Abstract
APC/β-catenin pathway perturbation is a common early event in colorectal carcinogenesis and is affected by calcium and vitamin D in basic science studies. To assess the effects of calcium and vitamin D on APC, β-catenin, and E-cadherin expression in the normal appearing colorectal mucosa of sporadic colorectal adenoma patients, we conducted a randomized, double-blinded, placebo-controlled 2×2 factorial clinical trial. Pathology-confirmed colorectal adenoma cases were treated with 2 g/day elemental calcium and/or 800 IU/day vitamin D3 versus placebo over 6 months (N=92; 23/group). Overall APC, β-catenin, and E-cadherin expression and distributions in colon crypts in normal-appearing rectal mucosa biopsies were detected by standardized automated immunohistochemistry and quantified by image analysis. In the vitamin D3-supplemented group relative to placebo, the proportion of APC in the upper 40% of crypts (ϕh APC) increased 21% (p=0.01), β-catenin decreased 12% (p=0.18), E-cadherin increased 72% (p=0.03), and the ϕh APC/β-catenin ratio (APC/β-catenin score) increased 31% (p=0.02). In the calcium-supplemented group ϕh APC increased 10% (p=0.12), β-catenin decreased 15% (p=0.08), and the APC/β-catenin score increased 41% (p=0.01). In the calcium/vitamin D3 supplemented group β-catenin decreased 11% (p=0.20), E-cadherin increased 51% (p=0.08), and the APC/β-catenin score increased 16% (p=0.26). These results support 1) that calcium and vitamin D modify APC, β-catenin, and E-cadherin expression in humans in directions hypothesized to reduce risk for colorectal neoplasms, 2) calcium and vitamin D as potential chemopreventive agents against colorectal neoplasms, and 3) the potential of APC, β-catenin, and E-cadherin expression as modifiable, pre-neoplastic risk biomarkers for colorectal neoplasms.
Keywords: calcium, vitamin D, colonic neoplasms, biomarkers, clinical trial
Introduction
Colorectal cancer (CRC), the second leading cause of cancer deaths in the United States (1), is responsible for approximately 8% of all cancer deaths worldwide (2, 3). The etiology of sporadic CRC is predominately rooted in dietary and lifestyle behaviors (2, 4), suggesting that it may be preventable. The molecular basis of colorectal carcinogenesis is becoming clearer (4); however, there are no validated, treatable, pre-neoplastic biomarkers of risk for colorectal neoplasms.
Malfunction of the APC/β-catenin signaling pathway is an early and common event in the pathogenesis of colorectal neoplasms. Impaired APC function occurs in approximately 80-90% of sporadic CRCs(5), resulting in the increased potential of β-catenin to translocate to the nucleus and activate target genes responsible for promoting cell proliferation and inhibiting differentiation (5-7). E-cadherin may also antagonize β-catenin nuclear expression by sequestering β-catenin to its cytoplasmic tail, linking E-cadherin to actin filaments and promoting cell adhesion and differentiation (5, 7). We reported that APC expression (especially the proportion of APC in the upper 40% of colorectal crypts (ϕh APC)), β-catenin expression, and the ϕh APC/β-catenin ratio (APC/β-catenin score) in normal colorectal mucosa may be valid, potentially modifiable, pre-neoplastic biomarkers of risk for colorectal neoplasms (8).
Convincing evidence from experimental and observational studies and randomized, placebo-controlled clinical trials suggests that calcium and vitamin D have chemopreventive effects against colorectal neoplasms (9). The beneficial effects of calcium may partially be attributed to its binding of toxic secondary bile acids and ionized fatty acids, and/or by directly inhibiting proliferation and promoting differentiation and apoptosis (9). Vitamin D signaling may induce cell-cycle arrest and promote differentiation and apoptosis directly through vitamin D-mediated gene transcription and indirectly through modifying growth factors, and play roles in promoting oxidative DNA damage repair, inhibiting angiogenesis, and regulating immune cell function (9). Also, prospective cohort studies have consistently found higher total calcium intake to be associated with lower risk for colorectal neoplasms (9), calcium supplementation reduces colorectal adenoma recurrence (modified by vitamin D status) (10), and higher circulating 25(OH)-vitamin D (25(OH)D) is inversely associated with colorectal neoplasms (9, 11, 12).
Evidence from animal models (13, 14) and in vitro (15-17) studies suggest that the chemopreventive effects of calcium and vitamin D may, in part, include modification of the APC/β-catenin signaling pathway. However, to our knowledge there are no reported human in vivo investigations on the effects of supplemental calcium and vitamin D3 on the expression of APC, β-catenin, and E-cadherin in the normal colorectal mucosa. To address this, as reported herein, we conducted a pilot, randomized, double-blind, placebo-controlled 2 × 2 factorial chemoprevention clinical trial of supplemental calcium and vitamin D3, alone and in combination, versus placebo over 6 months, to estimate the efficacy of these agents on APC, β-catenin, and E-cadherin expression in the normal colorectal mucosa.
Study Participants and Methods
Participant population
A detailed description of the study protocol for recruitment procedures and detailed specific exclusions was published previously (18). Briefly, eligible participants were 30 to 75 years of age, in general good health, and had a history of at least one pathology-confirmed adenomatous colorectal polyp within the past 36 months. Exclusions from participation included contraindications to calcium or vitamin D supplementation or rectal biopsy procedures, and medical conditions, habits, or medication usage that potentially could interfere with the study. Participants were recruited from patients attending the Digestive Diseases Clinic of the Emory Clinic, Emory University.
Clinical trial protocol
Between April 2005 and January 2006, 522 potentially eligible patients were identified through initial screening of electronic medical records; of these, 244 (43%) were contacted, and of these 105 (47%) attended the eligibility visit to be interviewed, sign a consent form, complete questionnaires, and provide blood samples (18). Diet was assessed using a semi-quantitative Willett Food Frequency Questionnaire (19). Medical and pathology records were reviewed. Following a 30-day placebo run-in trial, 92 (88%) participants with no significant perceived side effects and who took at least 80% of their assigned tablets underwent a baseline rectal biopsy were randomly assigned, stratified on sex and nonsteroidal anti-inflammatory drug (NSAID) use, to the following four treatment groups: placebo (n = 23), 2.0 g elemental calcium supplementation (as calcium carbonate in equal doses twice daily; n = 23), 800 IU vitamin D3 supplementation (400 IU twice daily; n = 23), and 2.0 g elemental calcium plus 800 IU vitamin D3 supplementation (n = 23). Additional details and rationale for the doses and forms of calcium and vitamin D3 supplements were previously published (18). Participants were instructed to maintain their usual diet and not take any new nutritional supplements they were not taking at the time of entry into the study. All aspects of the trial were approved by the Institutional Review Board of Emory University.
During the 6-month treatment period, participants attended follow-up visits 2 and 6 months after randomization. At follow-up visits participants completed questionnaires and were interviewed about adherence and adverse events. At the 6-month follow-up, participants again underwent a venipuncture and rectal biopsy. All visits for a given participant were scheduled for the same time of day to control for potential circadian variation. Dietary, lifestyle, and other factors hypothesized to modify biomarker expression in normal colon mucosa were assessed at baseline and at 6-months follow-up. Participants were asked to abstain from aspirin use seven days prior to each biopsy. Participants were not required to be fasting for their visits and did not take a bowel cleansing preparation or enema.
Six approximately one millimeter thick biopsy specimens were taken from the normal-appearing rectal mucosa 10 cm above the level of the external anal aperture through a short rigid sigmoidoscope using a jumbo cup flexible biopsy forceps mounted on a semi-rigid rod. No biopsies were taken within 4.0 cm of a polypoid lesion. Biopsies were placed onto a strip of bibulous paper and immediately placed in phosphate buffered saline (PBS), oriented, transferred to 10% normal buffered formalin for 24 hours, and then transferred to 70% ethanol. Then, within a week, the biopsies were processed and embedded in paraffin blocks (2 blocks of 3 biopsies per participant, per biopsy visit).
Immunohistochemistry protocol
Five slides with 4 levels of 3 micron-thick biopsy sections taken 40 microns apart were prepared for each antigen, yielding a total of 20 levels for each antigen. Antigen retrieval was performed by placing the slides in a preheated Pretreatment Module (Lab Vision Corp.) with 100x Citrate Buffer (pH 6.0; DAKO S1699, DAKO Corp.) and steaming them for 40 min. Following antigen retrieval, slides were immunohistochemically processed in a DAKO Automated Immunostainer (DAKO Corp.) using a labeled streptavidin-biotin method for APC (Calbiochem, OP80; 1:70 dilution), β-catenin (Transduction Laboratories 610154; 1:300 dilution), and E-cadherin (Zymed 33-4000; 1:50 dilution). No slides were counterstained. After processing, slides were coverslipped with a Leica CV5000 Coverslipper (Leica Microsystems, Inc.). The negative and positive control slides were treated identically to the patients’ slides except that antibody diluent was used rather than primary antibody on the negative slides.
Protocol for quantifying labeling densities of immunohistochemically detected biomarkers in normal colon crypts (“scoring”)
A detailed description of the protocol used to quantify biomarker labeling optical densities (“biomarker expression”) in normal colon crypts was previously described (18). Briefly, a scorable crypt was defined as an intact crypt extending from the muscularis mucosa to the colon lumen (20). Prior to “scoring”, the negative and positive control slides were checked for staining adequacy. The major equipment and software for the image analysis procedures included: personal computer, light microscope (Olympus BX40, Olympus Corporation, Japan) with appropriate filters and attached digital light microscope camera (Polaroid DMC Digital Light Microscope Camera, Polaroid Corporation, USA), digital drawing board, ImagePro Plus image analysis software (Media Cybernetics, Inc., MD), our in-house developed plug-in software for colorectal crypt analysis, and Microsoft Access 2003 relational database software (Microsoft Corporation, WA).
Evaluation of biomarker expression consisted of the same technician cleaning all slides, selecting the two of the three biopsies with the most scorable crypts per biopsy, creating background correction images for each slide scored, capturing 16-bit grayscale images of crypts at 200x magnification, and tracing the border of the “hemicrypt” (one half of the crypt). The program then divided the outlined hemicrypt into equally spaced segments that corresponded to the average width of colonocytes, and measured the optical density of the labeling across the entire hemicrypt and within each segment, adjusting for the background. The technician then repeated this process for the adjacent hemicrypt, and proceeded to the next crypt, level, biopsy, and/or slide. The goal was to score 16 to 20 hemicrypts per biopsy visit for each biomarker (Figure 1).
Figure 1.
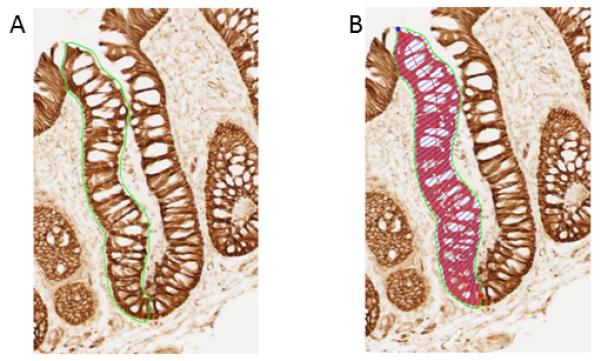
Quantitative image analysis. A, finding and tracing the hemicrypt; B, automated sectioning and quantification of β-catenin labeling optical density (figure adapted from reference 8).
Reliability control was performed by selecting samples of previously analyzed slides to be re-analyzed by the technician, who was blinded to the selection. Intra-reader reliability was greater than 0.90 for APC, β-catenin, and E-cadherin.
Protocol for measuring serum vitamin D levels
Serum 25-OH-vitamin D and 1,25-(OH)2-vitamin D were measured by Dr. Bruce W. Hollis at the Medical University of South Carolina using a RIA method as previously described (21, 22). Serum samples for baseline and follow-up visits for all subjects were assayed together, ordered randomly, and labeled to mask treatment group, follow-up visit, and quality control replicates. The average intra-assay coefficient of variation was 2.3% and 6.2% for serum 25-OH-vitamin D and for 1,25-(OH)2-vitamin D, respectively.
Statistical analysis
All statistical analyses were performed using SAS 9.3 statistical software (SAS Institute Inc.). A P value ≤ 0.05 (two-sided) was considered statistically significant. Treatment groups were assessed for comparability of characteristics at baseline and at final follow-up by the Fisher’s exact test for categorical variables and analysis of variance (ANOVA) for continuous variables. Slide scoring reliability was analyzed using intra-class correlation coefficients.
The mean labeling optical density expression of each biomarker on each study participant, at baseline and 6-month follow-up, was calculated by summing the biomarker’s expression for all analyzed crypts and dividing by the total number of analyzed crypts. Biomarker expression was transformed to adjust for possible staining batch effects by dividing an individual’s mean biomarker expression by their batch mean biomarker expression (18). To evaluate distinct functional zones of crypts, measures of crypt biomarker distribution selected a priori were the upper 40% of the crypts (differentiation zone), the lower 60% of the crypts (proliferation zone), and the ratio of the upper 40% of crypts to the whole crypt to (ϕh). An APC/β-catenin score was calculated by dividing an individual’s ϕh APC by their β-catenin expression in the whole crypt (ϕh APC expression/β-catenin expression). E-cadherin was not included in the APC/β-catenin score because during carcinogenesis malfunctioning regulation of β-catenin by APC occurs most often earlier than E-cadherin down-regulation (23). We hypothesize that a higher score is associated with reduced potential of β-catenin to promote proliferation.
The distributions of batch standardized APC, β-catenin, and E-cadherin labeling optical densities along the full length of the crypts were graphically plotted and evaluated using the LOESS procedure. First, each hemicrypt was standardized to 50 sections. Then, the average of each section across all crypts was predicted by the LOESS model separately for each patient and then for each treatment group by visit. The results were graphically plotted along with the smoothing lines. Although the plots illustrate the distribution of expression, they do not provide a complete analysis of treatment effects because they do not account for changes in the placebo group.
Primary analyses were based on assigned treatment at the time of randomization, regardless of adherence status (intent-to-treat analysis). Treatment effects were evaluated by assessing the differences in the transformed biomarker expression from baseline to the 6-month follow-up between participants in the active treatment groups and those in the placebo group by a repeated-measures linear MIXED effects model. The model included the intercept, follow-up visit effects (baseline and follow-up), treatment groups, and interactions between treatment groups and the follow-up visit effect. Because optical density is measured in arbitrary units, to provide perspective on the magnitude of the treatment effects, we also calculated the relative effect. The relative effect was calculated as the (treatment group at follow-up/treatment group at baseline)/(placebo group at follow-up/placebo group at baseline). The relative effect provides a conservative estimate of the proportional change in the treatment group relative to that in the placebo group, and its interpretation is somewhat analogous to that of an odds ratio (e.g., a relative effect of 2.0 would mean that the proportional change in the treatment group was two times that in the placebo group).
Sensitivity analysis
To investigate the sensitivity of our analyses to missing data, we used multiple imputation to impute biomarker expression for study participants who did not have scorable crypts or were lost to follow-up. To create a monotone missing pattern, we used a Markov Chain Monte Carlo method to impute a value for the few observations that were missing on biomarkers measured at baseline, based on an assumption of multivariate normality (SAS, V 9.3, Proc MI). Once a monotone missing pattern was created, we used a regression approach to impute the remaining values(24). Age and sex were included as covariates for imputation to create six completed data sets that we then analyzed as previously described.
Results
Characteristics of study participants
Baseline characteristics of study participants did not significantly differ by treatment group (Table 1). The mean age of study participants was 61 years, 70% were men, 71% were white, and 20% had a family history of colorectal cancer in a first degree relative. Most participants were non-smokers, college graduates, and overweight.
Table 1. Selected baseline characteristics of the study participantsa (n=92).
| Characteristics | Treatment Group |
P-valueb | |||
|---|---|---|---|---|---|
| Placebo (n=23) |
Calcium (n=23) |
Vitamin D (n=23) |
Calcium + Vit. D (n=23) |
||
| Demographics, medical history, habits, anthropometrics | |||||
| Age, years | 58.5 (8.2) | 61.9 (8.2) | 60.2 (8.1) | 62.1 (7.5) | 0.39 |
| Men (%) | 70 | 70 | 70 | 70 | 1.00 |
| White (%) | 74 | 83 | 65 | 61 | 0.39 |
| College graduate (%) | 65 | 61 | 57 | 44 | 0.53 |
| History of colorectal cancer in 1° relative (%) | 17 | 30 | 17 | 13 | 0.60 |
| Take NSAIDc regularlyd (%) | 22 | 13 | 9 | 22 | 0.60 |
| If woman (n = 28), taking estrogens (%) | 4 | 9 | 4 | 4 | 1.00 |
| Current smoker (%) | 9 | 4 | 0 | 0 | 0.61 |
| Take multivitamin (%) | 30 | 30 | 26 | 39 | 0.86 |
| Body mass index (BMI), kg/m2 | 30.6 (7.2) | 29.4 (5.5) | 28.9 (5.56) | 31.6 (6.0) | 0.44 |
| Mean dietary intakes e | |||||
| Total energy intake, kcal/d | 1,596 (528) | 1,788 (691) | 1,848 (821) | 1,845 (752) | 0.59 |
| Totalf calcium, mg/d | 625 | 678 | 753 | 733 | 0.75 |
| Totalf vitamin D, IU/d | 279 | 326 | 348 | 401 | 0.50 |
| Total fat, gm/d | 66 | 66 | 61 | 65 | 0.59 |
| Dietary fiber, gm/d | 15 | 16 | 16 | 15 | 0.97 |
| Alcohol intake, gm/d | 8 | 10 | 13 | 9 | 0.84 |
| Total serum vitamin D | |||||
| 25-OH-vitamin D, ng/mL | 20.4 (7.6) | 25.7 (7.6) | 21.0 (8.3) | 20.9 (9.7) | 0.12 |
Data are given as means (SD) unless otherwise specified.
By Fisher’s exact χ2 test for categorical variables, and ANOVA for continuous variables.
Nonsteroidal anti-inflammatory drug.
At least once a week.
All nutrients energy adjusted using residual method.
Diet plus supplements.
Adherence to visit attendance averaged 92% and did not significantly differ among the four treatment groups. On average, at least 80% of pills were taken by 93% of participants at the first follow-up visit and by 84% of participants at the final follow-up visit. No adverse events were attributed to study procedures or treatments. Seven participants (8%) were lost to follow-up. Dropouts included one person from the vitamin D3 supplementation group and two from each of the other three groups (18).
Baseline serum 25-OH-vitamin D and 1,25-(OH)2-vitamin D levels did not differ between the four treatment groups. At the conclusion of the study, serum 25-OH-vitamin D levels had increased 60% (p<0.0001) and 56% (p<0.0001) in the vitamin D3 and calcium/vitamin D3 groups, respectively, relative to placebo; however, mean serum 25-OH-vitamin D concentrations remained below 32 ng/ml in all treatment groups (18). There was no evidence of treatment effect modification by obesity status (body mass index ≥30).
APC
A graphical comparison of APC crypt expression distribution at baseline and 6-month follow-up indicated that APC expression decreased in approximately the lower 40% of the crypt and increased in the upper 60% of the crypt (Figure 2A). As shown in Table 2, following 6 months of treatment, APC expression increased in the vitamin D3 treatment group 25% (p=0.14) in the full length of crypts, 48% (p=0.03) in the upper 40% of crypts, 11% in the lower 60% of crypts (p=0.47), and 21% (p=0.01) in the ϕh of crypts, relative to the placebo group. In the calcium group APC expression decreased 2% (p=0.91) in the full length of crypts, increased 7% (p=0.66) in the upper 40% of crypts, decreased 10% (p=0.51) in the lower 60% of crypts, and increased 10% (p=0.12) in the ϕh of crypts, relative to the placebo group. APC expression tended to increase in the calcium/vitamin D3 less than in the vitamin D3 group, and these findings were not statistically significant (Table 2A).
Figure 2.
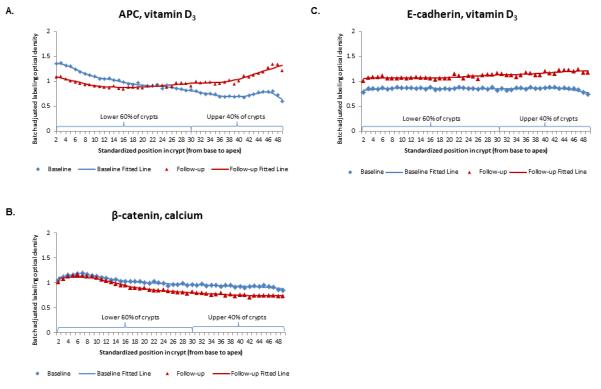
Representative examples of labeling expression distribution of (A) APC, (B) βcatenin, and (C) E-cadherin along normal colorectal crypts by treatment group at baseline and 6-month follow-up.
Table 2. Expression of APC, β-catenin, E-cadherin, and the APC/β-catenin scorea in the normal-appearing colorectal mucosa.
| Treatment Group |
Baseline |
6-mo follow-up |
Absolute Rx effect |
Relative Effectd |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Std Err | P | n | Mean | Std Err | P | Rx effectb | Std Err | P c | ||
| A. APC | ||||||||||||
| Whole crypts | ||||||||||||
| Placebo | 22 | 1.09 | 0.08 | 21 | 0.97 | 0.08 | 0.00 | . | . | 1.00 | ||
| Calcium | 23 | 1.07 | 0.08 | 0.90 | 21 | 0.94 | 0.08 | 0.80 | −0.02 | 0.15 | 0.91 | 0.98 |
| Vitamin D | 23 | 0.90 | 0.08 | 0.10 | 22 | 1.00 | 0.08 | 0.80 | 0.22 | 0.14 | 0.14 | 1.25 |
| Ca + Vit. D | 23 | 0.97 | 0.08 | 0.29 | 21 | 0.91 | 0.08 | 0.61 | 0.06 | 0.15 | 0.68 | 1.06 |
| Upper 40% of crypts | ||||||||||||
| Placebo | 22 | 0.39 | 0.04 | 21 | 0.41 | 0.04 | 0.00 | . | . | 1.00 | ||
| Calcium | 23 | 0.40 | 0.04 | 0.91 | 21 | 0.44 | 0.04 | 0.51 | 0.03 | 0.06 | 0.66 | 1.07 |
| Vitamin D | 23 | 0.30 | 0.04 | 0.07 | 22 | 0.46 | 0.04 | 0.31 | 0.15 | 0.06 | 0.03 | 1.48 |
| Ca + Vit. D | 23 | 0.36 | 0.04 | 0.47 | 21 | 0.41 | 0.04 | 0.39 | 0.04 | 0.06 | 0.58 | 1.10 |
| Lower 60% of crypts | ||||||||||||
| Placebo | 22 | 0.68 | 0.05 | 21 | 0.57 | 0.05 | 0.00 | . | . | 1.00 | ||
| Calcium | 23 | 0.70 | 0.05 | 0.81 | 21 | 0.53 | 0.05 | 0.53 | −0.06 | 0.09 | 0.51 | 0.90 |
| Vitamin D | 23 | 0.60 | 0.05 | 0.24 | 22 | 0.56 | 0.05 | 0.84 | 0.07 | 0.09 | 0.47 | 1.11 |
| Ca + Vit. D | 23 | 0.60 | 0.05 | 0.24 | 21 | 0.52 | 0.05 | 0.49 | 0.03 | 0.09 | 0.73 | 1.04 |
| ϕh e | ||||||||||||
| Placebo | 22 | 0.35 | 0.01 | 21 | 0.42 | 0.01 | 0.00 | . | . | 1.00 | ||
| Calcium | 23 | 0.34 | 0.01 | 0.06 | 21 | 0.46 | 0.01 | 0.06 | 0.04 | 0.03 | 0.12 | 1.10 |
| Vitamin D | 23 | 0.31 | 0.01 | 0.10 | 22 | 0.46 | 0.01 | 0.05 | 0.07 | 0.03 | 0.01 | 1.21 |
| Ca + Vit. D | 23 | 0.35 | 0.01 | 0.86 | 21 | 0.44 | 0.01 | 0.30 | 0.01 | 0.03 | 0.64 | 1.03 |
| B. β-catenin | ||||||||||||
| Whole crypts | ||||||||||||
| Placebo | 21 | 0.99 | 0.04 | 18 | 0.98 | 0.04 | 0.00 | . | . | 1.00 | ||
| Calcium | 22 | 1.01 | 0.04 | 0.69 | 19 | 0.85 | 0.05 | 0.04 | −0.15 | 0.08 | 0.08 | 0.85 |
| Vitamin D | 21 | 0.94 | 0.04 | 0.45 | 21 | 0.82 | 0.04 | 0.03 | −0.11 | 0.08 | 0.18 | 0.88 |
| Ca + Vit. D | 22 | 1.04 | 0.04 | 0.38 | 17 | 0.92 | 0.05 | 0.37 | −0.11 | 0.08 | 0.20 | 0.89 |
| Upper 40% of crypts | ||||||||||||
| Placebo | 21 | 0.38 | 0.02 | 18 | 0.37 | 0.02 | 0.00 | . | . | 1.00 | ||
| Calcium | 22 | 0.40 | 0.02 | 0.52 | 19 | 0.33 | 0.02 | 0.07 | −0.06 | 0.03 | 0.06 | 0.85 |
| Vitamin D | 21 | 0.36 | 0.02 | 0.36 | 21 | 0.33 | 0.02 | 0.12 | −0.02 | 0.03 | 0.61 | 0.95 |
| Ca + Vit. D | 22 | 0.39 | 0.02 | 0.69 | 17 | 0.36 | 0.02 | 0.61 | −0.02 | 0.03 | 0.50 | 0.94 |
| Lower 60% of crypts | ||||||||||||
| Placebo | 21 | 0.61 | 0.02 | 18 | 0.62 | 0.03 | 0.00 | . | . | 1.00 | ||
| Calcium | 22 | 0.63 | 0.02 | 0.44 | 19 | 0.56 | 0.02 | 0.11 | −0.08 | 0.05 | 0.09 | 0.87 |
| Vitamin D | 21 | 0.58 | 0.02 | 0.38 | 21 | 0.54 | 0.02 | 0.02 | −0.05 | 0.05 | 0.27 | 0.91 |
| Ca + Vit. D | 22 | 0.63 | 0.02 | 0.58 | 17 | 0.59 | 0.03 | 0.46 | −0.05 | 0.05 | 0.36 | 0.93 |
| ϕh e | ||||||||||||
| Placebo | 21 | 0.39 | 0.01 | 18 | 0.38 | 0.01 | 0.00 | . | . | 1.00 | ||
| Calcium | 22 | 0.38 | 0.01 | 0.87 | 19 | 0.36 | 0.01 | 0.16 | −0.01 | 0.01 | 0.31 | 0.97 |
| Vitamin D | 21 | 0.39 | 0.01 | 0.94 | 21 | 0.38 | 0.01 | 0.81 | 0.00 | 0.01 | 0.80 | 1.01 |
| Ca + Vit. D | 22 | 0.38 | 0.01 | 0.88 | 17 | 0.37 | 0.01 | 0.67 | 0.00 | 0.01 | 0.81 | 0.99 |
| C. E-cadherin | ||||||||||||
| Whole crypts | ||||||||||||
| Placebo | 21 | 1.13 | 0.12 | 19 | 1.04 | 0.13 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 1.23 | 0.12 | 0.69 | 21 | 1.09 | 0.12 | 0.78 | −0.05 | 0.25 | 0.85 | 0.96 |
| Vitamin D | 21 | 0.82 | 0.12 | 0.05 | 18 | 1.29 | 0.13 | 0.17 | 0.56 | 0.25 | 0.03 | 1.72 |
| Ca + Vit. D | 22 | 0.91 | 0.12 | 0.14 | 19 | 1.26 | 0.13 | 0.23 | 0.46 | 0.25 | 0.08 | 1.51 |
| Upper 40% of crypts | ||||||||||||
| Placebo | 21 | 0.44 | 0.05 | 19 | 0.42 | 0.05 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 0.47 | 0.05 | 0.70 | 21 | 0.45 | 0.05 | 0.70 | 0.00 | 0.10 | 0.99 | 1.01 |
| Vitamin D | 21 | 0.33 | 0.05 | 0.09 | 18 | 0.56 | 0.05 | 0.06 | 0.25 | 0.10 | 0.02 | 1.78 |
| Ca + Vit. D | 22 | 0.37 | 0.05 | 0.26 | 19 | 0.55 | 0.05 | 0.09 | 0.20 | 0.10 | 0.05 | 1.56 |
| Lower 60% of crypts | ||||||||||||
| Placebo | 21 | 0.64 | 0.07 | 19 | 0.60 | 0.08 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 0.69 | 0.07 | 0.68 | 21 | 0.63 | 0.07 | 0.78 | −0.01 | 0.15 | 0.93 | 0.98 |
| Vitamin D | 21 | 0.48 | 0.07 | 0.12 | 18 | 0.75 | 0.08 | 0.19 | 0.31 | 0.16 | 0.05 | 1.68 |
| Ca + Vit. D | 22 | 0.55 | 0.07 | 0.38 | 19 | 0.70 | 0.08 | 0.39 | 0.19 | 0.15 | 0.23 | 1.35 |
| ϕh e | ||||||||||||
| Placebo | 21 | 0.41 | 0.02 | 19 | 0.40 | 0.02 | 0.00 | . | . | 1.00 | ||
| Calcium | 21 | 0.41 | 0.02 | 0.92 | 21 | 0.41 | 0.02 | 0.66 | 0.01 | 0.03 | 0.71 | 1.03 |
| Vitamin D | 21 | 0.40 | 0.02 | 0.61 | 18 | 0.45 | 0.02 | 0.06 | 0.05 | 0.03 | 0.10 | 1.14 |
| Ca + Vit. D | 22 | 0.40 | 0.02 | 0.60 | 19 | 0.46 | 0.02 | 0.01 | 0.07 | 0.03 | 0.03 | 1.18 |
| D. APC/β-catenin Score | ||||||||||||
| Placebo | 21 | 0.37 | 0.03 | 18 | 0.44 | 0.03 | 0.00 | . | . | 1.00 | ||
| Calcium | 22 | 0.35 | 0.03 | 0.49 | 18 | 0.58 | 0.03 | 0.003 | 0.16 | 0.06 | 0.01 | 1.41 |
| Vitamin D | 21 | 0.36 | 0.03 | 0.83 | 20 | 0.57 | 0.03 | 0.004 | 0.13 | 0.06 | 0.02 | 1.31 |
| Ca + Vit. D | 22 | 0.37 | 0.03 | 0.84 | 17 | 0.50 | 0.03 | 0.18 | 0.07 | 0.06 | 0.26 | 1.16 |
APC/β-catenin score = ϕh APC/β-catenin expression in the whole crypt, where ϕh APC = ratio of APC expression in the upper 40% of the crypts to the whole crypt.
Rx effect (treatment effect) = [(treatment group follow-up) − (treatment group baseline)] − [(placebo group follow-up) − (placebo group baseline)].
P value for difference between each active treatment group and placebo group from repeated-measures MIXED model.
Relative effect = [(treatment group follow-up)/(treatment group baseline)]/[(placebo follow-up)/(placebo baseline)]; interpretation similar to that for an odds ratio (e.g., a relative effect of 1.7 indicates a 70% proportional increase in the treatment group relative to that in the placebo group).
ϕh = proportion of expression in the distribution zone (i.e. ratio of expression in upper 40% to expression in whole crypt)
β-catenin
A graphical evaluation of β-catenin crypt expression distribution at baseline and 6-month follow-up indicated that β-catenin expression did not change in approximately the lower 20% of the crypt, but steadily decreased towards the crypt apex (Figure 2B). As shown in Table 2, following 6 months of treatment, β-catenin expression decreased along the full length of crypts by 15% (p=0.08), 12% (p=0.18), and 11% (p=0.20) in the calcium, vitamin D3 and calcium/vitamin D3 groups, respectively, relative to the placebo group. The findings in the upper 40% and lower 60% of crypts did not appreciably differ from those observed in the full length of crypts. There were no apparent treatment effects on β-catenin expression in the ϕh of crypts.
E-cadherin
A graphical evaluation of E-cadherin crypt expression distribution at baseline and 6-month follow-up indicated that E-cadherin expression uniformly increased along the full length of the crypt (Figure 2C). As shown in Table 2, following 6 months of treatment, E-cadherin expression increased in the vitamin D3 group 72% (p=0.03) in the full length of crypts, 78% (p=0.02) in the upper 40% of crypts, 68% (p=0.05) in the lower 60% of crypts, and 14% (p=0.10) in the ϕh of crypts. E-cadherin expression also increased in the calcium/vitamin D3 group, but less so than in the vitamin D3 group, except in the ϕh of crypts where E-cadherin expression increased 18% (p=0.03). In the calcium group E-cadherin did not appreciably change relative to the placebo group (Table 2C).
APC/β-catenin score
The APC/β-catenin score increased 41% (p=0.01), 31% (p=0.02), and 16% (p=0.26) in the calcium, vitamin D3, and calcium/vitamin D3 groups, respectively, relative to the placebo group (Table 2D).
Sensitivity analysis
There were no apparent differences in findings following imputation of missing observations (Supplemental material Table 1).
Discussion
The results of this pilot, randomized, placebo-controlled clinical trial provides the first human in vivo evidence that supplemental calcium and vitamin D3, alone or in combination, may increase APC and E-cadherin expression and the APC/β-catenin score and decrease β-catenin expression in the normal colorectal mucosa of sporadic adenoma patients. These finding support the hypothesis that the anti-carcinogenic effects calcium and vitamin D, alone or in combination, may in part operate by modifying the APC/β-catenin signaling pathway. These findings are relevant because, in light of our previous report of differences in APC and β-catenin expression between persons with incident sporadic adenomas and persons with no past or current adenomas, they 1) provide further support that APC and β-catenin expression and the APC/β-catenin score in the normal colorectal mucosa may be modifiable, pre-neoplastic biomarkers of risk for colorectal adenomas, and 2) provide human in vivo mechanistic evidence of the possible protective effects of calcium and vitamin D3 against colorectal neoplasms.
APC and β-catenin are appealing candidates for being pre-neoplastic biomarkers of risk because malfunctioning of the APC/β-catenin signaling pathway is a common and early event in the colorectal neoplastic transition (5). In normal colorectal mucosa APC, axin, glycogen synthase kinase 3, and casein kinase negatively regulate Wnt signaling by forming the “β-catenin destruction” complex, and, in the absence of Wnt signaling, phosphorylate and promote the degradation of free β-catenin (5). Normal functioning of the APC gene is inhibited in approximately 80-90% of sporadic CRC, resulting in increased potential for β-catenin to translocate to the nucleus and activate Wnt target genes (5). In normal colorectal mucosa APC, β-catenin, and E-cadherin are all strongly expressed—APC primarily in the cytoplasm, and E-cadherin and β-catenin primarily at the cell membrane. During the adenoma-carcinoma sequence APC and E-cadherin expression markedly decrease (although the decrease in E-cadherin tends to occur in later stages) (25-27), and β-catenin expression appears to steadily increase and translocate from the membrane to the cytoplasm and eventually into the nucleus (25, 28). We previously proposed that the APC/β-catenin score may represent the potential of β-catenin to translocate to the nucleus and promote proliferative signaling (8). We found the APC/β-catenin score in the normal colorectal mucosa of sporadic colorectal adenoma patients to be statistically significantly lower than in the normal colorectal mucosa of healthy controls; and that ϕh APC and β-catenin expression and the APC/β-catenin score may be modifiable as suggested by their being associated with lifestyle and dietary risk factors for colorectal neoplasms (8).
The etiology of CRC is heavily influenced by modifiable dietary and lifestyle behaviors. Dietary-induced epigenetic modifications to the APC/β-catenin signaling pathway may initiate or be a “second hit” in the adenoma-carcinoma pathway. Calcium and vitamin D are two promising chemopreventive agents that may act against colorectal neoplasms; however, the mechanisms by which they operate are not entirely clear (9). Colorectal cancer cell line studies suggest that calcium and 1,25(OH)2D up-regulate E-cadherin expression and promote the translocation of β-catenin from the nucleus and cytoplasm to the plasma membrane (15-17, 29). Mice fed a diet comparable to a typical “Western” diet had increased β-catenin and Tcf gene expression and decreased APC gene expression; however, supplementation of the “Western” diet with increased dietary calcium and vitamin D decreased β-catenin and Tcf gene expression, but had no apparent effect on APC gene expression (13). In a transmissible murine colonic hyperplasia model high dietary calcium modestly reduced total β-catenin expression (14). A diet supplemented with the vitamin D analog 1α(OH)D5 inhibited β-catenin nuclear expression in azoxymethane-treated mice (30), and endometrial E-cadherin expression increased in mice fed a diet high in vitamin D3 (31). Investigations of the in vivo effects of calcium and/or vitamin D on APC expression are limited (13), but dietary modification of APC expression is supported by reports that diets moderately deficient in B-vitamin methyl donors reduced APC expression and increased β-catenin/Tcf signaling in rodents (32, 33).
Our results are consistent with the hypothesis that calcium and vitamin D reduce cell proliferation and promote differentiation in the colorectal mucosa. To our knowledge this is the first human in vivo study to suggest that calcium and/or vitamin D3 may affect APC expression in the normal colorectal mucosa; however, the mechanism by which calcium and/or vitamin D3 may modify APC expression remains unclear. We did not evaluate β-catenin localization, but consistent with the observed increase in APC expression, particularly ϕh APC, we observed decreased β-catenin expression and an increased APC/β-catenin score in all three active treatment groups, suggesting that calcium and vitamin D3 treatment may decrease the potential of β-catenin to promote proliferative signaling. These results are in line with our reports that suggested that calcium and/or vitamin D3 treatment reduced hTERT expression (a potential cofactor in the β-catenin transcriptional complex (34)) and increased p21 expression (which is negatively regulated by β-catenin/TCF signaling (35)) (36). These results also corroborate our report that the APC/β-catenin score may be a valid, potentially modifiable, pre-neoplastic biomarker of risk for colorectal neoplasms, providing in vivo evidence that supplemental calcium and/or vitamin D3 may promote a greater APC/β-catenin score in the normal rectal mucosa.
Conflicting with previous in vitro studies (15, 16) we did not observe increased E-cadherin expression in the calcium group. The explanation for this lack of consistency in findings is not clear and may be a chance finding; however, the difference may be attributed to our investigation evaluating the effects of calcium on E-cadherin expression in the normal mucosa of free living humans rather than in vitro, or that the ability of calcium to increase E-cadherin expression may be limited to neoplastic mucosa.
Contrary to our hypothesis and what has been reported in some studies (10, 37), the estimated treatment effect of calcium plus vitamin D3 was not greater than that of either the calcium or vitamin D3 alone in increasing ϕh APC, E-cadherin, or the APC/β-catenin score, or in decreasing β-catenin. We previously reported that calcium combined with vitamin D3 may mitigate treatment effects of calcium and vitamin D3 alone on colorectal mucosa markers of apoptosis and differentiation (18, 36). There are several plausible explanations for why this was observed, the first being that these could have been chance findings given the small sample size of our study. At least one study reported that calcium and vitamin D individually suppressed tumorigenesis in rodents, but the combination of the two was ineffective (38). We previously observed that calcium combined with vitamin D3 increased CYP24A1 expression (39), which may reduce the effects of 1,25(OH)2D in the colorectal mucosa. A large clinical trial of colorectal adenoma recurrence suggested that calcium supplementation was primarily effective among people with 25(OH)D concentrations greater than the median in the study population (29.1 ng/ml) (10). In our study population only participants in the vitamin D3 group reached 25(OH)D concentrations greater than 29.1 ng/ml (18), suggesting the possibility of a threshold effect.
We previously reported that calcium and vitamin D3 supplementation in this same trial favorably modified the expression of markers of calcium and vitamin D metabolism (39), proliferation (36), differentiation (36), apoptosis (18), mismatch repair (40), and oxidative DNA damage (41) in the normal human colorectal mucosa. Our current results, taken together with our previous findings, support the hypothesized effects of calcium and vitamin D on favorably modulating the molecular phenotype of the normal colorectal mucosa and reducing risk for colorectal neoplasms.
Our study had several limitations. First, it was a pilot study with a relatively small sample size, increasing the role of chance observations and limiting our ability to perform stratified analyses. We were unable to evaluate β-catenin sub-cellular localization; however, our previous findings (8) suggested that sporadic colorectal adenoma cases relative to normal controls may have greater total β-catenin expression in the normal colorectal mucosa. We propose that the APC/β-catenin score may represent the potential of β-catenin to promote proliferative signaling, and needs to be investigated in basic science studies. Also, we only examined the rectal mucosa and therefore treatment effects in other parts of the colon remain unknown. Another limitation is that we measured protein expression but not protein activity, and, therefore, could not correlate changes in expression with changes in protein activity. Finally, these markers are not proven biomarkers of risk; however, evidence from our pilot case-control study suggests that APC, β-catenin expression, and the APC/β-catenin score may be pre-neoplastic biomarkers of risk.
The strengths of this study include 1) that it is, to our knowledge, the first randomized, double-blind, placebo-controlled clinical trial to test the effects of supplemental calcium and vitamin D3, alone and in combination, on components of the APC/β-catenin signaling pathway in the normal colorectal epithelium in sporadic adenoma patients, 2) the high protocol adherence by study participants, and 3) the automated immunostaining and newly-designed image analysis software to quantify the crypt distribution of the expression of APC, β-catenin, and E-cadherin, resulting in high biomarker measurement reliability.
In summary, the results of this pilot, randomized, placebo-controlled clinical-trial provide human in vivo evidence that supplemental calcium and vitamin D3, alone and in combination, may increase APC and E-cadherin expression and the APC/β-catenin score and decrease β-catenin expression in the normal colorectal mucosa of sporadic colorectal adenoma cases. These results suggest that the anti-carcinogenetic effects of supplemental calcium and vitamin D3 may, in part, depend on the ability of these agents to favorably modulate the expression of APC, β-catenin, and E-cadherin and thus, possibly, inhibit proliferative β-catenin signaling. Taken together with our previous findings, APC (especially ϕh APC) and β-catenin expression, the APC/β-catenin score, and E-cadherin may be modifiable, pre-neoplastic biomarkers of risk for colorectal neoplasms and warrant further investigation. Finally, our results support further investigation of calcium and vitamin D3 as chemopreventive agents against colorectal neoplasms.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute, National Institutes of Health (R01 CA104637 and R03 CA115230 to R.M.B.); Georgia Cancer Coalition Distinguished Scholar award (to R.M.B.); the Franklin Foundation. The National Cancer Institute, the Georgia Cancer Coalition, and the Franklin Foundation had no influence on the design of the study; the collection, analysis, and interpretation of the data; the decision to submit the manuscript for publication; or the writing of the manuscript.
Footnotes
Conflict of Interest: The authors disclose no potential conflicts of interest
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- 3.Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Potter JD. Colorectal Cancer: Molecules and Populations. J Natl Cancer Inst. 1999;91:916–32. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 5.Goss KH, Groden J. Biology of the Adenomatous Polyposis Coli Tumor Suppressor. Journal of Clinical Oncology. 2000;18:1967–79. doi: 10.1200/JCO.2000.18.9.1967. [DOI] [PubMed] [Google Scholar]
- 6.Markowitz SD, Bertagnolli MM. Molecular Basis of Colorectal Cancer. New England Journal of Medicine. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willert K, Jones KA. Wnt signaling: is the party in the nucleus? Genes & Development. 2006;20:1394–404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 8.Ahearn TU, Shaukat A, Flanders WD, Seabrook ME, Bostick RM. Markers of the APC/β-Catenin Signaling Pathway as Potential Treatable, Preneoplastic Biomarkers of Risk for Colorectal Neoplasms. Cancer Epidemiology Biomarkers & Prevention. 2012;21:969–79. doi: 10.1158/1055-9965.EPI-12-0126. [DOI] [PubMed] [Google Scholar]
- 9.Bostick RM, Goodman M, Sidelnikov E. Calcium and Vitamin D. Springer Science + Business Media, LLC; New York: 2009. [Google Scholar]
- 10.Grau MV, Baron JA, Sandler RS, Haile RW, Beach ML, Church TR, et al. Vitamin D, Calcium Supplementation, and Colorectal Adenomas: Results of a Randomized Trial. J Natl Cancer Inst. 2003;95:1765–71. doi: 10.1093/jnci/djg110. [DOI] [PubMed] [Google Scholar]
- 11.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal Vitamin D Status for Colorectal Cancer Prevention: A Quantitative Meta Analysis. American Journal of Preventive Medicine. 2007;32:210–6. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Fedirko V, Bostick RM, Goodman M, Flanders WD, Gross MD. Blood 25-Hydroxyvitamin D3 Concentrations and Incident Sporadic Colorectal Adenoma Risk: A Pooled Case-Control Study. Am J Epidemiol. 2010 doi: 10.1093/aje/kwq157. kwq157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, et al. Dietary Induction of Colonic Tumors in a Mouse Model of Sporadic Colon Cancer. Cancer Research. 2008;68:7803–10. doi: 10.1158/0008-5472.CAN-08-1209. [DOI] [PubMed] [Google Scholar]
- 14.Umar S, Morris AP, Kourouma F, Sellin JH. Dietary pectin and calcium inhibit colonic proliferation in vivo by differing mechanisms. Cell Proliferation. 2003;36:361–75. doi: 10.1046/j.1365-2184.2003.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhagavathula N, Hanosh AW, Nerusu KC, Appelman H, Chakrabarty S, Varani J. Regulation of E-cadherin and β-catenin by Ca2+ in colon carcinoma is dependent on calcium-sensing receptor expression and function. International Journal of Cancer. 2007;121:1455–62. doi: 10.1002/ijc.22858. [DOI] [PubMed] [Google Scholar]
- 16.Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular Calcium and Calcium Sensing Receptor Function in Human Colon Carcinomas: Promotion of E-Cadherin Expression and Suppression of {beta}-Catenin/TCF Activation. Cancer Res. 2003;63:67–71. [PubMed] [Google Scholar]
- 17.Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium Sensing Receptor in Human Colon Carcinoma: Interaction with Ca2+ and 1,25-Dihydroxyvitamin D3. Cancer Res. 2005;65:493–8. [PubMed] [Google Scholar]
- 18.Fedirko V, Bostick RM, Flanders WD, Long Q, Shaukat A, Rutherford RE, et al. Effects of Vitamin D and Calcium Supplementation on Markers of Apoptosis in Normal Colon Mucosa: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cancer Prev Res. 2009;2:213–23. doi: 10.1158/1940-6207.CAPR-08-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu G, Hu X, Chakrabarty S. Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner: Downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU. International Journal of Cancer. 2010;126:631–9. doi: 10.1002/ijc.24762. [DOI] [PubMed] [Google Scholar]
- 20.Bostick RM, Fosdick L, Lillemoe TJ, Overn P, Wood JR, Grambsch P, et al. Methodological findings and considerations in measuring colorectal epithelial cell proliferation in humans. Cancer Epidemiology Biomarkers & Prevention. 1997;6:931–42. [PubMed] [Google Scholar]
- 21.Hollis BW. Quantitation of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D by radioimmunoassay using radioiodinated tracers. Methods Enzymol. 1997;282:174–86. doi: 10.1016/s0076-6879(97)82106-4. [DOI] [PubMed] [Google Scholar]
- 22.Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL. Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem. 1996;42:586–92. [PubMed] [Google Scholar]
- 23.Elzagheid A, Algars A, Bendardaf R, Lamlum H, Ristamaki R, Collan Y, et al. E-cadherin expression pattern in primary colorectal carcinomas and their metastases reflects disease outcome. World J Gastroenterol. 2006;12:4304–9. doi: 10.3748/wjg.v12.i27.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schafer JL. Analysis of Incomplete Multivariate Data. Chapman and Hall/CRC; New York: 1997. [Google Scholar]
- 25.Iwamoto M, Ahnen DJ, Franklin WA, Maltzman TH. Expression of β-catenin and full-length APC protein in normal and neoplastic colonic tissues. Carcinogenesis. 2000;21:1935–40. doi: 10.1093/carcin/21.11.1935. [DOI] [PubMed] [Google Scholar]
- 26.Day RM, Hao X, Ilyas M, Daszak P, Talbot IC, Forbes A. Changes in the expression of syndecan-1 in the colorectal adenoma-carcinoma sequence. Virchows Archiv. 1999;434:121–5. doi: 10.1007/s004280050315. [DOI] [PubMed] [Google Scholar]
- 27.Pap Z, Pávai Z, Dénes L, Kovalszky I, Jung J. An Immunohistochemical Study of Colon Adenomas and Carcinomas: E-cadherin, Syndecan-1, Ets-1. Pathology & Oncology Research. 2009 doi: 10.1007/s12253-009-9157-x. [DOI] [PubMed] [Google Scholar]
- 28.Mi B, Wang X, Bai Y, Gong W, Geng Y, Wang J, et al. Beta-catenin expression is altered in dysplastic and nondysplastic aberrant crypt foci of human colon. Appl Immunohistochem Mol Morphol. 2009;17:294–300. doi: 10.1097/PAI.0b013e318194525c. [DOI] [PubMed] [Google Scholar]
- 29.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J Cell Biol. 2001;154:369–88. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murillo G, Mehta RG. Chemoprevention of chemically-induced mammary and colon carcinogenesis by 1α-hydroxyvitamin D5. The Journal of Steroid Biochemistry and Molecular Biology. 2005;97:129–36. doi: 10.1016/j.jsbmb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 31.Yu W, Cline M, Maxwell LG, Berrigan D, Rodriguez G, Warri A, et al. Dietary Vitamin D Exposure Prevents Obesity-Induced Increase in Endometrial Cancer in Pten+/− Mice. Cancer Prevention Research. 2010;3:1246–58. doi: 10.1158/1940-6207.CAPR-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Choi S-W, Crott JW, Keyes MK, Jang H, Smith DE, et al. Mild Depletion of Dietary Folate Combined with Other B Vitamins Alters Multiple Components of the Wnt Pathway in Mouse Colon. The Journal of Nutrition. 2007;137:2701–8. doi: 10.1093/jn/137.12.2701. [DOI] [PubMed] [Google Scholar]
- 33.Liu Z, Ciappio ED, Crott JW, Brooks RS, Nesvet J, Smith DE, et al. Combined inadequacies of multiple B vitamins amplify colonic Wnt signaling and promote intestinal tumorigenesis in BAT-LacZ×Apc1638N mice. The FASEB Journal. 2011;25:3136–45. doi: 10.1096/fj.11-184143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park J-I, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, et al. The β-Catenin/TCF-4 Complex Imposes a Crypt Progenitor Phenotype on Colorectal Cancer Cells. Cell. 2002;111:241–50. doi: 10.1016/s0092-8674(02)01014-0. [DOI] [PubMed] [Google Scholar]
- 36.Fedirko V, Bostick RM, Flanders WD, Long Q, Sidelnikov E, Shaukat A, et al. Effects of Vitamin D and Calcium on Proliferation and Differentiation In Normal Colon Mucosa: a Randomized Clinical Trial. Cancer Epidemiology Biomarkers & Prevention. 2009;18:2933–41. doi: 10.1158/1055-9965.EPI-09-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. The American Journal of Clinical Nutrition. 2007;85:1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 38.Pence BC, Buddingh F. Inhibition of dietary fat-promoted colon carcinogenesis in rats by supplemental calcium or vitamin D3. Carcinogenesis. 1988;9:187–90. doi: 10.1093/carcin/9.1.187. [DOI] [PubMed] [Google Scholar]
- 39.Ahearn TU, McCullough ML, Flanders WD, Long Q, Sidelnikov E, Fedirko V, et al. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on markers of their metabolism in normal mucosa of colorectal adenoma patients. Cancer Res. 2011;71:413–23. doi: 10.1158/0008-5472.CAN-10-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sidelnikov E, Bostick RM, Flanders WD, Long Q, Fedirko V, Shaukat A, et al. Effects of calcium and vitamin D on MLH1 and MSH2 expression in rectal mucosa of sporadic colorectal adenoma patients. Cancer Epidemiol Biomarkers Prev. 2010;19:1022–32. doi: 10.1158/1055-9965.EPI-09-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedirko V, Bostick RM, Long Q, Flanders WD, McCullough ML, Sidelnikov E, et al. Effects of supplemental vitamin D and calcium on oxidative DNA damage marker in normal colorectal mucosa: a randomized clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19:280–91. doi: 10.1158/1055-9965.EPI-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


