Abstract
This meta-analysis evaluates alterations of neurometabolites in schizophrenia and bipolar disorder. PubMed was searched to find controlled studies evaluating N-acetylaspartate (NAA), Choline (Cho) and Creatine (Cr) assessed with 1H-MRS (proton magnetic resonance spectroscopy) in patients with schizophrenia and bipolar disorder up to September 2010. Random effects meta-analyses were conducted to estimate pooled standardized mean differences. I2 statistic was used to quantify inconsistencies. Subgroup analyses were conducted to explore potential explanations for inconsistencies. 146 studies with 5643 participants were included in the systematic review. NAA levels were affected in schizophrenia and bipolar disorder. Decreased levels in the basal ganglia and frontal lobe were the most consistent findings in schizophrenia, decreased levels in the basal ganglia were the most consistent findings in bipolar disorder. Cho and Cr levels were not altered in either disorder. Findings for Cr were most consistent in the thalamus, frontal lobe and dorsolateral prefrontal cortex in schizophrenia and the basal ganglia and frontal lobe in bipolar disorder. Findings for Cho were most consistent in the thalamus, frontal lobe and anterior cingulate cortex in schizophrenia and basal ganglia in bipolar disorder. Large, carefully designed studies are needed to better estimate the extent of alterations in neurometabolites.
Keywords: Magnetic resonance spectroscopy (MRS), N-acetylaspartate, Creatine (Cr), Choline (Cho)
1. INTRODUCTION
Emil Kraepelin was the first to establish dementia praecox and manic-depressive insanity as dichotomous model, which has been utilized in conceptualization of schizophrenia and bipolar disorder ever since (Crow, 1990a; Heckers, 2008). However, emerging data suggest a relationship between schizophrenia and bipolar disorder, even a conceptualization of these disorders as polar ends of a continuum of the same mental illness is proposed (Crow, 1990b).
Family studies and twin studies have shown co-aggregation between schizophrenia and bipolar disorder. Shared genetic susceptibility has been reported in both candidate gene studies and whole-genome linkage analyses (Dalby et al. 1986; Bramon and Sham, 2001; Craddock and Owen, 2005; Lichtenstein et al., 2009; Purcell et al., 2009; Van Snellenberg and de Candia, 2009). A meta-analysis in magnetic resonance imaging (MRI) studies suggested gray matter reductions in paralimbic structures implicated in emotional processing in bipolar disorder, with a more extensive reduction in schizophrenia, not only affecting paralimbic structures, but also limbic and neocortical structures (Ellison-Wright and Bullmore, 2010).
Magnetic Resonance Spectroscopy (MRS) is increasingly being applied to characterize tissue-based chemical or metabolic abnormalities in psychiatric disorders. It is a non-invasive technique that measures chemical composition of tissues, energy metabolism, neurotransmitter levels, and neuronal integrity in vivo. It detects magnetic resonance signals produced by atomic nuclei located within molecules in living tissue. Quantification of MRS signal amplitude can provide an estimate for concentrations of signal generating molecules. (Kreis et al., 1993; Dager et al., 2008; Alger, 2010). The peak integral is proportional to the number of resonating nuclei. However, metabolite quantification is affected by high variability as the signal has low sensibility and multiple processing steps are performed. To take these variations in account, a reference signal is obtained. The reference signal is generally generated by a metabolite, water or a chemical compound in a phantom object. The reference signal often is classified as either “internal” or “external”, with internal meaning that the reference signal is generated by a metabolite or by water within the brain, and external meaning that the reference signal is generated by a phantom that is outside the brain. Resulting metabolite levels are then reported in two different ways. The first method is to report metabolite ratios, referring to Creatine (Cr) (e.g. NAA/Cr); the second method is to report absolute concentrations. These absolute concentrations are referenced to either brain water content or an external metabolic phantom with known metabolite concentrations and usually reported as mmol/l or institutional units (i.u.) (reviewed in Bagory et al., 2007).
1H-MRS (proton magnetic resonance spectroscopy) is the most widely applied technique studying alterations of neurometabolites in psychiatric disorders. Metabolites measured with 1H-MRS include N-acetyl aspartate (NAA), a metabolite that is thought to reflect neuronal integrity and is exclusively found in the brain; Cr, a putative marker of phosphate metabolism; Trimethylamines/ choline containing compounds (Cho), indicating breakdown of cell membranes and cellular turnover; and neurotransmitters such as Gamma-aminobutyric acid, glutamine, glutamate, with glutamate being the most abundant amino acid and excitatory neurotransmitter in the brain (Miller, 1991; Keshavan et al. 2000).
Abnormalities of neurometabolites in various regions of the brain have been implicated in the pathophysiology of both schizophrenia and bipolar disorder. Meta-analytic evidence in schizophrenia suggests that NAA may be reduced in the hippocampus and the frontal lobe (grey and white matter). Similar findings were reported in a systematic review performed in bipolar disorder, showing decreased NAA levels in euthymic bipolar patients in the hippocampus and frontal lobe as well. (Steen et al., 2005; Yildiz-Yesiloglu and Ankerst, 2006). Decrease of NAA is thought reflect neuronal or axonal loss or mitochondrial dysfunction (Meyerhoff et al., 1993; Sager et al., 2001), implying structural abnormalities on a molecular/neuronal level in both disorders. Cr has long thought to be a stable neurometabolite, and has been widely used as internal reference in MRS studies. However, several studies found reduced Cr levels in the dorsolateral prefrontal cortex, hippocampus and basal ganglia in both bipolar disorder and schizophrenia (Deicken et al., 2003; Ohrmann et al. 2005; Frey et al., 2007; Ruesch et al., 2008). These reports not only suggest alterations in the cellular energy metabolism but also question the validity of using Cr as internal reference. Conflictive results are also found for Cho; levels have been reported to be decreased in some studies in the basal ganglia, hippocampus and DLPFC in schizophrenia while others report an increase (Maier et al., 1996; Stanley et al. 1996; Bustillo et al., 2002; Ohrmann et al., 2005; Bustillo et al., 2008; Ruesch et al., 2008). In bipolar disorder, different studies suggest an increase, decrease or no change in Cho levels in the DLPFC, increased and decreased levels in the hippocampus and increased or unchanged levels in the ACC (Deicken et al., 2003; Michael et al., 2003; Brambilla et al., 2005; Frye et al., 2007a; Iosifescu et al. 2009; Colla et al., 2009).
We sought to systematically review all controlled studies of brain metabolite levels measured by 1H-MRS, to estimate the extent to which NAA, Cr, and Cho are altered in schizophrenia and bipolar disorder, to seek explanations (other than chance) by which studies in this field have yielded inconsistent results, and to examine if data supports the conception of a continuum or a dichotomy of bipolar disorder and schizophrenia.
2. METHODS
2.1 Eligibility Criteria
Eligible studies were clinical trials evaluating brain metabolites with 1H-MRS in patients with schizophrenia, schizoaffective disorder, schizophreniform disorder, or bipolar disorder according to DSM-III, DSM-III-R, or DSM-IV criteria.
Studies published in languages other than English, reported neurometabolites other than NAA, Cho or Cr, did not include a healthy control group, postmortem studies and studies enrolling adolescent subjects (younger than age 18) or geriatric subjects (older than 65) were excluded.
2.2 Literature Search
NVK and MAR performed a literature search in PubMed for 1H-MRS studies in schizophrenia and bipolar disorder for the timeframe up to September 2010 using the following key words: “Schizophrenia”, “Bipolar Disorder”, “Manic depressive disorder”, and “Magnetic Resonance Spectroscopy (MRS)”. The reference lists of included studies were inspected for additional eligible studies.
2.3 Study Selection
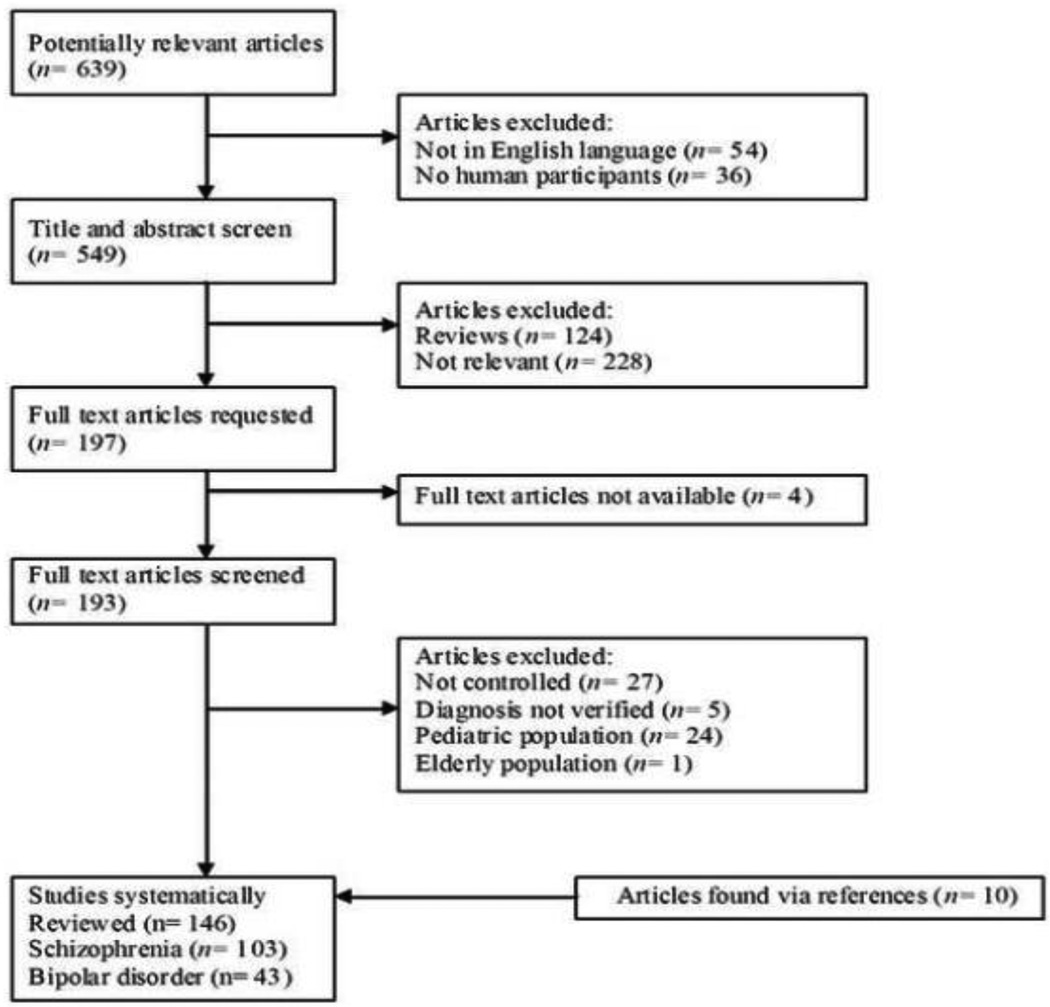
NVK and MAR reviewed titles and abstracts retrieved from the search and selected potentially eligible studies for full text review. Full text articles were then requested and assessed for eligibility. Figure 1 describes the outcomes at each level of our study identification process.
Fig. 1.
2.4. Author Contact
If relevant data were not reported in the article, we attempted to contact authors via e-mail to obtain this information. If no initial response was received, a second e-mail was sent two weeks later. If we did not receive a response then, the study was excluded from the meta-analysis. We contacted 76 authors for additional information. We received information from 13% of the authors. A total of 146 studies were included in the systematic review.
2.5. Data Extraction
We extracted the following data from each study: year of publication, number of participants (patients and healthy controls), illness duration (first episode vs. chronic illness), mood state (manic, depressed, euthymic), use of psychotropic medication (currently on medication vs. off medication vs. never treated and or minimally treated), MRS data acquisition parameters (MRSI vs. single-voxel (SV), field strength, location of voxel placement, use of a internal or external reference), absolute metabolite levels (mM or institutional units), and metabolite ratios.
2.6. Outcome Measures
Our primary outcome variables were absolute metabolite levels reported as mmol/l or i.u. (NAA, Cr, and Cho) as well as metabolite ratios (NAA/Cr and Cho/Cr) in the following regions of the brain: frontal lobe (given that the frontal lobe is a large and functionally complex region, we decided to analyze the ACC and DLPFC separately when authors specified that they studied these specific regions), parietal lobe, temporal lobe, occipital lobe, hippocampus, thalamus, basal ganglia, and cerebellum.
2.7. Statistical Analyses
2.7.1. Meta-analyses
We conducted meta-analyses for studies including subjects with bipolar disorder and schizophrenia meeting criteria as outlined above across all mentioned regions of the brain; however, not all regions had adequate number of studies to conduct a formal meta-analysis. If individual study results were reported separately for the left and right hemisphere, the left hemisphere data was included in the analysis, as it is the dominant hemisphere in most subjects. (Analyses were then re-run with right hemisphere data and compared with the initial analysis, given there was no significant difference between results of left and right hemisphere, this data is not shown).
To avoid double counting of the control group we only included subjects with chronic schizophrenia and not first episode psychosis when results were reported separately for both groups, but only one control group was reported. Chronic schizophrenia subjects were included because diagnostic stability likely is higher in this group.
To limit heterogeneity, eight studies that reported values (either in the original publication or via author contact) were excluded from analysis with absolute metabolite data, because they reported peak areas and did not report a internal water reference or external phantom of metabolites for spectra obtained (Buckley et al., 1994; Nasrallah et al., 1994; Shioiri et al., 1996; Kegeles et al., 2000; Ohara et al., 2000; Delamillieure et al., 2002; Szulc et al., 2007; Tang et al., 2007). One study was excluded because it reported relaxation times as opposed to concentrations (Ongur et al., 2010a). Eight studies were excluded because they reported the same subjects, or clearly had high overlap in subjects that have been published in other studies already included in the meta-analysis (Maier et al., 1995; Deicken et al., 1999; Deicken et al., 2000; van Elst et al., 2005; Ohrmann et al., 2007; Theberge et al., 2007; Ongur et al., 2008; Wood et al., 2009).
Data were analyzed with Review Manager 5.0.25 (Collaboration, 2008). We expected a high level of heterogeneity in the studies included in this meta-analysis; therefore, we conducted meta-analyses using the DerSimonian and Laird random-effects model to estimate effect sizes as standardized mean difference and its 95% confidence interval (CI) (DerSimonian and Laird, 1986).
We further quantified the extent to which observed inconsistency corresponded to between-study differences using the I2 statistic which measures the percentage of total variation across studies due to methodological or treatment heterogeneity rather than chance. Inconsistency is low when I2 is less than 25%, moderate when I2 is between 25% and 75%, and high when I2 is greater than 75% (Higgins et al., 2003).
2.7.2. Quantitative comparison of schizophrenia and bipolar disorder
We performed secondary meta-analyses, grouping both studies conducted in bipolar disorder and schizophrenia together in the same analysis. To assess differences in metabolite levels based on diagnosis, we then did a subgroup analysis with subgroups being defined as bipolar disorder and schizophrenia.
2.7.3. Subgroup analyses
A priori hypotheses examining potential heterogeneity across studies included differences in magnetic resonance field strength (low field strength: 1.5T and 2T vs. high field strenght: 3T and 4T), duration of illness (first episode vs. chronic disease), mood state (manic vs. depressed vs. euthymic), and medication status (current treatment vs. off medication vs. never/minimal treatment). A subgroup analysis performs separate meta-analyses in each subgroup. SMD in each subgroup is obtained, I2 statistics are performed to assess heterogeneity within the subgroup and χ2 statistics are done to explore differences between subgroups. Subgroup analyses were performed if inconsistency was moderate to high, and if there were at least two studies to include in each subgroup.
2.7.4. Assessment of publication bias
We planned to conduct a funnel plot to explore publication bias, but did not have an adequate number of studies to perform analysis. When only a limited number of studies are included, accurate identification of publication bias is practically due to chance. Resulting problems are subjectivity in the visual interpretation of the results, technical feasibility, and remaining uncertainty (Lau et al., 2006).
3. RESULTS
3.1. Study Characteristics
Table 1a and 1b give the characteristics of the studies. A total of 4182 subjects (2067 patients and 2115 healthy controls) were included in the 103 studies conducted in patients with schizophrenia, with a median study size of 36 participants (range: 13–115). In the 43 studies with bipolar disorder, 1461 subjects (738 patients and 721 healthy controls) were included, with a median study size of 34 participants (range: 13–64).
Table 1.
| a: Studies included in systematic review, schizophrenia | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | n sz/hc |
Illness duration |
Antipsych otic treatment |
MRS | Tesl a |
Data processing | use of reference |
Region studied | Absolute value data available |
Ratio data available |
| Ando et al. | 2002 | 7/7 | chronic | current | SV | 1.5 | peak area | lenticular nucleus | incomplete* | ||
| Auer et al. | 2001 | 32/17 | chronic | current | SV | 1.5 | institutional units | internal water | thalamus, parietal white matter | X | |
| Aydin et al. | 2007 | 28/28 | chronic | current | SV | 1.5 | concentration | Internal water | cingulate gyrus | X | |
| Aydin et al. | 2008 | 14/30 | first episode | never | SV | 1.5 | concentration | internal water | whole brain | X | |
| Bartha et al. | 1997 | 10/10 | first episode | never | SV | 1.5 | concentration | internal water | medial prefrontal cortex, ACC | ||
| Bartha et al. | 1999 | 11/11 | first episode | never/minimal | SV | 1.5 | concentration | internal water | left mesial temporal lobe | incomplete | |
| Bertolino et al. | 1996 | 10/10 | chronic | current | MRSI | 1.5 | peak area | hippocampus, DLPFC, thalamus, putamen, superior temporal gyrus, orbitofrontal cortex, posterior cingulate, ACC, occipital cortex, centrum semiovale, prefrontal white matter | incomplete | ||
| Bertolino et al. | 2000a | 13/13 | chronic | varies | MRSI | 1.5 | peak area | DLPFC | |||
| Bertolino et al. | 2000b | 9/7 | chronic | never/off | MRSI | 1.5 | peak area | DLPFC | |||
| Bertolino et al. | 2003b | 24/24 | first episode | minimal | MRSI | 1.5 | peak area | hippocampus, DLPFC | incomplete | ||
| Block et al. | 2000 | 25/19 | chronic | current | SV | 1.5 | peak area | frontal lobe, BG | X | ||
| Buckley et al. | 1994 | 28/20 | varies | varies | SV | 1.5 | ?concentration | frontal lobe, temporal lobe | X | ||
| Bustillo et al. | 2001 | 19/21 | chronic | current | SV | 1.5 | concentration | internal water | frontal lobe, caudate nucleus | X | |
| Bustillo et al. | 2002a | 11/11 | first episode | never | SV | 1.5 | concentration | internal water | frontal lobe, occipital lobe | X | |
| Bustillo et al. | 2002b | 10/10 | first episode | never/minimal | SV | 1.5 | concentration | Internal water | caudate nucleus | X | |
| Bustillo et al. | 2008 | 32/21 | early | never/minimal | SV | 1.5 | concentration | internal water | frontal obe, occipital lobe, cerebellum, caudate nucleus | X | |
| Bustillo et al. | 2010 | 14/10 | early | never/minimal | SV | 4 | concentration | internal water | frontal white matter, thalamus, ACC | incomplete | |
| Callicott et al. | 1998 | 47/66 | chronic | current | MRSI | 1.5 | peak area | mesial temporal cortex, hippocampus | |||
| Callicott et al. | 2000a | 13/18 | chronic | unclear | MRSI | 1.5 | peak area | superior temporal gyrus, DLPFC, ACC, posterior cingulate, occipital cortex, frontal white matter, putamen, hippocampus, thalamus | |||
| Callicott et al. | 2000b | 36/73 | chronic | varies | MRSI | 1.5 | peak area | DLPFC, hippocampus | |||
| Choe et al. | 1994 | 23/10 | chronic | never | SV | 1.5 | peak area | prefrontal white matter | incomplete | ||
| Choe et al. | 1996 | 55/20 | chronic | off | SV | 1.5 | peak area | prefrontal cortex | incomplete | ||
| Cecil et al. | 1999 | 8/14 | first episode | never | SV | 1.5 | peak area | DLPFC, temporal lobe | |||
| Deicken et al. | 1997a | 24/15 | chronic | current | MRSI | 1.5 | concentration | internal water | frontal lobe | X | |
| Deicken et al. | 1997b | 26/16 | chronic | varies | MRSI | 1.5 | concentration | internal water | ACC | X | |
| Deicken et al. | 1998 | 30/18 | chronic | varies | MRSI | 1.5 | concentration | internal water | hippocampus | X | X |
| Deicken et al. | 1999 | 23/18 | chronic | current | MRSI | 1.5 | concentration | internal water | hippocampus | X | |
| Deicken et al. | 2000 | 17/10 | chronic | current | MRSI | 1.5 | concentration | internal water | thalamus | X | incomplete |
| Deicken et al. | 2001 | 20/15 | chronic | current | MRSI | 1.5 | concentration | internal water | cerebellum | X | |
| Delamillieure et al. | 2000a | 27/24 | unclear | unclear | SV | 1.5 | peak area | thalamus | incomplete | ||
| Delamillieure et al. | 2000b | 17/21 | chronic | current | SV | 1.5 | peak area | medial prefrontal cortex | incomplete | ||
| Delamillieure et al. | 2002 | 17/14 | chronic | current | SV | 1.5 | peak area | prefrontal cortex, thalamus, hippocampus | X* | X* | |
| Eluri et al. | 1998 | 12/8 | chronic | current | SV | 1.5 | peak area | cerebellum, pons | incomplete | ||
| Ende et al. | 2000 | 19/16 | chronic | current | MRSI | 1.5 | absolute integral values | ACC | X | ||
| Ende et al. | 2001 | 15/15 | chronic | current | MRSI | 1.5 | absolute integral values | thalamus | X | ||
| Ende et al. | 2003 | 13/13 | chronic | current | MRSI | 1.5 | absolute integral values | thalamus, hippocampus, BG | X | incomplete | |
| Ende et al. | 2005 | 13/14 | chronic | unclear | MRSI | 1.5 | ?concentration | internal water | cerebellum, dentate nucleus | ||
| Fannon et al. | 2003 | 11/25 | first episode | off | SV | 1.5 | internal water | prefrontal cortex, hippocampus, BG | incomplete | ||
| Fujimoto et al | 1996 | 14/12 | chronic | current | SV | 2 | peak area | BG | X | ||
| Fukuzako et al. | 1995 | 15/15 | chronic | current | SV | 2 | peak area | frontal lobe, medial temporal lobe | X | ||
| Fukuzako et al. | 1999 | 40/40 | chronic | current | SV | 2 | peak area | medial temporal lobe | incomplete | ||
| Fukuzako et al. | 2000 | 64/51 | chronic | current | SV | 2 | peak area | hippocampus | incomplete | ||
| Galinska et al. | 2009 | 30/19 | first episode | never | SV | 1.5 | ?concentration | internal water | frontal lobe, temporal lobe, thalamus | X* | incomplete |
| Gimenez et al. | 2003 | 11/11 | first episode | never | SV | 1.5 | peak area | striatum | |||
| Goto et al. | 2009 | 18/18 | unclear | current | SV | 3 | frontal lobe, BG, parieto-occipital lobe | incomplete* | |||
| Hagino et al. | 2002 | 13/13 | chronic | current | SV | 1.5 | peak area | inferior frontal cortex, thalamus | X | ||
| Heimberg et al. | 1998 | 24/39 | chronic | varies | SV | 1.5 | peak area | internal water | BG, frontal cortex, temporal cortex, thalamus | incomplete | |
| Jakary et al. | 2005 | 22/22 | chronic | current | MRSI | 1.5 | concentration | internal water | thalamus | X | |
| Klaer et al. | 2010 | 29/44 | chronic | current | SV | 3 | concentration | Internal water | hippocampus | X | |
| Kegeles et al. | 2000 | 10/10 | chronic | varies | SV | 1.5 | peak area | hippocampus | X* | X | |
| Lim et al. | 1998 | 10/9 | chronic | current | MRSI | 1.5 | concentration | AA, Cho and Cr | grey matter, white matter | X | incomplete |
| Lutkenhoff et al. | 2010 | 14/13 | chronic | unclear | SV | 3 | concentration | internal water | mesial prefrontal cortex, prefrontal white matter, hippocampus | X | |
| Maier et al. | 1995 | 25/32 | chronic | unclear | SV | 1.5 | concentration | internal water | hippocampus | X* | |
| Maier et al. | 1996 | 26/38 | chronic | current | SV | 1.5 | concentration | internal water | hippocampus | X | |
| Martinez-Granados et al. | 2008 | 49/37 | chronic | current | MRSI | 1.5 | peak area | thalamus | X | ||
| Molina et al. | 2005 | 17/15 | chronic | current | SV | 1.5 | peak area | DLPFC | X* | ||
| Molina et al. | 2006 | 34/20 | chronic | current | SV | 1.5 | peak area | DLPFC | X* | ||
| Molina et al | 2007 | 11/10 | chronic | current | SV | 1.5 | peak area | DLPFC | X* | ||
| Moore et al. | 2002 | 20/20 | chronic | current | SV | 1.5 | peak area | mesial temporal lobe | incomplete | ||
| Nasrallah et al. | 1994 | 11/11 | chronic | current | SV | 1.5 | peak area | hippocampus | incomplete | ||
| Ohara et al. | 2000 | 10/10 | chronic | varies | SV | 1.5 | peak area | lenticular nucleus | X | ||
| Ohrmann et al. | 2005 | 21/21 | chronic | current | SV | 1.5 | concentration | internal water | DLPFC | X | |
| Ohrmann et al. | 2007 | 20/20 | chronic | current | SV | 1.5 | concentration | internal water | DLPFC | X | |
| Ohrmann et al. | 2008 | 43/37 | chronic | current | SV | 1.5 | concentration | internal water | ACC, DLPFC | X | |
| Olbrich et al. | 2008 | 9/32 | first episode | naive | SV | 2 | concentration | internal water | DLPFC, hippocampus | X | |
| Omori et al. | 2000 | 20/16 | chronic | varies | SV | 1.5 | peak area | thalamus, frontal lobe | incomplete | ||
| Ongur et al. | 2010b | 21/19 | chronic | current | SV | 4 | concentration | internal water | ACC, parieto-occipital cortex | X | |
| Ongur et al. | 2009 | 15/22 | chronic | current | SV | 4 | institutional units | internal water | ACC, parieto-occipital cortex | incomplete | |
| Pae et al. | 2004 | 24/20 | varies | naïve/off | SV | 1.5 | peak area | frontal lobe | incomplete | ||
| Pajonk et al. | 2010 | 8/8 | chronic | current | SV | 1.5 | peak area | hippocampus | incomplete | ||
| Premkumar et al. | 2010 | 30/15 | chronic | current | SV | 1.5 | concentration | internal water | ACC | X | |
| Reid et al. | 2010 | 26/23 | chronic | current | SV | 3 | ACC | X* | |||
| Rowland et al. | 2009 | 20/11 | chronic | current | SV | 3 | concentration | internal water | frontal lobe, inferior parietal lobe | X | |
| Ruesch et al. | 2008 | 29/31 | chronic | current | SV | 2 | concentration | internal water | hippocampus, DLPFC | X* | X* |
| Sanches et al. | 2008 | 38/38 | chronic | current | SV | 1.5 | peak area | frontal lobe, ACC, perirolandic fissure | |||
| Sarramea et al. | 2008 | 14/15 | chronic | current | SV | 1.5 | peak area | cingulate gyrus | X | ||
| Sharma et al. | 1992 | 4/9 | chronic | current | SV | 1.5 | peak area | basal ganglia, occipital cortex | incomplete | ||
| Shimizu et al. | 2007 | 19/18 | chronic | current | SV | 1.5 | peak area | posterior cingulate gyrus | incomplete | ||
| Shirayama et al. | 2010 | 19/18 | chronic | current | SV | 3 | concentration | internal water | medial prefrontal cortex | X | X |
| Shioiri et al. | 1996 | 21/21 | chronic | current | SV | 1.5 | peak area | BG | X | X | |
| Sigmundsson et al. | 2003 | 25/26 | chronic | current | SV | 1.5 | concentration | internal water | DLPFC | X | |
| Stanley et al. | 1996 | 13/25 | first episode | never | SV | 1.5 | concentration | internal water | DLPFC | X* | X* |
| Steel et al. | 2001 | 10/10 | chronic | current | SV | 2 | institutional units | internal water | frontal lobe | incomplete | |
| Szulc et al. | 2007 | 58/21 | chronic | current | SV | 1.5 | ?concentration | internal water | frontal lobe, temporal lobe, thalamus | X | incomplete |
| Tanaka et al. | 2006 | 14/13 | chronic | current | SV | 1.5 | concentration | NAA phantom | frontal lobe | X | incomplete |
| Tang et al. | 2007 | 40/42 | chronic | varies | SV | 3 | peak area | DLPFC, medial temporal cortex, occipital cortex | incomplete | incomplete | |
| Tayoshi et al. | 2009 | 30/25 | chronic | current | SV | 3 | concentration | internal water | ACC, BG | incomplete* | X* |
| Theberge et al. | 2002 | 21/21 | first episode | never | SV | 4 | concentration | internal water | ACC, thalamus | X* | X* |
| Theberge et al. | 2003 | 21/21 | chronic | current | SV | 4 | concentration | internal water | ACC, thalamus | X* | X* |
| Theberge et al. | 2004a | 9/8 | chronic | current | SV | 4 | concentration | internal water | ACC, thalamus | ||
| Theberge et al. | 2004b | 18/18 | first episode | never | SV | 4 | concentration | internal water | ACC, thalamus | incomplete* | incomplete* |
| Theberge et al. | 2007 | 13/16 | first episode | never | SV | 4 | concentration | internal water | ACC, thalamus | X* | X* |
| Tibbo et al. | 2000 | 12/12 | chronic | current | SV | 3 | peak area | cerebellum | incomplete | ||
| Tunc-Skarka et al. | 2009 | 23/29 | chronic | varies | SV | 3 | concentration | internal water | frontal white matter | X | X* |
| van Elst et al. | 2005 | 21/32 | chronic | current | SV | 2 | concentration | internal water | DLPFC, hippocampus | X | |
| Weber-Fahr et al. | 2002 | 15/15 | chronic | current | MRSI | 1.5 | concentration | internal water | hippocampus | ||
| Wobrock et al. | 2008 | 14/24 | chronic | current | SV | 1.5 | relative concentration | internal capsule | X | ||
| Wood et al. | 2003 | 56/21 | first episode | current | SV | 1.5 | peak area | temporal lobe, DLPFC | X | ||
| Wood et al. | 2007 | 15/14 | chronic | current | SV | 3 | concentration | internal water | ACC | X* | |
| Wood et al. | 2008 | 19/19 | chronic | current | SV | 3 | concentration | internal water | temporal lobe | X* | |
| Wood et al. | 2009 | 30/18 | first episode | never/minimal | SV | 3 | concentration | internal water | temporal lobe | X* | |
| Yamasue et al. | 2003 | 16/15 | chronic | current | SV | 1.5 | concentration | in vitro spectra | putamen | X | X* |
| Yurgelun-Todd et al. | 1996 | 16/14 | chronic | current | SV | 1.5 | peak area | mesial temporal lobe | incomplete | ||
| b: Studies included in systematic review, bipolar disorder | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | n bp/hc |
Mood state | Mood stabilizing treatment |
MRS | Tesl a |
Data processing | use of reference |
Region studied | Absolute value data available |
Ratio data available |
| Amaral et al. | 2006 | 13/15 | euthymic | current | SV | 1.5 | ratio | ACC | incomplete | ||
| Atmaca et al. | 2006 | 12/12 | unclear | unclear | MRSI | 1.5 | ratio | hippocampus | X | ||
| Atmaca et al. | 2007 | 30/10 | varies | varies | MRSI | 1.5 | ratio | hippocampus | X | ||
| Bertolino et al. | 2003a | 17/17 | varies | varies | MRSI | 1.5 | ratio | Thalamus, putamen, hippocampus, inferior frontal gyrus, DLPFC, ACC, posterior cingulated, centrum semiovale, prefrontal white matter, superior temporal gyrus | incomplete | ||
| Bhagwagar et al. | 2007 | 16/18 | euthymic | off meds | SV | 3 | ratio | parieto-occipital cortex | incomplete | ||
| Brambilla et al. | 2005 | 10/32 | unclear | varies | SV | 1.5 | concentration | internal water | DLPFC | X | X |
| Cecil et al. | 2002 | 17/21 | Manic/mixed | varies | SV | 1.5 | concentration | internal water | frontal lobe grey and white matter | X | |
| Colla et al. | 2009 | 21/19 | Manic/mixed | current | SV | 3 | concentration | internal water | hippocampus | X | |
| Dager et al. | 2004 | 32/26 | depressed/ mixed | off meds | MRSI | 1.5 | concentration | internal water | thalamus, putamen, cingulate gyrus, caudate nucleus, frontal white matter, parietal white matter, occipital lobe | ||
| Deicken et al. | 2001 | 15/15 | euthymic | varies | MRSI | 1.5 | institutional units | internal water | thalamus | X | |
| Deicken et al. | 2003 | 15/20 | euthymic | varies | SV | 1.5 | institutional units | internal water | hippocampus | X | |
| Frey et al. | 2005 | 10/10 | manic/mixed | varies | SV | 1.5 | concentration | internal water | DLPFC | X | |
| Frey et al. | 2007 | 32/32 | varies | off meds | SV | 1.5 | concentration | internal water | DLPFC | X | |
| Friedman et al. | 2004 | 21/12 | varies | off meds | MRSI | 1.5 | concentration | internal water | grey matter, white matter | ||
| Frye et al. | 2007a | 16/17 | manic | current | SV | 3 | concentration | internal water | ACC, basal ganglia, occipito-parietal white matter | X | incomplete |
| Frye et al. | 2007b | 23/12 | depressed | varies | SV | 1.5 | concentration | internal water | ACC | X | incomplete |
| Hamakawa et al. | 1998 | 18/20 | varies | varies | SV | 1.5 | concentration | external phantom | basal ganglia | X | X |
| Hamakawa et al. | 1999 | 23/20 | varies | varies | SV | 1.5 | concentration | external phantom | frontal lobe | X | |
| Hajek et al. | 2008 | 14/21 | unclear | unclear | SV | 1.5 | concentration | internal water | dorsal frontal lobe | X | |
| Iosifescu et al. | 2009 | 18/10 | euthymic | current | SV | 4 | institutional units | internal water | hippocampus | X | |
| Kato et al. | 1996 | 19/19 | euthymic | varies | SV | 1.5 | ratio | basal ganglia | incomplete | ||
| Kaufman et al. | 2009 | 13/11 | varies | current | MRSI | 4 | concentration | internal water | basal ganglia, whole brain | X | |
| Michael et al. | 2003 | 8/8 | manic | unclear | SV | 1.5 | concentration | internal water | ACC, DLPFC | X* | |
| Michael et al. | 2009 | 6/8 | varies | current | SV | 1.5 | concentration | internal water | DLPFC | X | |
| Mahli et al. | 2007 | 9/9 | hypomanic/euthymic | current | SV | 1.5 | concentration | internal water | ACC, basal ganglia, frontal white matter | incomplete | |
| Molina et al. | 2007 | 13/10 | euthymic | current | SV | 1.5 | ratio | DLPFC | X* | ||
| Moore et al. | 2000 | 12/9 | depressed | off meds | SV | 1.5 | arbitrary units | internal water | frontal lobe, parietal lobe, occipital lobe, temporal lobe | ||
| Moore et al. | 2000b | 9/14 | depressed | current | MRSI | 1.5 | ratio | ACC | |||
| Ohara et al. | 1998 | 10/10 | euthymic | varies | SV | 1.5 | ratio | basal ganglia | X | ||
| Ongur et al. | 2008 | 15/22 | manic | current | SV | 4 | arbitrary units | internal water | ACC, parieto-occipital cortex | X | |
| Ongur et al. | 2009 | 15/22 | manic | current | SV | 4 | arbitrary units | internal water | ACC, parieto-occipital cortex | ||
| Ongur et al. | 2010a | 15/20 | manic | varies | SV | 4 | relaxation times | internal water | ACC, parieto-occipital cortex | X | |
| Port et al. | 2008 | 21/21 | varies | off meds | MRSI | 3 | concentration | internal water | basal ganglia | ||
| Sarramea et al. | 2008 | 15/17 | euthymic | varies | SV | 1.5 | ratio | cingulum | X | ||
| Scherk et al. | 2008 | 13/13 | euthymic | varies | SV | 1.5 | ratio | hippocampus, thalamus, putamen | X | ||
| Scherk et al. | 2009a | 30/16 | euthymic | current | SV | 1.5 | ratio | putamen | X | ||
| Scherk et al. | 2009b | 33/29 | euthymic | current | SV | 1.5 | ratio | ACC, DLPFC | X | ||
| Senaratne et al. | 2009 | 12/12 | euthymic | current | SV | 3 | concentration | internal water | hippocampus, orbitofrontal lobe, occipital lobe | incomplete | |
| Sharma et al. | 1992 | 4/9 | manic | current | SV | 1.5 | ratio | basal ganglia, occipital cortex | X | ||
| Silverstone et al. | 2002 | 14/18 | euthymic | current | SV | 3 | ratio | external standard | temporal lobe | incomplete | |
| Silverstone et al. | 2003 | 25/18 | euthymic | current | SV | 3 | ratio | frontal lobe, temporal lobe | incomplete | ||
| Silverstone et al. | 2003 | 9/11 | euthymic | current | SV | 3 | concentration | internal water | frontal lobe, temporal lobe | incomplete | |
| Winsberg et al. | 2000 | 20/20 | euthymic | off meds | SV | 1.5 | ratio | DLPFC | X | ||
| Wu et al. | 2004 | 25/18 | euthymic | current | SV | 3 | concentration | internal water | frontal lobe, temporal lobe | incomplete | |
data obtained from author directly
X: data is available and included in meta-analysis. Studies with no X have not reported actual values
Incomplete: only some data available
84% of studies investigating schizophrenia included chronically ill patients, with 80% of studies including patients currently on antipsychotic medications. 33% of studies researching bipolar disorder included subjects in various mood states, 45% included euthymic patients only, three studies depressed patients only, and five studies included exclusively manic subjects. 45% of studies were conducted in patients currently on psychotropic medications.
A total of 104 studies used MRS with 1.5T field strength, eight studies with 2T, 21 studies with 3T, and 13 studies with 4T field strength.
3.2. Meta-Analyses
In schizophrenia, available data allowed conducting meta-analyses of absolute metabolite levels (NAA, Cr, and Cho) in the following regions of the brain: frontal lobe (n= 11) (separate analyses shown for ACC (n= 10) and DLPFC (n= 6)), hippocampus (n= 7), thalamus (n= 8), and basal ganglia (n= 6). Data were insufficient to perform analyses in both temporal lobe and cerebellum.
Meta-analyses for NAA/Cr ratios were performed in the hippocampus (n= 8), thalamus (n= 9), basal ganglia (n= 8), frontal lobe (n= 16), and temporal lobe (n= 7). Analyses for Cho/Cr ratios were performed in all the aforementioned regions except temporal lobe, due to lack of sufficient number of studies to include. There were not a sufficient number of studies to conduct meta-analysis in the ACC.
In bipolar disorder, sufficient data were available to conduct meta-analyses of absolute metabolite levels (NAA, Cr, and Cho) in the frontal lobe (n= 7) (separate analyses shown for ACC (n= 5) and DLPFC (n= 5)), hippocampus (n= 4), and basal ganglia (n= 4). Data were insufficient to perform analyses in the temporal lobe and thalamus.
Meta-analyses for NAA/Cr were conducted in the hippocampus (n= 4), basal ganglia (n= 7), ACC (n= 5), and DLPFC (n= 6). For Cho/Cr ratios analyses were conducted in the hippocampus (n= 3), basal ganglia (n= 6), ACC (n= 6), and DLPFC (n= 5). There were not sufficient numbers of studies to conduct meta-analyses in the thalamus, frontal lobe, and temporal lobe.
3.2.1. Absolute metabolite value data
Results in schizophrenia demonstrated significantly decreased levels of NAA in the thalamus [SMD = −0.62 (CI −1.12 to −0.13); p= 0.01], and frontal lobe [SMD = −0.44 (CI −0.65 to −0.23); p< 0.001]. Cr and Cho levels did not differ in any of the regions investigated (Figure 2a). NAA data were consistent over studies in basal ganglia and the frontal lobe. Cr data were consistent in frontal lobe, DLPFC and thalamus. Cho data were consistent in the thalamus, frontal lobe and ACC. Inconsistency was moderate to high in all metabolite levels in the hippocampus. Inconsistencies were evident also in thalamus (NAA), basal ganglia (Cr, Cho), ACC (NAA, Cr) and DLPFC (NAA, Cho).
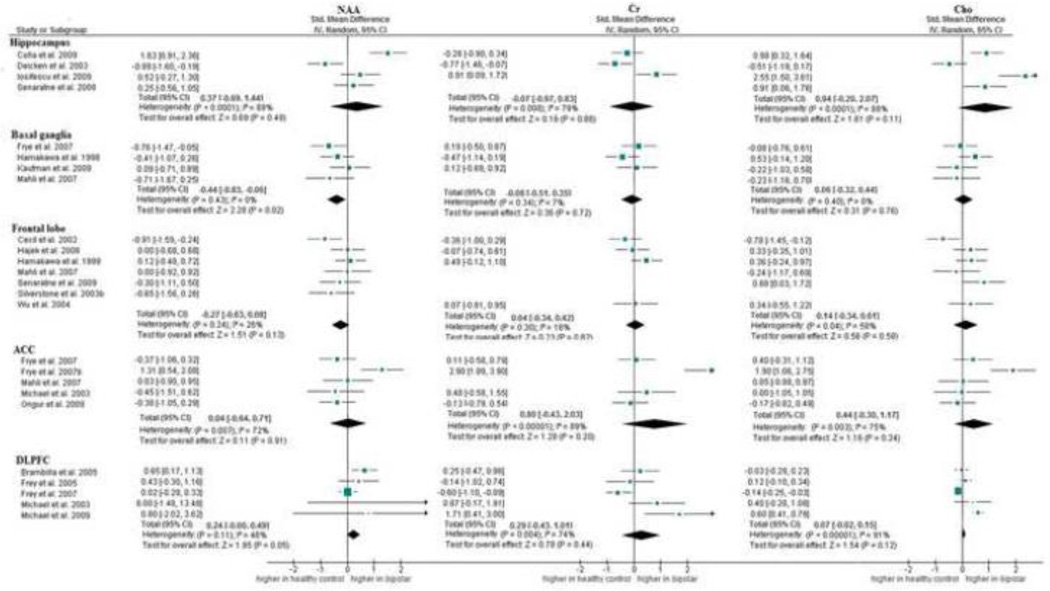
Fig. 2.
In bipolar disorder (Figure 2b), results showed significantly decreased levels of NAA in the basal ganglia compared to healthy controls [SMD = −0.44 (CI −0.83 to −0.06); p= 0.02]. Increased NAA levels approached significance in the DLPFC [SMD = 0.24 (CI 0.00 to 0.49); p= 0.05]. Cr and Cho levels did not differ in any of the regions investigated (Figure 2b). Results were consistent over studies in the basal ganglia for all metabolites and in the frontal lobe for Cr levels. Results showed moderate to high inconsistency in all metabolite levels in hippocampus, ACC, and DLPFC. Inconsistency for Cho in the frontal lobe was moderate.
3.2.2. Metabolite ratio data
Results in schizophrenia demonstrated lower levels of NAA/Cr in the hippocampus (p< 0.01), thalamus (p< 0.01), frontal lobe (p< 0.01), and temporal lobe (p< 0.01), but not in the basal ganglia or the DLPFC. Cho/Cr was significantly lower in the hippocampus (p= 0.03). No differences were found in any other region. Inconsistency between studies was low for NAA/Cr in the thalamus and frontal lobe and for Cho/Cr in the hippocampus. However, results for ratio data were moderately inconsistent in the hippocampus and basal ganglia, and highly inconsistent in the temporal lobe (Table 2a).
Table 2.
| a: Meta-analysis with ratio data, schizophrenia | |||||
|---|---|---|---|---|---|
| Region | number of studies |
Metabolite | SMD [95% CI] | p value |
Heterogeneity (I2) |
| Hippocampus | 8 | NAA/Cr | −0.72 [−1.20, −0.25] | <0 .01 | 74% |
| 5 | Cho/Cr | −0.28 [−0.54, −0.02] | 0.03 | 0% | |
| Thalamus | 9 | NAA/Cr | −037 [−0.58, −0.17] | <0 .01 | 6% |
| 6 | Cho/Cr | −0.02 [−0.34, 0.30] | 0.91 | 42% | |
| Basal ganglia | 8 | NAA/Cr | −0.16 [−0.46, 0.13] | 0.28 | 32% |
| 6 | Cho/Cr | 0.13 [−0.22, 0.48] | 0.47 | 37% | |
| Frontal lobe | 16 | NAA/Cr | −0.22 [−0.39, −0.06] | < 0.01 | 0% |
| 13 | Cho/Cr | 0.09 [−0.24, 0.41] | 0.61 | 68% | |
| DLPFC | 3 | NAA/Cr | 0.14 [−0.72, 1.00] | 0.75 | 86% |
| 2 | Cho/Cr | −0.15 [−0.73, 0.42] | 0.60 | 58% | |
| Temporal lobe | 7 | NAA/Cr | −0.64 [−1.09, −0.19] | <0 .01 | 77% |
| b: Meta-analysis with ratio data, bipolar disorder | |||||
|---|---|---|---|---|---|
| Region | number of studies |
Metabolite | SMD [95% CI] | p value |
Heterogeneity (I2) |
| Hippocampus | 4 | NAA/Cr | −0.96 [−1.37, −0.55] | <0.01 | 0% |
| 3 | Cho/Cr | −0.37 [−0.84, 0.11] | 0.13 | 0% | |
| Basal ganglia | 7 | NAA/Cr | −0.02 [−0.55, 0.51] | 0.95 | 69% |
| 6 | Cho/Cr | −0.04 [−0.62, 0.54] | 0.89 | 70% | |
| ACC | 5 | NAA/Cr | −0.59 [−1.18, 0.01] | 0.06 | 75% |
| 6 | Cho/Cr | 0.00 [−0.00, 0.01] | 0.52 | 43% | |
| DLPFC | 6 | NAA/Cr | −0.03 [−0.42, 0.36] | 0.88 | 49% |
| 5 | Cho/Cr | 0.05 [−0.23, 0.33] | 0.74 | 6% | |
In bipolar disorder, significantly lower NAA/Cr ratios were seen in the hippocampus (p< 0.01). Inconsistency was low for Cho/Cr only in the hippocampus and DLPFC (Table 2b).
3.2.3. Absolute versus ratio data
In schizophrenia, data for NAA levels and NAA/Cr ratios were relatively consistent in areas examined. Absolute Cho levels were not affected in patients with schizophrenia, but Cho/Cr levels in the hippocampus were significantly lower in schizophrenia compared to healthy controls.
In bipolar disorder, absolute NAA levels were found to be significantly decreased in the basal ganglia, but these findings could not be confirmed with NAA/Cr ratio data. NAA/Cr ratios were only decreased in the hippocampus, while absolute NAA levels were not decreased in the hippocampus.
3.2.4. Quantitative comparison of schizophrenia and bipolar disorder
We found significant lower NAA levels in the hippocampus (χ2= 21.96; df= 1; p< 0.001) and DLPFC (χ2= 11.84; df= 1; p< 0.001) in subjects with schizophrenia compared to subjects with bipolar disorder. Cho levels in the hippocampus (χ2= 10.17; df= 1; p< 0.001) and Cr levels in the ACC (χ2= 7.35; df= 1; p< 0.001) were also significantly lower in schizophrenia than in bipolar disorder. No significant differences in metabolite levels between the disorders were found in any other region studied.
3.2.5. Subgroup analyses
3.2.5.1. First episode vs. chronic schizophrenia
In schizophrenia, all studies that were conducted in first episode subjects enrolled them while off medication. Further, all studies that were conducted in subjects with chronic schizophrenia enrolled subjects currently treated with medication. We were therefore unable to attribute differences between subgroups to either duration of illness or medication status alone.
While initial analyses in schizophrenia showed a significant decrease of NAA in the thalamus with moderate inconsistency, subgroup analyses demonstrated that the decrease was attributable to studies conducted in chronic schizophrenia/subjects on medication [SMD= −0.77, p<0.01]. In first episode psychosis/subjects of medication, there was no significant decrease of NAA compared to healthy controls [SMD= −0.13; p= 0.86]. Differences between subgroups were significant [χ2= 10.90; p< 0.01]. Inconsistencies found in the basal ganglia and DLPFC could not be explained by difference in illness duration. Data were not sufficient to conduct subgroup analyses in the hippocampus and ACC.
3.2.5.2. Manic vs depressive episode vs euthymic mood state
Data were insufficient to perform subgroup analyses based on mood states.
3.2.5.3. Medication status
For studies conducted in subjects with schizophrenia, results are as described above. Data was insufficient to perform subgroup analyses based on medication status in bipolar disorder.
3.2.5.4. Field strength
In the thalamus, studies conducted with low field strength demonstrated significant decrease of absolute NAA concentrations in schizophrenia (SMD= −0.89, p< 0.01) with inconsistencies across studies remaining moderate, while studies conducted with high field strength did not show any difference compared to healthy controls, with low inconsistency (SMD= −0.17, p= 0.86). There was a significant difference between studies conducted with low vs. high field strength [χ2= 11.03; p< 0.01]. This means that studies conducted with high field strength did not find differences, and these findings were consistent across studies. Findings with low field strength did show decrease in NAA but inconsistency was higher between studies. Inconsistencies in hippocampus and ACC could not be explained by differences in field strength, SMDs between studies conducted with high vs. low field strength did not significantly differ, heterogeneity between studies in subgroups remained high. Data was not sufficient to perform subgroup analyses in basal ganglia and DLPFC.
While none of the subgroups significantly differed in from healthy controls, there was a significant difference between studies conducted with low vs high field strength in the ACC in bipolar disorder. (NAA (absolute values): χ2= 5.82; p= 0.02, Cr (absolute values): χ2= 16.02; p< 0.01]. When only studies with low field strength vs studies with high field strength were grouped together, inconsistency between studies was low. This means that inconsistencies between groups can be explained by differences in field strength. Inconsistencies in the frontal lobe in both absolute and ratio data could not be explained by differences in field strength. Data was not sufficient to perform subgroup analyses in the hippocampus and DLPFC.
4. DISCUSSION
4.1. Findings
While our meta-analysis failed to reveal significant abnormalities in either Cr or Cho levels, we found several abnormalities in NAA levels in schizophrenia and bipolar disorder.
NAA levels appear to be globally decreased (hippocampus, thalamus, frontal and temporal lobe) in patients with schizophrenia compared with healthy controls, which is consistent with previous reports of a subtle decrease of NAA of about 5% overall (Steen, et al., 2005). Brugger previously suggested decreased NAA levels in the thalamus as trait marker of schizophrenia, as he did not find any significant difference between first episode psychosis and chronic schizophrenia (Brugger et al., 2011). However, our analysis suggests that NAA levels in the thalamus are only decreased in chronic schizophrenia but not first episode patients. These differences in results may be partly accounted for by inclusion of a study in pediatric population (O'Neill et al., 2004) and a study published in Turkish language (Basoglu et al., 2006) by Brugger, but at this time it is unclear if findings can be generalized to first episode patients.
NAA levels in bipolar disorder appear to be decreased in the basal ganglia. Data for the DLPFC show that NAA levels in bipolar disorder are increased compared to healthy controls. While the finding of an increase in NAA appears counterintuitive, a recent study suggests a close correlation between NAA and glutamate levels in healthy subjects (Waddell et al., 2011). Glutamate levels are consistently found to be elevated in bipolar disorder (Yuksel and Ongur, 2010), which could imply that the increase of NAA may be related to an increase of glutamate levels. Our understanding of the physiology/ pathophysiology of NAA remains limited, relationships between and interactions with other neurometabolites and resulting pathological implications are poorly researched. In conclusion, alterations of NAA levels may reflect a much more complex underlying process than simply neuronal viability.
In a comparison of effect sizes of alterations in metabolite levels we found significant differences in NAA in the DLPFC between schizophrenia and bipolar disorder. While levels in bipolar disorder were significantly increased compared to healthy controls, decrease in NAA did not reach significance in schizophrenia. However, a bidirectional effect appears to be emerging. While not as clear, a similar pattern is noticeable in NAA and Cho in the hippocampus. No statistically significant increase in NAA in the hippocampus in bipolar disorder was found, but levels were decreased at a trend level in schizophrenia; results differed significantly between the two groups. Cho in the hippocampus and Cr in the ACC did not significantly differ from healthy controls in schizophrenia and bipolar disorder. However, Cho in the hippocampus and Cr in the ACC were significantly lower in schizophrenia and bipolar disorder. It appears that, although alterations in neurometabolites share several commonalities, there appear to be several differences, which stands in contrast to the conceptualization of schizophrenia and bipolar disorder as a continuum of the same disorder. However, the validity of these findings is limited, as there was no direct comparison in metabolite levels. Also, the total number of subjects reported in studies conducted in schizophrenia (1329 patients and 1394 healthy controls in 103 studies) differs from the ones conducted bipolar disorder (738 patients and 721 healthy controls in 43 studies). While this may be affecting outcomes, subgroup analysis compares effect sizes between groups. Effect size calculations take into account the number of subjects included in the analysis. Many studies did not specify that they have matched their samples for age and gender, which may have confounded results. To date, only a few studies have directly examined neurometabolite level differences between the disorders. Ongur did not find differences in Cr between subjects with schizophrenia and bipolar disorder (Ongur et al., 2009). In the ACC, NAA/Cr was found to be lower in schizophrenia and Cho/Cr was higher when compared to bipolar disorder (Sarramea et al., 2000); others reported decrease in NAA that were similar in both disorders (Molina et al., 2007). More data needs to become available before firm conclusions can be drawn.
4.2. Limitations
The evidence of alterations in brain metabolite levels consists of small studies in different regions of the brain with different patient populations receiving different or no treatment for their disorder. To detect a 10% change in NAA levels with 80% power, a sample size of 39 subjects and 39 controls was suggested; assuming these parameters, no studies conducted in bipolar disorder and only five studies in schizophrenia were adequately powered (Steen et al., 2005). While we attempted to ameliorate the effect of publication and outcome reporting bias by contacting authors, but only 13% provided requested data.
To avoid double counting effects, we excluded studies that included subjects reported elsewhere from analysis. However, it is possible that data remains in analyses that have been published in two sources. For the same reason, we decided to only include one reported dataset when two different sets of schizophrenic/bipolar subjects (e.g. first episode and chronic schizophrenia, manic and euthymic subjects) but only one healthy control group were included in a study. While this approach may lead to decreased power in overall analysis, we believe it is crucial to do so in order to avoid an overstating of the precision of results (Senn, 2009).
To reduce inconsistencies we excluded studies from data analysis if no internal or external concentration reference was used in data acquisition.
We have not attempted to weigh studies by methodological quality, as there is no objective way to assess this from published study methodology descriptions. However, it should be noted that studies conducted with magnets with high field strength that have a priori defined measures of spectral quality, spectral fitting and partial volume correction and include subjects that are not on medications with healthy controls that are matched by age, gender and socioeconomic status are considered to be of highest methodological quality.
We only examined NAA, Cr, and Cho in our systematic review. Since high field strength magnets are available, glutamate, glutamine, and GABA have become metabolites of interest. These metabolites have been implicated in the pathophysiology of both schizophrenia and bipolar disorder (Ongur, et al., 2008; Bustillo et al., 2010; Ongur et al., 2010b; Reid et al., 2010). Given the limited number of studies conducted examining glutamate, we decided that a meta-analytical approach would be premature and not include glutamate, glutamine and GABA in our analysis.
High remaining inconsistency in different areas of the brain makes results harder to interpret as factors that have not been controlled for could have a significant influence on the results. Further data will need to be obtained to clarify these findings, and other potential confounding factors need to be explored. Inconsistencies may be partly attributable to a number of variables that differ in the populations studied.
One of the variables that differ between populations and potentially confounds results is medication status. Many studies enrolled subjects currently treated with antipsychotic or moodstabilizers, potentially confounding our results. 80% of all studies conducted in schizophrenia included subjects on medication, while only 45% of subjects in bipolar trials were currently treated. Modulation of neurometabolite levels with both classes of drugs were demonstrated in the rat model. A consistent upregulation of NAA, with distinct regional patterns of activation depending on the agent given was the most robust finding (McLoughlin et al., 2009). In humans, antipsychotic medications are reported to increase NAA levels, even after a short period of treatment (Bertolino et al., 2001; Szulc et al., 2005). While some studies reported fewer effects on NAA levels in typical antipsychotics than atypical antipsychotics, others were unable to replicate these findings (Braus et al., 2001; Braus et al., 2002; Szulc, et al., 2007; Bustillo et al., 2008). Valproic acid was found to increase NAA/Cr in bipolar patients; lithium, but not valproic acid, was reported to increase NAA in euthymic bipolar patients (Silverstone et al., 2003; Atmaca et al., 2007). We were unable to draw conclusions on the influence of psychotropic medication on neurometabolites as data was not sufficient to do subgroup analyses.
Another potentially confounding clinical factor is mood state of subjects included. A small study suggested that Cr in the frontal lobe was decreased when the same subjects were in a depressed state compared to euthymia Hamkawa et al., 1999). Severity of depression was positively correlated with elevation of Cho levels in the cingulate, suggesting a mood state dependent alteration (Moore et al., 2000) A recent systematic review found alteration of glutamate levels in bipolar disorder are increased independent of mood state (Yuksel and Ongur, 2010). Our analysis did not allow drawing conclusions about mood state dependent alterations of neurometabolites, as not enough data was available to conduct subgroup analysis.
Several technical variations that may contribute to remaining inconsistencies. Differences in voxel size and voxel placement as well as grey and white matter contributions to selected voxels across studies may have contributed to inconsistencies. Studies used various definitions for areas of interest. Especially in studies with voxel placement within the frontal lobe, authors often identified their area of interest globally as “frontal lobe”. Others placed and defined their area of interest specifically within the ACC or DLPFC, both located within the frontal lobe. Where areas of interest within the frontal lobe were identified, we decided to analyze them separately, in an attempt to avoid overgeneralization of results in this functionally complex region. However, a potential limitation is that studies that actually did place their voxel completely or partly in one of these areas but did not state this in their method section were included in the general frontal lobe analysis which may have skewed the results. Further, MRS quantification is affected by signal to noise ratio (SNR), the quality of spectra form some regions is higher than others. Results show the moderate to high inconsistency in all metabolite levels in hippocampus, inconsistencies were evident also in thalamus, which may be attributable to lower SNR. T1/T2 is different at different field strength and affects metabolite quantification, it has also been demonstrated that T2 changes are associated with psychotic disorders, which may affect outcomes (Ongur et al. 2010a).
Some studies acquired single-voxel MRS while others used MRSI. Single-voxel MRS benefits from ease of implementation and quantitation, with only a single spectrum to process. MRSI has the advantage of acquiring data from multiple voxels in a single measurement, allowing investigation of different brain regions and tissue types. However, the spectrum at a given voxel will have contributions from neighboring voxels, and it is difficult to get a good shim and water suppression over a larger volume.
1H-MRS studies generally report a combined NAA and N-acetylaspartyl-glutamate (NAAG) peak, this peak is composed of approximately 90% NAA and 10% NAAG (Pouwels et al., 1997; Edden et al., 2007). NAAG is a peptide that is synthesized from NAA and glutamate and abundant in the nervous system, NAAG has been implied to be altered in both schizophrenia and bipolar disorder. It is possible that some of the alterations in NAA levels can be contributed to actual abnormalities of NAAG.
Use of a concentration reference is necessary to account for spatial field inhomogeneities as well as field variations across scanning sessions. Metabolite ratios provide a measure of relative concentrations and are easy to obtain, many studies used Cr as an internal reference. However, this method has the disadvantage of not definitively distinguishing between the numerator and denominator metabolite changes. To calculate metabolite concentrations, an internal or external reference is needed. Some studies used an unsuppressed internal water reference spectrum. Another approach is the use of an external reference solution, but this method is sensitive to inhomogeneities because the reference solution is separated from the volume of interest in the brain.
4.3. Research implications
We found a global decrease of NAA levels in schizophrenia. Of the regions studied, altered NAA levels in bipolar disorder seem to be limited to the basal ganglia and frontal lobe, with decreased levels in the ACC and increased levels in the DLPFC. Cr and Cho levels do not appear to be significantly affected any of the studied areas in either schizophrenia or bipolar disorder. While absolute metabolite levels and ratio data appeared to be consistent in schizophrenia, data were more conflictive in bipolar disorder.
Heterogeneity of data remains large in both schizophrenia and bipolar disorder. We were able to explain some of the inconsistencies. Specifically, high field strength magnets appear to detect more subtle alterations in metabolite levels, but factors that have not been controlled for are probably accounting for the majority of inconsistent findings. Likely confounding factors are not only variability in the patient populations studied but also data acquisition parameters.
Large, carefully designed studies are needed to better estimate the extent of alterations in brain metabolite levels in patient and to determine if MRS could be established as a tool to help differentiate schizophrenia from bipolar disorder.
ACKNOWLEDGEMENTS
DISCLOSURE/ CONFLICT OF INTEREST
ACL has received research funds from the National Institute of Mental Health grant R01 MH081014, the National Institute of Health grant R01 NCI141663, and an investigator-initiated grant from Pfizer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alger JR. Quantitative proton magnetic resonance spectroscopy and spectroscopic imaging of the brain: a didactic review. Topics in Magnetic Resonance Imaging. 2010;21(2):115–128. doi: 10.1097/RMR.0b013e31821e568f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral JA, Tamada RS, Issler CK, Caetano SC, Cerri GG, de Castro CC, Lafer B. A 1HMRS study of the anterior cingulate gyrus in euthymic bipolar patients. Human Psychopharmacology. 2006;21(4):215–220. doi: 10.1002/hup.761. [DOI] [PubMed] [Google Scholar]
- Ando K, Takei N, Matsumoto H, Iyo M, Isoda H, Mori N. Neural damage in the lenticular nucleus linked with tardive dyskinesia in schizophrenia: a preliminary study using proton magnetic resonance spectroscopy. Schizophrenia Researcj. 2002;57(2–3):273–279. doi: 10.1016/s0920-9964(01)00290-0. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Poyraz AK, Tezcan E, Ogur E. Hippocampal 1H MRS in first-episode bipolar I patients. Progress in Neuropsychopharmacology and Biological Psychiatry. 2006;30(7):1235–1239. doi: 10.1016/j.pnpbp.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Ogur E, Tezcan E. Hippocampal 1H MRS in patients with bipolar disorder taking valproate versus valproate plus quetiapine. Psychological Medicine. 2007;37(1):121–129. doi: 10.1017/S0033291706008968. [DOI] [PubMed] [Google Scholar]
- Auer DP, Wilke M, Grabner A, Heidenreich JO, Bronisch T, Wetter TC. Reduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentation. Schizophrenia Research. 2001;52(1–2):87–99. doi: 10.1016/s0920-9964(01)00155-4. [DOI] [PubMed] [Google Scholar]
- Aydin K, Ucok A, Cakir S. Quantitative proton MR spectroscopy findings in the corpus callosum of patients with schizophrenia suggest callosal disconnection. American Journal of Neuroradiology. 2007;28(10):1968–1974. doi: 10.3174/ajnr.A0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin K, Ucok A, Guler J. Altered metabolic integrity of corpus callosum among individuals at ultra high risk of schizophrenia and first-episode patients. Biological Psychiatry. 2008;64(9):750–757. doi: 10.1016/j.biopsych.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Bagory M, Durand-Dubief F, Ibarrola D, Confavreux C, Sappey-Marinier D. "Absolute" quantification in Magnetic Resonance Spectroscopy: validation of a clinical protocol in multiple sclerosis. Conference Proceedings IEEE Engineering in Medicine and Biology Society. 2007;2007:3458–34661. doi: 10.1109/IEMBS.2007.4353075. [DOI] [PubMed] [Google Scholar]
- Bartha R, Williamson PC, Drost DJ, Malla A, Carr TJ, Cortese L, Canaran G, Rylett RJ, Neufeld RW. Measurement of glutamate and glutamine in the medial prefrontal cortex of never-treated schizophrenic patients and healthy controls by proton magnetic resonance spectroscopy. Archives of General Psychiatry. 1997;54(10):959–965. doi: 10.1001/archpsyc.1997.01830220085012. [DOI] [PubMed] [Google Scholar]
- Bartha R, al-Semaan YM, Williamson PC, Drost DJ, Malla AK, Carr TJ, Densmore M, Canaran G, Neufeld RW. A short echo proton magnetic resonance spectroscopy study of the left mesial-temporal lobe in first-onset schizophrenic patients. Biological Psychiatry. 1999;45(11):1403–1411. doi: 10.1016/s0006-3223(99)00007-4. [DOI] [PubMed] [Google Scholar]
- Basoglu C, Cetin M, Oner O, Ebrinc S, Semiz UB, Kandilcioglu H, Silit E, Kizilkaya E. [Comparison of right thalamus and temporal cortex metabolite levels of drugnaive first-episode psychotic and chronic schizophrenia in patients] Turkish Journal of Psychiatry. 2006;17(2):85–91. [PubMed] [Google Scholar]
- Bertolino A, Nawroz S, Mattay VS, Barnett AS, Duyn JH, Moonen CT, Frank JA, Tedeschi D, Weinberger DR. Regionally specific pattern of neurochemical pathology in schizophrenia as assessed by multislice proton magnetic resonance spectroscopic imaging. American Journal of Psychiatry. 1996;153(12):1554–1563. doi: 10.1176/ajp.153.12.1554. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Breier A, Callicott JH, Adler C, Mattay VS, Shapiro M, Frank JA, Pickar D, Weinberger DR. The relationship between dorsolateral prefrontal neuronal N-acetylaspartate and evoked release of striatal dopamine in schizophrenia. Neuropsychopharmacology. 2000a;22(2):125–132. doi: 10.1016/S0893-133X(99)00096-2. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Esposito G, Callicott JH, Mattay VS, Van Horn JD, Frank JA, Berman KF, Weinberger DR. Specific relationship between prefrontal neuronal Nacetylaspartate and activation of the working memory cortical network in schizophrenia. American Journal of Psychiatry. 2000b;157(1):26–33. doi: 10.1176/ajp.157.1.26. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, Weinberger DR. The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biological Psychiatry. 2001;49(1):39–46. doi: 10.1016/s0006-3223(00)00997-5. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Frye M, Callicott JH, Mattay VS, Rakow R, Shelton-Repella J, Post R, Weinberger DR. Neuronal pathology in the hippocampal area of patients with bipolar disorder: a study with proton magnetic resonance spectroscopic imaging. Biological Psychiatry. 2003a;53(10):906–913. doi: 10.1016/s0006-3223(02)01911-x. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Sciota D, Brudaglio F, Altamura M, Blasi G, Bellomo A, Antonucci N, Callicott JA, Goldberg TE, Scarabino T, Weinberger DR, Nardini M. Working memory deficits and levels of N-acetylaspartate in patients with schizophreniform disorder. American Journal of Psychiatry. 2003b;160(3):483–489. doi: 10.1176/appi.ajp.160.3.483. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, Matthews PM, Cowen PJ. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biological Psychiatry. 2007;61(6):806–812. doi: 10.1016/j.biopsych.2006.08.048. [DOI] [PubMed] [Google Scholar]
- Block W, Bayer TA, Tepest R, Traber F, Rietschel M, Muller DJ, Schulze TG, Honer WG, Maier W, Schild HH, Falkai P. Decreased frontal lobe ratio of N-acetyl aspartate to choline in familial schizophrenia: a proton magnetic resonance spectroscopy study. Neuroscience Letters. 2000;289(2):147–151. doi: 10.1016/s0304-3940(00)01264-7. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Stanley JA, Nicoletti MA, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. 1H Magnetic resonance spectroscopy study of dorsolateral prefrontal cortex in unipolar mood disorder patients. Psychiatry Research. 2005;138(2):131–139. doi: 10.1016/j.pscychresns.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Bramon E, Sham PC. The common genetic liability between schizophrenia and bipolar disorder: a review. Current Psychiatry Reports. 2001;3(4):332–337. doi: 10.1007/s11920-001-0030-1. [DOI] [PubMed] [Google Scholar]
- Braus DF, Ende G, Weber-Fahr W, Demirakca T, Henn FA. Favorable effect on neuronal viability in the anterior cingulate gyrus due to long-term treatment with atypical antipsychotics: an MRSI study. Pharmacopsychiatry. 2001;34(6):251–253. doi: 10.1055/s-2001-18037. [DOI] [PubMed] [Google Scholar]
- Braus DF, Ende G, Weber-Fahr W, Demirakca T, Tost H, Henn FA. Functioning and neuronal viability of the anterior cingulate neurons following antipsychotic treatment: MR-spectroscopic imaging in chronic schizophrenia. European Neuropsychopharmacology. 2002;2(2):145–152. doi: 10.1016/s0924-977x(02)00003-2. [DOI] [PubMed] [Google Scholar]
- Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia-a systematic review and meta-analysis. Biological Psychiatry. 2011;69(5):495–503. doi: 10.1016/j.biopsych.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Moore C, Long H, Larkin C, Thompson P, Mulvany F, Redmond O, Stack JP, Ennis JT, Waddington JL. 1H-magnetic resonance spectroscopy of the left temporal and frontal lobes in schizophrenia: clinical, neurodevelopmental, and cognitive correlates. Biological Psychiatry. 1994;36(12):792–800. doi: 10.1016/0006-3223(94)90591-6. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Lauriello J, Rowland LM, Jung RE, Petropoulos H, Hart BL, Blanchard J, Keith SJ, Brooks WM. Effects of chronic haloperidol and clozapine treatments on frontal and caudate neurochemistry in schizophrenia. Psychiatry Research. 2001;107(3):135–149. doi: 10.1016/s0925-4927(01)00102-0. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Lauriello J, Rowland LM, Thomson LM, Petropoulos H, Hammond R, Hart B, Brooks WM. Longitudinal follow-up of neurochemical changes during the first year of antipsychotic treatment in schizophrenia patients with minimal previous medication exposure. Schizophr Research. 2002a;58(2–3):313–321. doi: 10.1016/s0920-9964(02)00210-4. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Lauriello J, Petropoulos H, Hammond R, Hart B, Brooks WM. High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. American Journal of Psychiatry. 2002b;159(1):130–133. doi: 10.1176/appi.ajp.159.1.130. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Jung R, Brooks WM, Qualls C, Hammond R, Hart B, Lauriello J. Proton magnetic resonance spectroscopy during initial treatment with antipsychotic medication in schizophrenia. Neuropsychopharmacology. 2008;33(10):2456–2466. doi: 10.1038/sj.npp.1301631. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, Hammond R, Brooks WM. 1H-MRS at 4 tesla in minimally treated early schizophrenia. Molecular Psychiatry. 2010;15(6):629–636. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Egan MF, Bertolino A, Mattay VS, Langheim FJ, Frank JA, Weinberger DR. Hippocampal N-acetyl aspartate in unaffected siblings of patients with schizophrenia: a possible intermediate neurobiological phenotype. Biological Psychiatry. 1998;44(10):941–950. doi: 10.1016/s0006-3223(98)00264-9. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Egan MF, Mattay VS, Langheim FJ, Weinberger DR. Selective relationship between prefrontal N-acetylaspartate measures and negative symptoms in schizophrenia. American Journal of Psychiatry. 2000a;157(10):1646–1651. doi: 10.1176/appi.ajp.157.10.1646. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, Goldberg TE, Weinberger DR. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cerebral Cortex. 2000b;10(11):1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Cecil KM, Lenkinski RE, Gur RE, Gur RC. Proton magnetic resonance spectroscopy in the frontal and temporal lobes of neuroleptic naive patients with schizophrenia. Neuropsychopharmacology. 1999;20(2):131–140. doi: 10.1016/S0893-133X(98)00063-3. [DOI] [PubMed] [Google Scholar]
- Cecil KM, DelBello MP, Morey R, Strakowski SM. Frontal lobe differences in bipolar disorder as determined by proton MR spectroscopy. Bipolar Disorders. 2002;4(6):357–365. doi: 10.1034/j.1399-5618.2002.02235.x. [DOI] [PubMed] [Google Scholar]
- Choe BY, Kim KT, Suh TS, Lee C, Paik IH, Bahk YW, Shinn KS, Lenkinski RE. 1H magnetic resonance spectroscopy characterization of neuronal dysfunction in drug-naive, chronic schizophrenia. Academic Radiology. 1994;1(3):211–216. doi: 10.1016/s1076-6332(05)80716-0. [DOI] [PubMed] [Google Scholar]
- Choe BY, Suh TS, Shinn KS, Lee CW, Lee C, Paik IH. Observation of metabolic changes in chronic schizophrenia after neuroleptic treatment by in vivo hydrogen magnetic resonance spectroscopy. Investigative Radiology. 1996;31(6):345–352. doi: 10.1097/00004424-199606000-00006. [DOI] [PubMed] [Google Scholar]
- Colla M, Schubert F, Bubner M, Heidenreich JO, Bajbouj M, Seifert F, Luborzewski A, Heuser I, Kronenberg G. Glutamate as a spectroscopic marker of hippocampal structural plasticity is elevated in long-term euthymic bipolar patients on chronic lithium therapy and correlates inversely with diurnal cortisol. Molecular Psychiatry. 2009;14(7):696–704. 647. doi: 10.1038/mp.2008.26. [DOI] [PubMed] [Google Scholar]
- Collaboration TC. Review Manager (RevMan) [computer program]. Version 5.0. 2008 [Google Scholar]
- Craddock N, Owen MJ. The beginning of the end for the Kraepelinian dichotomy. British Journal of Psychiatry. 2005;186:364–366. doi: 10.1192/bjp.186.5.364. [DOI] [PubMed] [Google Scholar]
- Crow TJ. The continuum of psychosis and its genetic origins. The sixty-fifth Maudsley lecture. British Journal of Psychiatry. 1990a;156:788–797. doi: 10.1192/bjp.156.6.788. [DOI] [PubMed] [Google Scholar]
- Crow TJ. In: The question of a genetic continuum for schizophrenia and affective disorder Depression in Schizophrenia. DeLisi LE, editor. Washington, DC: American Psychiatric Press; 1990b. pp. 81–98. [Google Scholar]
- Dager SR, Friedman SD, Parow A, Demopulos C, Stoll AL, Lyoo IK, Dunner DL, Renshaw PF. Brain metabolic alterations in medication-free patients with bipolar disorder. Archives of General Psychiatry. 2004;61(5):450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- Dager SR, Corrigan NM, Richards TL, Posse S. Research applications of magnetic resonance spectroscopy to investigate psychiatric disorders. Topics in Magnetic Resonance Imaging. 2008;19(2):81–96. doi: 10.1097/RMR.0b013e318181e0be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby JT, Morgan D, Lee ML. Schizophrenia and mania in identical twin brothers. Journal of Nervous and Mental Disease. 1986;174(5):304–308. [PubMed] [Google Scholar]
- Deicken RF, Zhou L, Corwin F, Vinogradov S, Weiner MW. Decreased left frontal lobe N-acetylaspartate in schizophrenia. American Journal of Psychiatry. 1997a;154(5):688–690. doi: 10.1176/ajp.154.5.688. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Zhou L, Schuff N, Weiner MW. Proton magnetic resonance spectroscopy of the anterior cingulate region in schizophrenia. Schizophrenia Research. 1997b;27(1):65–71. doi: 10.1016/S0920-9964(97)00082-0. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Zhou L, Schuff N, Fein G, Weiner MW. Hippocampal neuronal dysfunction in schizophrenia as measured by proton magnetic resonance spectroscopy. Biological Psychiatry. 1998;43(7):483–488. doi: 10.1016/S0006-3223(97)00490-3. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Pegues M, Amend D. Reduced hippocampal N-acetylaspartate without volume loss in schizophrenia. Schizophrenia Research. 1999;37(3):217–223. doi: 10.1016/s0920-9964(98)00173-x. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Johnson C, Eliaz Y, Schuff N. Reduced concentrations of thalamic N-acetylaspartate in male patients with schizophrenia. American Journal of Psychiatry. 2000;157(4):644–647. doi: 10.1176/appi.ajp.157.4.644. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Eliaz Y, Feiwell R, Schuff N. Increased thalamic N-acetylaspartate in male patients with familial bipolar I disorder. Psychiatry Research. 2001a;106(1):35–45. doi: 10.1016/s0925-4927(00)00083-4. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Feiwell R, Schuff N, Soher B. Evidence for altered cerebellar vermis neuronal integrity in schizophrenia. Psychiatry Research. 2001b;107(3):125–134. doi: 10.1016/s0925-4927(01)00103-2. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Pegues MP, Anzalone S, Feiwell R, Soher B. Lower concentration of hippocampal N-acetylaspartate in familial bipolar I disorder. American Journal of Psychiatry. 2003;160(5):873–882. doi: 10.1176/appi.ajp.160.5.873. [DOI] [PubMed] [Google Scholar]
- Delamillieure P, Constans J, Fernandez J, Brazo P, Dollfus S. Proton magnetic resonance spectroscopy (1H-MRS) of the thalamus in schizophrenia. European Psychiatry. 2000a;15(8):489–491. doi: 10.1016/s0924-9338(00)00522-8. [DOI] [PubMed] [Google Scholar]
- Delamillieure P, Fernandez J, Constans JM, Brazo P, Benali K, Abadie P, Vasse T, Thibaut F, Courtheoux P, Petit M, Dollfus S. Proton magnetic resonance spectroscopy of the medial prefrontal cortex in patients with deficit schizophrenia: preliminary report. American Journal of Psychiatry. 2000b;157(4):641–643. doi: 10.1176/appi.ajp.157.4.641. [DOI] [PubMed] [Google Scholar]
- Delamillieure P, Constans JM, Fernandez J, Brazo P, Benali K, Courtheoux P, Thibaut F, Petit M, Dollfus S. Proton magnetic resonance spectroscopy (1H MRS) in schizophrenia: investigation of the right and left hippocampus, thalamus, and prefrontal cortex. Schizophrenia Bulletin. 2002;28(2):329–339. doi: 10.1093/oxfordjournals.schbul.a006942. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Edden RAE, Pomper MG, Barker PB. In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 Tesla. Magnetic resonance in medicine. 2007;(6):977–982. doi: 10.1002/mrm.21234. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophrenia Research. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Eluri R, Paul C, Roemer R, Boyko O. Single-voxel proton magnetic resonance spectroscopy of the pons and cerebellum in patients with schizophrenia: a preliminary study. Psychiatry Research. 1998;84(1):17–26. doi: 10.1016/s0925-4927(98)00043-2. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Weber-Fahr W, Soher B, Maudsley AA, Henn FA. Effects of age, medication, and illness duration on the N-acetyl aspartate signal of the anterior cingulate region in schizophrenia. Schizophr Research. 2000;41(3):389–395. doi: 10.1016/s0920-9964(99)00089-4. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Henn FA. Lower concentration of thalamic nacetylaspartate in patients with schizophrenia: a replication study. American Journal of Psychiatry. 2001;158(8):1314–1316. doi: 10.1176/appi.ajp.158.8.1314. [DOI] [PubMed] [Google Scholar]
- Ende G, Braus DF, Walter S, Weber-Fahr W, Henn FA. Multiregional 1H-MRSI of the hippocampus, thalamus, and basal ganglia in schizophrenia. European Archives of Psychiatry and Clinicial Neuroscience. 2003;253(1):9–15. doi: 10.1007/s00406-003-0398-5. [DOI] [PubMed] [Google Scholar]
- Ende G, Hubrich P, Walter S, Weber-Fahr W, Kammerer N, Braus DF, Henn FA. Further evidence for altered cerebellar neuronal integrity in schizophrenia. American Journal of Psychiatry. 2005;162(4):790–792. doi: 10.1176/appi.ajp.162.4.790. [DOI] [PubMed] [Google Scholar]
- Fannon D, Simmons A, Tennakoon L, O'Ceallaigh S, Sumich A, Doku V, Shew C, Sharma T. Selective deficit of hippocampal N-acetylaspartate in antipsychoticnaive patients with schizophrenia. Biological Psychiatry. 2003;54(6):587–598. doi: 10.1016/s0006-3223(03)00185-9. [DOI] [PubMed] [Google Scholar]
- Frey BN, Folgierini M, Nicoletti M, Machado-Vieira R, Stanley JA, Soares JC, Kapczinski F. A proton magnetic resonance spectroscopy investigation of the dorsolateral prefrontal cortex in acute mania. Human Psychopharmacology. 2005;20(2):133–139. doi: 10.1002/hup.671. [DOI] [PubMed] [Google Scholar]
- Frey BN, Stanley JA, Nery FG, Monkul ES, Nicoletti MA, Chen HH, Hatch JP, Caetano SC, Ortiz O, Kapczinski F, Soares JC. Abnormal cellular energy and phospholipid metabolism in the left dorsolateral prefrontal cortex of medication-free individuals with bipolar disorder: an in vivo 1H MRS study. Bipolar Disorders. 2007;9(Suppl 1):119–127. doi: 10.1111/j.1399-5618.2007.00454.x. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Dager SR, Parow A, Hirashima F, Demopulos C, Stoll AL, Lyoo IK, Dunner DL, Renshaw PF. Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biological Psychiatry. 2004;56(5):340–348. doi: 10.1016/j.biopsych.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Frye MA, Watzl J, Banakar S, O'Neill J, Mintz J, Davanzo P, Fisher J, Chirichigno JW, Ventura J, Elman S, Tsuang J, Walot I, Thomas MA. Increased anterior cingulate/medial prefrontal cortical glutamate and creatine in bipolar depression. Neuropsychopharmacology. 2007a;32(12):2490–2499. doi: 10.1038/sj.npp.1301387. [DOI] [PubMed] [Google Scholar]
- Frye MA, Thomas MA, Yue K, Binesh N, Davanzo P, Ventura J, O'Neill J, Guze B, Curran JG, Mintz J. 0. Reduced concentrations of N-acetylaspartate (NAA) and the NAA-creatine ratio in the basal ganglia in bipolar disorder: a study using 3-Tesla proton magnetic resonance spectroscopy. Psychiatry Research. 2007b;154(3):259–265. doi: 10.1016/j.pscychresns.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Nakano T, Takano T, Takeuchi K, Yamada K, Fukuzako T, Akimoto H. Proton magnetic resonance spectroscopy of basal ganglia in chronic schizophrenia. Biological Psychiatry. 1996;40(1):14–18. doi: 10.1016/0006-3223(95)00316-9. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Takeuchi K, Hokazono Y, Fukuzako T, Yamada K, Hashiguchi T, Obo Y, Ueyama K, Takigawa M, Fujimoto T. Proton magnetic resonance spectroscopy of the left medial temporal and frontal lobes in chronic schizophrenia: preliminary report. Psychiatry Research. 1995;61(4):193–200. doi: 10.1016/0925-4927(95)02622-5. [DOI] [PubMed] [Google Scholar]
- Fukuzako H, Kodama S, Fukuzako T, Yamada K, Doi W, Sato D, Takigawa M. Subtype-associated metabolite differences in the temporal lobe in schizophrenia detected by proton magnetic resonance spectroscopy. Psychiatry Research. 1999;92(1):45–56. doi: 10.1016/s0925-4927(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Fukuzako H. Heritability heightens brain metabolite differences in schizophrenia. Journal of Neuropsychiatry and Clinical Neuroscience. 2000;12(1):95–97. doi: 10.1176/jnp.12.1.95. [DOI] [PubMed] [Google Scholar]
- Galinska B, Szulc A, Tarasow E, Kubas B, Dzienis W, Czernikiewicz A, Walecki J. Duration of untreated psychosis and proton magnetic resonance spectroscopy (1H-MRS) findings in first-episode schizophrenia. Medical Science Monitor. 2009;15(2):CR82–CR88. [PubMed] [Google Scholar]
- Gimenez M, Junque C, Perez M, Vendrell P, Baeza I, Salamero M, Merdader JM, Bernardo M. Basal ganglia N-acetylaspartate correlates with the performance in the procedural task 'Tower of Hanoi' of neuroleptic-naive schizophrenic patients. Neuroscience Letters. 2003;347(2):97–100. doi: 10.1016/s0304-3940(03)00698-0. [DOI] [PubMed] [Google Scholar]
- Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, Umene-Nakano W, Hayashi K, Oonari N, Korogi Y, Nakamura J. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophrenia Research. 2009;112(1–3):192–193. doi: 10.1016/j.schres.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Hagino H, Suzuki M, Mori K, Nohara S, Yamashita I, Takahashi T, Kurokawa K, Matsui M, Watanabe N, Seto H, Kurachi M. Proton magnetic resonance spectroscopy of the inferior frontal gyrus and thalamus and its relationship to verbal learning task performance in patients with schizophrenia: a preliminary report. Psychiatry and Clinical Neurosciences. 2002;56(5):499–507. doi: 10.1046/j.1440-1819.2002.01045.x. [DOI] [PubMed] [Google Scholar]
- Hajek T, Bernier D, Slaney C, Propper L, Schmidt M, Carrey N, MacQueen G, Duffy A, Alda M. A comparison of affected and unaffected relatives of patients with bipolar disorder using proton magnetic resonance spectroscopy. Journal of Psychiatry and Neuroscience. 2008;33(6):531–540. [PMC free article] [PubMed] [Google Scholar]
- Hamakawa H, Kato T, Murashita J, Kato N. Quantitative proton magnetic resonance spectroscopy of the basal ganglia in patients with affective disorders. European Archives of Psychiatry and Clinical Neurosciences. 1998;248(1):53–58. doi: 10.1007/s004060050017. [DOI] [PubMed] [Google Scholar]
- Hamakawa H, Kato T, Shioiri T, Inubushi T, Kato N. Quantitative proton magnetic resonance spectroscopy of the bilateral frontal lobes in patients with bipolar disorder. Psychological Medicine. 1999;29(3):639–644. doi: 10.1017/s0033291799008442. [DOI] [PubMed] [Google Scholar]
- Heckers S. Making progress in schizophrenia research. Schizophrenia Bulletin. 2008;34(4):591–594. doi: 10.1093/schbul/sbn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimberg C, Komoroski RA, Lawson WB, Cardwell D, Karson CN. Regional proton magnetic resonance spectroscopy in schizophrenia and exploration of drug effect. Psychiatry Research. 1998;83(2):105–115. doi: 10.1016/s0925-4927(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosifescu DV, Moore CM, Deckersbach T, Tilley CA, Ostacher MJ, Sachs GS, Nierenberg AA. Galantamine-ER for cognitive dysfunction in bipolar disorder and correlation with hippocampal neuronal viability: a proof-of-concept study. CNS Neuroscience and Therapeutics. 2009;15(4):309–319. doi: 10.1111/j.1755-5949.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakary A, Vinogradov S, Feiwell R, Deicken RF. N-acetylaspartate reductions in the mediodorsal and anterior thalamus in men with schizophrenia verified by tissue volume corrected proton MRSI. Schizophrenia Research. 2005;76(2–3):173–185. doi: 10.1016/j.schres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Kato T, Hamakawa H, Shioiri T, Murashita J, Takahashi Y, Takahashi S, Inubushi T. Choline-containing compounds detected by proton magnetic resonance spectroscopy in the basal ganglia in bipolar disorder. Journal of Psychiatry and Neuroscience. 1996;21(4):248–254. [PMC free article] [PubMed] [Google Scholar]
- Kaufman RE, Ostacher MJ, Marks EH, Simon NM, Sachs GS, Jensen JE, Renshaw PF, Pollack MH. Brain GABA levels in patients with bipolar disorder. Progress in Neuropsychopharmacology and Biological Psychiatry. 2009;33(3):427–434. doi: 10.1016/j.pnpbp.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Kegeles LS, Shungu DC, Anjilvel S, Chan S, Ellis SP, Xanthopoulos E, Malaspina D, Gorman JM, Mann JJ, Laruelle M, Kaufmann CA. Hippocampal pathology in schizophrenia: magnetic resonance imaging and spectroscopy studies. Psychiatry Research. 2000;98(3):163–175. doi: 10.1016/s0925-4927(00)00044-5. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Stanley JA, Pettegrew JW. Magnetic resonance spectroscopy in schizophrenia: methodological issues and findings--part II. Biological Psychiatry. 2000;48(5):369–380. doi: 10.1016/s0006-3223(00)00940-9. [DOI] [PubMed] [Google Scholar]
- Klar AA, Ballmaier M, Leopold K, Hake I, Schaefer M, Bruhl R. Interaction of hippocampal volume and N-acetylaspartate concentration deficits in schizophrenia: a combined MRI and 1H-MRS study. Neuroimage. 2010;53(1):51–57. doi: 10.1016/j.neuroimage.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magnetic Resonance in Medicine. 1993;30(4):424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. British Medical Journal. 2006;333(7568):597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373(9659):234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Adalsteinsson E, Spielman D, Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Proton magnetic resonance spectroscopic imaging of cortical gray and white matter in schizophrenia. Archives of General Psychiatry. 1998;55(4):346–352. doi: 10.1001/archpsyc.55.4.346. [DOI] [PubMed] [Google Scholar]
- Lutkenhoff ES, van Erp TG, Thomas MA, Therman S, Manninen M, Huttunen MO, Kaprio J, Lonngvist J, O'Neill J, Cannon TD. Proton MRS in twin pairs discordant for schizophrenia. Molecular Psychiatry. 2010;15(3):308–318. doi: 10.1038/mp.2008.87. [DOI] [PubMed] [Google Scholar]
- Maier M, Ron MA, Barker GJ, Tofts PS. Proton magnetic resonance spectroscopy: an in vivo method of estimating hippocampal neuronal depletion in schizophrenia. Psychological Medicine. 1995;25(6):1201–1209. doi: 10.1017/s0033291700033171. [DOI] [PubMed] [Google Scholar]
- Maier M, Ron MA. Hippocampal age-related changes in schizophrenia: a proton magnetic resonance spectroscopy study. Schizophrenia Research. 1996;22(1):5–17. doi: 10.1016/0920-9964(96)00044-8. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Ivanovski B, Wen W, Lagopoulos J, Moss K, Sachdev P. Measuring mania metabolites: a longitudinal proton spectroscopy study of hypomania. Acta Psychiatrica Scandinavia Supplement. 2007;434:57–66. doi: 10.1111/j.1600-0447.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- Martinez-Granados B, Brotons O, Martinez-Bisbal MC, Celda B, Marti-Bonmati L, Aguilar EJ, Gonzalez JC, Sanjuan J. Spectroscopic metabolomic abnormalities in the thalamus related to auditory hallucinations in patients with schizophrenia. Schizophrenia Research. 2008;104(1–3):13–22. doi: 10.1016/j.schres.2008.05.025. [DOI] [PubMed] [Google Scholar]
- McLoughlin GA, Ma D, Tsang TM, Jones DN, Cilia J, Hill MD, Robbins MJ, Benzel IM, Maycox PR, Holmes E, Bahn S. Analyzing the effects of psychotropic drugs on metabolite profiles in rat brain using 1H NMR spectroscopy. Journal of Proteome Research. 2009;8(4):1943–1952. doi: 10.1021/pr800892u. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, MacKay S, Bachman L, Poole N, Dillon WP, Weiner MW, Fein G. Reduced brain N-acetylaspartate suggests neuronal loss in cognitively impaired human immunodeficiency virus-seropositive individuals: in vivo 1H magnetic resonance spectroscopic imaging. Neurology. 1993;43(3 Pt 1):509–515. doi: 10.1212/wnl.43.3_part_1.509. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Ohrmann P, Gossling M, Arolt V, Heindel W, Pfleiderer B. Acute mania is accompanied by elevated glutamate/glutamine levels within the left dorsolateral prefrontal cortex. Psychopharmacology (Berl) 2003;168(3):344–346. doi: 10.1007/s00213-003-1440-z. [DOI] [PubMed] [Google Scholar]
- Michael N, Erfurth A, Pfleiderer B. Elevated metabolites within dorsolateral prefrontal cortex in rapid cycling bipolar disorder. Psychiatry Research. 2009;172(1):78–81. doi: 10.1016/j.pscychresns.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Miller BL. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR in biomedicine. 1991;4(2):47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Molina V, Sanchez J, Reig S, Sanz J, Benito C, Santamarta C, Pascau J, Sarramea F, Gispert JD, Misiego JM, Palomo T, Desco M. N-acetyl-aspartate levels in the dorsolateral prefrontal cortex in the early years of schizophrenia are inversely related to disease duration. Schizophrenia Research. 2005;73(2–3):209–219. doi: 10.1016/j.schres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Molina V, Sanz J, Sarramea F, Luque R, Benito C, Palomo T. No association between dorsolateral prefrontal gray matter deficit and N-acetyl aspartate ratios in schizophrenia. Neuropsychobiology. 2006;54(3):171–178. doi: 10.1159/000098653. [DOI] [PubMed] [Google Scholar]
- Molina V, Sanchez J, Sanz J, Reig S, Benito C, Leal I, Sarramea F, Rebolledo R, Palomo T, Desco M. Dorsolateral prefrontal N-acetyl-aspartate concentration in male patients with chronic schizophrenia and with chronic bipolar disorder. European Psychiatry. 2007;22(8):505–512. doi: 10.1016/j.eurpsy.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Moore CM, Breeze JL, Gruber SA, Babb SM, Frederick BB, Villafuerte RA, Stoll AL, Hennen J, Yurgelun-Todd DA, Cohen BM, Renshaw PF. Choline, myo-inositol and mood in bipolar disorder: a proton magnetic resonance spectroscopic imaging study of the anterior cingulate cortex. Bipolar Disorders. 2000;2(3 Pt 2):207–216. doi: 10.1034/j.1399-5618.2000.20302.x. [DOI] [PubMed] [Google Scholar]
- Moore CM, Bonello CM, Sherwood AR, Cohen BM, Renshaw PF, Yurgulen-Todd DA. Mesial temporal lobe Cho to Cr(PCr) ratio asymmetry in chronic schizophrenics. Schizophrenia Research. 2002;57(1):35–42. doi: 10.1016/s0920-9964(01)00302-4. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Hasanat K, Chen G, Seraji-Bozorgzad N, Wilds IB, Faulk MW, Koch S, Glitz DA, Jolkovsky L, Manji HK. Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2's neurotrophic effects? Biological Psychiatry. 2000;48(1):1–8. doi: 10.1016/s0006-3223(00)00252-3. [DOI] [PubMed] [Google Scholar]
- Nasrallah HA, Skinner TE, Schmalbrock P, Robitaille PM. Proton magnetic resonance spectroscopy (1H MRS) of the hippocampal formation in schizophrenia: a pilot study. British Journal of Psychiatry. 1994;165(4):481–485. doi: 10.1192/bjp.165.4.481. [DOI] [PubMed] [Google Scholar]
- O'Neill J, Levitt J, Caplan R, Asarnow R, McCracken JT, Toga AW, Alger JR. 1H MRSI evidence of metabolic abnormalities in childhood-onset schizophrenia. Neuroimage. 2004;21(4):1781–1789. doi: 10.1016/j.neuroimage.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Ohara K, Isoda H, Suzuki Y, Takehara Y, Ochiai M, Takeda H, Igarashi Y, Ohara K. Proton magnetic resonance spectroscopy of the lenticular nuclei in bipolar I affective disorder. Psychiatry Research. 1998;84(2–3):55–60. doi: 10.1016/s0925-4927(98)00050-x. [DOI] [PubMed] [Google Scholar]
- Ohara K, Isoda H, Suzuki Y, Takehara Y, Ochiai M, Takeda H, Hattori K, Igarashi Y, Ohara K. Proton magnetic resonance spectroscopy of lenticular nuclei in simple schizophrenia. Progress in Neuropsychopharmacology and Biological Psychiatry. 2000;24(4):507–519. doi: 10.1016/s0278-5846(00)00089-0. [DOI] [PubMed] [Google Scholar]
- Ohrmann P, Siegmund A, Suslow T, Spitzberg K, Kersting A, Arolt V, Heindel W, Pleiderer B. Evidence for glutamatergic neuronal dysfunction in the prefrontal cortex in chronic but not in first-episode patients with schizophrenia: a proton magnetic resonance spectroscopy study. Schizophrenia Research. 2005;73(2–3):153–157. doi: 10.1016/j.schres.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Ohrmann P, Siegmund A, Suslow T, Pedersen A, Spitzberg K, Kersting A. Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy study. Journal of Psychiatric Research. 2007;41(8):625–634. doi: 10.1016/j.jpsychires.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Ohrmann P, Kugel H, Bauer J, Siegmund A, Kolkebeck K, Suslow T, Wiedl KH, Rothermundt M, Arolt V, Pedersen A. Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophrenia Research. 2008;106(2–3):156–163. doi: 10.1016/j.schres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Rusch N, Buchert M, Thiel T, Hennig J, Ebert D, Van Elst LT. Frontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy study. World Journal of Biological Psychiatry. 2008;9(1):59–63. doi: 10.1080/15622970701227811. [DOI] [PubMed] [Google Scholar]
- Omori M, Murata T, Kimura H, Koshimoto Y, Kado H, Ishimori Y, Ito H, Wada Y. Thalamic abnormalities in patients with schizophrenia revealed by proton magnetic resonance spectroscopy. Psychiatry Research. 2000;98(3):155–162. doi: 10.1016/s0925-4927(00)00049-4. [DOI] [PubMed] [Google Scholar]
- Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biological Psychiatry. 2008;64(8):718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, Jensen JE, Cohen BM, Renshaw PF. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Research. 2009;172(1):44–48. doi: 10.1016/j.pscychresns.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, Jensen JE, Rouse ED, Cohen BM, Renshaw PF. T2 relaxation time abnormalities in bipolar disorder and schizophrenia. Magnetic Resonance in Medicine. 2010a;63(1):1–8. doi: 10.1002/mrm.22148. [DOI] [PubMed] [Google Scholar]
- Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biological Psychiatry. 2010b;68(7):667–670. doi: 10.1016/j.biopsych.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pae CU, Choe BY, Joo RH, Lim HK, Kim TS, Yoo SS, Choi BG, Kim JJ, Lee SJ, Lee C, Paik IH, Lee CU. Neuronal dysfunction of the frontal lobe in schizophrenia. Neuropsychobiology. 2004;50(3):211–215. doi: 10.1159/000079972. [DOI] [PubMed] [Google Scholar]
- Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, Kierer A, Mueller S, Oest M, Meyer T, Backens M, Schneider-Axmann T, Thornton AE, Honer WG, Falkai P. Hippocampal plasticity in response to exercise in schizophrenia. Archives of General Psychiatry. 2010;67(2):133–143. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- Port JD, Unal SS, Mrazek DA, Marcus SM. Metabolic alterations in medication-free patients with bipolar disorder: a 3T CSF-corrected magnetic resonance spectroscopic imaging study. Psychiatry Research. 2008;162(2):113–121. doi: 10.1016/j.pscychresns.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Premkumar P, Parbhakar VA, Fannon D, Lythgoe D, Williams SC, Kuipers E, Kumari V. N-acetyl aspartate concentration in the anterior cingulate cortex in patients with schizophrenia: a study of clinical and neuropsychological correlates and preliminary exploration of cognitive behaviour therapy effects. Psychiatry Research. 2010;182(3):251–260. doi: 10.1016/j.pscychresns.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels PJ, Frahm J. Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR in biomedicine. 1997;10(2):73–78. doi: 10.1002/(sici)1099-1492(199704)10:2<73::aid-nbm448>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MA, Stoeckel LE, White DM, Avsar KB, Bolding MS, Akella NS, Knowlton RC, den Hollander JA, Lahti AC. Assessments of function and biochemistry of the anterior cingulate cortex in schizophrenia. Biological Psychiatry. 2010;68(7):625–633. doi: 10.1016/j.biopsych.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland LM, Spieker EA, Francis A, Barker PB, Carpenter WT, Buchanan RW. White matter alterations in deficit schizophrenia. Neuropsychopharmacology. 2009;34(6):1514–1522. doi: 10.1038/npp.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch N, Tebartz van Elst L, Valerius G, Buchert M, Thiel T, Ebert D, Henning J, Olbrich HM. Neurochemical and structural correlates of executive dysfunction in schizophrenia. Schizophrenia Research. 2008;99(1–3):155–163. doi: 10.1016/j.schres.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Sager TN, Topp S, Torup L, Hanson LG, Egestad B, Moller A. Evaluation of CA1 damage using single-voxel 1H-MRS and un-biased stereology: Can non-invasive measures of N-acetyl-asparate following global ischemia be used as a reliable measure of neuronal damage? Brain Research. 2001;892(1):166–175. doi: 10.1016/s0006-8993(00)03274-1. [DOI] [PubMed] [Google Scholar]
- Sanches RF, Crippa JA, Hallak JE, de Sousa JP, Araujo D, Santos AC, Zuardi AW. Proton magnetic resonance spectroscopy of the frontal, cingulate and perirolandic cortices and its relationship to skin conductance in patients with schizophrenia. Brazilian Journal of Medical and Biological Research. 2008;41(12):1132–1141. doi: 10.1590/s0100-879x2008001200015. [DOI] [PubMed] [Google Scholar]
- Sarramea F, Luque R, Prieto D, Sau P, Albert C, Leal I. Biochemical changes in the cingulum in patients with schizophrenia and chronic bipolar disorder. European Archives of Psychiatry and Clinical Neuroscience. 2008;258(7):394–401. doi: 10.1007/s00406-008-0808-9. [DOI] [PubMed] [Google Scholar]
- Scherk H, Backens M, Schneider-Axmann T, Kemmer C, Usher J, Reith W, Falkai P, Gruber O. Neurochemical pathology in hippocampus in euthymic patients with bipolar I disorder. Acta Psychiatrica Scandinavia. 2008;117(4):283–288. doi: 10.1111/j.1600-0447.2007.01142.x. [DOI] [PubMed] [Google Scholar]
- Scherk H, Backens M, Schneider-Axmann T, Kraft S, Kemmer C, Usher J, Reith W, Falkai P, Meyer J, Gruber O. Dopamine transporter genotype influences N-acetyl-aspartate in the left putamen. World Journal of Biological Psychiatry. 2009a;10(4 Pt 2):524–530. doi: 10.1080/15622970701586349. [DOI] [PubMed] [Google Scholar]
- Scherk H, Backens M, Schneider-Axmann T, Usher J, Kemmer C, Reith W, Falkai P, Gruber O. Cortical neurochemistry in euthymic patients with bipolar I disorder. World Journal of Biological Psychiatry. 2009b;10(4):285–294. doi: 10.3109/15622970701472086. [DOI] [PubMed] [Google Scholar]
- Senaratne R, Milne AM, MacQueen GM, Hall GB. Increased choline-containing compounds in the orbitofrontal cortex and hippocampus in euthymic patients with bipolar disorder: a proton magnetic resonance spectroscopy study. Psychiatry Research. 2009;172(3):205–209. doi: 10.1016/j.pscychresns.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Senn SJ. Overstating the evidence: double counting in meta-analysis and related problems. BMC Medical Research Methodology. 2009;9:10. doi: 10.1186/1471-2288-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Venkatasubramanian PN, Barany M, Davis JM. Proton magnetic resonance spectroscopy of the brain in schizophrenic and affective patients. Schizophrenia Research. 1992;8(1):43–49. doi: 10.1016/0920-9964(92)90059-e. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Ochi S, Fukami G, Fujisaki M, Koike K, Okamura N, Ohgake S, Koizumi H, Matsuzawa D, Zhang L, Watanabe H, Nakazato M, Shinoda N, Komatsu N, Morita F, Iyo M. Posterior cingulate gyrus metabolic changes in chronic schizophrenia with generalized cognitive deficits. Journal of Psychiatric Research. 2007;41(1–2):49–56. doi: 10.1016/j.jpsychires.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Shioiri T, Hamakawa H, Kato T, Murashita J, Fujii K, Inubushi T, Takahasi S. Proton magnetic resonance spectroscopy of the basal ganglia in patients with schizophrenia: a preliminary report. Schizophrenia Research. 1996;22(1):19–26. doi: 10.1016/0920-9964(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Obata T, Matsuzawa D, Nonaka H, Kanazawa Y, Yoshitome E, Ikehira H, Hashimoto K, Iyo M. Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: a preliminary study. Neuroimage. 2010;49(3):2783–2790. doi: 10.1016/j.neuroimage.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Maier M, Toone BK, Williams SC, Simmons A, Greenwood K, Ron MA. Frontal lobe N-acetylaspartate correlates with psychopathology in schizophrenia: a proton magnetic resonance spectroscopy study. Schizophrenia Research. 2003;64(1):63–71. doi: 10.1016/s0920-9964(02)00533-9. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Wu RH, O'Donnell T, Ulrich M, Asghar SJ, Hanstock CC. Chronic treatment with both lithium and sodium valproate may normalize phosphoinositol cycle activity in bipolar patients. Human Psychopharmacology. 2002;17(7):321–327. doi: 10.1002/hup.420. [DOI] [PubMed] [Google Scholar]
- Silverstone PH, Wu RH, O'Donnell T, Ulrich M, Asghar SJ, Hanstock CC. Chronic treatment with lithium, but not sodium valproate, increases cortical N-acetylaspartate concentrations in euthymic bipolar patients. International Clinical Psychopharmacology. 2003;18(2):73–79. doi: 10.1097/00004850-200303000-00002. [DOI] [PubMed] [Google Scholar]
- Stanley JA, Williamson PC, Drost DJ, Rylett RJ, Carr TJ, Malla A, Thompson RT. An in vivo proton magnetic resonance spectroscopy study of schizophrenia patients. Schizophrenia Bulletin. 1996;22(4):597–609. doi: 10.1093/schbul/22.4.597. [DOI] [PubMed] [Google Scholar]
- Steel RM, Bastin ME, McConnell S, Marshall I, Cunningham-Owens DG, Lawrie SM, Johnstone EC, Best JJ. Diffusion tensor imaging (DTI) and proton magnetic resonance spectroscopy (1H MRS) in schizophrenic subjects and normal controls. Psychiatry Research. 2001;106(3):161–170. doi: 10.1016/s0925-4927(01)00080-4. [DOI] [PubMed] [Google Scholar]
- Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30(11):1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- Szulc A, Galinska B, Tarasow E, Dzienis W, Kubas B, Konarzewska B, Walecki J, Alathiaki AS, Czernikiewicz A. The effect of risperidone on metabolite measures in the frontal lobe, temporal lobe, and thalamus in schizophrenic patients. A proton magnetic resonance spectroscopy (1H MRS) 2005;38(5):214–219. doi: 10.1055/s-2005-873156. [DOI] [PubMed] [Google Scholar]
- Szulc A, Galinska B, Tarasow E, Kubas B, Dzienis W, Konarzewska B, Poplawska R, Bibulowicz D, Simonieko K, Walecki J. N-acetylaspartate (NAA) levels in selected areas of the brain in patients with chronic schizophrenia treated with typical and atypical neuroleptics: a proton magnetic resonance spectroscopy (1H MRS) study. Medical Science Monitor. 2007;13(Supplement 1):17–22. [PubMed] [Google Scholar]
- Tanaka Y, Obata T, Sassa T, Yoshitome E, Asai Y, Ikehira H, Suhara T, Okubo Y, Nishikawa T. Quantitative magnetic resonance spectroscopy of schizophrenia: relationship between decreased N-acetylaspartate and frontal lobe dysfunction. Psychiatry and Clinical Neurosciences. 2006;60(3):365–372. doi: 10.1111/j.1440-1819.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- Tang CY, Friedman J, Shungu D, Chang L, Ernst T, Stewart D, Hajianpour A, Carpenter D, Ng J, Mao X, Hof PR, Buchsbaum MS, Davis K, Gorman JM. Correlations between Diffusion Tensor Imaging (DTI) and Magnetic Resonance Spectroscopy (1H MRS) in schizophrenic patients and normal controls. BMC Psychiatry. 2007;7:25. doi: 10.1186/1471-244X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayoshi S, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, Iga J, Nakataki M, Ueno S, Harada M, Omori T. Metabolite changes and gender differences in schizophrenia using 3-Tesla proton magnetic resonance spectroscopy (1H-MRS) Schizophrenia Research. 2009;108(1–3):69–77. doi: 10.1016/j.schres.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Theberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, Neufeld RW, Rogers J, Pavlosky W, Schaefer B, Densmore M, Al-Semaan Y, Williamson PC. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. American Journal of Psychiatry. 2002;159(11):1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- Theberge J, Al-Semaan Y, Williamson PC, Menon RS, Neufeld RW, Rajakumar N, Schaefer B, Densmore M, Drost DJ. Glutamate and glutamine in the anterior cingulate and thalamus of medicated patients with chronic schizophrenia and healthy comparison subjects measured with 4.0-T proton MRS. American Journal of Psychiatry. 2003;160(12):2231–2233. doi: 10.1176/appi.ajp.160.12.2231. [DOI] [PubMed] [Google Scholar]
- Theberge J, Al-Semaan Y, Drost DJ, Malla AK, Neufeld RW, Bartha R, Manchanda R, Mennon R, Densmore M, Schaefer B, Williamson PC. Duration of untreated psychosis vs. N-acetylaspartate and choline in first episode schizophrenia: a 1H magnetic resonance spectroscopy study at 4.0 Tesla. Psychiatry Research. 2004a;131(2):107–114. doi: 10.1016/j.pscychresns.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Theberge J, Al-Semaan Y, Jensen JE, Williamson PC, Neufeld RW, Menon RS, Schaefer B, Densmore M, Drost DJ. Comparative study of proton and phosphorus magnetic resonance spectroscopy in schizophrenia at 4 Tesla. Psychiatry Research. 2004b;132(1):33–39. doi: 10.1016/j.pscychresns.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Theberge J, Williamson KE, Aoyama N, Drost DJ, Manchanda R, Malla AK, Northcott S, Menon RS, Neufeld RW, Rajakumar N, Pavlosky W, Densmore M, Schaefer B, Williamson PC. Longitudinal grey-matter and glutamatergic losses in first-episode schizophrenia. British Journal of Psychiatry. 2007;191:325–334. doi: 10.1192/bjp.bp.106.033670. [DOI] [PubMed] [Google Scholar]
- Tibbo P, Hanstock CC, Asghar S, Silverstone P, Allen PS. Proton magnetic resonance spectroscopy (1H-MRS) of the cerebellum in men with schizophrenia. Journal of Psychiatry and Neuroscience. 2000;25(5):509–512. [PMC free article] [PubMed] [Google Scholar]
- Tunc-Skarka N, Weber-Fahr W, Hoerst M, Meyer-Lindenberg A, Zink M, Ende G. MR spectroscopic evaluation of N-acetylaspartate's T2 relaxation time and concentration corroborates white matter abnormalities in schizophrenia. Neuroimage. 2009;48(3):525–531. doi: 10.1016/j.neuroimage.2009.06.061. [DOI] [PubMed] [Google Scholar]
- van Elst LT, Valerius G, Buchert M, Thiel T, Rusch N, Bubl E, Hennig J, Ebert D, Olbrich HM. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biological Psychiatry. 2005;58(9):724–730. doi: 10.1016/j.biopsych.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Van Snellenberg JX, de Candia T. Meta-analytic evidence for familial coaggregation of schizophrenia and bipolar disorder. Archives of General Psychiatry. 2009;66(7):748–755. doi: 10.1001/archgenpsychiatry.2009.64. [DOI] [PubMed] [Google Scholar]
- Waddell KW, Zanjanipour P, Pradhan S, Xu L, Welch EB, Joers JM, Martin PR, Avison MJ, Gore JC. Anterior cingulate and cerebellar GABA and Glu correlations measured by (1)H J-difference spectroscopy. Magnetic Resonance Imaging. 2011;29(1):19–24. doi: 10.1016/j.mri.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Fahr W, Ende G, Braus DF, Bachert P, Soher BJ, Henn FA, Buchel C. A fully automated method for tissue segmentation and CSF-correction of proton MRSI metabolites corroborates abnormal hippocampal NAA in schizophrenia. Neuroimage. 2002;16(1):49–60. doi: 10.1006/nimg.2002.1057. [DOI] [PubMed] [Google Scholar]
- Winsberg ME, Sachs N, Tate DL, Adalsteinsson E, Spielman D, Ketter TA. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biological Psychiatry. 2000;47(6):475–481. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- Wobrock T, Kamer T, Roy A, Vogeley K, Schneider-Axmann T, Wagner M, Maier W, Rietschel M, Schulze TG, Scherk H, Schild HH, Block W, Traber F, Tepest R, Honer WG, Falkai P. Reduction of the internal capsule in families affected with schizophrenia. Biological Psychiatry. 2008;63(1):65–71. doi: 10.1016/j.biopsych.2007.02.026. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger G, Velakoulis D, Phillips LJ, McGorry PD, Yung AR, Desmon P, Pantelis C. Proton magnetic resonance spectroscopy in first episode psychosis and ultra high-risk individuals. Schizophrenia Bulletin. 2003;29(4):831–843. doi: 10.1093/oxfordjournals.schbul.a007049. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Yucel M, Wellard RM, Harrison BJ, Clarke K, Fornito A, Velakoulis D, Pantelis C. Evidence for neuronal dysfunction in the anterior cingulate of patients with schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. Schizophrenia Research. 2007;94(1–3):328–331. doi: 10.1016/j.schres.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Wellard RM, Proffitt T, McConchie M, Velakoulis D. A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naive and early-treated first episode psychosis. Schizophrenia Research. 2008;102(1–3):163–170. doi: 10.1016/j.schres.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Berger GE, Wellard RM, Proffitt TM, McConchie M, Berk M, McGorry PD, Pantelis C. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiology of Disease. 2009;33(3):354–357. doi: 10.1016/j.nbd.2008.11.018. [DOI] [PubMed] [Google Scholar]
- Wu RH, O'Donnell T, Ulrich M, Asghar SJ, Hanstock CC, Silverstone PH. Brain choline concentrations may not be altered in euthymic bipolar disorder patients chronically treated with either lithium or sodium valproate. Annals of General Hospital Psychiatry. 2004;3(1):13. doi: 10.1186/1475-2832-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H, Fukui T, Fukuda R, Kasai K, Iwanami A, Kato N, Kato T. Drug-induced parkinsonism in relation to choline-containing compounds measured by 1H-MR spectroscopy in putamen of chronically medicated patients with schizophrenia. International Journal of Neuropsychopharmacology. 2003;6(4):353–360. doi: 10.1017/S1461145703003687. [DOI] [PubMed] [Google Scholar]
- Yildiz-Yesiloglu A, Ankerst DP. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Progress in Neuropsychopharmacology and Biol Psychiatry. 2006;30(6):969–995. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Yuksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biological Psychiatry. 2010;68(9):785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Renshaw PF, Gruber SA, Ed M, Waternaux C, Cohen BM. Proton magnetic resonance spectroscopy of the temporal lobes in schizophrenics and normal controls. Schizophrenia Research. 1996;19(1):55–59. doi: 10.1016/0920-9964(95)00071-2. [DOI] [PubMed] [Google Scholar]