for neurobiology of depressive symptoms and psychiatric manifestation of neurodegenerative processes in general.
Acknowledgements
This study was made possible by grant support from NINDS NS16375, NIH-NCRR P41-RR015241, R21AG033774, P50AG005146. The National Center for Research Resources (NCRR) is a component of the National Institutes of Health (NIH). The contents of the paper are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Dr. Paul G. Unschuld has been supported by NIH-T32MH015330. We thank Terri Brawner, Ivana Kusevic and Kathleen Kahl of the F.M.Kirby Research Center and Guillermo Verduzco of the Division of Psychiatric Neuroimaging for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Drs. Paul G. Unschuld, Suresh E. Joel, Sarah A. Reading, Kenishi Oishi, Arnold Bakker, Russell L. Margolis, Susan S. Bassett, Adam Rosenblatt, Susumu Mori, Christopher A. Ross, Graham W. Redgrave and Julie McEntee, Megan Shanahan reported no biomedical financial interests or potential conflicts of interest. Equipment used in the study is manufactured by Philips. Dr. Peter van Zijl is a paid lecturer for Philips Healthcare and an inventor of technology that is licensed to Philips. This arrangement has been approved by Johns Hopkins University in accordance with its conflict of interest policies. Dr. Pekar serves as Manager of the F.M. Kirby Research Center for Functional Brain Imaging, which receives research support from Philips HealthCare.
References
- Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L, Mathews VP, Kalnin A, Lowe MJ. Antidepressant effect on connectivity of the mood-regulating circuit: an FMRI study. Neuropsychopharmacology. 2005;30(7):1334–1344. doi: 10.1038/sj.npp.1300725. [DOI] [PubMed] [Google Scholar]
- Aylward EH. Change in MRI striatal volumes as a biomarker in preclinical Huntington's disease. Brain Research Bulletin. 2007;72(2–3):152–158. doi: 10.1016/j.brainresbull.2006.10.028. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Nopoulos PC, Ross CA, Langbehn DR, Pierson RK, Mills JA, Johnson HJ, Magnotta VA, Juhl AR, Paulsen JS. Longitudinal change in regional brain volumes in prodromal Huntington disease. Journal of Neurology, Neurosurgery & Psychiatry. 2010 doi: 10.1136/jnnp.2010.208264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Computation. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Benoit G, Fortin L, Lemelin S, Laplante L, Thomas J, Everett J. Selective attention in major depression: clinical retardation and cognitive inhibition. Canadian Journal of Psychiatry. 1992;46(1):41–52. [PubMed] [Google Scholar]
- Berrios GE, Wagle AC, Markova IS, Wagle SA, Ho LW, Rubinsztein DC, Whittaker J, Ffrench-Constant C, Kershaw A, Rosser A, Bak T, Hodges JR. Psychiatric symptoms and CAG repeats in neurologically asymptomatic Huntington's disease gene carriers. Psychiatry Research. 2001;102(3):217–225. doi: 10.1016/s0165-1781(01)00257-8. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Brandt J, Inscore AB, Ward J, Shpritz B, Rosenblatt A, Margolis RL, Ross CA. Neuropsychological deficits in Huntington's disease gene carriers and correlates of early "conversion". The Journal of Neuropsychiatry & Clinical Neurosciences. 2008;20(4):466–472. doi: 10.1176/appi.neuropsych.20.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassen S, Kalisch R, Weber-Fahr W, Braus DF, Buchel C. Ventromedial prefrontal cortex processing during emotional evaluation in late-life depression: a longitudinal functional magnetic resonance imaging study. Biological Psychiatry. 2008;64(4):349–355. doi: 10.1016/j.biopsych.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Bright P, Jaldow E, Kopelman MD. The National Adult Reading Test as a measure of premorbid intelligence: a comparison with estimates derived from demographic variables. Journal of the International Neuropsychological Society. 2002;8(6):847–854. doi: 10.1017/s1355617702860131. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Human Brain Mapping. 2008a;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and "default" hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Human Brain Mapping. 2008b;29(11):1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Behavioral and psychiatric symptoms associated with Huntington's disease. Advances in Neurology. 1995;65:179–186. [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain Structure and Function. 2009;213(6):525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC. Psychiatric symptoms in Huntington's disease before diagnosis: the predict-HD study. Biological Psychiatry. 2007;62(12):1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- Duyao M, Ambrose C, Myers R, Novelletto A, Persichetti F, Frontali M, Folstein S, Ross C, Franz M, Abbott M, et al. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nature Genetics. 1993;4(4):387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Edwards AL. An Introduction to Linear Regression and Correlation. New York, NY: W. H. Freeman & Co.; 1984. [Google Scholar]
- Fellows LK. Advances in understanding ventromedial prefrontal function: the accountant joins the executive. Neurology. 2007;68(13):991–995. doi: 10.1212/01.wnl.0000257835.46290.57. [DOI] [PubMed] [Google Scholar]
- Fisher AO. Frequency Distribution of the Values of the Correlation Coefficient in Samples from an Indefinitely Large Population. Biometrika. 1915;10(4):522–529. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Folstein SE, Folstein MF, McHugh PR. Psychiatric Syndromes in Huntington's disease. Advances in Neurology. 1979;23:281–289. [Google Scholar]
- Folstein SE, Jensen B, Leigh RJ, Folstein MF. The measurement of abnormal movement: methods developed for Huntington's disease. Neurobehavioral toxicology and teratology. 1983;5(6):605–609. [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Commentary and opinion: II. Statistical parametric mapping: ontology and current issues. Journal of Cerebral Blood Flow & Metabolism. 1995;15(3):361–370. doi: 10.1038/jcbfm.1995.45. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS. Functional connectivity: the principal-component analysis of large (PET) data sets. Journal of Cerebral Blood Flow & Metabolism. 1993;13(1):5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H, Reiss AL, Schatzberg AF. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62(5):429–437. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm S, Boesiger P, Beck J, Schuepbach D, Bermpohl F, Walter M, Ernst J, Hell D, Boeker H, Northoff G. Altered negative BOLD responses in the default-mode network during emotion processing in depressed subjects. Neuropsychopharmacology. 2009;34(4):932–843. doi: 10.1038/npp.2008.81. [DOI] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Holcomb P, Soraci S, Yurgelun-Todd D. Stroop performance in normal control subjects: an fMRI study. Neuroimage. 2002;16(2):349–360. doi: 10.1006/nimg.2002.1089. [DOI] [PubMed] [Google Scholar]
- Gusella JF, Wexler NS, Conneally PM, Naylor SL, Anderson MA, Tanzi RE, Watkins PC, Ottina K, Wallace MR, Sakaguchi AY, et al. A polymorphic DNA marker genetically linked to Huntington's disease. Nature. 1983;306(5940):234–238. doi: 10.1038/306234a0. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Lopez-Sola M, Hernandez-Ribas R, Deus J, Ortiz H, Soriano-Mas C, Yucel M, Pantelis C, Cardoner N. Consistency and functional specialization in the default mode brain network. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(28):9781–9786. doi: 10.1073/pnas.0711791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HD_Collaborative_Research_Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Henley SM, Wild EJ, Hobbs NZ, Warren JD, Frost C, Scahill RI, Ridgway GR, MacManus DG, Barker RA, Fox NC, Tabrizi SJ. Defective emotion recognition in early HD is neuropsychologically and anatomically generic. Neuropsychologia. 2008;46(8):2152–2160. doi: 10.1016/j.neuropsychologia.2008.02.025. [DOI] [PubMed] [Google Scholar]
- Hobbs NZ, Pedrick AV, Say MJ, Frost C, Dar Santos R, Coleman A, Sturrock A, Craufurd D, Stout JC, Leavitt BR, Barnes J, Tabrizi SJ, Scahill RI. The structural involvement of the cingulate cortex in premanifest and early Huntington's disease. Movement Disorders. 2011;26(9):1684–1690. doi: 10.1002/mds.23747. [DOI] [PubMed] [Google Scholar]
- Holm S. A Simple Sequentially Rejective Bonferroni Test Procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- Huang H, Ceritoglu C, Li X, Qiu A, Miller MI, van Zijl PC, Mori S. Correction of B0 susceptibility induced distortion in diffusion-weighted images using large-deformation diffeomorphic metric mapping. Magnetic Resonance Imaging. 2008;26(9):1294–1302. doi: 10.1016/j.mri.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AR. Scoring the Stroop test. ACTA Psychologica (Amsterdam) 1965;24(5):398–408. doi: 10.1016/0001-6918(65)90024-7. [DOI] [PubMed] [Google Scholar]
- Jensen AR, Rohwer WD., Jr. The Stroop color-word test: a review. ACTA Psychologica (Amsterdam) 1966;25(1):36–93. doi: 10.1016/0001-6918(66)90004-7. [DOI] [PubMed] [Google Scholar]
- Joel SE, Caffo BS, van Zijl PC, Pekar JJ. On the relationship between seed-based and ICA-based measures of functional connectivity. Magnetic Resonance in Medicine. 2011 doi: 10.1002/mrm.22818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Stout JC, Solomon AC, Langbehn DR, Aylward EH, Cruce CB, Ross CA, Nance M, Kayson E, Julian-Baros E, Hayden MR, Kieburtz K, Guttman M, Oakes D, Shoulson I, Beglinger L, Duff K, Penziner E, Paulsen JS. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington's disease. Brain. 2007;130(Pt 7):1732–1744. doi: 10.1093/brain/awm107. [DOI] [PubMed] [Google Scholar]
- Julien CL, Thompson JC, Wild S, Yardumian P, Snowden JS, Turner G, Craufurd D. Psychiatric disorders in preclinical Huntington's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(9):939–943. doi: 10.1136/jnnp.2006.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58(11):843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Killian GA, Holzman PS, Davis JM, Gibbons R. Effects of psychotropic medication on selected cognitive and perceptual measures. Journal of Abnormal Psychology. 1984;93(1):58–70. doi: 10.1037//0021-843x.93.1.58. [DOI] [PubMed] [Google Scholar]
- Kirkwood SC, Siemers E, Viken R, Hodes ME, Conneally PM, Christian JC, Foroud T. Longitudinal personality changes among presymptomatic Huntington disease gene carriers. Neuropsychology, Neuropsychiatry, and Behavioral Neurology. 2002;15(3):192–197. [PubMed] [Google Scholar]
- Klöppel S, Henley SM, Hobbs NZ, Wolf RC, Kassubek J, Tabrizi SJ, Frackowiak RS. Magnetic resonance imaging of Huntington's disease: preparing for clinical trials. Neuroscience. 2009;164(1):205–219. doi: 10.1016/j.neuroscience.2009.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöppel S, Stonnington CM, Petrovic P, Mobbs D, Tuscher O, Craufurd D, Tabrizi SJ, Frackowiak RS. Irritability in pre-clinical Huntington's disease. Neuropsychologia. 2010;48(2):549–557. doi: 10.1016/j.neuropsychologia.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. The Journal of Neuroscience. 2008;28(47):12341–12348. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clinical Genetics. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Laxton AW, Tang-Wai DF, McAndrews MP, Zumsteg D, Wennberg R, Keren R, Wherrett J, Naglie G, Hamani C, Smith GS, Lozano AM. A phase I trial of deep brain stimulation of memory circuits in Alzheimer's disease. Annals of Neurology. 2010;68(4):521–534. doi: 10.1002/ana.22089. [DOI] [PubMed] [Google Scholar]
- Lemelin S, Baruch P, Vincent A, Everett J, Vincent P. Distractibility and processing resource deficit in major depression. Evidence for two deficient attentional processing models. The Journal of Nervous and Mental Disease. 1997;185(9):542–548. doi: 10.1097/00005053-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping. 2007;28(11):1251–1266. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychological Bulletin. 1991;109(2):163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- MacLeod CM, MacDonald PA. Interdimensional interference in the Stroop effect: uncovering the cognitive and neural anatomy of attention. Trends in Cognitive Sciences. 2000;4(10):383–391. doi: 10.1016/s1364-6613(00)01530-8. [DOI] [PubMed] [Google Scholar]
- Majer M, Ising M, Kunzel H, Binder EB, Holsboer F, Modell S, Zihl J. Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychological Medicine. 2004;34(8):1453–1463. doi: 10.1017/s0033291704002697. [DOI] [PubMed] [Google Scholar]
- Marder K, Zhao H, Myers RH, Cudkowicz M, Kayson E, Kieburtz K, Orme C, Paulsen J, Penney JB, Jr., Siemers E, Shoulson I. Rate of functional decline in Huntington's disease. Huntington Study Group. Neurology. 2000;54(2):452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- Marshall J, White K, Weaver M, Flury Wetherill L, Hui S, Stout JC, Johnson SA, Beristain X, Gray J, Wojcieszek J, Foroud T. Specific psychiatric manifestations among preclinical Huntington disease mutation carriers. Arch Neurol. 2007;64(1):116–121. doi: 10.1001/archneur.64.1.116. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of PsychiatryÊ. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Jung TP, Makeig S, Brown G, Kindermann SS, Lee TW, Sejnowski TJ. Spatially independent activity patterns in functional MRI data during the stroop color-naming task. Proceedings of the National Academy of Sciences of the United States of America. 1998a;95(3):803–810. doi: 10.1073/pnas.95.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Human Brain Mapping. 1998b;6(3):160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MI, Trouve A, Younes L. On the metrics and euler-lagrange equations of computational anatomy. Annual Review of Biomedical Engineering. 2002;4:375–405. doi: 10.1146/annurev.bioeng.4.092101.125733. [DOI] [PubMed] [Google Scholar]
- Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. American Journal of PsychiatryÊ. 1980;137(9):1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R. Ventricular enlargement as a possible measure of Alzheimer's disease progression validated using the Alzheimer's disease neuroimaging initiative database. Brain. 2008;131(Pt 9):2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Magnotta VA, Mikos A, Paulson H, Andreasen NC, Paulsen JS. Morphology of the cerebral cortex in preclinical Huntington's disease. American Journal of PsychiatryÊ. 2007;164(9):1428–1434. doi: 10.1176/appi.ajp.2007.06081266. [DOI] [PubMed] [Google Scholar]
- Nopoulos PC, Aylward EH, Ross CA, Mills JA, Langbehn DR, Johnson HJ, Magnotta VA, Pierson RK, Beglinger LJ, Nance MA, Barker RA, Paulsen JS. Smaller intracranial volume in prodromal Huntington's disease: evidence for abnormal neurodevelopment. Brain. 2011 doi: 10.1093/brain/awq280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, Hsu JT, Miller MI, van Zijl PC, Albert M, Lyketsos CG, Woods R, Toga AW, Pike GB, Rosa-Neto P, Evans A, Mazziotta J, Mori S. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. Neuroimage. 2009;46(2):486–499. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso S, Lamberty GJ, Garvey MJ, Robinson RG. Cognitive impairment in the euthymic phase of chronic unipolar depression. The Journal of Nervous and Mental Disease. 1997;185(12):748–754. doi: 10.1097/00005053-199712000-00005. [DOI] [PubMed] [Google Scholar]
- Paulsen JS. Functional imaging in Huntington's disease. Experimental Neurology. 2009;216(2):272–277. doi: 10.1016/j.expneurol.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen JS, Magnotta VA, Mikos AE, Paulson HL, Penziner E, Andreasen NC, Nopoulos PC. Brain structure in preclinical Huntington's disease. Biological Psychiatry. 2006;59(1):57–63. doi: 10.1016/j.biopsych.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Nehl C, Hoth KF, Kanz JE, Benjamin M, Conybeare R, McDowell B, Turner B. Depression and stages of Huntington's disease. The Journal of Neuropsychiatry & Clinical Neurosciences. 2005;17(4):496–502. doi: 10.1176/jnp.17.4.496. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Nopoulos PC, Aylward E, Ross CA, Johnson H, Magnotta VA, Juhl A, Pierson RK, Mills J, Langbehn D, Nance M. Striatal and white matter predictors of estimated diagnosis for Huntington disease. Brain Research Bulletin. 2010;82(3–4):201–207. doi: 10.1016/j.brainresbull.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penney JB, Jr., Vonsattel JP, MacDonald ME, Gusella JF, Myers RH. CAG repeat number governs the development rate of pathology in Huntington's disease. Annals of Neurology. 1997;41(5):689–692. doi: 10.1002/ana.410410521. [DOI] [PubMed] [Google Scholar]
- Perlbarg V, Bellec P, Anton JL, Pelegrini-Issac M, Doyon J, Benali H. CORSICA: correction of structured noise in fMRI by automatic identification of ICA components. Magnetic Resonance Imaging. 2007;25(1):35–46. doi: 10.1016/j.mri.2006.09.042. [DOI] [PubMed] [Google Scholar]
- Perlis RH, Smoller JW, Mysore J, Sun M, Gillis T, Purcell S, Rietschel M, Nothen MM, Witt S, Maier W, Iosifescu DV, Sullivan P, Rush AJ, Fava M, Breiter H, Macdonald M, Gusella J. Prevalence of incompletely penetrant Huntington's disease alleles among individuals with major depressive disorder. American Journal of PsychiatryÊ. 2010;167(5):574–579. doi: 10.1176/appi.ajp.2009.09070973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JR, Sheldon FC, Prince SE, Calhoun VD, Doraiswamy PM. Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology. 2011;76(6):511–517. doi: 10.1212/WNL.0b013e31820af94e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ravnkilde B, Videbech P, Clemmensen K, Egander A, Rasmussen NA, Rosenberg R. Cognitive deficits in major depression. Scandinavian Journal of Psychology. 2002;43(3):239–251. doi: 10.1111/1467-9450.00292. [DOI] [PubMed] [Google Scholar]
- Reading SA, Dziorny AC, Peroutka LA, Schreiber M, Gourley LM, Yallapragada V, Rosenblatt A, Margolis RL, Pekar JJ, Pearlson GD, Aylward E, Brandt J, Bassett SS, Ross CA. Functional brain changes in presymptomatic Huntington's disease. Annals of Neurology. 2004;55(6):879–883. doi: 10.1002/ana.20121. [DOI] [PubMed] [Google Scholar]
- Reading SA, Yassa MA, Bakker A, Dziorny AC, Gourley LM, Yallapragada V, Rosenblatt A, Margolis RL, Aylward EH, Brandt J, Mori S, van Zijl P, Bassett SS, Ross CA. Regional white matter change in pre-symptomatic Huntington's disease: a diffusion tensor imaging study. Psychiatry Research. 2005;140(1):55–62. doi: 10.1016/j.pscychresns.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Reppermund S, Zihl J, Lucae S, Horstmann S, Kloiber S, Holsboer F, Ising M. Persistent cognitive impairment in depression: the role of psychopathology and altered hypothalamic-pituitary-adrenocortical (HPA) system regulation. Biological Psychiatry. 2007;62(5):400–406. doi: 10.1016/j.biopsych.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Vernon SW. The Center for Epidemiologic Studies Depression Scale: its use in a community sample. American Journal of PsychiatryÊ. 1983;140(1):41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65(5):745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Lee SY, Bender AC, Zaleta AK, Vangel M, Yu P, Fischl B, Pappu V, Onorato C, Cha JH, Salat DH, Hersch SM. Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical "disconnection". Neuroimage. 2010;49(4):2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. The Lancet Neurology. 2011;10(1):83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- Sämann PG, Wehrle R, Hoehn D, Spoormaker VI, Peters H, Tully C, Holsboer F, Czisch M. Development of the Brain's Default Mode Network from Wakefulness to Slow Wave Sleep. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhq295. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America. 2010a;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biological Psychiatry. 2010b;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(47):18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson G, Calhoun VD. Functional neural circuits for mental timekeeping. Human Brain Mapping. 2007;28(5):394–408. doi: 10.1002/hbm.20285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18(6):643–662. [Google Scholar]
- Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, Borowsky B, Tobin AJ, Rosas HD, Johnson H, Reilmann R, Landwehrmeyer B, Stout JC. Biological and clinical manifestations of Huntington's disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. The Lancet Neurology. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, Landwehrmeyer GB, Fox NC, Johnson H, Hicks SL, Kennard C, Craufurd D, Frost C, Langbehn DR, Reilmann R, Stout JC. Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: the 12-month longitudinal analysis. The Lancet Neurology. 2011;10(1):31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- Thieben MJ, Duggins AJ, Good CD, Gomes L, Mahant N, Richards F, McCusker E, Frackowiak RS. The distribution of structural neuropathology in pre-clinical Huntington's disease. Brain. 2002;125(Pt 8):1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- Thu DC, Oorschot DE, Tippett LJ, Nana AL, Hogg VM, Synek BJ, Luthi-Carter R, Waldvogel HJ, Faull RL. Cell loss in the motor and cingulate cortex correlates with symptomatology in Huntington's disease. Brain. 2010;133(Pt 4):1094–1110. doi: 10.1093/brain/awq047. [DOI] [PubMed] [Google Scholar]
- Trichard C, Martinot JL, Alagille M, Masure MC, Hardy P, Ginestet D, Feline A. Time course of prefrontal lobe dysfunction in severely depressed in-patients: a longitudinal neuropsychological study. Psychological Medicine. 1995;25(1):79–85. doi: 10.1017/s0033291700028105. [DOI] [PubMed] [Google Scholar]
- Van de Moortele PF, Cerf B, Lobel E, Paradis AL, Faurion A, Le Bihan D. Latencies in fMRI time-series: effect of slice acquisition order and perception. NMR in Biomedicine. 1997;10(4–5):230–236. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<230::aid-nbm470>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- van de Ven VG, Formisano E, Prvulovic D, Roeder CH, Linden DE. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Human Brain Mapping. 2004;22(3):165–178. doi: 10.1002/hbm.20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijn E, Kingma EM, van der Mast RC. Psychopathology in verified Huntington's disease gene carriers. The Journal of Neuropsychiatry & Clinical Neurosciences. 2007;19(4):441–448. doi: 10.1176/jnp.2007.19.4.441. [DOI] [PubMed] [Google Scholar]
- van Kesteren MT, Fernandez G, Norris DG, Hermans EJ. Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(16):7550–7555. doi: 10.1073/pnas.0914892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassos E, Panas M, Kladi A, Vassilopoulos D. Higher levels of extroverted hostility detected in gene carriers at risk for Huntington's disease. Biological Psychiatry. 2007;62(12):1347–1352. doi: 10.1016/j.biopsych.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Gammelgaard L, Egander A, Clemmensen K, Rasmussen NA, Gjedde A, Rosenberg R. The Danish PET/depression project: performance on Stroop's test linked to white matter lesions in the brain. Psychiatry Research. 2004;130(2):117–130. doi: 10.1016/j.pscychresns.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Walker FO. Huntington's disease. Lancet. 2007;369(9557):218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- Windischberger C, Lanzenberger R, Holik A, Spindelegger C, Stein P, Moser U, Gerstl F, Fink M, Moser E, Kasper S. Area-specific modulation of neural activation comparing escitalopram and citalopram revealed by pharmaco-fMRI: a randomized cross-over study. Neuroimage. 2010;49(2):1161–1170. doi: 10.1016/j.neuroimage.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Wolf H, Kruggel F, Hensel A, Wahlund LO, Arendt T, Gertz HJ. The relationship between head size and intracranial volume in elderly subjects. Brain Research. 2003;973(1):74–80. doi: 10.1016/s0006-8993(03)02552-6. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, Schonfeldt-Lecuona C, Ecker D, Landwehrmeyer B. Aberrant connectivity of lateral prefrontal networks in presymptomatic Huntington's disease. Experimental Neurology. 2008a;213(1):137–144. doi: 10.1016/j.expneurol.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Sambataro F, Vasic N, Schonfeldt-Lecuona C, Ecker D, Landwehrmeyer B. Altered frontostriatal coupling in pre-manifest Huntington's disease: effects of increasing cognitive load. European Journal of Neurology. 2008b;15(11):1180–1190. doi: 10.1111/j.1468-1331.2008.02253.x. [DOI] [PubMed] [Google Scholar]
- World_Medical_Association. Declaration of Helsinki. Law, Medicine & Health Care. 1991;19(3–4):264–265. [PubMed] [Google Scholar]
- Zihl J, Gron G, Brunnauer A. Cognitive deficits in schizophrenia and affective disorders: evidence for a final common pathway disorder. ActaÊPsychiatrica Scandinavica. 1998;97(5):351–357. doi: 10.1111/j.1600-0447.1998.tb10014.x. [DOI] [PubMed] [Google Scholar]
- Zimbelman JL, Paulsen JS, Mikos A, Reynolds NC, Hoffmann RG, Rao SM. fMRI detection of early neural dysfunction in preclinical Huntington's disease. Journal of the International Neuropsychological Society. 2007;13(5):758–769. doi: 10.1017/S1355617707071214. [DOI] [PubMed] [Google Scholar]